Abstract
A key barrier against developing preventive and therapeutic human immunodeficiency virus (HIV) vaccines is the inability of viral envelope glycoproteins to elicit broad and potent neutralizing antibodies. However, in the presence of fusion inhibitor enfuvirtide, we show that the nonneutralizing antibodies induced by the HIV type 1 (HIV-1) gp41 N-terminal heptad repeat (NHR) domain (N63) exhibit potent and broad neutralizing activity against laboratory-adapted HIV-1 strains, including the drug-resistant variants, and primary HIV-1 isolates with different subtypes, suggesting the potential of developing gp41-targeted HIV therapeutic vaccines.
TEXT
Development of prophylactic and therapeutic HIV vaccines is urgently needed. Thus far, HIV vaccine development has been impeded by the high sequence variation of HIV envelope glycoproteins (Env) and their inability to induce broad and potent neutralizing antibodies (1). Human immunodeficiency virus type 1 (HIV-1) Env transmembrane subunit gp41, which contains the fusion peptide (FP) and the N- and C-terminal heptad repeats (NHR and CHR) (Fig. 1A), plays an important role in mediating fusion between the viral envelope and target cell membrane. After binding of gp120 to the cellular receptor CD4 and coreceptor CXCR4 or CCR5, gp41 changes conformation by inserting FP into the target cell membrane, resulting in the exposure of the NHR domain and formation of a six-helix bundle (6-HB) between the NHR and CHR domains, bringing the viral and cellular membranes into close proximity for fusion. Peptides derived from the gp41 CHR domain, such as SJ-2176 (2), T20 (3), and C34 (4, 5), can bind to the NHR domain to form 6-HB, thus blocking gp41-mediated membrane fusion (6, 7). T20 (brand name, Fuzeon; generic name, enfuvirtide) was approved by the U.S. FDA as the first HIV fusion inhibitor for treatment of HIV infection. However, its clinical use is limited by the requirement of a high dosage and multiple injections.
FIG 1.
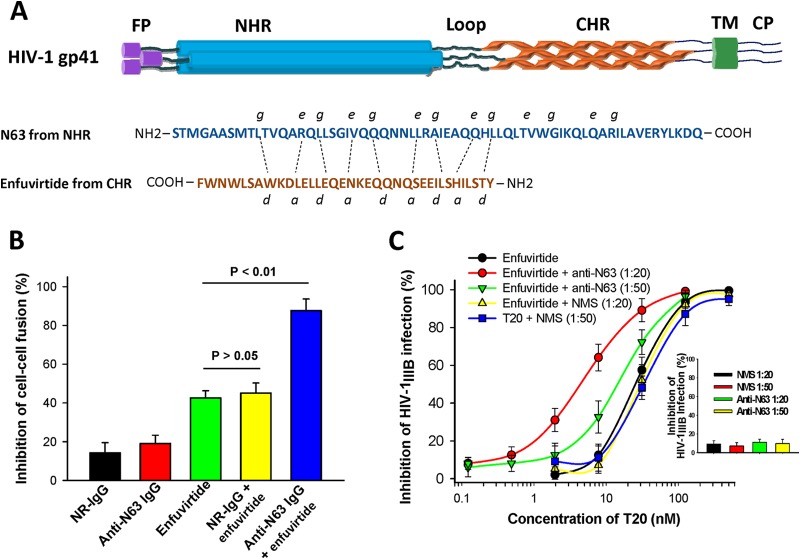
(A) The molecular structure of the HIV-1 gp41 and the sequences of enfuvirtide peptide and N63 protein. Enfuvirtide was synthesized using a standard Fmoc solid-phase synthetic method by Jill Biochemical Inc. (Shanghai, China) with 98% purity. N63 protein was expressed in Escherichia coli with expression vector pGEX-6p-N63His-pp-PDI and purified by Ni column chromatography as previously described (19). TM, transmembrane. (B) Effect of inhibitory activity of anti-N63 IgG (200 nM) in the presence or absence of enfuvirtide (10 nM) on cell-cell fusion mediated by HIV-1IIIB Env on chronically infected H9 (H9/HIV-1IIIB) cells. NR-IgG and rabbit anti-N63 IgG were purified with protein A and protein G affinity columns, respectively, from normal rabbit sera and sera of rabbits immunized with 100 μg of N63 protein plus Freund's adjuvant as previously described (20). The effect of the inhibitory activity of the antibodies and peptide on HIV-1 Env-mediated cell-cell fusion was determined as previously described (16). (C) The neutralizing activity of mouse anti-N63 sera at a 1:20 dilution and a 1:50 dilution in the presence of enfuvirtide at graded concentrations against HIV-1IIIB infection in MT-2 cells was determined as previously described (16). Normal mouse serum (NMS) and mouse anti-N63 serum alone were used as controls. Each sample was tested in triplicate, and the data are presented as means ± standard deviations (SD) (bar).
Compared with gp120, gp41, particularly, its NHR domain, has a relatively conserved sequence. We and others have shown that antibodies induced by NHR trimer exhibited low neutralizing antibody response in immunized animals (8, 9), while those induced by the monomeric NHR fragments have no neutralizing activity, possibly because of the rapid transition from the prehairpin intermediate state to the posthairpin fusion-active state, i.e., because of kinetic restriction (10, 11) (Fig. 2). Golding et al. (12) demonstrated that antibodies specific to the HIV-1 gp41 NHR lacked neutralizing activity at 37°C. However, they could inhibit HIV-1 Env-mediated cell-cell fusion after the effector/target cells and antibodies were incubated at the suboptimal temperature (31.5°C) for 1 h before the cocultures were transferred to 37°C, suggesting that a lower incubation temperature could slow down the transition from the prehairpin intermediate state to the posthairpin fusion state, thus allowing IgG sufficient time to bind the gp41 NHR domain and block membrane fusion.
FIG 2.
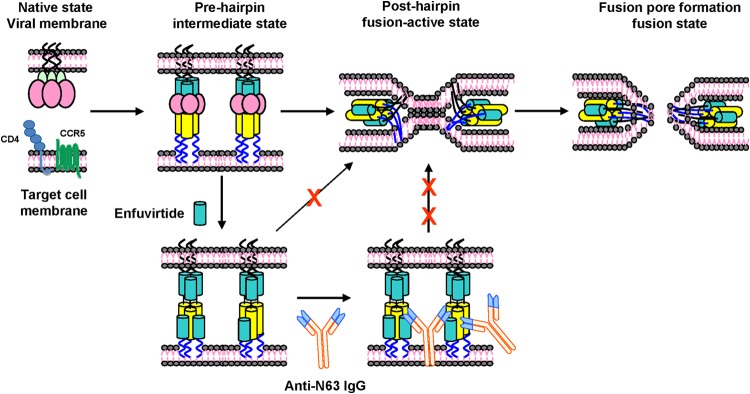
Schematic representation of the cooperative effect of combining enfuvirtide and NHR-specific antibodies in activity against gp41-mediated membrane fusion. Once gp120 binds to CD4 and a coreceptor such as CCR5, the gp41 fusion peptide (FP) inserts into the target cell membrane, resulting in the exposure of gp41 N- and C-terminal heptad repeat (NHR and CHR) trimers (prehairpin intermediate state). The NHR and CHR helices interact with each other to form a 6-HB (posthairpin fusion-active state), which pulls the viral envelope and target membrane together, resulting in fusion pore formation. In the prehairpin fusion intermediate state, enfuvirtide binds to the gp41 NHR trimer, leading to the inhibition of viral gp41 6-HB formation and virus-cell fusion. Binding of enfuvirtide to the gp41 NHR trimer extends the temporal window of the prehairpin fusion intermediate, allowing the gp41 NHR-specific antibodies access to the viral gp41 NHR domain to block membrane fusion and neutralize HIV-1 infection. Moreover, antibody binding to the gp41 NHR may stabilize interaction of enfuvirtide with the NHR trimer, thus increasing the potency of enfuvirtide with respect to inhibition of HIV-1 fusion with the target cell membrane.
In the present study, we generated antibodies in rabbits and mice immunized with N63 peptide corresponding to the gp41 NHR domain and tested their neutralizing activity against HIV-1 in the presence or absence of enfuvirtide.
At 200 nM, normal rabbit IgG (NR-IgG) or anti-N63 IgG alone exhibited no significant inhibition effect on cell-cell fusion mediated by the Env of HIV-1IIIB on chronically infected H9 (H9/HIV-1IIIB) cells. Enfuvirtide at 10 nM alone displayed about 42% inhibition, while the combination of enfuvirtide (10 nM) and NR-IgG (200 nM) showed inhibitory activity similar to that of enfuvirtide alone (P > 0.05). However, by combining anti-N63 IgG with enfuvirtide mixed in a molar ratio of 1:20 (enfuvirtide/anti-N63 IgG), about 88% inhibition was observed (Fig. 1B). We then compared the inhibitory activities of mouse anti-N63 antisera and normal mouse sera (NMS) at 1:20 and 1:50 dilutions in the absence or presence of enfuvirtide at graded concentrations. NMS alone and mouse anti-N63 sera alone showed no significant inhibition of HIV-1IIIB infection in MT-2 cells (insert in Fig. 1C). However, while the combination of enfuvirtide and NMS at 1:20 and 1:50 dilutions showed a low level of inhibitory activity commensurate with that of enfuvirtide alone, the combination of enfuvirtide and anti-N63 serum at 1:20 and 1:50 dilutions exhibited significantly higher inhibitory activity than that of the enfuvirtide/NMS combination. Moreover, the combined enfuvirtide/anti-N63 treatment displayed greater inhibition at the dilution of 1:20 than at 1:50, thus demonstrating dose dependency (Fig. 1C). These results suggest that anti-gp41 NHR antibodies gain inhibitory activity against HIV-1 Env-mediated cell-cell fusion and HIV-1 infection in the presence of the HIV fusion inhibitor enfuvirtide.
Subsequently, we assessed the effect of the inhibitory activity of anti-N63 IgG combined with enfuvirtide at a molar ratio of 1:20 (enfuvirtide/anti-N63 IgG) on infection of HIV-1IIIB (X4) and HIV-1Bal (R5) in MT-2 and CEMx174 5.25M7 cells, respectively, using NR-IgG and SARS-CP1, an irrelevant peptide derived from the HR2 region of S2 subunit of severe acute respiratory syndrome coronavirus (SARS-CoV) spike protein (13), as the controls. The IC50 (concentration causing 50% inhibition) and combination index (CI) were calculated using the CalcuSyn program (2, 12), as previously described (14, 15). As shown in Table 1, anti-N63 IgG tested alone showed no anti-HIV-1 activity at concentrations as high as 3,000 nM. In combination with enfuvirtide, however, anti-N63 IgG exhibited potent neutralizing activity against HIV-1IIIB and HIV-1Bal infection, with IC50s of 72.61 ± 2.02 and 89.16 ± 4.77 nM and dose reductions of >40.32-fold (CI, 0.16 ± 0.02) and >32.65-fold (CI, 0.26 ± 0.03), respectively, suggesting a strong synergistic effect. In contrast, the normal rabbit antibody IgG, when combined with enfuvirtide, exhibited no HIV-1 neutralizing activity at the concentration of 3,000 nM. The irrelevant peptide SARS-CP1, when tested alone or in combination with enfuvirtide, exhibited no inhibitory activity at a concentration as high as 500 nM.
TABLE 1.
Effect of inhibitory activity of rabbit anti-N63 IgG in the absence or presence of enfuvirtide on infection by HIV-1 strains, including drug-resistant variantsa
| Inhibitor A | CI | IC50 (nM) |
Dose reduction (fold) | Inhibitor B | IC50 (nM) |
Dose reduction (fold) | ||
|---|---|---|---|---|---|---|---|---|
| Alone | In mixture | Alone | In mixture | |||||
| HIV-1 IIIB strain (B, X4) | ||||||||
| Enfuvirtide | 0.16 ± 0.02 | 22.63 ± 2.95 | 3.63 ± 0.11 | 5.23 | Anti-N63 IgG | >3,000 | 72.61 ± 2.02 | >40.32 |
| SARS-CP1 | NC | >500 | >500 | Anti-N63 IgG | >3,000 | >3,000 | ||
| HIV-1 Bal strain (B, R5) | ||||||||
| Enfuvirtide | 0.26 ± 0.03 | 18.24 ± 1.05 | 4.46 ± 0.02 | 3.09 | Anti-N63 IgG | >3,000 | 89.16 ± 4.77 | >32.65 |
| SARS-CP1 | NC | >500 | >500 | Anti-N63 IgG | >3,000 | >3,000 | ||
| AZT-resistant HIV-1 (catalog no. 964)b | ||||||||
| Enfuvirtide | 0.16 ± 0.01 | 17.04 ± 1.96 | 2.68 ± 0.04 | 5.36 | Anti-N63 IgG | >3,000 | 53.5 ± 0.64 | >55.07 |
| SARS-CP1 | NC | >500 | >500 | Anti-N63 IgG | >3,000 | >3,000 | ||
| Nevirapine-resistant HIV-1 (N119 [catalog no. 1392])b | ||||||||
| Enfuvirtide | 0.18 ± 0.01 | 35.82 ± 0.98 | 5.62 ± 0.67 | 5.37 | Anti-N63 IgG | >3,000 | 112.36 ± 12.68 | >25.70 |
| SARS-CP1 | NC | >500 | >500 | Anti-N63 IgG | >3,000 | >3,000 | ||
| HIV-1 RTMDR1/MT-2 (catalog no. 2529)b | ||||||||
| Enfuvirtide | 0.18 ± 0.02 | 14.64 ± 1.25 | 2.42 ± 0.54 | 5.05 | Anti-N63 IgG | >3,000 | 48.34 ± 10.78 | >61.06 |
| SARS-CP1 | NC | >500 | >500 | Anti-N63 IgG | >3,000 | >3,000 | ||
| T20-resistant HIV-1 strain (pNL4-3 gp41) (36G/V38A [catalog no. 9490])b | ||||||||
| Enfuvirtide | 0.20 ± 0.01 | 1,340 ± 38.89 | 139.59 ± 4.27 | 8.60 | Anti-N63 IgG | >3,000 | 2,791 ± 84.85 | >0.10 |
| SARS-CP1 | NC | >500 | >500 | Anti-N63 IgG | >3,000 | >3,000 | ||
NC, not calculable. The effect of the inhibitory activity of enfuvirtide and anti-N63 IgG purified from sera of rabbits immunized with N63, alone or in combination, on HIV-1 infection was determined as previously described (16). Briefly, MT-2 cells (for X4 HIV-1IIIB and drug-resistant strains) or CEMx174 5.25M7 cells (for the R5 HIV-1Bal strain) were infected with an HIV-1 strain at 100 50% tissue culture infective doses (TCID50) in 200 μl culture medium in the absence or presence of an inhibitor at graded concentrations (the starting concentrations of enfuvirtide and anti-N63 IgG in the mixture were 150 and 3,000 nM, respectively) overnight, followed by replacing the culture supernatants with fresh medium. On day 4 postinfection, culture supernatants were collected for testing p24 by enzyme-linked immunosorbent assay (ELISA). Each sample was tested in triplicate. The experiments were repeated three times, and the data are presented as means ± standard deviations (SD). The average variability between the results of the replicate experiments was about 8%. The HIV-1 RTMDR1/MT-2 strain is resistant to AZT, dideoxyinosine (ddI), nevirapine, and other nonnucleoside reverse transcriptase inhibitors (17). The mole ratio of the T20/anti-N63 IgG combination is 1:20. The strength of synergism is indicated by the CI values (CI < 0.1, very strong synergism; 0.1 to 0.3, strong synergism; 0.3 to 0.7, synergism; 0.7 to 0.85, moderate synergism; 0.85 to 0.90, slight synergism) (18). (Fold) dose reduction was calculated using the following formula (14): (IC50 of an inhibitor tested alone/IC50 of the same inhibitor tested in combination with another inhibitor). SARS-CP1, an irrelevant peptide derived from the HR2 region of SARS-CoV spike protein, was used as a control.
These drug-resistant strains were obtained from the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH, and their catalog numbers are included in parentheses.
Similarly, in the presence of enfuvirtide, anti-N63 IgG was able to neutralize infection by zidovudine (AZT)-resistant strains, nevirapine-resistant strains, and the multidrug-resistant stain HIV-1RTMDR1/MT-2 with IC50s of about 53.5 ± 0.64, 121.36 ± 12.68, and 48.34 ± 10.78 nM and dose reductions of >55.07-, >25.70-, and >61.06-fold, respectively (CI, 0.16 ± 0.01, 0.18 ± 0.01, and 0.18 ± 0.02, respectively). Interestingly, enfuvirtide did not significantly increase the neutralizing activity of anti-N63 IgG against the enfuvirtide-resistant strain. However, in the presence of anti-N63 IgG, enfuvirtide became much more effective against the enfuvirtide-resistant strain, with a dose reduction of 8.60-fold (IC50, 1,340 ± 38.89 versus 139.59 ± 4.27 nM). Again, NR-IgG and SARS-CP1, alone or in their combinations with enfuvirtide and anti-N63 IgG, respectively, exhibited no inhibitory activity against these drug-resistant HIV-1 variants.
Finally, we tested the effect of the inhibitory activity of anti-N63 IgG in the absence or presence of enfuvirtide on infection by HIV-1 primary isolates with different subtypes and tropisms. As shown in Table 2, the combination of anti-N63 IgG with enfuvirtide at the molar ratio of 1:20 (T20/anti-N63 IgG) exhibited a very strong synergistic antiviral effect against all the isolates tested, with CI values in a range of 0.14 to 0.26 and dose reductions ranging from 2.9-fold to 7.2-fold for enfuvirtide and from >28-fold to >60-fold for anti-N63 IgG. These results confirm that, with the addition of enfuvirtide, anti-N63 IgG could also inhibit infection by primary HIV-1 isolates.
TABLE 2.
Effect of inhibitory activity of rabbit anti-N63 IgG in the absence or presence of enfuvirtide on infection by primary HIV-1 isolatesa
| Inhibitor A | CI | IC50 (nM) |
Dose reduction (fold) | Inhibitor B | IC50 (nM) |
Dose reduction (fold) | ||
|---|---|---|---|---|---|---|---|---|
| Alone | In mixture | Alone | In mixture | |||||
| 92UG029 (subtype A, X4) | ||||||||
| Enfuvirtide | 0.26 ± 0.02 | 16.29 ± 0.82 | 4.13 ± 0.41 | 2.94 | Anti-N63 IgG | >3,000 | 82.69 ± 8.27 | >35.28 |
| 94US_33931N (subtype B, R5) | ||||||||
| Enfuvirtide | 0.20 ± 0.01 | 25.44 ± 1.82 | 4.82 ± 0.47 | 4.28 | Anti-N63 IgG | >3,000 | 96.25 ± 9.48 | >30.17 |
| 93IN101 (subtype C, R5) | ||||||||
| Enfuvirtide | 0.15 ± 0.01 | 20.60 ± 2.68 | 2.91 ± 0.17 | 6.08 | Anti-N63 IgG | >3,000 | 58.09 ± 3.39 | >50.64 |
| 92UG024 (subtype D, X4) | ||||||||
| Enfuvirtide | 0.20 ± 0.01 | 12.61 ± 2.06 | 2.45 ± 0.54 | 4.15 | Anti-N63 IgG | >3,000 | 48.97 ± 10.75 | >60.26 |
| 92TH009 (subtype A/E, R5) | ||||||||
| Enfuvirtide | 0.19 ± 0.02 | 24.14 ± 2.43 | 4.16 ± 0.08 | 4.80 | Anti-N63 IgG | >3,000 | 83.21 ± 1.73 | >35.05 |
| 93/BR/020 (subtype F, X4 and R5) | ||||||||
| Enfuvirtide | 0.18 ± 0.02 | 17.14 ± 2.18 | 2.99 ± 0.73 | 4.73 | Anti-N63 IgG | >3,000 | 59.78 ± 14.58 | >49.18 |
| BCF02 (group O, R5) | ||||||||
| Enfuvirtide | 0.14 ± 0.01 | 42.69 ± 3.34 | 5.18 ± 0.18 | 7.24 | Anti-N63 IgG | >3,000 | 103.48 ± 3.50 | >27.99 |
The inhibitory activity of enfuvirtide and anti-N63 IgG purified from sera of rabbits immunized with N63, alone or in combination, on infection by primary HIV-1 isolates in CEMx174 5.25M7 cells using p24 assay as described above. All HIV-1 strains were obtained from the NIH AIDS Research and Reference Reagent Program. Each sample was tested in triplicate. The experiments were repeated three times, and the data are presented as means ± SD. The average variability between the results of the repeated experiments was about 9%.
Therefore, in the presence of enfuvirtide, nonneutralizing antibodies specific to the gp41 NHR domain gain neutralizing activity against HIV-1 Env-mediated membrane fusion and infection by both laboratory-adapted HIV-1 strains, including drug-resistant variants, and primary HIV-1 isolates with different subtypes circulating in the world. Correspondingly, in the presence of these nonneutralizing antibodies, enfuvirtide becomes more potent against both enfuvirtide-sensitive and enfuvirtide-resistant HIV-1 strains. To explain this cooperativity, it is hypothesized that binding of enfuvirtide to the gp41 NHR domain may significantly prolong the lifetime of the gp41 prehairpin intermediate and extend its temporal window, thus providing sufficient time for the gp41 NHR-specific antibodies to bind with the viral gp41 NHR domain to block virus-cell fusion (Fig. 2). At the same time, binding of the antibodies to the NHR may potentiate the interaction between enfuvirtide and NHR trimer, thereby enhancing the antiviral activity of enfuvirtide against HIV-1 strains, including those resistant to enfuvirtide and other antiretroviral drugs. Therefore, antigens derived from the HIV-1 gp41 NHR domain do have potential for development as therapeutic vaccines for the treatment of HIV-1 infection when used in combination with HIV fusion inhibitors such as enfuvirtide.
ACKNOWLEDGMENTS
We thank the NIH AIDS Reagent Program (ARP) for providing all the HIV-1 strains used in this study.
This work was supported by grants from the National Natural Science Foundation of China (81173098 and 81361120378 to S.J. and 81102476 and 81373456 to L.L.), the National Grand Program on Key Infectious Disease Control (2012ZX10001008-002 to L.L.), and the Shanghai Pujiang Program (13PJD004 to L.L.).
REFERENCES
- 1.Koff WC. 2012. HIV vaccine development: challenges and opportunities towards solving the HIV vaccine-neutralizing antibody problem. Vaccine 30:4310–4315. doi: 10.1016/j.vaccine.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 2.Jiang S, Lin K, Strick N, Neurath AR. 1993. HIV-1 inhibition by a peptide. Nature 365:113. [DOI] [PubMed] [Google Scholar]
- 3.Wild CT, Shugars DC, Greenwell TK, McDanal CB, Matthews TJ. 1994. Peptides corresponding to a predictive alpha-helical domain of human immunodeficiency virus type 1 gp41 are potent inhibitors of virus infection. Proc Natl Acad Sci U S A 91:9770–9774. doi: 10.1073/pnas.91.21.9770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan DC, Fass D, Berger JM, Kim PS. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263–273. doi: 10.1016/S0092-8674(00)80205-6. [DOI] [PubMed] [Google Scholar]
- 5.Lu M, Blacklow SC, Kim PS. 1995. A trimeric structural domain of the HIV-1 transmembrane glycoprotein. Nat Struct Biol 2:1075–1082. doi: 10.1038/nsb1295-1075. [DOI] [PubMed] [Google Scholar]
- 6.Bagai S, Lamb RA. 1997. A glycine to alanine substitution in the paramyxovirus SV5 fusion peptide increases the initial rate of fusion. Virology 238:283–290. doi: 10.1006/viro.1997.8858. [DOI] [PubMed] [Google Scholar]
- 7.Chan DC, Kim PS. 1998. HIV entry and its inhibition. Cell 93:681–684. doi: 10.1016/S0092-8674(00)81430-0. [DOI] [PubMed] [Google Scholar]
- 8.Bianchi E, Joyce JG, Miller MD, Finnefrock AC, Liang X, Finotto M, Ingallinella P, McKenna P, Citron M, Ottinger E, Hepler RW, Hrin R, Nahas D, Wu C, Montefiori D, Shiver JW, Pessi A, Kim PS. 2010. Vaccination with peptide mimetics of the gp41 prehairpin fusion intermediate yields neutralizing antisera against HIV-1 isolates. Proc Natl Acad Sci U S A 107:10655–10660. doi: 10.1073/pnas.1004261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qi Z, Pan C, Lu H, Shui Y, Li L, Li X, Xu X, Liu S, Jiang S. 2010. A recombinant mimetics of the HIV-1 gp41 prehairpin fusion intermediate fused with human IgG Fc fragment elicits neutralizing antibody response in the vaccinated mice. Biochem Biophys Res Commun 398:506–512. doi: 10.1016/j.bbrc.2010.06.109. [DOI] [PubMed] [Google Scholar]
- 10.de Rosny E, Vassell R, Wingfield PT, Wild CT, Weiss CD. 2001. Peptides corresponding to the heptad repeat motifs in the transmembrane protein (gp41) of human immunodeficiency virus type 1 elicit antibodies to receptor-activated conformations of the envelope glycoprotein. J Virol 75:8859–8863. doi: 10.1128/JVI.75.18.8859-8863.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore JP, Parren PWHI, Burton DR. 2001. Genetic subtypes, humoral immunity, and human immunodeficiency virus type 1 vaccine development. J Virol 75:5721–5729. doi: 10.1128/JVI.75.13.5721-5729.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golding H, Zaitseva M, de Rosny E, King LR, Manischewitz J, Sidorov I, Gorny MK, Zolla-Pazner S, Dimitrov DS, Weiss CD. 2002. Dissection of human immunodeficiency virus type 1 entry with neutralizing antibodies to gp41 fusion intermediates. J Virol 76:6780–6790. doi: 10.1128/JVI.76.13.6780-6790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu S, Xiao G, Chen Y, He Y, Niu J, Escalante CR, Xiong H, Farmar J, Debnath AK, Tien P, Jiang S. 2004. Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors. Lancet 363:938–947. doi: 10.1016/S0140-6736(04)15788-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pan C, Cai L, Lu H, Qi Z, Jiang S. 2009. Combinations of the first and next generation HIV fusion inhibitors exhibit highly potent synergistic effect against enfuvirtide-sensitive and resistant HIV-1 strains. J Virol 83:7862–7872. doi: 10.1128/JVI.00168-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xu W, Wang Q, Yu F, Lu L, Jiang S. 2014. Synergistic effect resulting from combinations of a bifunctional HIV-1 antagonist with antiretroviral drugs. J Acquir Immune Defic Syndr 67:1–6. doi: 10.1097/QAI.0000000000000265. [DOI] [PubMed] [Google Scholar]
- 16.Tong P, Lu Z, Chen X, Wang Q, Yu F, Zou P, Yu X, Li Y, Lu L, Chen YH, Jiang S. 2013. An engineered HIV-1 gp41 trimeric coiled coil with increased stability and anti-HIV-1 activity: implication for developing anti-HIV microbicides. J Antimicrob Chemother 68:2533–2544. doi: 10.1093/jac/dkt230. [DOI] [PubMed] [Google Scholar]
- 17.Larder BA, Kellam P, Kemp SD. 1993. Convergent combination therapy can select viable multidrug-resistant HIV-1 in vitro. Nature 365:451–453. doi: 10.1038/365451a0. [DOI] [PubMed] [Google Scholar]
- 18.Chou TC. 2006. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev 58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Pan C, Li Y, Lu H, He W, Jiang S. 2012. A bivalent recombinant protein inactivates HIV-1 by targeting the gp41 prehairpin fusion intermediate induced by CD4 D1D2 domains. Retrovirology 9:104. doi: 10.1186/1742-4690-9-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du L, Zhao G, He Y, Guo Y, Zheng BJ, Jiang S, Zhou Y. 2007. Receptor-binding domain of SARS-CoV spike protein induces long-term protective immunity in an animal model. Vaccine 25:2832–2838. doi: 10.1016/j.vaccine.2006.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]