ABSTRACT
Most blinding ocular herpetic disease is due to reactivation of herpes simplex virus 1 (HSV-1) from latency rather than to primary acute infection. No herpes simplex vaccine is currently available for use in humans. In this study, we used the HLA-A*02:01 transgenic (HLA Tg) rabbit model of ocular herpes to assess the efficacy of a therapeutic vaccine based on HSV-1 gD epitopes that are recognized mainly by CD8+ T cells from “naturally” protected HLA-A*02:01-positive, HSV-1-seropositive healthy asymptomatic (ASYMP) individuals (who have never had clinical herpes disease). Three ASYMP CD8+ T-cell epitopes (gD53–61, gD70–78, and gD278–286) were linked with a promiscuous CD4+ T-cell epitope (gD287–317) to create 3 separate pairs of CD4-CD8 peptides, which were then each covalently coupled to an Nε-palmitoyl-lysine moiety, a Toll-like receptor 2 (TLR-2) ligand. This resulted in the construction of 3 CD4-CD8 lipopeptide vaccines. Latently infected HLA Tg rabbits were immunized with a mixture of these 3 ASYMP lipopeptide vaccines, delivered as eye drops in sterile phosphate-buffered saline (PBS). The ASYMP therapeutic vaccination (i) induced HSV-specific CD8+ T cells that prevent HSV-1 reactivation ex vivo from latently infected explanted trigeminal ganglia (TG), (ii) significantly reduced HSV-1 shedding detected in tears, (iii) boosted the number and function of HSV-1 gD epitope-specific CD8+ T cells in draining lymph nodes (DLN), conjunctiva, and TG, and (iv) was associated with fewer exhausted HSV-1 gD-specific PD-1+ TIM-3+ CD8+ T cells. The results underscore the potential of an ASYMP CD8+ T-cell epitope-based therapeutic vaccine strategy against recurrent ocular herpes.
IMPORTANCE Seventy percent to 90% of adults harbor herpes simplex virus 1 (HSV-1), which establishes lifelong latency in sensory neurons of the trigeminal ganglia. This latent state sporadically switches to spontaneous reactivation, resulting in viral shedding in tears. Most blinding herpetic disease in humans is due to reactivation of HSV-1 from latency rather than to primary acute infection. To date, there is no licensed therapeutic vaccine that can effectively stop or reduce HSV-1 reactivation from latently infected sensory ganglia and the subsequent shedding in tears. In the present study, we demonstrated that topical ocular therapeutic vaccination of latently infected HLA transgenic rabbits with a lipopeptide vaccine that contains exclusively human “asymptomatic” CD8+ T-cell epitopes successfully decreased spontaneous HSV-1 reactivation, as judged by a significant reduction in spontaneous shedding in tears. The findings should guide the clinical development of a safe and effective T-cell-based therapeutic herpes vaccine.
INTRODUCTION
A staggering 1 billion individuals worldwide currently carry herpes simplex virus 1 (HSV-1) which causes a wide range of diseases throughout their lives (1–5). Following ocular or oro-facial primary infection, HSV-1 establishes latency in sensory neurons of the trigeminal ganglia (TG) (6). Most herpetic disease is due to viral reactivations from latency rather than to primary acute infection (7, 8). Sporadic spontaneous reactivation of HSV-1 from latently infected TG, which leads to return of infectious virus to the eye and produces viral shedding in tears, occurs in asymptomatic individuals and can cause recurrent herpes stromal keratitis (HSK), a blinding ocular disease (9). Current antiviral drug therapies (e.g., acyclovir and derivatives) reduce recurrent herpetic disease by ∼45% and do not eliminate virus reactivation (10). An effective immunotherapeutic vaccine able to prevent HSV-1 reactivation from latently infected neurons of TG, the root of the disease, would be a powerful and cost-effective means to prevent viral shedding in tears and reduce recurrent herpetic diseases and blindness (reviewed in reference 1).
A major gap in our current knowledge of ocular herpes infection and immunity is how we can prevent or significantly reduce HSV-1 shedding in tears due to spontaneous reactivation. The virus, the latently infected neuron, and the host immunosurveillance all appear to be involved in the regulation of the HSV-1 latency/reactivation cycle (11). The present study focuses mainly on the role of host immunosurveillance, and particularly the role of HSV-1 human epitope-specific CD8+ T cells, in protection against virus reactivation from latently infected TG (in vitro) and viral shedding in tears (in vivo). HSV-specific CD8+ T cells producing gamma interferon (IFN-γ) and granzyme B (GzmB) appear to decrease induced HSV-1 reactivation in vitro in explanted mouse TG (11). Unfortunately, in vivo reactivation and spontaneous HSV-1 shedding and recurrent eye disease are extremely rare in mice (12–14), so the relevance of these findings to in vivo HSV-1 spontaneous reactivation remains to be determined. Traditional vaccines, although protective against primary acute infection in mice, have failed therapeutically in clinical trials (15, 16) One “common denominator” among previously failed clinical trials is that they used either the whole virus or whole HSV proteins (e.g., HSV glycoprotein D [gD]), which deliver protective epitopes, nonprotective epitopes, and maybe even pathogenic epitopes (i.e., infection- or disease-enhancing epitopes) (reviewed in reference 17). Thus, although these traditional vaccines were intended to target only HSV-specific protective immunity, antigen processing might have also generated HSV-derived epitopes that elicit nonprotective responses and possibly even harmful responses (1). We recently found that symptomatic (SYMP) patients (with a history of numerous episodes of recurrent ocular herpes disease) tend to develop CD8+ T cells that strongly recognize a subset of HSV-1 gD epitopes that differs from HSV-1 gD epitopes that are strongly recognized by CD8+ T cells from HSV-1-seropositive healthy asymptomatic (ASYMP) individuals (who have never had clinical herpes disease), and vice versa (1–5). The former epitopes are designated “symptomatic” (SYMP) epitopes, and the latter are designated “asymptomatic” (ASYMP) epitopes. In the present study, we hypothesized that, compared to that with human HSV-1 gD SYMP epitopes, therapeutic immunization with human HSV-1 gD ASYMP epitopes will reduce shedding of reactivated infectious viral particles in tears and lessen recurrent ocular herpetic disease.
To test this hypothesis, we used our recently developed HLA-A*02:01 transgenic rabbit (HLA Tg rabbit) model of ocular herpes (9), which, similar to humans, (i) develops spontaneous (i.e., not induced) HSV-1 reactivation and recurrent ocular herpetic disease (9) and (ii) mounts “humanized” CD8+ T-cell responses to human epitopes (9). We report that therapeutic immunization of latently infected HLA Tg rabbits with a mixture of three human HSV-1 gD ASYMP epitopes, but not with HSV-1 gD SYMP epitopes, induced strong, polyfunctional local HSV-specific CD8+ T-cell responses, which were associated with a decrease of spontaneous recurrent ocular shedding in tears and a reduction of recurrent corneal herpetic disease. To our knowledge, the findings demonstrate, for the first time, the safety, immunogenicity, and protective efficacy of an ASYMP epitope therapeutic ocular herpes vaccine strategy.
MATERIALS AND METHODS
HLA-A*02:01 transgenic rabbits.
A colony of human leukocyte antigen (HLA) transgenic (Tg) rabbits maintained at the University of California Irvine were used for all experiments. HLA Tg rabbits were derived from New Zealand White rabbits (18). The HLA Tg rabbits retain their endogenous rabbit major histocompatibility complex (MHC) locus and express human HLA-A*02:01 under the control of its normal promoter (18). Prior to this study, the expression of HLA-A*02:01 molecules on the peripheral blood mononuclear cells (PBMC) of each HLA Tg rabbit was confirmed by fluorescence-activated cell sorter (FACS) analysis. Briefly, rabbit PBMC were stained with 2 μl of anti-HLA-A2 monoclonal antibody (MAb) (clone BB7.2; BD-Pharmingen, USA) at 4°C for 30 min. The cells were washed and analyzed by flow cytometry using an LSRII instrument (Becton Dickinson, Mountain View, CA). The acquired data were analyzed with FlowJo software (TreeStar, Ashland, OR). Rabbits with high-level HLA expression in fewer than 90% of PBMC were not used in these studies. Thus, all of the HLA rabbits used had similar high-level expression of HLA-A*02:01. This avoided potential bias due to variability of HLA-A*0201 molecule levels in different animals. New Zealand White rabbits (non-Tg control rabbits), purchased from Western Oregon Rabbit Co. (WORC), were used as controls.
HSV-1.
The McKrae strain of herpes simplex virus 1 (HSV-1) was used in this study. The virus was triple plaque purified and prepared as previously described (7, 8, 19).
SYMP and ASYMP lipopeptide vaccines.
Three different CD4-CD8 lipopeptide constructs were synthesized by Mgenex Biosciences, San Diego, CA. Each ASYMP lipopeptide vaccine contains one fixed CD4+ T-cell epitope (gD287–317) and three variable ASYMP CD8+ T cells epitopes (gD53–61, gD70–78, and gD278–286) from HSV-1 gD (see Fig. 2A and B). Each SYMP lipopeptide vaccine contain one fixed CD4+ T-cell epitope (gD287–317) and three variable SYMP CD8+ T cells epitopes (gD95–103, gD153–161, or gD253–261) from HSV-1 gD. All peptides and lipopeptides were purified by high-pressure liquid chromatography (HPLC), with a purity of 95% to 98%.
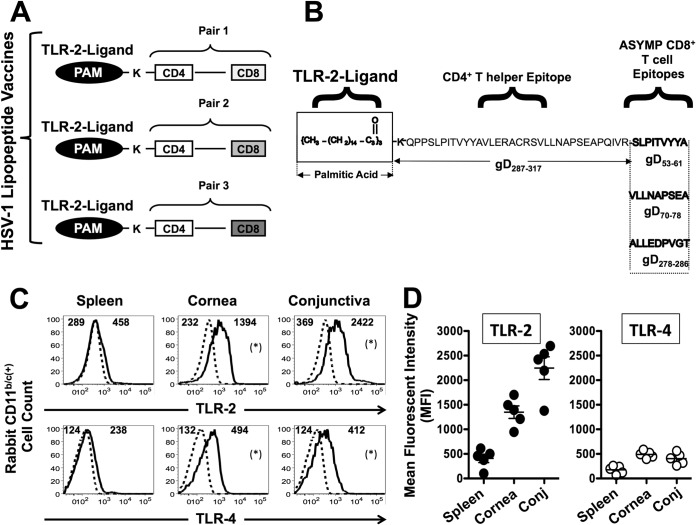
FIG 2.
ASYMP HSV-1 gD lipopeptide vaccine targeting ocular surface-derived APCs highly expressing TLR-2. (A) Schematic representation of prototypes of human ASYMP CD4-CD8 lipopeptide vaccines. The C-terminal end of a promiscuous CD4+ T-cell peptide epitope (gD287–317) was covalently joined with the N-terminal end of one of three different HSV-1 ASYMP gD CD8 T-cell epitopes: gD53–61, gD70–78, or gD278–286. The N-terminal end of each resulting CD4-CD8 peptide was extended by a lysine covalently linked to a TLR-2 ligand (one molecule of palmitic acid [PAM]). This results in 3 separate pairs of ASYMP CD4-CD8 lipopeptides. (B) Amino acid sequence of each CD4+ and CD8+ T-cell epitope. (C) Representative FACS data showing that high levels of Toll-like receptor 2 (TLR-2) were expressed by rabbit cornea- and conjunctiva-derived CD11b/c+ antigen-presenting cells (APCs) isolated from naive conjunctiva (106 per assay) first surface stained with 1 μg/ml of anti-rabbit CD11b/c-FITC MAb and then intracellularly stained with 3 μg/ml of PE-labeled MAbs specific to either TLR-2 or TLR-4 (thick black lines) or with a PE-labeled isotype control MAb (dotted black lines). The numbers above each histogram plot represent the intensity of expression of each TLR, represented by mean fluorescence intensity (MFI). (D) Average expression of TLR-2 (black circles) and TLR-4 (white circles) in spleens, corneas, and conjunctivas of five rabbits. *, P < 0.05 compared to the MFI of TLR-2 expression to the isotype control. The results are representative of two independent experiments.
Establishment and selection of latently HSV-1-infected HLA Tg rabbits and therapeutic immunization.
We selected and infected HLA Tg rabbits with the highest expression of HLA-A*02:01 molecules. The higher expression of HLA-A*02:01 molecules is expected to (i) force rabbit CD8+ T cells to make use of the human HLA-A*02:01 molecules at both the thymic educational and peripheral effector levels (9) and (ii) minimize the competition of rabbits' own MHC class I molecules with the human HLA-A*02:01-restricted responses (9). Our group has used HSV-1 strain McKrae in the rabbit model for 40 years. McKrae has a high spontaneous reactivation rate in rabbit eyes and, unlike most other strains, does not require corneal scarification for efficient ocular infection. This eliminates problems related to scoring eye disease due to corneal damage by scratches.
Without corneal scarification, female HLA Tg rabbits (8 to 10 weeks) were ocularly infected by eye drops (both eyes) of 5 μl of tissue culture medium containing 2 × 105 PFU of HSV-1 strain McKrae on day 0, as we previously described (20). At the dose used (2 × 105 PFU/eye) all surviving rabbits harbor latent virus in both TG with high spontaneous reactivation and shedding in tears of both eyes (7, 8, 19, 21, 22).
Acute ocular infection was confirmed by HSV-1-positive tear film cultures collected on days 3 to 4 postinfection (p.i.). Prior to therapeutic vaccination, the latently infected rabbits were divided into three similar groups of 20 animals, based on severity of the acute infection (similar virus levels in tears and similar severity of corneal disease during the acute phase of HSV-1 infection), as we have previously done (7–9, 19). Thus, the test and control groups have animals with similar spectra of acute virus replication and eye disease. On day 28, once latency was well established, two drops (25 μl each) of SYMP or ASYMP gD lipopeptide–phosphate-buffered saline (PBS) formulation or control (PBS alone) was administered topically to both eyes (see Fig. 3). This was repeated on day 49 (first boost) and day 70 (second boost) p.i. Rabbits were treated in accordance with Association for Research in Vision and Ophthalmology (ARVO), Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC), and National Institutes of Health (NIH) guidelines.
FIG 3.
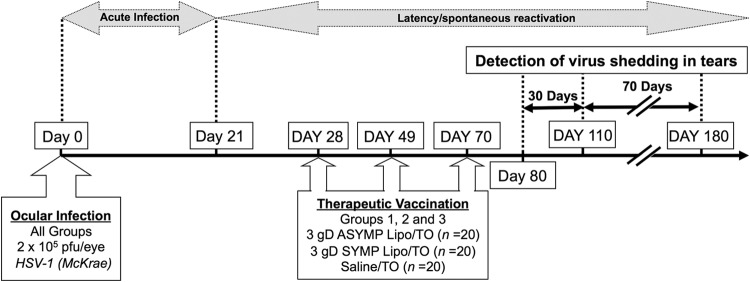
Immunotherapeutic regimen of HLA transgenic rabbits latently infected with McKrae.
PBMC isolation.
Twenty milliliters of blood was drawn from each rabbit into yellow-top Vacutainer tubes (Becton Dickinson, USA). The serum was isolated and centrifuged for 10 min at 800 × g. The peripheral blood mononuclear cells (PBMC) were isolated by gradient centrifugation using leukocyte separation medium (Cellgro, USA). The cells were washed in PBS and resuspended in complete culture medium consisting of RPMI 1640 medium containing 10% fetal bovine serum (FBS) (Bio-Products, Woodland, CA, USA) supplemented with 1× penicillin–l-glutamine–streptomycin, 1× sodium pyruvate, 1× nonessential amino acids, and 50 μM 2-mercaptoethanol (Life Technologies, Rockville, MD, USA). Aliquots of freshly isolated PBMC were also cryopreserved in 90% fetal calf serum (FCS) and 10% dimethyl sulfoxide (DMSO) in liquid nitrogen for future testing.
Flow cytometry analysis.
PBMC were analyzed by flow cytometry. The following anti-rabbit and anti-human antibodies were used: mouse anti-rabbit CD8 (clone MCA1576F; AbD Serotec), mouse anti-rabbit CD4 (clone MCA799F; AbD Serotec), hamster anti-mouse PD-1 (clone J43; BD-Pharmingen), anti-human Tim-3 (clone F38-2E2; BioLegend), anti-human/mouse granzyme B (clone GB1; BioLegend), rat anti-mouse IFN-γ (clone XMG1.2; BD-Pharmingen), mouse anti-human CD45RB (clone MEM-55; AbD Serotec), mouse anti-rabbit CD25 (clone KEI-alpha1; AbD Serotec), and rat anti-mouse CD11b (clone M1/70.15; AbD Serotec). For surface staining, MAbs against various cell markers were added to a total of 1 × 106 cells in phosphate-buffered saline containing 1% FBS and 0.1% sodium azide (fluorescence-activated cell sorter [FACS] buffer) and left for 45 min at 4°C. After washing with FACS buffer, cells were permeabilized for 20 min on ice using the Cytofix/Cytoperm kit (BD Biosciences) and then washed twice with Perm/Wash buffer (BD Bioscience). For intracellular staining, GzmB, Toll-like receptor 2 (TLR-2) and TLR-4 MAbs were added to the cells and incubated for 45 min on ice in the dark. Cells were washed again with Perm/Wash and FACS buffer and fixed in PBS containing 2% paraformaldehyde (Sigma-Aldrich, St. Louis, MO). For the measurement of IFN-γ, cells were in vitro stimulated with SYMP and ASYMP peptides. Briefly, 1 × 106 cells were transferred into 96-well flat bottom plates and stimulated with SYMP and ASYMP peptides (10 μg/ml in 200 μl complete culture medium) in the presence of BD Golgi stop (10 μg/ml) for 6 h at 37°C. Phytohemagglutinin (PHA) (5 μg/ml) (Sigma) and no peptide were used as positive and negative controls, respectively. At the end of the incubation period, the cells were transferred to a 96-well round-bottom plate and washed once with FACS buffer. Surface staining and intracellular staining were done as described above. A total of 50,000 events were acquired with the LSRII (Becton Dickinson, Mountain View, CA), followed by analysis using FlowJo software (TreeStar, Ashland, OR).
Preparation of single-cell suspensions from rabbit conjunctiva.
The bulbar and palpebral conjunctiva tissue from rabbit eyes was excised and collected in Hanks balanced salt solution. Conjunctiva tissues were spun down at 1,600 rpm for 5 min at 4°C and digested with collagenase type I (GIBCO, Carlsbad, CA) at 3 mg/ml for 3 h at 37°C with occasional vortexing every 15 min. The digested tissue suspension was passed through a 70-μm nylon cell strainer and spun down for 5 min at 4°C. This process was repeated two times. The final pellet was resuspended in Hanks balanced salt solution, passed through a 40-μm cell strainer, and spun down for 5 min at 4°C. This process was repeated two times. The final pellet was resuspended in complete RPMI 1640 medium and kept on ice. The live cells were counted using a hemocytometer.
Tetramer assay.
Rabbits were euthanized, draining lymph nodes (DLN), conjunctiva, and trigeminal ganglia (TG) were individually harvested, and single-cell suspensions were prepared. Cells were analyzed for the frequency of CD8+ T cells specific to each of the three immunizing CD8+ T-cell epitopes using the corresponding HLA-A2-peptide/tetramer as we previously described (4, 23, 24). A human beta-2-microglobulin was incorporated in the tetramers, as no rabbit beta-2-microglobulins are currently available. Briefly, the cells were first incubated with 1 μg/ml of phycoerythrin (PE)-labeled HLA-A2-peptide/tetramer at 37°C for 30 to 45 min. The cells were washed twice and stained with 1 μg/ml of fluorescein isothiocyanate (FITC)-conjugated mouse anti-rabbit CD8 MAb (clone MCA1576F; Serotec). After 2 additional washings, cells were fixed with 1% formaldehyde in phosphate-buffered saline. A total of 50,000 events were acquired with the LSRII (Becton Dickinson, Mountain View, CA), followed by analysis using FlowJo software (TreeStar, Ashland, OR). The absolute numbers of gD-peptide-specific CD8+ T cells were calculated using the following formula: (number of events in CD8+/tetramer+ cells) × (number of events in gated lymphocytes)/(number of total events acquired).
Quantification of infectious virus.
Tears from both eyes were collected by swabbing with a Dacron swab (type 1; Spectrum Laboratories, Los Angeles, CA) daily for 30 days postimmunization and then every other day from day 58 to 128 postimmunization. Individual swabs were transferred to a 2-ml sterile cryogenic vial containing 500 μl culture medium and stored at −80°C until use. The HSV-1 titers in tear samples were determined by standard plaque assays on rabbit skin (RS) cells as previously described (20).
Detecting HSV-1 reactivation from ex vivo TG cultures.
Following bilateral HSV-1 corneal infection, TG from SYMP and ASYMP vaccinated HLA Tg rabbits were removed on day 30 p.i. and the TG cell suspensions dispersed, as previously described (11). Dissociated TG cells were suspended in Dulbecco modified Eagle medium (DMEM) containing complete culture medium, as described above. A third of each suspension was removed for phenotypic and functional analysis of CD8+ T cells. The remaining cells were split into three aliquots that were supplemented with anti-CD8, anti-CD4 MAb or isotype IgG control (at 100 μg/ml). Pools of 2 TG were then dispersed into 10 cultures (0.2 TG/culture well). Individual culture wells were monitored for reactivation by serially testing supernatant fluid for live virus using a standard plaque assay on RS cell monolayers, as we previously described (3, 25).
Statistical analyses.
Data for each assay were compared by analysis of variance (ANOVA) and Student's t test using GraphPad Prism version 5 (GraphPad, La Jolla, CA). Differences between the groups were identified by ANOVA and multiple-comparison procedures, as we previously described (24). Data are expressed as the mean ± standard deviation (SD). Results were considered statistically significant at a P value of <0.05.
RESULTS
Selection of HLA-A*02:01 Tg rabbits for preclinical evaluation of HSV-1 ASYMP CD4-CD8 lipopeptide therapeutic vaccines against ocular herpes.
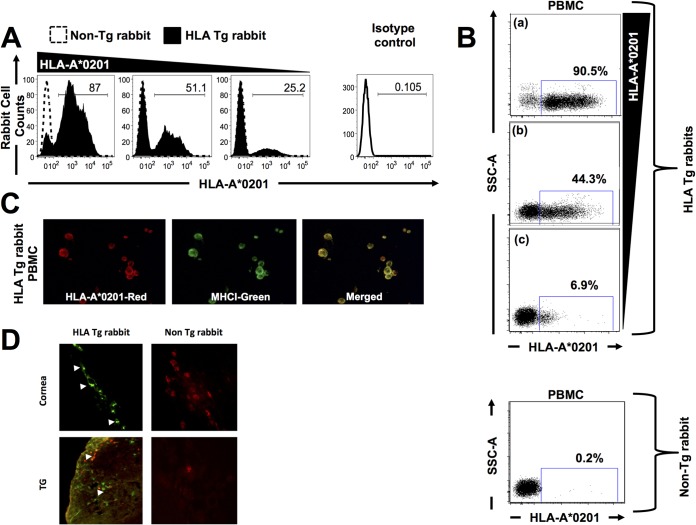
We first selected HLA Tg rabbits with the highest expression of HLA-A*02:01 molecules, since expression of the rabbits' own MHC class I molecules might interfere with the human HLA-A*02:01-restricted responses (9). High and specific expression of HLA-A*02:01 molecules was detected by FACS (Fig. 1A and B) and by immunostaining of PBMC (Fig. 1C), corneas (Fig. 1D), and trigeminal ganglia (TG) (Fig. 1D) of HLA Tg rabbits. As expected, no HLA-A*02:01 expression was detected in wild-type (nontransgenic) rabbits (negative controls). The specificity of anti-human HLA-A*02:01 antibody was confirmed using an isotype IgG control. These results strongly suggest ubiquitous expression of HLA-A*02:01 molecules in these HLA Tg rabbits. While up to 90.5% of PBMC express HLA-A*02:01 molecules, the level of expression appeared to vary among different rabbits (Fig. 1A and B). The higher expression of HLA-A*02:01 molecules in selected HLA Tg rabbits is expected to force rabbit CD8+ T cells to make use of the human HLA-A*02:01 molecules at both the thymic selection and peripheral effector levels (9).
FIG 1.
Detection of HLA-A*02:01 molecules in PBMC, corneas, and trigeminal ganglia of HLA transgenic rabbits. (A and B) Peripheral blood mononuclear cells (PBMC) from either HLA transgenic (HLA Tg) rabbits or wild-type nontransgenic rabbits were stained with PE-conjugated anti-HLA-A2 MAb, (clone BB7.2) and analyzed by flow cytometry. (C) Coexpression of HLA-A*02:01 (red) and rabbit MHC class I (green) on the surface of a PBMC derived from an HLA Tg rabbit and detected by fluorescence microscopy (magnification, ×20). PBMC from the HLA Tg rabbit were double stained with two different primary antibodies, HLA-A2.1 MAb (BB7.2) and a rabbit MHCI, followed by staining with secondary antibodies conjugated with either Alexa Fluor 594 (red) or Alexa Fluor 488 (green), respectively. Merged yellow signals indicate the colocalization of these two molecules on the surface of PBMC. (D) Corneal and TG sections from either HLA transgenic rabbits or wild-type nontransgenic rabbits were immunostained with FITC (green)-conjugated BB7.2 MAb (anti-human HLA-A*02:01) and analyzed by fluorescence microscopy (see Materials and Methods). Cell nuclei are shown in red.
Construction of HSV-1 CD4-CD8 lipopeptide vaccines bearing human ASYMP CD8+ T-cell epitopes: choice of TLR-2 ligand-peptide conjugate vaccines for topical ocular (TO) delivery.
Three asymptomatic (ASYMP) CD8+ T-cell epitopes (gD53–61, gD70–78, and gD278–286) were selected from the HSV-1 glycoprotein gD, based on their strong recognition by T cells from “naturally” protected HLA-A*02:01-positive healthy ASYMP individuals (who, despite being HSV-1 seropositive, have never had clinical herpes disease) (4, 23). Since the induction of effector and maintenance of memory CD8+ T cells requires CD4+ T-cell help (1, 2), it was important to link each CD8+ T-cell epitope with a CD4+ T-cell epitope. Therefore, one promiscuous CD4+ T-cell epitope (gD287–317) was linearly joined with each ASYMP human CD8+ T-cell epitope (gD53–61, gD70–78, or gD278–286) to make 3 different pairs of ASYMP CD4-CD8 epitope peptides (Fig. 2A and B).
To enhance their immunogenic potential without having to add an external immunoadjuvant, which might be toxic to the eyes, each pair of ASYMP CD4-CD8 epitope peptides was covalently linked at the N-terminal end to an Nε-palmitic acid-lysine moiety (PAM) (Fig. 2A and B). We choose the Nε-palmitic acid-lysine residue (a TLR-2 ligand) to better target the ASYMP CD4-CD8 peptide vaccine to the local ocular mucosal immune system, since, as shown in Fig. 2C and D, high levels of Toll-like receptor 2 (TLR-2), but not Toll-like receptor 4 (TLR-4), were expressed by rabbit cornea- and conjunctiva-derived CD11b/c+ antigen-presenting cells (APCs) (26). The final three TLR-2 ligand-peptide conjugate constructs (i.e., CD4-CD8 lipopeptide vaccines) were made using a chemoselective ligation method (25), which provides higher yield, purity, and solubility than the traditional method of lipopeptide synthesis. Unlike the “first generation of lipopeptides,” which were synthesized using classic solid-phase methods in which the fatty acyl moiety was introduced directly onto the crude peptide backbone before its purification, the lipopeptide vaccines used in this study were constructed by synthesizing and purifying the peptide and then ligating the lysine-lipid moiety with the peptide backbone. This process yields lipopeptides that are fully soluble in PBS at concentrations up to 3 mg/ml.
As negative controls, three different pairs of symptomatic (SYMP) CD4-CD8 lipopeptide vaccines were also constructed using the same approach and the same CD4+ T-cell epitope. These SYMP lipopeptide vaccines contain human gD SYMP epitopes (gD95–103, gD153–161, or gD253–261) that were recognized mainly by CD8+ T cells from SYMP patients (with a history of numerous episodes of recurrent ocular herpetic disease) (4, 23, 24).
Therapeutic immunization of latently infected HLA Tg rabbits with a mixture of three HSV-1 ASYMP CD4-CD8 lipopeptide vaccines decreases spontaneous viral shedding in tears.
In an initial experiment, we performed a dose-response study using 50-, 100-, or 200-μg doses of each lipopeptide following topical ocular (TO) (eye drop) administration in HLA Tg rabbits. There were no obvious vaccine-related severe side effects with any of the lipopeptides at any dose, as evaluated by weight loss or local inflammation at the delivery-ocular site or obvious corneal or conjunctival irritations. Accordingly, subsequent experiments were performed using the middle dose of 100 μg.
A cohort of 60 HLA Tg rabbits with bilateral HSV-1 latent infections of the TG was established by infecting both eyes with HSV-1 (2 × 105 PFU, strain McKrae), as described in Materials and Methods. The latently infected HLA Tg rabbits were then divided into three groups of age-matched animals (n = 20 each) and received TO (eye drop) administration of a mixture of 3 HSV-1 gD ASYMP lipopeptide vaccines delivered in PBS (test), a mixture of 3 HSV-1 gD SYMP lipopeptide vaccines delivered in PBS (negative control), or PBS alone (mock vaccinated) on days 28, 49, and 70 postinfection, as illustrated in Fig. 3. Since the level of expression appeared to vary among different rabbits (Fig. 1A and B) and to avoid any biases of protection by the level of HLA expression, the HLA Tg rabbits in each group with the highest levels (>90%) of expression of HLA-A*02:01 molecules were equally distributed in each group. Virus shedding in tears (due to spontaneous reactivation) was monitored daily for 30 days starting 10 days after the final immunization (i.e., from day 80 to day 110 postinfection) by collecting tear films from both eyes (1,200 tear films; 600 rabbit days) and individually plating on indicator cells to assay for the presence of reactivated (recurrent) virus, as described in Materials and Methods.
As shown in Table 1, the ASYMP lipopeptide-vaccinated group had a significant decrease in spontaneous reactivation (judged by shedding of reactivated infectious virus in tears) during the initial 30-day monitoring period compared to that in the mock-vaccinated group. This result was obtained regardless of whether the analysis was done using cumulative days in which rabbits were virus positive (shed virus in tears), the number of eyes with at least one episode of spontaneous reactivation, or the number of rabbits with at least one episode of spontaneous reactivation (P < 0.0001, P < 0.0001, and P ≤ 0.0008, respectively). In contrast, the SYMP lipopeptide-vaccinated group was not significantly different from the mock-vaccinated group in regard to spontaneous reactivation (P > 0.05). During the initial 30-day monitoring period, the amount of infectious virus in the virus-positive tear samples was also determined by standard plaque assays. The maximum amount of infectious virus for each eye that shed virus was reduced in the ASYMP-immunized group compared to the mock and SYMP groups (P < 0.003) (Fig. 4A). These results demonstrate that immunization with human ASYMP CD8+ T-cell epitopes, but not with SYMP CD8+ T-cell epitopes, decreased the level of spontaneously reactivated HSV-1 detected in tears of latently infected HLA Tg rabbits.
TABLE 1.
Therapeutic immunization with HSV-1 “asymptomatic” CD4-CD8 lipopeptide vaccine protected latently infected HLA Tg rabbits against shedding of reactivated virus in tearsa
| Treatment | Cumulative no. of days in which rabbits were virus positive/total rabbit daysb | No. of positive eyes/total no. of eyesc | No. of positive rabbits/total no. of eyesd |
|---|---|---|---|
| ASYMP lipopeptide vaccine | 30/1,200 (P < 0.0001) | 8/40 (P < 0.0001) | 5/20 (P < 0.0008) |
| SYMP lipopeptide vaccine | 90/1,200 (P > 0.05) | 38/40 (P > 0.05) | 19/20 (P > 0.05) |
| Mock | 80/1,200 | 40/40 | 20/20 |
HLA Tg rabbits were inoculated with 2 × 105 PFU of HSV-1 strain McKrae in both eyes. At 28 days after virus inoculation, when latency was well established, 20 rabbits were immunized with a mixture of 3 ASYMP CD4-CD8 gD lipopeptide human epitopes, with a mixture of 3 SYMP CD4-CD8 gD lipopeptide human epitopes, or with PBS alone 3 times at 3-week intervals as described in Fig. 3. The presence of infectious spontaneously reactivated virus in tears was determined by collecting tears from all eyes daily for 30 days beginning 1 day after the final immunization.
Rabbits were scored positive if either eye contained reactivated virus on the day of collection. P values were determined by chi-square test.
Total number of eyes with at least one virus-positive culture/total number of eyes. P values were determined by the Fisher exact test.
Total number of rabbits with at least one virus-positive eye culture/total number of rabbits. P values were determined by the Fisher exact.
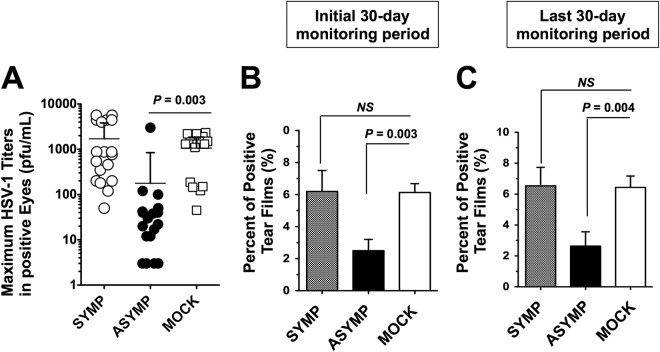
FIG 4.
Therapeutic immunization of latently infected HLA Tg rabbits with HSV-1 ASYMP lipopeptide vaccine decreases virus spontaneous virus shedding in tears. Ocularly infected HLA Tg rabbits were immunized with a mixture of 3 SYMP lipopeptide vaccines or a mixture of 3 ASYMP lipopeptide vaccines delivered as eye drops in sterile PBS or with PBS alone (mock), as illustrated in Fig. 3. HSV-1 shedding detected in tears and reduced recurrent ocular herpetic disease were monitored for 100 days postimmunization, as described in Materials and Methods. (A) Maximal viral loads detected during the initial 30-day monitoring period in eye swabs. (B) Percentage of positive tears during the initial 30-day monitoring period. (C) Percentage of positive tears during the last 30-day monitoring period.
To determine the longevity of the ASYMP lipopeptide-vaccine, we continued to collect tears every other day for an additional 70-day monitoring period, as illustrated in Fig. 3. During the initial 30-day monitoring period, 6.1% of the tear films from control mock-vaccinated eyes contained reactivated virus (positive/total eye cultures) (Fig. 4B). In contrast, only 2.4% of the tear films from ASYMP lipopeptide-vaccinated eyes contained recurrent virus. This was significantly less than in the mock-vaccinated group (P < 0.003). On the other hand, in the SYMP lipopeptide-vaccinated group, 6.6% of the tear films contained recurrent virus (Fig. 4B). Moreover, during the last 30-day monitoring period, 6.5% of the tear films from control mock-vaccinated eyes contained reactivated virus (positive/total eye cultures) (Fig. 4C). In contrast, only 2.6% of the tear films from ASYMP lipopeptide-vaccinated eyes contained recurrent virus. On the other hand, in the SYMP lipopeptide-vaccinated group, 7.1% of the tear films contained recurrent virus (Fig. 4C). These results do the following: (i) indicate that the ASYMP lipopeptide vaccine reduced spontaneous reactivation by 4- to 5-fold, which is much higher than previously reported in rabbits that were immunized with partial or whole gD protein, which delivers both SYMP and ASYMP CD8+ T-cell epitopes (7, 8, 19); (ii) provide tangible evidence that therapeutic immunization using an ASYMP CD8+ T-cell epitope-based vaccine strategy is successful in decreasing spontaneous HSV-1 reactivation, as judged by a significant reduction in shedding of reactivated infectious virus in tears; and (iii) suggest that the ASYMP lipopeptide vaccine, but not the SYMP lipopeptide vaccine, induced long-lasting protective immunity against virus reactivation and shedding of the virus in tears that lasted throughout a period of 100 days postvaccination in HLA Tg rabbits. One hundred days in a rabbit's life span is roughly equivalent to 10 years in a human's life span.
Altogether, these results underscore the potential of ASYMP lipopeptide therapeutic vaccines using human ASYMP CD8+ T-cell epitopes in decreasing detectable reactivated virus in tears and hence recurrent herpetic diseases.
Therapeutic vaccination with HSV-1 ASYMP CD4-CD8 lipopeptide vaccine boosts the number and function of local HSV-specific CD8+ T cells in protected HLA Tg rabbits.
We next determined whether the observed protection against recurrent ocular herpes is associated with enhancement of local HSV-specific CD8+ T-cell responses following therapeutic immunization with ASYMP vaccine. Antibody depletion of CD8+ T cells is often used in mice to determine if a protective immunity is CD8+ T-cell dependent. Unfortunately, at this time, in vivo CD8+ T-cell depletion studies are not feasible in rabbits because of the lack of a suitable in vivo depleting antibody. Thus, we determined correlations of the number and the function of local HSV-1 gD epitope-specific CD8+ T cells with reduction of spontaneous viral shedding in tears.
Forty latently infected HLA Tg rabbits were immunized with the ASYMP vaccine on days 28, 49, and 70 postinfection, as illustrated in Fig. 3. Tears were collected daily for 30 days, beginning 10 days after the final immunization, and the presence of reactivated (recurrent) virus was determined by collecting and individually plating tear films from both eyes on indicator cells, as described above. The animals were then divided into two groups based on the absence (protected) and presence (unprotected) of virus in tear films. Protected rabbits (n = 10) had no detected virus shedding in tear films from either eye. In contrast, unprotected rabbits shed virus at least once in one or both eyes (n = 10). The frequencies of HSV-1 gD epitope-specific CD8+ T cells were determined in TG, conjunctivas, and draining cervical and periocular draining lymph nodes (DLN) of protected HLA Tg rabbits and compared to those in unprotected HLA Tg rabbits using tetramer assays.
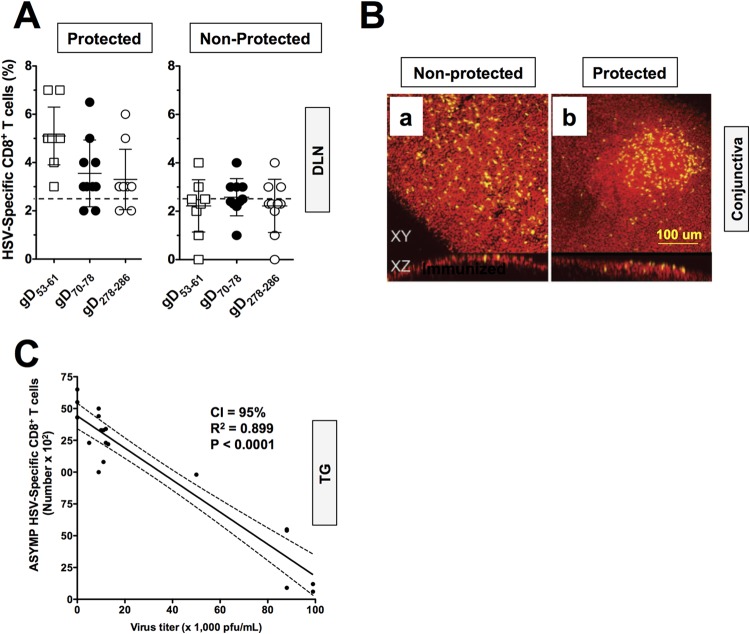
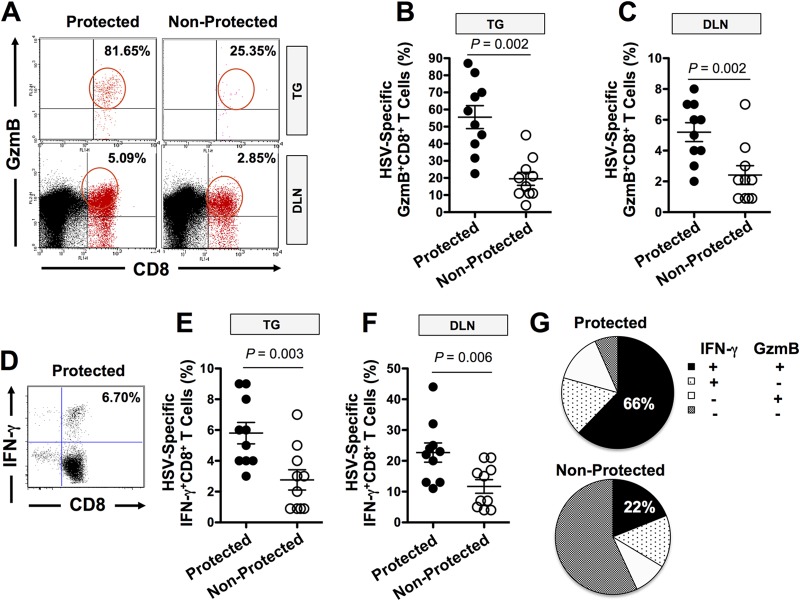
An increase in the number of CD8+ T cells of up to 2-fold was detected in DLN of protected compared to DLN of unprotected HLA Tg rabbits (Fig. 5A). Specifically, after ASYMP lipopeptide immunization, an average of 5.1% of HLA-A*02:01/HSV-gD53–61, 3.3% of HLA-A*02:01/HSV-gD70–78, and 3.1% of HLA-A*02:01/HSV-gD278–286 tetramer-positive CD8+ T cells were detected in the DLN of protected HLA Tg rabbits, compared to only 2.1%, 2.3%, and 2.1%, respectively, in the DLN of unprotected HLA Tg rabbits. Few CD8+ T cells were detected using an irrelevant OVA tetramer, indicating the specificity of the staining (0.2% to 0.3%) (data not shown). As expected, immunized nontransgenic wild-type New Zealand rabbits had insignificant HLA-restricted CD8+ T cells (0.3% to 0.6%) (data not shown) confirming that HSV-restricted CD8+ T cells detected in HLA Tg rabbits were HLA-A*02:01 restricted. An increase in the number of HSV-restricted CD8+ T cells of 2- to 3-fold was also detected in the conjunctivas and corneas of ASYMP lipopeptide-immunized and protected compared to unprotected HLA Tg rabbits (data not shown). Interestingly, aggregates of CD8+ T cells were visualized by microscopy in the conjunctival epithelia of protected rabbits, suggesting an ongoing antigenic stimulation. Figure 5B shows a representative image of a CD8+ T-cell aggregate in “protected” conjunctiva (panel b) compared to dispersed CD8+ T cells in unprotected conjunctiva (panel a). Moreover, high numbers of TG-resident HSV-1 gD epitope-specific CD8+ T cells positively and significantly correlated with low viral shedding in tears of ASYMP lipopeptide-vaccinated and protected HLA Tg rabbits (P < 0.0001) (Fig. 5C). These results point to a boost in the number of local HSV-specific CD8+ T cells by ASYMP lipopeptide vaccine (cells that were originally primed by the virus during acute infection and/or reactivation) in protected, but not in unprotected, HLA Tg rabbits following ASYMP therapeutic vaccination.
FIG 5.
Local HSV-1 gD epitope-specific CD8+ T cells boosted by the ASYMP therapeutic lipopeptide vaccine in latently infected HLA Tg rabbits. Forty latently infected HLA Tg rabbits were immunized with the ASYMP vaccine on days 28, 49, and 70 postinfection, as illustrated in Fig. 3. Tears were collected daily for 30 days beginning 10 days after the final immunization, and the rabbits were divided into two groups. Protected rabbits (n = 10) had no detected virus shedding in either eye. Unprotected rabbits shed virus at least once in one or both eyes (n = 10). The frequency and absolute numbers of CD8+ T cells specific to each HSV-1 gD epitopes in protected versus unprotected HLA Tg rabbits were determined following therapeutic immunization with the ASYMP CD4-CD8 lipopeptide vaccine. CD8+ T cells were counted from DLN from the protected versus unprotected rabbits used for Fig. 4. (A) The cell suspensions were immunostained with an FITC-labeled MAb specific to rabbit CD8 and with a PE-labeled human HLA-A*0201/tetramer specific to each of the three human ASYMP CD8+ T-cell epitopes. The numbers show the percentage of tetramer-positive/CD8+ T cells specific to the gD53–61, gD70–78, or gD278–286 epitope from each protected and unprotected rabbit. (B) Conjunctiva sections from either protected or unprotected HLA Tg rabbits were immunostained with MAb specific to rabbit CD8+ and analyzed by fluorescence microscopy (see Materials and Methods). Panel b shows representative data for CD8+ T cells aggregate in “protected” conjunctiva compared to dispersed CD8+ T cells in unprotected conjunctiva in panel a. Yellow represents stained CD8+ T cells. (C) Correlation of the average number of tetramer-positive/CD8+ T cells in TG specific to three human CD8+ T-cell gD epitopes (gD53–61, gD70–78, and gD278–286) with virus titers detected in tears of latently infected and ASYMP lipopeptide-vaccinated HLA Tg rabbits. The results are representative of two independent experiments.
Correlation of the function of local HSV-specific CD8+ T cells with protection against virus reactivation from TG was also determined. TG, corneas, conjunctivas, and DLN were excised from protected and unprotected HLA Tg rabbits and cell suspensions prepared. The function of CD8+ T cells specific to gD epitopes was detected using specific tetramer together with intracellular staining with GzmB and IFN-γ, as described in Materials and Methods. There was significantly more HSV-specific GzmB-positive CD8+ T cells (Fig. 6A, B, C, and G) and IFN-γ-producing CD8+ T cells (Fig. 6D to F) in both TG and DLN of protected HLA Tg rabbits than in those of unprotected rabbits. A similar result was obtained when corneas and conjunctivas of protected HLA Tg rabbits were compared to those of unprotected rabbits (data not shown). Two-thirds (66%) of HSV-1 gD epitope-specific CD8+ T cells in protected HLA Tg rabbits expressed at least two functions (i.e., production of IFN-γ and cytotoxic activity measured by expression of GzmB) (Fig. 6G). In contrast, fewer than a third (22%) of the HSV-1 gD epitope-specific memory CD8+ T cells in unprotected HLA Tg rabbits expressed two functions. Thus, high proportions of polyfunctional HSV-specific CD8+ T cells coproducing IFN-γ and GzmB (two immune effectors known to be associated with protection against ocular herpes [1–5]) appeared to be associated with protection in HLA Tg rabbits.
FIG 6.
HSV-1 ASYMP CD4-CD8 lipopeptide vaccine-induced CD8+ T-cell responses in protected and unprotected HLA Tg rabbits. (A and D) Representative data for GzmB (A) and IFN-γ (D) and CD8+ T-cell responses in TG and DLN of ASYMP CD4-CD8 lipopeptide-immunized protected and unprotected HLA Tg rabbits. (B, C, E, and F) Individual responses for week 4 in TG and DLN of ASYMP CD4-CD8 lipopeptide-immunized protected and unprotected HLA Tg rabbits. A one-way ANOVA was used to calculate the P values for the comparison of the magnitude of the CD8+ T-cell responses. Horizontal lines indicate medians and vertical lines interquartile ranges. (G) Proportions, at week 4, of HSV-1 gD epitope-specific CD8+ T cells (i.e., cells having at least one function) that make any given combination of the two functions (GzmB cytotoxicity and IFN-γ). The proportions of HSV-1 gD epitope-specific CD8+ T cells capable of simultaneously secreting IFN-γ (IFN+) and expressing cytotoxic activity (GzmB+) in protected versus unprotected HLA Tg rabbits are shown.
Altogether, these results suggest that local cytotoxic and IFN-γ-producing HSV-specific CD8+ T cells might play a role against virus reactivation from TG and, hence, in subsequent reduction of viral shedding/replication in eyes.
HSV-specific CD8+ T cells from unprotected symptomatic HLA Tg rabbits are phenotypically and functionally exhausted.
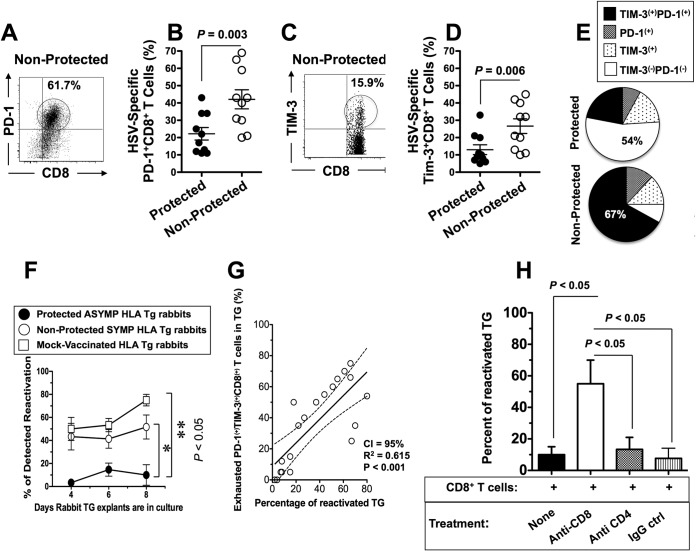
In a final experiment, we explored whether the lack of protection seen in SYMP HLA Tg rabbits would be associated with a dysfunction (i.e., the exhaustion) of HSV-specific CD8+ T cells. We compared the expression of PD-1 and TIM-3, two T-cell-coinhibitory receptors and useful markers to differentiate T cells with the exhausted phenotype, by HSV-1 gD-specific CD8+ T cells from TG of protected (i.e., ASYMP) versus unprotected (i.e., SYMP) HLA Tg rabbits (6). We found significantly higher frequencies of HSV-1 gD epitope-specific CD8+ T cells from TG of unprotected HLA Tg rabbits expressing PD-1 (P < 0.05) (Fig. 7A and B) and TIM-3 (P < 0.05) (Fig. 7C and D) than from TG of protected HLA Tg rabbits. The majority (67%) of HSV-gD epitope-specific CD8+ T cells in TG of unprotected HLA Tg rabbits coexpressed both PD-1 and TIM-3 (Fig. 7E). In contrast, only 22% of HSV-gD epitope-specific CD8+ T cells in TG of protected HLA Tg rabbits coexpressed both PD-1 and TIM-3 (Fig. 7E).
FIG 7.
HSV-specific CD8+ T cells from unprotected symptomatic HLA Tg rabbits are phenotypically and functionally exhausted. (A and B) Representative dot plot (A) and mean (B) of percentages of HSV-1 gD53–61-specific PD-1+ CD8+ T cells in protected (ASYMP) and unprotected (SYMP) HLA Tg rabbits. (C and D) representative dot plot (C) and mean (D) of percentages of HSV-1 gD53–61-specific TIM3+ CD8+ T cells in protected (ASYMP) and unprotected (SYMP) HLA Tg rabbits. (E) Proportions of exhausted HSV-1 gD epitope-specific CD8+ T cells expressing PD-1, TIM3, and both PD-1 and TIM3 in unprotected SYMP HLA Tg rabbits. (F) Percentages of reactivated TG in protected versus unprotected HLA Tg rabbits. (G) Correlation between the percentages of exhausted PD-1+ TIM3+ CD8+ T cells and percentages of reactivated TG. (H) Percentages of reactivated TG in protected HLA Tg rabbits in the presence and absence of anti-CD8 and anti-CD4 MAbs and IgG controls. The results are representative of 2 independent experiments. The indicated P values, calculated using one-way ANOVA, show statistical significance of differences between protected and nonprotected HLA Tg rabbits.
Since phenotypic exhaustion may not always translate into functional exhaustion, we compared the functional abilities of CD8+ T cells from protected versus unprotected HLA Tg rabbits to stop or reduce HSV-1 reactivation ex vivo from infected explanted TG. Compared to DLN, the TG (a nonlymphoid organ) does not contain a sufficient number of CD8+ T cells for in vitro functional testing of blocking of HSV-1 reactivation from explanted TG. Instead of TG-derived CD8+ T cells, we therefore used CD8+ T cells from autologous DLN that were harvested from protected versus unprotected HLA Tg rabbits. CD8+ T cells from protected HLA Tg rabbits, but not those from unprotected HLA Tg rabbits, significantly decreased induced HSV-1 reactivation in vitro in explanted autologous TG (P < 0.05) (Fig. 7F). As expected, similar to explanted TG without any added T cells, CD8+ T cells from infected and mock-vaccinated HLA Tg rabbits and from wild-type nontransgenic rabbits failed to decrease induced HSV-1 reactivation from explanted TG. This suggests that HSV-specific CD8+ T cells induced in HLA Tg rabbits by the vaccine decreased virus reactivation in explanted autologous TG in a major histocompatibility complex (MHC)-dependent manner. A high frequency of HSV-specific PD-1+ TIM-3+ CD8+ exhausted T cells positively correlated with high percentages of TG with reactivated virus (Fig. 7G). Monoclonal antibody blockade of CD8+ T cells, but not of CD4+ T cells, in vitro before preincubation with explanted TG reversed the functional ability of ASYMP CD8+ T cells to reduce virus reactivation (Fig. 7H).
Altogether, these results (i) confirm the ability of HSV-specific CD8+ T cells from protected ASYMP HLA Tg rabbits to reduce virus reactivation from latently infected TG, (ii) indicate that HSV-specific CD8+ T cells from unprotected rabbits are phenotypically and functionally exhausted, (iii) demonstrate that a high frequency of exhausted HSV-specific CD8+ T cells is associated with an inability to reduce virus reactivation from infected TG, and (iv) support the HLA-A*02:01 Tg rabbit as a useful animal model for investigating the underlying mechanisms by which TG-resident CD8+ T cells specific to human HSV-1 CD8+ T-cell epitopes mediate control of spontaneous virus reactivation and recurrent ocular herpetic disease.
DISCUSSION
Following ocular infection, HSV-1 establishes a lifelong latent state in sensory neurons of the TG. Throughout life, this latent state occasionally spontaneously switches to reactivation, resulting in viral shedding in tears that can lead to herpetic stromal keratitis (HSK), a major cause of blinding disease (9). Despite spontaneous reactivation and shedding of virus in tears, the majority of infected individuals remain asymptomatic (ASYMP), while a nonnegligible number of infected individuals (e.g., 20,000 individuals per year in the United States alone) are symptomatic (SYMP) with recurrent, painful, and potentially blinding ocular herpetic lesions (1–5). The SYMP and ASYMP groups are different with regard to the specificity, the magnitude and the nature of their HSV-1 epitope-specific CD8+ T cells (1–5, 27). Thus, a therapeutic vaccine that converts the CD8+ T-cell profile in previously SYMP patients to mimic the presumably protective profile seen in ASYMP individuals may decrease HSV-induced recurrent ocular disease. Since recurrent herpetic ocular disease likely requires the appearance of reactivated virus in tears, a vaccine that decreases the frequency of reactivated virus in tears should also decrease recurrent disease. In the present study, we demonstrated that topical ocular delivery (by eye drops) of lipopeptide vaccines bearing human ASYMP CD8+ T-cell gD epitopes, which are recognized mainly by CD8+ T cells from HSV-1-seropositive healthy ASYMP individuals who have never had clinical herpes disease (6, 7), reduced shedding of reactivated infectious virus in tears of latently infected HLA Tg rabbits (9). This lipopeptide vaccine excludes SYMP epitopes that are recognized by CD8+ T cells from SYMP individuals with a history of numerous episodes of recurrent ocular herpes disease. The ASYMP epitope therapeutic vaccine boosted both the number and function of local HSV-1 gD epitope-specific CD8+ T cells in DLN, conjunctivas, and TG and was associated with a significant reduction in HSV-1 reactivation from latently infected TG as judged by a significant reduction in detectable virus shedding in tears. These results strongly suggest that during latency HSV-1 gD-specific CD8+ T cells directed against ASYMP epitopes play a role in decreasing reactivation. To our knowledge, this is the first report of a successful human CD8+ T-cell epitope-based therapeutic vaccine able to significantly reduce shedding in tears of reactivated virus in any animal model.
Past clinical vaccine trials that used partial or whole HSV-1 gD protein containing both SYMP and ASYMP T-cell epitopes failed to prevent or reduce recurrent herpes (1, 15, 28, 29). These protein-based vaccines were intended to deliver only protective herpes epitopes, but the antigen processing of HSV-1 proteins might also generate epitopes that elicit nonprotective and possibly even harmful (e.g., infection- or disease-enhancing) immune responses. Moreover, these therapeutic vaccines were administered parenterally (e.g., intramuscularly or subcutaneously) and failed to generate significant local T-cell immunity at or near the site of latent infection (i.e., TG) and/or the site at which reactivated virus replicates (i.e., the eye). Our previous preclinical studies in rabbits demonstrated that local immunity is necessary for prevention of HSV-1 reactivation and replication (7, 8, 19). To induce local mucosal immunity, the next generation of needle-free mucosal vaccines is being rationally designed according to rules that govern the way in which the epitopes are recognized and processed by antigen-presenting cells (APCs) and stimulate the mucosal immune system. In the last decade, there has been an interest in targeting toll-like receptors (TLRs) at the mucosal surfaces to induce protective immunity against transmitted infectious diseases, including herpes (reviewed in references 24 and 26). Thus, our recent studies have investigated the patterns of TLRs expressed by APCs from the ocular mucosal surface and have shown that both dendritic cells (DCs) and monocytes/macrophages, derived from the conjunctiva and cornea, abundantly express TLR-2 (26) (Fig. 2C and D). Thus, our ASYMP epitope CD8+ T-cell peptide-based vaccine was covalently linked to a TLR-2 ligand (an Nε-palmitoyl-lysine moiety) and delivered to the mucosal surface of the eye (by eye drops) for efficient delivery and stimulation of local ocular mucosal T-cell responses. An essential feature of lipopeptide immunogenicity is the lipid component, which is thought to enhance antigen uptake, to redirect the peptide epitope to endogenous pathways of antigen presentation, and to induce DC maturation, allowing efficient priming of naive T cells (25, 30, 31). We report that therapeutic vaccination of latently infected HLA Tg rabbits with human HSV-1 CD8+ T-cell epitopes linked to a TLR-2 ligand induced long-lasting protection against recurrent herpes throughout the first 100 days of the monitoring period. One hundred days in a rabbit's life span is roughly equivalent to 10 human years. The HSV-1 ASYMP gD epitope-based lipopeptide vaccine used in this study offers potential advantages over traditional protein vaccines, including high specificity in eliciting protective ASYMP immunity, while avoiding unwanted SYMP immunopathological responses (25, 30, 32, 33). We recently found that immunization with a SYMP HLA-restricted CD8+ T-cell epitope selected from the HSV-1 gK protein exacerbates ocular herpetic disease (34). The self-adjuvanting lipopeptide-based vaccine also offers additional advantages over conventional whole protein-based vaccine approaches, including safety and the capacity of eliciting highly specific immune responses without external and potentially toxic adjuvants (3, 25). Lipopeptide vaccines engineered in this study using chemoselective ligation are simple to produce under good manufacturing practice (GMP) conditions and offer a relatively low-cost epitope delivery system for clinical trials.
Since the “natural” immune responses induced by primary HSV-1 infection do not prevent reactivation from latency, a successful therapeutic vaccine will have to induce an immune response that is qualitatively different and/or quantitatively stronger than naturally produced immunity (35, 36). CD8+ T cells have been found surrounding neurons of latently infected human TG and rabbit TG (11, 37). HSV-specific CD8+ T cells reduce HSV-1 reactivation ex vivo in explanted mouse TG (38) and rabbit TG, as shown in the present study. Based on these in vitro findings, several reports have suggested that boosting HSV-specific CD8+ T cells at the site of latent infection (TG) may reduce reactivation, thereby reducing HSV-1 shedding in tears and HSV-induced recurrent ocular herpetic disease (35, 39, 40). Thus, many vaccine strategies are focused on boosting the number and function of TG-resident CD8+ T cells, hoping to reduce or block future attempts of HSV-1 reactivation. Paradoxically, increasing evidence of a harmful role for CD8+ T cells in the pathology of ocular herpetic disease is also emerging (9). We recently proposed a new concept whereby the wide and different spectrum of herpes diseases, ranging from rare asymptomatic to frequent distressing symptomatic outbreaks, is reflected in different clones of host CD8+ T cells reacting to different sets of HSV-1-specific epitopes from one or several HSV proteins (reviewed in reference 1). Results from the present study support this hypothesis by demonstrating that a therapeutic vaccine, based on human gD epitopes that were recognized mainly by CD8+ T cells from ASYMP patients (4, 23, 24), decreased spontaneous ocular shedding in tears of latently infected HLA Tg rabbits.
A therapeutic herpes vaccine is not expected to “cure” latency by eliminating the latent virus or to eliminate latently infected neurons. This is because during latency there is no viral antigen production, and no viral antigens are detectable on the cell surface. A therapeutic herpes vaccine will likely function by suppressing virus replication and spread once spontaneous reactivation has occurred or by targeting neurons in which the virus has begun to reactivate and which are therefore expressing small amounts of viral antigens. The latter scenario (of targeting early reactivation events) has been referred to as targeting “attempted reactivation” (11). For simplicity, in this study we talk about “preventing or decreasing spontaneous reactivation” when what we really mean is “preventing or decreasing the amount of infectious virus detectable in the eye (tears) following spontaneous reactivation of HSV-1 in TG.” It should be noted that preventing shedding of HSV-1 in tears is important for decreasing HSV-1-induced recurrent disease. It may also be important for preventing spread of virus to others. A therapeutic vaccine that reduces virus shedding in tears may therefore also be useful for increasing “herd immunity.”
The choice of the right animal model currently is a crucial first step in herpes vaccine development (1). The lack of a reliable and proper animal model of spontaneous reactivation that leads to measurable spontaneous virus shedding and recurrent herpetic disease has severely limited the progress in developing a therapeutic ocular herpes vaccine (reviewed in reference 41). The focus of this study was not to prevent primary ocular infection and disease but to develop a therapeutic vaccine that decreases spontaneous herpesvirus shedding in tears and thus should decrease recurrent ocular disease. Thus, a critical question was which animal model would be the most appropriate to assess the efficacy of a human T-cell epitope-based therapeutic vaccine against recurrent ocular herpes infection and disease. The ideal animal model should mount HLA-restricted immune responses specific to human epitopes while mimicking as many aspects of herpesvirus shedding and recurrent disease as possible (42). Mice have been the animal model of choice for most immunologists, and results from mice have yielded tremendous insights into the role of T cells in protection against primary herpes infection (4, 11–13, 23, 43, 44). However, mice present eye disease that is different from that in humans (45, 46), and they do not mount “humanized” HLA-restricted T-cell responses specific to human epitopes (reviewed in reference 41). Although HLA Tg mice can develop T-cell responses to human epitopes, unlike in humans, spontaneous HSV-1 reactivation either does not occur at all or occurs at very low levels (the so-called molecular reactivation) in mice (14). In contrast, spontaneous HSV-1 reactivations occur sporadically in latently infected rabbits, and reactivated infectious virus does return to the eye, can be detected in tear films, and can produce recurrent eye disease (7, 8, 19, 21, 22). Moreover, virus shedding occurs in latently infected rabbits at a rate similar to that in humans (∼10% of tears contain infectious virus) (7, 8, 19, 21, 22). Severe recurrent eye disease also occurs at a rate similar to that in humans. Unfortunately, this rate is much less than 1% of eyes. Thus, the rabbit studies described in this report were unable to directly investigate recurrent eye disease, and decreasing HSV-1 reactivation/shedding, which should decrease recurrent eye disease, was used instead. Direct ocular infection of rabbits results in 100% of surviving animals developing latency in the ophthalmic branches of both TG with a high rate of spontaneous viral reaction (7, 8, 19). From a practical standpoint, rabbit corneas, conjunctivas, and TG are significantly larger than those of mice and offer plentiful amounts of tissues for phenotypic and functional characterization of HSV-specific T cells using individual tissues (21, 22). In addition, compared to those in mice, the surfaces of the rabbit and human eyes are relatively immunologically isolated from systemic immune responses (47). Microanatomy and immunohistological studies indicate that the rabbit ocular mucosal immune system is comparable to that of humans and has a typical follicular ultrastructure with an abundance of “conjunctival lymphoid follicles” (CLF), whereas no lymphoid tissue was identified in mice (33). To overcome the hurdle that rabbits do not mount T-cell responses specific to human HLA-restricted human epitopes, we recently introduced a novel “humanized” HLA Tg rabbit model of ocular herpes where one major player of rabbit CD8+ T cells is replaced by the identical player taken from the human counterpart (i.e., human leukocyte antigen [HLA] class I molecules) (9, 41). This novel HLA Tg rabbit mounts CD8+ T-cell responses specific to HLA-restricted epitopes similar to humans. Since expression of the rabbit's own MHC class I molecules might interfere with the human HLA-A*02:01-restricted responses (9) and to avoid a possible bias introduced by the level of expression of HLA-A*0201 molecules, all HLA rabbits in these studies were preselected as described in Materials and Methods to have similar high expression of HLA-A*02:01 molecules, and therefore all groups of rabbits had similar and uniform high levels of HLA-A*0201 expression. This model also develops spontaneous HSV-1 reactivation with rates similar to those in humans. We previously reported that all gD epitopes that are recognized by CD8+ T cells from HSV-1-infected HLA Tg rabbits are also recognized by CD8+ T cells from HLA-A*0201-positive HSV-seropositive humans (9). Thus, the HLA Tg rabbit model appears to be a useful preclinical model for testing of potential T-cell epitope-based therapeutic vaccine candidates against spontaneous reactivation and recurrent ocular disease. Whether the vaccine results obtained in the HLA Tg rabbit model will correlate with vaccine results in humans remains to be determined in future studies.
Since expression of the HLA Tg rabbits' own MHC class I molecules might interfere with the human HLA-A*02:01-restricted CD8+ T-cell responses (9), in the present study we selected HLA Tg rabbits with high expression of HLA-A*02:01 and low expression of rabbit class I molecules. The higher expression of HLA-A*02:01 molecules is expected to force rabbit CD8+ T cells to make use of the human molecules at both the thymic selection and peripheral effector levels (9). The results reported here support that concept by showing that the selected HLA Tg rabbits did develop strong functional HLA-A*02:01-restricted CD8+ T-cell responses following immunization with human CD8+ T-cell epitopes. Altogether, these results support the HLA-A*02:01 Tg rabbits as a useful animal model for the assessment of the therapeutic efficacy of HLA-A*02:01-restricted epitope-based vaccines against recurrent ocular herpes.
Our ASYMP lipopeptide therapeutic vaccine appeared to reduce spontaneous reactivation by 4- to 5-fold. This is much higher than the 57% reduction we previously reported in rabbits (7, 8, 19) and the ∼58% reduction previously reported in humans (15, 16) using partial or whole gD protein. However, this protection was not full, and there is still room for improvement. The ASYMP lipopeptide therapeutic vaccine did boost the number and function of local HSV-specific CD8+ T cells in protected HLA Tg rabbits, while the number and function of local HSV-specific CD8+ T cells were significantly lower in unprotected HLA Tg rabbits. Interestingly, we found higher frequencies of HSV-1 gD epitope-specific PD1+ CD8+ and TIM-3+ CD8+ T cells in vaccinated unprotected HLA Tg rabbits than in vaccinated protected HLA Tg rabbits, suggesting exhaustion (i.e., dysfunction) of local CD8+ T cells. T cells upregulate multiple inhibitory receptors, including PD-1, TIM-3, 2B4, CTLA-4, CD160, and LAG-3, in response to persistent antigen stimulation (6). Although continuous production of antigens does not occur during latent HSV-1 infections, antigenic stimulation during sporadic reactivations of HSV-1 from latency may lead to T-cell exhaustion (6). Our results suggest that full or partial exhaustion of local CD8+ T cells (e.g., in TG-resident CD8+ T cells directly exposed to virus reactivation) led to loss of protection and may contribute to symptomatic herpes disease. The underlying mechanisms remain to be determined, but it is conceivable that the differences between function versus exhaustion of herpesvirus-specific CD8+ T cells in protected versus unprotected rabbits is related to antigen processing and antigen load. Our finding are in agreement with previous studies in other systems showing higher frequencies of CD8+ T cells expressing PD-1 and TIM-3 associated with virus replication and disease progression (48–51). Our results also highlight the therapeutic potential of blocking PD-1-PDL1, TIM-3-GAL-9, and other immune checkpoints for activating potent CD8+ T-cell responses against recurrent herpes. Thus, we are currently testing whether blockade of PD-1-PDL1 and/or TIM-3-GAL-9 pathways, using specific MAb therapy in combination with therapeutic ASYMP epitope vaccination, will restore the function of exhausted HSV-specific CD8+ T cells in latently infected HLA Tg rabbits.
In conclusion, there are four principal findings in this report. First, a human herpes therapeutic vaccine that exclusively contains human ASYMP CD8+ T-cell epitopes derived from HSV-1 gD provides protection in HLA-A*02:01 Tg rabbits against spontaneous ocular shedding. Second, topical ocular mucosal delivery (i.e., by eye drops) of self-adjuvanting lipopeptides extended by a TLR-2 agonist (palmitic acid moiety) in latently infected HLA Tg rabbits boosts the number and function of local CD8+ T-cell responses in conjunctivas, corneas, TG and DLN that are associated with protection. Third, a high percentage of exhausted HSV-1 ASYMP epitope-specific PD1+ TIM-3+ CD8+ T cells were detected in unprotected HLA Tg rabbits, suggesting that activation of PD-1-PD-L1- and TIM-3-GAL-9-negative T-cell costimulatory pathways contributes to loss of protection. Fourth, the study validates the HLA-A*02:01 Tg rabbit model of recurrent ocular herpes for preclinical testing of future therapeutic herpes vaccine candidates bearing human ASYMP CD8+ T-cell epitopes against spontaneous ocular shedding. Overall, this preclinical therapeutic vaccine study in HLA-A*02:01 Tg rabbits paves the way for the clinical testing of ASYMP CD8+ T-cell epitope-based therapeutic vaccines against recurrent ocular herpes.
ACKNOWLEDGMENTS
This work is supported by Public Health Service research grants EY019896, EY14900, and EY024618 from the NIH, The Discovery Eye Foundation, The Discovery Center for Eye Research, and Research to Prevent Blindness.
We thank Alison Deckhut Augustine and Dale Long from the NIH Tetramer Facility (Emory University, Atlanta, GA) for providing the tetramers used in this study.
We declare that no conflict of interest exists.
REFERENCES
- 1.Kuo T, Wang C, Badakhshan T, Chilukuri S, BenMohamed L. 2014. The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine. Vaccine 34:715–729. doi: 10.1016/j.vaccine.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Samandary S, Kridane-Miledi H, Sandoval JS, Choudhury Z, Langa-Vives F, Spencer D, Chentoufi AA, Lemonnier FA, BenMohamed L. 2014. Associations of HLA-A, HLA-B and HLA-C alleles frequency with prevalence of herpes simplex virus infections and diseases across global populations: implication for the development of an universal CD8+ T-cell epitope-based vaccine. Hum Immunol 75:715–729. doi: 10.1016/j.humimm.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, Diaz OR, Gottimukkala C, Kalantari M, Villacres MC, Scarfone VM, McKinney DM, Sidney J, Sette A, Nesburn AB, Wechsler SL, BenMohamed L. 2013. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol 191:5124–5138. doi: 10.4049/jimmunol.1301415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chentoufi AA, Zhang X, Lamberth K, Dasgupta G, Bettahi I, Nguyen A, Wu M, Zhu X, Mohebbi A, Buus S, Wechsler SL, Nesburn AB, BenMohamed L. 2008. HLA-A*0201-restricted CD8+ cytotoxic T lymphocyte epitopes identified from herpes simplex virus glycoprotein D. J Immunol 180:426–437. doi: 10.4049/jimmunol.180.1.426. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Dervillez X, Chentoufi AA, Badakhshan T, Bettahi I, Benmohamed L. 2012. Targeting the genital tract mucosa with a lipopeptide/recombinant adenovirus prime/boost vaccine induces potent and long-lasting CD8+ T cell immunity against herpes: importance of MyD88. J Immunol 189:4496–4509. doi: 10.4049/jimmunol.1201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chentoufi AA, Kritzer E, Tran MV, Dasgupta G, Lim CH, Yu DC, Afifi RE, Jiang X, Carpenter D, Osorio N, Hsiang C, Nesburn AB, Wechsler SL, BenMohamed L. 2011. The herpes simplex virus 1 latency-associated transcript promotes functional exhaustion of virus-specific CD8+ T cells in latently infected trigeminal ganglia: a novel immune evasion mechanism. J Virol 85:9127–9138. doi: 10.1128/JVI.00587-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. 1998. Therapeutic periocular vaccination with a subunit vaccine induces higher levels of herpes simplex virus-specific tear secretory immunoglobulin A than systemic vaccination and provides protection against recurrent spontaneous ocular shedding of virus in latently infected rabbits. Virology 252:200–209. doi: 10.1006/viro.1998.9454. [DOI] [PubMed] [Google Scholar]
- 8.Nesburn AB, Burke RL, Ghiasi H, Slanina SM, Wechsler SL. 1998. A therapeutic vaccine that reduces recurrent herpes simplex virus type 1 corneal disease. Invest Ophthalmol Vis Sci 39:1163–1170. [PubMed] [Google Scholar]
- 9.Chentoufi AA, Dasgupta G, Christensen ND, Hu J, Choudhury ZS, Azeem A, Jester JV, Nesburn AB, Wechsler SL, BenMohamed L. 2010. A novel HLA (HLA-A*0201) transgenic rabbit model for preclinical evaluation of human CD8+ T cell epitope-based vaccines against ocular herpes. J Immunol 184:2561–2571. doi: 10.4049/jimmunol.0902322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herpetic Eye Disease Study Group. 1998. Acyclovir for the prevention of recurrent herpes simplex virus eye disease. N Engl J Med 339:300–306. doi: 10.1056/NEJM199807303390503. [DOI] [PubMed] [Google Scholar]
- 11.Knickelbein JE, Khanna KM, Yee MB, Baty CJ, Kinchington PR, Hendricks RL. 2008. Noncytotoxic lytic granule-mediated CD8+ T cell inhibition of HSV-1 reactivation from neuronal latency. Science 322:268–271. doi: 10.1126/science.1164164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wuest T, Farber J, Luster A, Carr DJ. 2006. CD4+ T cell migration into the cornea is reduced in CXCL9 deficient but not CXCL10 deficient mice following herpes simplex virus type 1 infection. Cell Immunol 243:83–89. doi: 10.1016/j.cellimm.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conrady CD, Zheng M, Stone DU, Carr DJ. 2012. CD8+ T cells suppress viral replication in the cornea but contribute to VEGF-C-induced lymphatic vessel genesis. J Immunol 189:425–432. doi: 10.4049/jimmunol.1200063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feldman LT, Ellison AR, Voytek CC, Yang L, Krause P, Margolis TP. 2002. Spontaneous molecular reactivation of herpes simplex virus type 1 latency in mice. Proc Natl Acad Sci U S A 99:978–983. doi: 10.1073/pnas.022301899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belshe PB, Leone PA, Bernstein DI, Wald A, Levin MJ, Stapleton JT, Gorfinkel I, Morrow RLA, Ewell MG, Stokes-Riner A, Dubin G, Heineman TC, Schulte JM, Deal CD. 2012. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med 366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stanberry LR, Spruance SL, Cunningham AL, Bernstein DI, Mindel A, Sacks S, Tyring S, Aoki FY, Slaoui M, Denis M, Vandepapeliere P, Dubin G. 2002. Glycoprotein-D-adjuvant vaccine to prevent genital herpes. N Engl J Med 347:1652–1661. doi: 10.1056/NEJMoa011915. [DOI] [PubMed] [Google Scholar]
- 17.Kuo T, Wang C, Badakhshan T, Chilukuri S, BenMohamed L. 2014. The challenges and opportunities for the development of a T-cell epitope-based herpes simplex vaccine. Vaccine 32:6733–6745. doi: 10.1016/j.vaccine.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu J, Peng X, Schell TD, Budgeon LR, Cladel NM, Christensen ND. 2006. An HLA-A2.1-transgenic rabbit model to study immunity to papillomavirus infection. J Immunol 177:8037–8045. doi: 10.4049/jimmunol.177.11.8037. [DOI] [PubMed] [Google Scholar]
- 19.Nesburn AB, Slanina S, Burke RL, Ghiasi H, Bahri S, Wechsler SL. 1998. Local periocular vaccination protects against eye disease more effectively than systemic vaccination following primary ocular herpes simplex virus infection in rabbits. J Virol 72:7715–7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nesburn AB, Ramos TV, Zhu X, Asgarzadeh H, Nguyen V, BenMohamed L. 2005. Local and systemic B cell and Th1 responses induced following ocular mucosal delivery of multiple epitopes of herpes simplex virus type 1 glycoprotein D together with cytosine-phosphate-guanine adjuvant. Vaccine 23:873–883. doi: 10.1016/j.vaccine.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Webre JM, Hill JM, Nolan NM, Clement C, McFerrin HE, Bhattacharjee PS, Hsia V, Neumann DM, Foster TP, Lukiw WJ, Thompson HW. 2012. Rabbit and mouse models of HSV-1 latency, reactivation, and recurrent eye diseases. J Biomed Biotechnol 2012:612316. doi: 10.1155/2012/612316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill JM, Nolan NM, McFerrin HE, Clement C, Foster TP, Halford WP, Kousoulas KG, Lukiw WJ, Thompson HW, Stern EM, Bhattacharjee PS. 2012. HSV-1 latent rabbits shed viral DNA into their saliva. Virol J 9:221. doi: 10.1186/1743-422X-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chentoufi AA, Binder NR, Berka N, Durand G, Nguyen A, Bettahi I, Maillere B, BenMohamed L. 2008. Asymptomatic human CD4+ cytotoxic T-cell epitopes identified from herpes simplex virus glycoprotein B. J Virol 82:11792–11802. doi: 10.1128/JVI.00692-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Chentoufi AA, Dasgupta G, Nesburn AB, Wu M, Zhu X, Carpenter D, Wechsler SL, You S, BenMohamed L. 2009. A genital tract peptide epitope vaccine targeting TLR-2 efficiently induces local and systemic CD8+ T cells and protects against herpes simplex virus type 2 challenge. Mucosal Immunol 2:129–143. doi: 10.1038/mi.2008.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.BenMohamed L, Wechsler SL, Nesburn AB. 2002. Lipopeptide vaccines—yesterday, today, and tomorrow. Lancet Infect Dis 2:425–431. doi: 10.1016/S1473-3099(02)00318-3. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta G, Chentoufi AA, You S, Falatoonzadeh P, Urbano LA, Akhtarmalik A, Nguyen K, Ablabutyan L, Nesburn AB, BenMohamed L. 2011. Engagement of TLR2 reverses the suppressor function of conjunctiva CD4+CD25+ regulatory T cells and promotes herpes simplex virus epitope-specific CD4+CD25- effector T cell responses. Invest Ophthalmol Vis Sci 52:3321–3333. doi: 10.1167/iovs.10-6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava R, Khan AA, Spencer D, Vahed H, Lopes PP, Thai NT, Wang C, Pham TT, Huang J, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. 2015. HLA-A02:01-restricted epitopes identified from the herpes simplex virus tegument protein VP11/12 preferentially recall polyfunctional effector memory CD8+ T cells from seropositive asymptomatic individuals and protect humanized HLA-A*02:01 transgenic mice against ocular herpes. J Immunol 194:2232–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knipe DM, Corey L, Cohen JI, Deal CD. 2014. Summary and recommendations from a National Institute of Allergy and Infectious Diseases (NIAID) workshop on “Next Generation Herpes Simplex Virus Vaccines.” Vaccine 32:1561–1562. doi: 10.1016/j.vaccine.2014.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Awasthi S, Friedman HM. 2014. Status of prophylactic and therapeutic genital herpes vaccines. Curr Opin Virol 6C:6–12. doi: 10.1016/j.coviro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 30.BenMohamed L, Belkaid Y, Loing E, Brahimi K, Gras-Masse H, Druilhe P. 2002. Systemic immune responses induced by mucosal administration of lipopeptides without adjuvant. Eur J Immunol 32:2274–2281. doi:. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Ramos TV, Gras-Masse H, Kaplan BE, BenMohamed L. 2004. Lipopeptide epitopes extended by Ne-palmitoyl lysine moiety increases uptake and maturation of dendritic cells through a Toll-like receptor 2 pathway and triggers a Th1-dependent protective immunity. Eur J Immunol 34:1142–1149. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Issagholian A, Berg EA, Fishman JB, Nesburn AB, BenMohamed L. 2005. Th-cytotoxic T-lymphocyte chimeric epitopes extended by Nepsilon-palmitoyl lysines induce herpes simplex virus type 1-specific effector CD8+ Tc1 responses and protect against ocular infection. J Virol 79:15289–15301. doi: 10.1128/JVI.79.24.15289-15301.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nesburn AB, Bettahi I, Zhang X, Zhu X, Chamberlain W, Afifi RE, Wechsler SL, BenMohamed L. 2006. Topical/mucosal delivery of sub-unit vaccines that stimulate the ocular mucosal immune system. Ocul Surf 4:178–187. doi: 10.1016/S1542-0124(12)70164-7. [DOI] [PubMed] [Google Scholar]
- 34.Mott KR, Chentoufi AA, Carpenter D, BenMohamed L, Wechsler SL, Ghiasi H. 2009. The role of a glycoprotein K (gK) CD8+ T-cell epitope of herpes simplex virus on virus replication and pathogenicity. Invest Ophthalmol Vis Sci 50:2903–2912. doi: 10.1167/iovs.08-2957. [DOI] [PubMed] [Google Scholar]
- 35.Cunningham AL, Diefenbach RJ, Miranda-Saksena M, Bosnjak L, Kim M, Jones C, Douglas MW. 2006. The cycle of human herpes simplex virus infection: virus transport and immune control. J Infect Dis 194(Suppl 1):S11–18. doi: 10.1086/505359. [DOI] [PubMed] [Google Scholar]
- 36.Gebhardt BM, Halford WP. 2005. Evidence that spontaneous reactivation of herpes virus does not occur in mice. Virol J 2:67–73. doi: 10.1186/1743-422X-2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Verjans GM, Hintzen RQ, van Dun JM, Poot A, Milikan JC, Laman JD, Langerak AW, Kinchington PR, Osterhaus AD. 2007. Selective retention of herpes simplex virus-specific T cells in latently infected human trigeminal ganglia. Proc Natl Acad Sci U S A 104:3496–3501. doi: 10.1073/pnas.0610847104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Derfuss T, Segerer S, Herberger S, Sinicina I, Hufner K, Ebelt K, Knaus HG, Steiner I, Meinl E, Dornmair K, Arbusow V, Strupp M, Brandt T, Theil D. 2007. Presence of HSV-1 immediate early genes and clonally expanded T-cells with a memory effector phenotype in human trigeminal ganglia. Brain Pathol 17:389–398. doi: 10.1111/j.1750-3639.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghiasi H, Cai S, Perng GC, Nesburn AB, Wechsler SL. 2000. Both CD4+ and CD8+ T cells are involved in protection against HSV-1 induced corneal scarring. Br J Ophthalmol 84:408–412. doi: 10.1136/bjo.84.4.408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Noisakran S, Carr DJ. 2000. Plasmid DNA encoding IFN-alpha 1 antagonizes herpes simplex virus type 1 ocular infection through CD4+ and CD8+ T lymphocytes. J Immunol 164:6435–6443. doi: 10.4049/jimmunol.164.12.6435. [DOI] [PubMed] [Google Scholar]
- 41.Dasgupta G, BenMohamed L. 2011. Of mice and not humans: how reliable are animal models for evaluation of herpes CD8(+)-T cell-epitopes-based immunotherapeutic vaccine candidates? Vaccine 29:5824–5836. doi: 10.1016/j.vaccine.2011.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dasgupta G, Nesburn AB, Wechsler SL, BenMohamed L. 2010. Developing an asymptomatic mucosal herpes vaccine: the present and the future. Future Microbiol 5:1–4. doi: 10.2217/fmb.09.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dasgupta G, Chentoufi AA, Nesburn AB, Wechsler SL, BenMohamed L. 2009. New concepts in herpes simplex virus vaccine development: notes from the battlefield. Expert Rev Vaccines 8:1023–1035. doi: 10.1586/erv.09.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaddipati S, Estrada K, Rao P, Jerome AD, Suvas S. 2015. IL-2/anti-IL-2 antibody complex treatment inhibits the development but not the progression of herpetic stromal keratitis. J Immunol 194:273–282. doi: 10.4049/jimmunol.1401285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Willey DE, Trousdale MD, Nesburn AB. 1984. Reactivation of murine latent HSV infection by epinephrine iontophoresis. Invest Ophthalmol Vis Sci 25:945–950. [PubMed] [Google Scholar]
- 46.Margolis TP, Elfman FL, Leib D, Pakpour N, Apakupakul K, Imai Y, Voytek C. 2007. Spontaneous reactivation of herpes simplex virus type 1 in latently infected murine sensory ganglia. J Virol 81:11069–11074. doi: 10.1128/JVI.00243-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nesburn AB, Bettahi I, Dasgupta G, Chentoufi AA, Zhang X, You S, Morishige N, Wahlert AJ, Brown DJ, Jester JV, Wechsler SL, BenMohamed L. 2007. Functional Foxp3+ CD4+ CD25(Bright+) “natural” regulatory T cells are abundant in rabbit conjunctiva and suppress virus-specific CD4+ and CD8+ effector T cells during ocular herpes infection. J Virol 81:7647–7661. doi: 10.1128/JVI.00294-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Channappanavar R, Twardy BS, Suvas S. 2012. Blocking of PDL-1 interaction enhances primary and secondary CD8 T cell response to herpes simplex virus-1 infection. PLoS One 7:e39757. doi: 10.1371/journal.pone.0039757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. 2010. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med 207:2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jin HT, Anderson AC, Tan WG, West EE, Ha SJ, Araki K, Freeman GJ, Kuchroo VK, Ahmed R. 2010. Cooperation of Tim-3 and PD-1 in CD8 T-cell exhaustion during chronic viral infection. Proc Natl Acad Sci U S A 107:14733–14738. doi: 10.1073/pnas.1009731107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fourcade J, Sun Z, Benallaoua M, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Kuchroo V, Zarour HM. 2010. Upregulation of Tim-3 and PD-1 expression is associated with tumor antigen-specific CD8+ T cell dysfunction in melanoma patients. J Exp Med 207:2175–2186. doi: 10.1084/jem.20100637. [DOI] [PMC free article] [PubMed] [Google Scholar]