Abstract
Fanconi syndrome results from a generalized abnormality of the proximal tubules of the kidney and owing to phosphate depletion can cause hypophosphatemic osteomalacia. Adefovir dipivoxyl (ADV) effectively suppresses hepatitis B virus replication but exhibits nephrotoxicity when administered at a low dosage. We report two cases of Fanconi syndrome induced by ADV at 10 mg/day to call for regular screening for evidence of proximal tubular dysfunction and detailed bone metabolic investigations for prompt detection of ADV nephrotoxicity is critically important to ensure timely drug withdrawal before the development of irreversible tubulointerstitial injury.
Keywords: Adefovir, Fanconi syndrome, hypophosphatemia, nephrotoxicity
INTRODUCTION
Fanconi syndrome results from generalized dysfunction of the proximal renal tubule leading to impaired reabsorption defects in amino acids, glucose, sodium, potassium, bicarbonate, and phosphorus.[1] Phosphate wasting owing to chronic renal loss and the inadequate synthesis of 1,25(OH)2 Vitamin D together produce hypophosphatemia, glycosuria with normal glucose level, aminoaciduria, hypouricemia, and hypokalemia. The symptoms include fatigue, muscle weakness, bone pain, fracture, and bone deformity.[2] Numerous drugs are associated with the development of acquired Fanconi syndrome including antiretrovirals, aminoglycosides, and salicylates.[3]
Recently, the nephrotoxicity of adefovir dipivoxyl (ADV), including Fanconi syndrome, secondary to antiretroviral therapy in HIV patients was reported.[4] ADV is excreted unchanged in the urine through glomerular filtration, and tubular secretion and administration of 60 mg daily and above have been associated with nephrotoxicity.[5] But, as a potent nucleotide analog against both the wild-type and lamivudine (LAM)-resistant hepatitis B virus (HBV),[6,7] ADV has been found nephrotoxicity at a 10 mg daily dose in HBV patients.[8,9,10] Thus, the renal safety of ADV attract new concern.
Here, we diagnosed acquired Fanconi syndrome conjunction with severe hypophosphatemic osteomalacia in 2 chronic hepatitis B-positive patients on prolonged low-dose (10 mg daily) ADV therapy.
CASE REPORTS
Case 1
The first patient was a 56-year-old male with a 1-year history of progressive bone pain involving his knees, ankles, lower back and ribs with difficulty in walking. He had a 7-year history of chronic hepatitis caused by HBV infection and had received LAM therapy for 2-year. Because the virus developed resistance to LAM, he had been receiving adefovir at a daily dose of 10 mg/day for 60 months before the development of these symptoms. The patient was not previously on any medication or herbal remedy known to affect skeletal health or result in nephrotoxicity. The laboratory results showed the feature of proximal renal tubule dysfunction, particularly severe hypophosphatemia [Table 1]. Bone scintigraphy revealed multifocal lesions including multiple ribs, costochondral junctions, costovertebral junctions, sacrum, both posterior iliac bones, both proximal tibia, right calcaneus, and the left second metatarsophalangeal joint area, which were suggestive of metabolic bone disorder (99mTc-hydroxymethylene diphosphonate whole-body bone scintigraphy showed multiple foci of increased radiotracer uptake in the thoracic spine, the sacroiliac region, the rib cage, the shoulders, the knees, and the ankles) [Figure 1]. Bailing capsule, a kind of cordyceps sinensis preparation was used to improve the kidney function. The patient refused phosphate supplementation for personal reason. Even so bone pain was significantly reduced, and laboratory findings [Table 1] returned to normal within months of discontinuation of ADV and supplementation with calcium carbonate, and cholecalciferol.
Table 1.
Renal parameters at baseline and during follow-up
Figure 1.
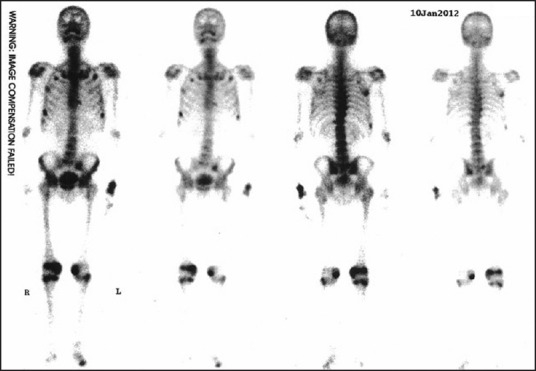
99mTc-hydroxymethylene diphosphonate scintigraphy demonstrates significant abnormal uptake in calvaria, maxilla, both scapulae, ribs
Case 2
The second patient was a 57-year-old male who presented with a 3-year history of generalized bone pain involving neck, shoulders, hip and knees, which resulted in an antalgic gait. He had a history of chronic hepatitis B-induced cirrhosis for which LAM 100 mg daily was commenced in 7-year before he was admitted into the hospital. ADV at a daily dose of 10 mg daily was added in owing to increasing serum aminotransferases and hepatitis B DNA levels, suggestive of LAM resistance. The patient had been receiving adefovir for 4 years before these symptoms come out. The patient was also not previously on any medication or herbal remedy known to affect skeletal health or result in nephrotoxicity. As presenting with diffuse musculoskeletal pain, the patient was first diagnosed as seronegative spondyloarthropathy in an outpatient department. After admitted into the hospital, hypophosphatemia and urinary phosphate wasting was confirmed by regular test. Laboratory investigation results on admission are included in Table 1. A diagnosis of hypophosphatemic osteomalacia in the context of Fanconi syndrome secondary to adefovir therapy was made. Dual energy X-ray absorptiometry and X-rays revealed diffused decrease in bone density. There was no clinical evidence of an infectious, inflammatory, or malignant process. Radiography showed ischemic necrosis of the femoral head on both sides (right, Garden III fracture; left, Garden III fracture) and femoral neck fractures [Figure 2a].[11] Magnetic resonance imaging of both hip joints showed fractures across the right and left femoral neck and bone edema, which had low-intensity on T1-weighted images and high-intensity on T2-weighted images [Figure 2b]. So the patient was diagnosed as Fanconi syndrome and pathological femoral neck fracture associated with osteomalacia induced by low-dose ADV treatment. Adefovir was, therefore, ceased and entecavir commenced at a daily dose of 1 mg. The symptoms were improved by supplementation with elemental phosphate, calcium carbonate, calcitriol and bailing capsule after several months. But there was no significant improvement in kidney function [Table 1].
Figure 2.
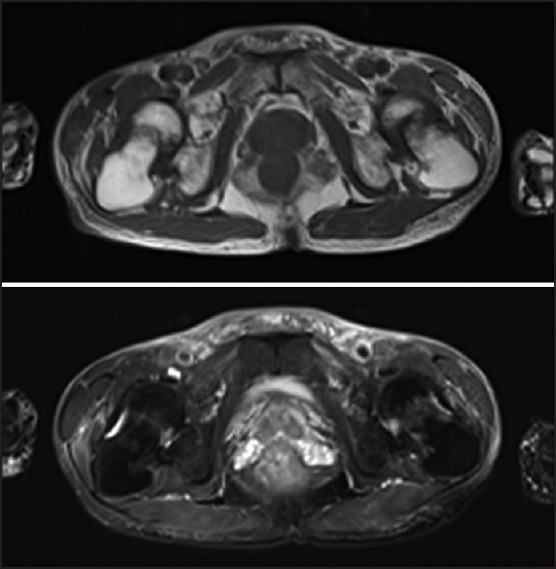
Transverse section T1-weighted image demonstrates low-intensity femoral neck fractures and the T2-weighted image shows high-intensity bone edema in both femoral necks
DISSCUSSION
As for these two patients, with normal levels of 1,25-dihydroxyvitamin D3 and urinalysis and 24 h urine collection confirmed phosphate wasting, we considered that the impaired phosphate reabsorption could have been caused by dysfunction of the proximal renal tubule dysfunction, not by deficiency of vitamin D. Increased levels of potassium, glucose, N-acetylglucosaminidase and β2-microglobulin of urine samples collected over 24 h also supported our conclusion. ADV was considered the most likely culprit and was replaced by the entecavir. The quick resolution of serum phosphate levels suggests a close association between ADV and tubular kidney function.
The mechanism of action has not been clearly identified. All nucleoside analogs have the potential ability of inhibition of human DNA polymerase gamma involved in mitochondrial DNA replication, which can lead to varying clinical manifestations of mitochondrial toxicity.[12] The pathophysiology of adefovir-related nephrotoxicity is multifactorial, initially dependent upon its entry into renal tubular cells through the human renal organic anion transporter-I (hOAT-1).[13,14] The importance of the hOAT-1 transporter was shown by a reduction in adefovir nephrotoxicity through its inhibition in patients on nonsteroidal anti-inflammatory agents.[14]
Adefovir was initially developed as an antiretroviral agent for HIV but was abandoned due to the high rate of nephrotoxicity with higher doses. But the side effect is rarely reported at a dose of 10 mg daily, which was approved for the treatment of chronic hepatitis B in 2002. Although these two patients had taken lamivudin for several years, which also has nephrotoxicity, both of them developed typical symptoms of osteomalacia several year after they cease the suspicious drug. So we concluded the acquired Fanconi's syndrome was secondary to adefovir therapy. Among the nucleoside analogs approved for use in hepatitis B, entecavir, with a favorable safety profile and low incidence of resistance,[15] has demonstrated little evidence of mitochondrial toxicity in humans.[13] So we prescribe entecavir instead for HBV therapy. It's worth noting that among all the nephrotoxicity cases caused by adefovir were almost middle or old people and predominantly male, we conjecture host cofactors such as age, gender, and medical comorbidities, may also influence the risk of nephrotoxicity.
The clinical pattern of renal toxicity is dose-dependent and usually presents as reversible proximal renal tubular toxicity, characterized by slight rises in serum creatinine levels and decreases in serum phosphate levels. For the first patient, he got complete remission both in symptom and laboratory indicators after the therapy is stopped rapidly. The second case who was delayed for misdiagnose as AS, and loss the opportunity of reversing. The agent not only caused frank renal insufficiency and troublesome renal tubular acidosis, but also hypophosphatemia, and femoral neck fractures occurred. We noted a distinct increase in serum bone-specific alkaline phosphatase levels in both patients during the weeks after adefovir cessation and the administration of supplemental phosphate and Vitamin D. This occurred in the absence of abnormalities in the other hepatic enzymes. We, therefore, interpret this change as a reflection of skeletal recovery in response to the removal of the nephrotoxin and the supplementation of phosphate, a crucial substrate for bone mineralization.
Despite large clinical trials advocating the safety of ADV at 10 mg daily, long-term use of this agent may be nephrotoxic in rare cases. To the best of our knowledge, there have been less than 10 reported cases of Fanconi syndrome with prolonged ADV use, even less has secondary necrosis of the femoral head. The long-term consequences of proximal tubular dysfunction in patients treated with ADV deserve further study, and there is increasing evidence that ADV is associated with Fanconi syndrome. As they can be initially clinically silent yet lead to serious medical problems. Because osteomalacia often presents with diffuse bone pain, the disease can be confused with various musculoskeletal diseases, clinicians prescribing this drug should be aware of this potential complication, so these patients should have regular monitoring of hypophosphatemia, hypoalbuminemia and proteinuria, including β2-microglubulin as an early indicator of tubulopathy.[2] A proactive surveillance and their prompt management are recommended in case of the development of irreversible tubulointerstitial injury and necrosis of the femoral head.
AUTHOR'S CONTRIBUTIONS
Xiao-Bing Wang contributed in the conception of the work, drafting and revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Xiao-Chun Zhu contributed in the conception of the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Xiao-Ying Huang contributed in the conception of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Wen-Jing Ye contributed in the analysis of data for the work, conducting the study, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
Liang-Xing Wang contributed in the conception and design of the work, revising the draft, approval of the final version of the manuscript, and agreed for all aspects of the work.
ACKNOWLEDGEMENTS
This study was supported by the Science and Technology Project of Wenzhou (Y20140048).
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Clarke BL, Wynne AG, Wilson DM, Fitzpatrick LA. Osteomalacia associated with adult Fanconi's syndrome: Clinical and diagnostic features. Clin Endocrinol (Oxf) 1995;43:479–90. doi: 10.1111/j.1365-2265.1995.tb02621.x. [DOI] [PubMed] [Google Scholar]
- 2.Sirac C, Bridoux F, Essig M, Devuyst O, Touchard G, Cogné M. Toward understanding renal Fanconi syndrome: Step by step advances through experimental models. Contrib Nephrol. 2011;169:247–61. doi: 10.1159/000313962. [DOI] [PubMed] [Google Scholar]
- 3.Choudhury D, Ahmed Z. Drug-associated renal dysfunction and injury. Nat Clin Pract Nephrol. 2006;2:80–91. doi: 10.1038/ncpneph0076. [DOI] [PubMed] [Google Scholar]
- 4.Earle KE, Seneviratne T, Shaker J, Shoback D. Fanconi's syndrome in HIV+ adults: Report of three cases and literature review. J Bone Miner Res. 2004;19:714–21. doi: 10.1359/jbmr.2004.19.5.714. [DOI] [PubMed] [Google Scholar]
- 5.Kahn J, Lagakos S, Wulfsohn M, Cherng D, Miller M, Cherrington J, et al. Efficacy and safety of adefovir dipivoxil with antiretroviral therapy: A randomized controlled trial. JAMA. 1999;282:2305–12. doi: 10.1001/jama.282.24.2305. [DOI] [PubMed] [Google Scholar]
- 6.Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, et al. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology. 2000;32:129–34. doi: 10.1053/jhep.2000.8626. [DOI] [PubMed] [Google Scholar]
- 7.Bárcena R, Del Campo S, Moraleda G, Casanovas T, Prieto M, Buti M, et al. Study on the efficacy and safety of adefovir dipivoxil treatment in post-liver transplant patients with hepatitis B virus infection and lamivudine-resistant hepatitis B virus. Transplant Proc. 2005;37:3960–2. doi: 10.1016/j.transproceed.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 8.Izzedine H, Kheder-Elfekih R, Housset P, Sarkozy C, Brocheriou I, Deray G. Adefovir dipivoxil-induced acute tubular necrosis and Fanconi syndrome in a renal transplant patient. AIDS. 2009;23:544–5. doi: 10.1097/QAD.0b013e32832407f7. [DOI] [PubMed] [Google Scholar]
- 9.Jung YK, Yeon JE, Choi JH, Kim CH, Jung ES, Kim JH, et al. Fanconi's syndrome associated with prolonged adefovir dipivoxil therapy in a hepatitis B virus patient. Gut Liver. 2010;4:389–93. doi: 10.5009/gnl.2010.4.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Minemura M, Tokimitsu Y, Tajiri K, Nakayama Y, Kawai K, Kudo H, et al. Development of osteomalacia in a post-liver transplant patient receiving adefovir dipivoxil. World J Hepatol. 2010;2:442–6. doi: 10.4254/wjh.v2.i12.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garden RS. Low-angle fixation in fractures of the femoral neck. JBone Joint Surg Br. 1961;43-B:647–63. [Google Scholar]
- 12.Tanji N, Tanji K, Kambham N, Markowitz GS, Bell A, D’agati VD. Adefovir nephrotoxicity: Possible role of mitochondrial DNA depletion. Hum Pathol. 2001;32:734–40. doi: 10.1053/hupa.2001.25586. [DOI] [PubMed] [Google Scholar]
- 13.Ho ES, Lin DC, Mendel DB, Cihlar T. Cytotoxicity of antiviral nucleotides adefovir and cidofovir is induced by the expression of human renal organic anion transporter 1. J Am Soc Nephrol. 2000;11:383–93. doi: 10.1681/ASN.V113383. [DOI] [PubMed] [Google Scholar]
- 14.Mulato AS, Ho ES, Cihlar T. Nonsteroidal anti-inflammatory drugs efficiently reduce the transport and cytotoxicity of adefovir mediated by the human renal organic anion transporter 1. J Pharmacol Exp Ther. 2000;295:10–5. [PubMed] [Google Scholar]
- 15.Asselah T, Lada O, Boyer N, Martinot M, Marcellin P. Treatment of chronic hepatitis B. Gastroenterol Clin Biol. 2008;32:749–68. doi: 10.1016/j.gcb.2008.07.001. [DOI] [PubMed] [Google Scholar]


