Abstract
Viruses are being redefined as more than just pathogens. They are also critical symbiotic partners in the health of their hosts. In some cases, viruses have fused with their hosts in symbiogenetic relationships. Mutualistic interactions are found in plant, insect, and mammalian viruses, as well as with eukaryotic and prokaryotic microbes, and some interactions involve multiple players of the holobiont. With increased virus discovery, more mutualistic interactions are being described and more will undoubtedly be discovered.
INTRODUCTION
While viruses have long had a very bad name as pathogens, and there are certainly many devastating human, animal, and plant diseases attributed to viruses, viruses are not all bad (1). Recent studies highlight the amazing intricacy of virus-host interactions that have evolved over long periods of time and involve interactions between the hosts and other entities, including other symbiotic microbes and vectors for transmission.
Bacteria are accepted as not just pathogens but also vital partners of eukaryotic life; it is clear that viruses are also essential to life (2). Over the past several years, more and more examples of beneficial viruses have been reported. In some cases, mutualistic symbioses have led to symbiogenesis, the fusion of entities to create a new entity. Mutualism in plant and insect viruses has been well documented, and more recently, mutualistic viruses have been described in mammalian health.
In this age of virus discovery, we are beginning to appreciate the enormous diversity of viruses, far beyond what we originally thought. Undoubtedly, many more will be understood as beneficial. This short review is not meant to be exhaustive but rather highlights some of the recent and dramatic examples of beneficial viruses, demonstrating why viruses need to be taken seriously not just as pathogens but as integrated members of the holobiont.
Symbiosis and symbiogenesis.
Viruses have been recognized as symbiotic members of their hosts' microbial community (1). Symbiosis was first described in the late 19th century to explain lichen and was thought to be an oddity rather than the norm; now we recognize that all life is symbiotic. Symbiotic relationships can take many forms, from antagonistic to mutualistic, and viruses, like other symbionts, lie on a continuum that can shift with environmental changes (3, 4). Symbiotic relationships can lead to symbiogenesis, the fusion of two entities to create a new species, and the extent of virus-like sequences in the genomes of just about everything is evidence of the viral symbiogenesis that has shaped modern genomes (5). From an evolutionary perspective, symbiogenesis should follow a mutualistic symbiotic relationship, but this is not necessarily clear with viral symbiogenesis.
In some cases, the line between virus and host is blurred. For example, the polydnaviruses of the endoparasitoid braconid and ichneumonid wasps have integrated most of the virus genes into the wasp genome, leaving the virus particles to encapsidate wasp genes that suppress the immune system of the caterpillar hosts of the parasitoid wasps (6). It is not clear that the virus and wasp are separate entities any longer, and this could be considered an example of mutualistic symbiosis in the process of becoming symbiogenetic.
In some cases, entire viruses genomes are integrated into the host genome, but these can exogenize and establish infections under some conditions. The badnavirus Banana streak virus, a pararetrovirus that has endogenized into the banana genome on multiple occasions (7), can exogenize and become active under various types of stress (8). Endogenization may provide a selective advantage to the host, acting as a method of immunization, as with endogenous pararetroviruses in tomato plants (9) and proposed for mammalian endogenous retroviruses (1), but this is not clear in banana plants. Banana streak virus may be an example of antagonistic symbiogenesis involving a selfish viral element that manages to hide in the host genome most of the time.
Other endogenous retroviruses have played a clear role in the evolution of their hosts. The mammalian genes for syncytin, essential in the establishment of the placenta, are retroviral env genes of viruses endogenized on several different occasions (10) and even function differently in ruminants versus other mammals (11). Many other symbiogenic viruses are integrated into the host genome. The role of these viruses, including many viruses beyond the retro- and pararetroviruses, is occasionally known. The endogenization events are often ancient, and these elements are considered viral fossils that can help us understand the deep evolution of viruses (12).
Mutualistic viruses and plants.
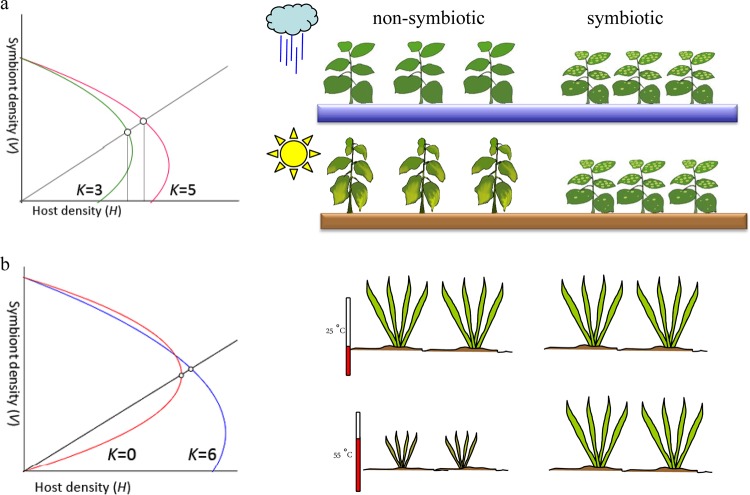
In plants, viruses can ameliorate the effects of abiotic stress. Few plants can grow in the high soil temperatures found in the geothermal soils of Yellowstone National Park. However, one plant is commonly found in those hot soils, a tropical panic grass. The grass is colonized by a fungal endophyte that is, in turn, infected with a virus. All three, virus, fungus, and plant, are required for survival in soils with temperatures of >50°C (13). Viruses can directly impact plants under abiotic stress as well. Several acute plant viruses conferred drought tolerance on a number of plants in greenhouse studies, and in at least one instance, virus infection also improves tolerance to cold (14) (Fig. 1).
FIG 1.
A simple model of biotic stress impacts on quality-selected mutualistic symbioses. The host isocline is a curve that intersects the x axis at the environmental carrying capacity (value K). The viral symbiont isocline is a line with a slope of the maximum titer, when the growth rate of the virus is much lower than the decay rate. The host and virus isoclines intersect at an equilibrium point that a pair of symbiotic partners can approach. (a) When an acute plant-pathogenic virus infects a plant (plant virus-plant host), the plant becomes tolerant to drought stress; without stress, the uninfected plant does better, but under stress, a decrease in K encourages a pathogenic virus to become mutualistic. (b) In a three-way mutualistic symbiosis (fungal virus-fungal endophyte-plant), the virus has no negative impact on the plant under normal conditions and is required for survival at high soil temperatures. Extremely low K and low viral virulence result in a stronger mutualistic symbiosis effect. Reproduced with permission from reference 3.
Plant viruses also impact biotic stress factors. In white clover, infection with White clover mosaic virus makes the plant less attractive to fungus gnats (15). Zucchini yellow mosaic virus infects wild gourds and reduces the production of volatile compounds that attract beetles to the plants. The beetles are vectors of a bacterial wilt pathogen, so the virus reduces transmission of the wilt bacteria (16). These relationships are never black and white; there are costs and benefits, and the players must find a balance. Viruses can be on a continuum between mutualism and antagonism, and where they fall depends on the environment (3, 4).
Plants are very often infected with asymptomatic persistent viruses that differ somewhat from persistent viruses in other systems. These viruses are vertically transmitted at nearly 100% rates; no horizontal transmission has been demonstrated (17). They remain with their hosts for very long periods of time, perhaps thousands of years. White clover cryptic virus, a persistent virus ubiquitous in white clover, suppresses the formation of nitrogen-fixing nodules when adequate nitrogen is present in the soil (18), saving the plant from producing a costly organ when it is not needed. The biology of most other persistent plant viruses remains unknown, but such long associations and high levels of vertical transmission imply beneficial relationships.
Beneficial insect viruses.
Besides the polydnaviruses discussed above, other beneficial insect viruses have recently been described. For example, Helicoverpa armigera densovirus 1 increases developmental rates in both the larva and pupa of its host, the cotton bollworm, and lengthens the life span and increases fecundity in female bollworms. As the bollworm is a serious crop pathogen, control measures are used in cotton fields, including the biopesticide Bacillus thuringiensis and biocontrol with a polyhedrosis virus. In field studies, the densovirus increased resistance to these agents (19), making it a bane for cotton farmers but a boon for the bollworm host. Another insect densovirus infects the rosy apple aphid. Virus infection results in the development of winged aphids that are smaller and have lower fecundity than their uninfected counterparts that do not develop wings. However, wings are an advantage to aphids when the plant becomes crowded. The virus is horizontally transmitted in aphid colonies using the plant as a vector. Since the virus does not replicate in the plant, it remains at a low level, but as the colony size increases, the odds that a nymph will acquire the virus increases, and eventually, an infected, winged aphid develops to move off and start a new colony (20).
A number of plant viruses can have dramatic impacts on their insect vectors (recently reviewed in reference 4). Some viruses infect both plants and insects, and while these are often deleterious to the plants, they can provide advantages to the insects. Tomato spotted wilt virus infects both plants and thrips, tiny insects that vector the virus between plants. The virus suppresses the antifeeding compounds produced by the plants in response to thrip damage, making virus-infected plants a better host for juvenile thrips than noninfected, thrip-damaged plants. The virus effect can extend to other insects; spider mites also do better on virus-infected plants, even though they are not a host for the virus (4 and references therein).
Good viruses in mammalian health.
Humans infected with GB virus C (GBV-C), also known as hepatitis G virus, do not show any clinical symptoms. However, HIV-positive patients who also have GBV-C show slower disease progression. Several effects of GBV-C on HIV have been shown in clinical studies and in vitro, including downregulation of cell receptors for HIV entry, reduced replication of HIV, effects on interferon synthesis, and interactions with interleukin pathways (21).
The importance of gut bacteria in digestion and gut architecture is well established. A recent study with mice showed that Murine norovirus establishes a latent infection in mice. In germfree or antibiotic-treated mice, the norovirus can provide many beneficial functions that bacteria provide, including intestinal morphological characteristics and lymphocyte function (22).
Mammalian viruses can provide immunity to infection by bacterial pathogens, as shown in mice infected with a gammaherpesvirus that increases resistance to Listeria monocytogenes and Yersinia pestis. Latent herpesviruses also affect natural killer (NK) cells, an important line of defense against pathogens and cancer because they kill virus-infected cells and tumor cells, in addition to producing cytokines like interferon. While NK cells do not require specific sensitization (i.e., they can kill cells on first encounter), they do require arming before they can efficiently produce their cytotoxic effects. This arming function is provided by a latent herpesvirus in mice (23).
The guts of humans and other mammals are rich in viruses. These are largely still in the discovery phase. Many of the gut viruses are bacteriophage, although eukaryotic viruses are also part of the human virome, and these include both human viruses and viruses of eukaryotic symbionts (23). For the most part, little is known about how these viruses impact their hosts, but their ubiquitous presence implies functions. In some cases, phage may be regulating the populations of resident bacteria or they could affect the expression of bacterial genes that are involved in host digestion (24). In a recent study, bacteriophage were shown to adhere to mucous membranes in many different metazoan hosts. Mucous membranes are found at the point of entry of many bacterial pathogens. The phage are poised to provide the first line of defense against bacteria invading the metazoan host by infection and lysis. The metazoan host is benefitted by this early attack of potential pathogens, and viruses are benefitted by access to new hosts (25).
Viruses of microbes.
In addition to providing benefits to the macrohosts of many microbes, viruses also directly benefit their microbial hosts. The killer viruses of yeasts and bacteria allow their hosts to invade new territories by killing off competitors while providing immunity to the virus-containing hosts (1). Phage also encode essential functions for bacteria, such as the production of toxins that allow them to invade their macrohosts, the horizontal gene transfer of essential elements, and in some cases, the ability to form biofilms (26). Viruses of other eukaryotic microbes have positive effects on the growth, fecundity, or persistence of their hosts (27).
Conclusions.
Our new understanding of the role of viruses in mutualistic symbiotic relationships with their hosts is expanding as our knowledge of the virome, through new sequencing technologies and bioinformatic strategies, is rapidly increasing. Viewing viruses in the context of ecology (28) provides a framework for a deeper understanding of the intertwined relationships of all life, including viruses. It is inevitable that more examples of mutualistic viruses will be revealed as we continue this exciting phase in virology of virus discovery.
ACKNOWLEDGMENTS
I acknowledge the support of the Pennsylvania State University College of Agricultural Science; National Science Foundation grants EF-0627108, EPS-0447262, IOS-0950579, and IOS-1157148; and United States Department of Agriculture grant OKLR-2007-01012.
REFERENCES
- 1.Roossinck MJ. 2011. The good viruses: viral mutualistic symbioses. Nat Rev Microbiol 9:99–108. doi: 10.1038/nrmicro2491. [DOI] [PubMed] [Google Scholar]
- 2.Villarreal LP, Witzany G. 2010. Viruses are essential agents within the roots and stems of the tree of life. J Theor Biol 262:698–710. doi: 10.1016/j.jtbi.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Bao X, Roossinck MJ. 2013. A life history view of mutualistic viral symbioses: quantity or quality for cooperation? Curr Opin Microbiol 16:514–518. doi: 10.1016/j.mib.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Roossinck MJ. Plants, viruses and the environment: ecology and mutualism. 479–480:271–277. doi: 10.1016/j.virol.2015.03.041. [DOI] [PubMed] [Google Scholar]
- 5.Koonin EV. 2006. On the origin of cells and viruses: a comparative-genomic perspective. Isr J Ecol Evol 52:299–318. doi: 10.1560/IJEE_52_3-4_299. [DOI] [Google Scholar]
- 6.Strand MR, Burke GR. 2014. Polydnaviruses: nature's genetic engineers. Annu Rev Virol 1:333–354. doi: 10.1146/annurev-virology-031413-085451. [DOI] [PubMed] [Google Scholar]
- 7.Gayral P, Iskra-Caruana M-L. 2009. Phylogeny of Banana streak virus reveals recent and repetitive endogenization in the genome of its banana host (Musa sp.). J Mol Evol 69:65–80. doi: 10.1007/s00239-009-9253-2. [DOI] [PubMed] [Google Scholar]
- 8.Iskra-Caruana M-L, Baurens F-C, Gayral P, Chabannes M. 2010. A four-partner plant-virus interaction: enemies can also come from within. Mol Plant Microbe Interact 23:1394–1402. doi: 10.1094/MPMI-05-10-0107. [DOI] [PubMed] [Google Scholar]
- 9.Staginnus C, Gregor W, Mette MF, Teo CH, Borroto-Fernández EG, Machado ML, Matzke M, Schwarzacher T. 2007. Endogenous pararetroviral sequences in tomato (Solanum lycopersicum) and related species. BMC Plant Biol 7:24. doi: 10.1186/1471-2229-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haig D. 2012. Retroviruses and the placenta. Curr Biol 22:R609–R613. doi: 10.1016/j.cub.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 11.Cornelis G, Heidmann O, Degrelle SA, Vernochet C, Lavialle C, Letzelter C, Bernard-Stoecklin S, Hassanin A, Mulot B, Guillomot M, Hue I, Heidman T, Dupressoir A. 2013. Captured retroviral envelope syncytin gene associated with the unique placental structure of higher ruminants. Proc Natl Acad Sci U S A 110:E828–E837. doi: 10.1073/pnas.1215787110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feschotte C, Gilbert C. 2012. Endogenous viruses: insights into viral evolution and impact on host biology. Nat Rev Genet 13:283–296. doi: 10.1038/nrg3199. [DOI] [PubMed] [Google Scholar]
- 13.Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ. 2007. A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science 315:513–515. doi: 10.1126/science.1136237. [DOI] [PubMed] [Google Scholar]
- 14.Xu P, Chen F, Mannas JP, Feldman T, Sumner LW, Roossinck MJ. 2008. Virus infection improves drought tolerance. New Phytol 180:911–921. doi: 10.1111/j.1469-8137.2008.02627.x. [DOI] [PubMed] [Google Scholar]
- 15.van Molken T, de Caluwe H, Hordijk CA, Leon-Reyes A, Snoeren TA, van Dam NM, Stuefer JF. 2012. Virus infection decreases the attractiveness of white clover plants for a non-vectoring herbivore. Oecologia 170:433–444. doi: 10.1007/s00442-012-2322-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shapiro LR, Salvaudon L, Mauck KE, Pulido H, DeMoraes CM, Stephenson AG, Mescher MC. 2013. Disease interactions in a shared host plant: effects of pre-existing viral infection on cucurbit plant defense responses and resistance to bacterial wilt disease. PLoS One 8:e77393. doi: 10.1371/journal.pone.0077393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roossinck MJ. 2012. Persistent plant viruses: molecular hitchhikers or epigenetic elements?, p 177–186. In Witzany G. (ed), Viruses: essential agents of life. Springer, Dordrecht, The Netherlands. [Google Scholar]
- 18.Nakatsukasa-Akune M, Yamashita K, Shimoda Y, Uchiumi T, Abe M, Aoki T, Kamizawa A, Ayabe S, Higashi S, Suzuki A. 2005. Suppression of root nodule formation by artificial expression of the TrEnodDR1 (coat protein of White clover cryptic virus 1) gene in Lotus japonicus. Mol Plant Microbe Interact 18:1069–1080. doi: 10.1094/MPMI-18-1069. [DOI] [PubMed] [Google Scholar]
- 19.Xu P, Liu Y, Graham RI, Wilson K, Wu K. 2014. Densovirus is a mutualistic symbiont of a global crop pest (Helicoverpa armigera) and protects against a baculovirus and Bt biopesticide. PLoS Pathog 10:e1004490. doi: 10.1371/journal.ppat.1004490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ryabov EV, Keane G, Naish N, Evered C, Winstanley D. 2009. Densovirus induces winged morphs in asexual clones of the rosy apple aphid, Dysaphis plantaginea. Proc Natl Acad Sci U S A 106:8465–8470. doi: 10.1073/pnas.0901389106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattarai N, Stapleton JT. 2012. GB virus C: the good boy virus? Trends Microbiol 20:124–130. doi: 10.1016/j.tim.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kernbauer E, Ding Y, Cadwell K. 2014. An enteric virus can replace the beneficial function of commensal bacteria. Nature 516:94–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Virgin HW. 2014. The virome in mammalian physiology and disease. Cell 157:142–150. doi: 10.1016/j.cell.2014.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Duerkop BA, Hooper LV. 2013. Resident viruses and their interactions with the immune system. Nat Immunol 14:654–659. doi: 10.1038/ni.2614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barr JJ, Auro R, Furlan M, Whiteson KL, Erb ML, Pogliano J, Stotland A, Wolkowicz R, Cutting AS, Doran KS, Salamon P, Youle M, Rohwer F. 2013. Bacteriophage adhering to mucus provide a non-host-derived immunity. Proc Natl Acad Sci U S A 110:10771–10776. doi: 10.1073/pnas.1305923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mai-Prochnow A, Hui JGK, Kjelleberg S, Rakonjac J, McDougald D, Rice SA. 10 February 2015. Big things in small packages: the genetics of filamentous phage and effects on fitness of their hosts. FEMS Microb Rev doi: 10.1093/femsre/fuu007. [DOI] [PubMed] [Google Scholar]
- 27.Márquez LM, Roossinck MJ. 2012. Do persistent RNA viruses fit the trade-off hypothesis of virulence evolution? Curr Opin Virol 2:556–560. doi: 10.1016/j.coviro.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Dennehy JJ. 2014. What ecologists can tell virologists. Annu Rev Microbiol 68:117–135. doi: 10.1146/annurev-micro-091313-103436. [DOI] [PubMed] [Google Scholar]