ABSTRACT
The N-terminal region of the foot-and-mouth disease virus (FMDV) 3D polymerase contains the sequence MRKTKLAPT (residues 16 to 24) that acts as a nuclear localization signal. A previous study showed that substitutions K18E and K20E diminished the transport to the nucleus of 3D and 3CD and severely impaired virus infectivity. These residues have also been implicated in template binding, as seen in the crystal structures of different 3D-RNA elongation complexes. Here, we report the biochemical and structural characterization of different mutant polymerases harboring substitutions at residues 18 and 20, in particular, K18E, K18A, K20E, K20A, and the double mutant K18A K20A (KAKA). All mutant enzymes exhibit low RNA binding activity, low processivity, and alterations in nucleotide recognition, including increased incorporation of ribavirin monophosphate (RMP) relative to the incorporation of cognate nucleotides compared with the wild-type enzyme. The structural analysis shows an unprecedented flexibility of the 3D mutant polymerases, including both global rearrangements of the closed-hand architecture and local conformational changes at loop β9-α11 (within the polymerase motif B) and at the template-binding channel. Specifically, in 3D bound to RNA, both K18E and K20E induced the opening of new pockets in the template channel where the downstream templating nucleotide at position +2 binds. The comparisons of free and RNA-bound enzymes suggest that the structural rearrangements may occur in a concerted mode to regulate RNA replication, processivity, and fidelity. Thus, the N-terminal region of FMDV 3D that acts as a nuclear localization signal (NLS) and in template binding is also involved in nucleotide recognition and can affect the incorporation of nucleotide analogues.
IMPORTANCE The study documents multifunctionality of a nuclear localization signal (NLS) located at the N-terminal region of the foot-and-mouth disease viral polymerase (3D). Amino acid substitutions at this polymerase region can impair the transport of 3D to the nucleus, reduce 3D binding to RNA, and alter the relative incorporation of standard nucleoside monophosphate versus ribavirin monophosphate. Structural data reveal that the conformational changes in this region, forming part of the template channel entry, would be involved in nucleotide discrimination. The results have implications for the understanding of viral polymerase function and for lethal mutagenesis mechanisms.
INTRODUCTION
Picornaviruses are positive-strand RNA viruses associated with a large number of human and animal diseases (1). They encode an RNA-dependent RNA polymerase (RdRp) termed 3D that catalyzes viral RNA synthesis in the infected cells (2). Inhibition of RdRp activity prevents genome replication and virus multiplication. Thus, RdRps are an important antiviral target. The crystal structures of a large number of picornaviral 3Ds unliganded and as replication initiation and elongation complexes, captured at different stages of the nucleotide incorporation process, have provided information on the functional properties of these enzymes (3–7). These structures show that both chain elongation and VPg uridylylation are performed without major conformational changes in the relative positions of individual subdomains. Instead, these enzymes use subtle palm rearrangements to shape the active site in a catalysis-competent conformation upon correct nucleoside triphosphate (NTP) binding.
The biochemical and structural studies with six different polymerase variants from foot-and-mouth disease virus (FMDV)-resistant mutants revealed two mechanisms of FMDV resistance to ribavirin: decreased ribavirin monophosphate (RMP) incorporation mediated by the 3D substitution M296I [3D(M296I)] and modulation of transition types (avoidance of the excess G → A and C → U transitions produced by ribavirin) when substitutions P44S and P169S were added to M296I [triple mutant 3D(SSI)] (8–11). In both 3D(M296I) and 3D(SSI) conformational rearrangements were observed in the flexible β9-α11 loop within the polymerase motif B (10–12). In addition, 3D(SSI) showed a modified conformation of amino acids M16 to K18 that form part of the template channel entry (10).
Interestingly, residues M16 to K18 belong to the sequence MRKTKLAPT (amino acids 16 to 24), which acts as a nuclear localization signal (NLS) (13). Substitutions K18E and K20E diminished the transport to the nucleus of transiently expressed 3D and 3CD and severely impaired virus infectivity; only direct revertants were rescued upon transfection of BHK-21 cells with viral RNAs encoding the relevant mutations (13). Because the same 3D region was altered in ribavirin-resistant FMDV mutants (10), it was interesting to investigate whether the substitutions that affect nuclear localization also have an influence on nucleotide recognition. Here, we report that substitutions K18E and K20E of FMDV 3D significantly modify biochemical activities of 3D and that the new properties are associated with important structural changes revealed by X-ray crystallography. Substitutions K18E and K20E impaired RNA binding, and, interestingly, they increased RMP incorporation relative to the that of the standard AMP or GMP nucleotide. A similar, albeit less drastic, effect was observed in 3D with the substitution K18A, K20A, or both (KAKA). The comparative analysis of three-dimensional structures revealed an unprecedented flexibility of the FMDV 3D polymerase (3Dpol), including both global rearrangements of the closed-hand architecture and local conformational changes at the β9-α11 loop and at the polymerase N terminus at the region that contains the substituted residues. Surprisingly, structural modifications at the polymerase N-terminal region can give rise to opposite phenotypes: either a remarkable increase or decrease of RMP incorporation. The results imply that subtle structural effects must be controlled in the design of mutagenic antiviral analogues.
MATERIALS AND METHODS
Molecular cloning, expression, and purification of FMDV 3D.
Wild-type (wt) and mutant FMDV 3Ds were expressed from the corresponding plasmid constructs and purified as previously described (10, 12, 14). Enzymes were >95% pure, according to analytical SDS-polyacrylamide gel electrophoresis (PAGE) and Coomassie brilliant blue staining.
Labeling and annealing of a symmetrical primer-template substrate (sym/sub).
RNA oligonucleotides (Dharmacon Research) were labeled at the 5′ end with 32P using [γ-32P]ATP (Amersham) and T4 polynucleotide kinase (New England BioLabs). Radioactive oligonucleotides were purified by G25 Sephadex chromatography (Mini Quick Spin Oligo columns; Roche) and annealed using described protocols (15, 16).
Gel mobility shift assay.
RNA binding of 3D was determined by a gel mobility shift assay (14). Briefly, sym/sub-AU (where AU indicates the templating nucleotides; sequence, 5′-CGUAGGGCCC-3′) (20 nM duplex) was incubated with different concentrations of 3D (0, 250, 500, 1,000, and 2,000 nM) for 10 min at 33°C in 100 mM morpholinepropanesulfonic acid (MOPS), pH 7.0, 20 mM Mg(CH3COO)2, 20 mM NaCl, 5% polyethylene glycol (wt/vol), and 50 μM UTP where indicated in Fig. 1C and the first paragraph of Results. Products were separated by nondenaturing PAGE (4%) with glycerol (4%) at 4°C and 200 V.
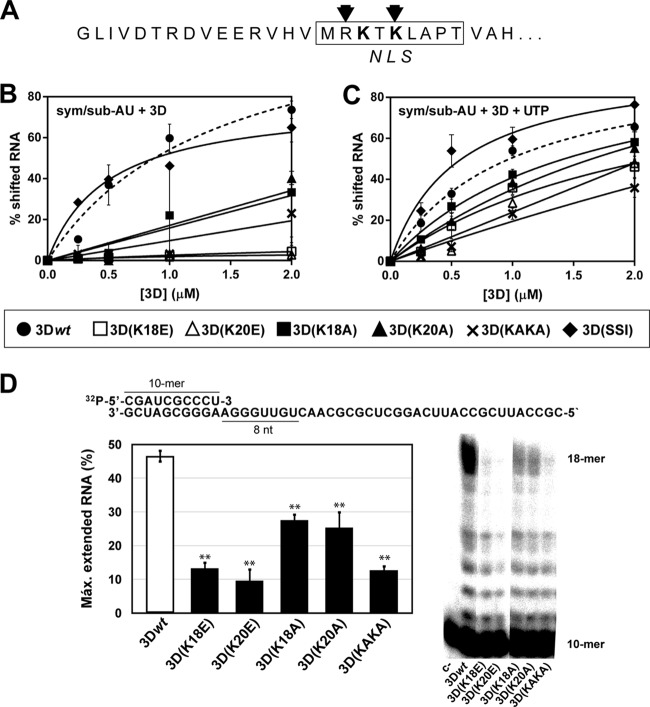
FIG 1.
RNA binding and processivity of wild-type and mutant 3Ds. (A) Amino acid sequence of the 27 amino-terminal residues of 3D. The box delimits the sequence identified as a nuclear localization signal (NLS) (13), the arrows indicate the amino acids that interact with RNA (12), and boldface highlights the substituted residues. (B) Electrophoretic mobility shift assay without UTP, where the indicated concentrations of 3D proteins were incubated with labeled sym/sub-AU (CGUAGGGCCC) RNA, as described in Materials and Methods. The products were separated electrophoretically in a 5% polyacrylamide gel; the percentage of shifted labeled RNA was plotted as a function of protein concentration, and the data points were fit to one-site binding (hyperbola). Binding of 3Dwt to RNA is represented as a dashed line. The values are the average of three determinations, and standard deviations are given. (C) Electrophoretic mobility shift assay with UTP. Reactions were performed as described in panel B except for the presence of 50 μM UTP during the incubation period. (D) The sequence of the heteropolymeric template (10-mer)-primer used in the processivity assay is given at the top; the sequence copied by 3D in the presence of ATP, UTP and CTP (absence of GTP) is underlined. The graph shows a representative electrophoretic separation (23% denaturing PAGE) of the products of elongation after a 5-min reaction with ATP, CTP, and UTP. The values represent the percentages of extended 18-mers measured relative to the total amount of elongated RNA (all bands shown in the electropherograms except the 10-mer). The values are the averages of three determinations, and standard deviations are given. Asterisks indicate the statistical significance of the difference with the value for 3Dwt (**, P < 0.0001; analysis of variance test).
Polymerization assays using heteropolymeric (sym/sub) and homopolymeric template-primers.
Incorporation of nucleoside monophosphates (NMPs) and RMP by wild-type and mutant 3Ds was measured using sym/sub RNAs as template-primers (15), according to published protocols (9, 10). Briefly, 3D (3 μM) was mixed with sym/sub RNA (0.5 μM duplex) in the presence of Mg(CH3COO)2 (15 mM), MOPS (30 mM, pH 7.0), NaCl (33 mM), and RNasin (1 U/μl) (Promega). When required, an excess of unlabeled sym/sub was added to the reaction mixture to trap polymerase molecules not bound to the labeled sym/sub. Reactions were stopped by the addition of EDTA (83 mM final concentration). The products were separated by 23% PAGE (7 M urea) and visualized by Phosphorimager (BAS-1500; Fuji) scanning. NMP and RMP incorporation was quantified with the program Tina 20 (version 2.08; Raytest Isotopenmessgeräte, GmbH). Polymerization using a homopolymeric template-primer involved quantification of UMP incorporation directed by poly(A)/oligo(dT), as previously described (14).
Processivity of RNA synthesis.
3D processivity was assayed by incubating 3D (2.5 μM) with a 45-residue RNA template (0.5 μM) (5′-CGCCAUUCGCCAUUCAGGCUGCGCAACUGUUGGGAAGGGCGAUCG-3′, annealed to the 32P-radiolabeled primer 5′-CGAUCGCCCU-3′) in 30 mM MOPS, pH 7.0, 15 mM Mg(CH3COO)2, 33 mM NaCl, and 1 U/μl RNasin, and incubated for 30 min at 37°C. The reactions were started by addition of ATP, UTP, and CTP (50 μM each) and allowed to proceed for 5 min before the addition of EDTA to 83 mM. Polymerization products were analyzed electrophoretically, as described for the polymerization assay, scanned with a Phosphorimager (BAS-1500; Fuji), and quantified with the program Tina 20. The processivity was calculated from the percentage of the maximum extended primer relative to the total amount of elongated RNA.
Rapid quench-flow experiments.
To measure incorporation of cognate nucleotides under pre-steady-state polymerization conditions, a rapid chemical quench-flow instrument (RQF-3; KinTek Co., College Park, PA) was used. The reactions were carried out at 37°C in 50 mM HEPES (pH 7.5), 5 mM MgCl2, and 10 mM 2-mercaptoethanol. FMDV 3D (0.5 μM active sites) was preincubated with the RNA template-primer (0.5 μM) and the next incoming NTP (10 μM) to allow formation of a 3D-RNA complex and incorporation of the first nucleotide. This reaction mixture was subsequently used for the measurement of the optimal polymerization rate constant (kpol) and binding affinity (Kd,NTP) of the NTP. The above reaction mixture (sample loop volume, 17.2 μl) was rapidly mixed with various concentrations of the NTP (sample loop volume, 17.4 μl). Reactions were allowed to proceed for different time periods (0.01 to 2 s) and were quenched with 0.5 M EDTA. For each NTP concentration, the reaction products were monitored for more than six time points. Polymerization products were resolved on a 23% PAGE–7 M urea gel, followed by scanning of the gel with a Typhoon FLA 9000 instrument (GE Healthcare). The corresponding bands were quantified with ImageJ, version 1.45s. The amount of product (P) for each NTP concentration was plotted against reaction time, and the data points were fit to a single exponential equation (equation 1) using nonlinear regression, by GraphPad Prism, version 4 (GraphPad, Inc.):
| (1) |
where A is the amplitude of the burst phase that represents the 3D-sym/sub complex at the start of the reaction, kobs is the observed burst rate constant for NTP incorporation, and t is the reaction time.
To obtain the dissociation constant Kd,NTP for NTP binding to the 3D-sym/sub complex, the observed burst rates (kobs) were plotted against NTP concentration, and the data were fit to the hyperbolic equation (equation 2) using nonlinear regression:
| (2) |
where kpol is the optimal rate of NTP incorporation.
VPg uridylylation.
To test VPg uridylylation, a synthetic peptide with the sequence of VPg1 of FMDV C-S8c1 (GPYAGPLERQRPLKVRAKLPRQE) was prepared by solid-phase peptide synthesis, purified by G25 Sephadex chromatography and high-performance liquid chromatography (HPLC), and analyzed by mass spectrometry, as previously described (4). VPg uridylylation assays were carried out using two protocols (17). Briefly, in the first protocol, 150 μM VPg, 5 μM 3D, and 20 nM RNA cre (oriC) were incubated with 0.16 μM 3CD in the presence of 5 mM MgCl2, 50 μM [α-32P]UTP, 0.4 mg/ml bovine serum albumin (New England BioLabs), and 8% glycerol in 30 mM MOPS at 37°C for 3 h. The reactions were stopped by addition of EDTA to a final concentration of 83 mM. In the second protocol, 20 nM RNA cre (oriC) was replaced by 40 ng/μl poly(A) as the template, 3CD was not added, and the reactions were allowed to proceed for 1 h in the presence of 0.6 mM MnCl2 instead of for 3 h in the presence of MgCl2. The products were analyzed by 20% PAGE, and the percentage of [α-32P]UTP incorporated into VPg was measured using a Phosphorimager (BAS-1500; Fuji) and Tina 20 software.
Crystallization and data collection.
Purified 3D mutants were stored in a buffer containing Tris-HCl (50 mM; pH 8.0), NaCl (500 mM), dithiothreitol (DTT; 0.8 mM), EDTA (0.8 mM), and glycerol (8%) at a concentration of 5 mg/ml. The oligonucleotide 5′-GCAUGGGCCC3-′ (NWG-Biotech) was annealed as described previously (12). Then, 3D was added slowly to the annealed oligonucleotide in the presence of 2 mM MgCl2 to reach an equimolar proportion. Crystals of unliganded mutant enzymes 3D (K18E), 3D(K20E), and 3D(K20A) and their complexes with RNA were obtained by hanging-drop vapor diffusion at 20°C with a precipitant/well solution containing 30% polyethylene glycol (PEG) 4000, 0.2 M Mg(CH3COO)2, 0.1 M MES [2-(N-morpholino) ethanesulfonic acid] pH 6.0, and 4% γ-butyrolactone. Crystals suitable for X-ray analysis appeared between 2 and 3 days. Crystals were then transferred to a cryo-protecting solution containing 20% glycerol in the crystallization buffer prior to cooling by immersion in liquid nitrogen.
Attempts to obtain the ternary complexes either by cocrystallization or by soaking with the nucleotide analogue ribavirin triphosphate (RTP) were unsuccessful; the substrates were not incorporated into the crystals, even when high nucleotide concentrations (up to 25 mM) and long incubation times (a few days) were used.
X-ray data were collected using synchrotron radiation. Diffraction images were processed with iMosflm (18) and XDS (19, 20) and internally scaled with Scala (in CCP4i) (21). Data collection statistics are given in Table 1.
TABLE 1.
Refinement statistics
| Parameter | Value for the protein or complex |
||||
|---|---|---|---|---|---|
| 3D(K18E) | 3D(K18E)-RNA | 3D(K20E) | 3D(K20E)-RNA | 3D(K20A)-RNA | |
| Data collection | |||||
| Beamline | SLSb | ID14.4 (ESRF) | PX1 (Soleil)c | ID14.4 (ESRF) | XALOC (Alba) |
| Resolution (Å) | 30.0–2.00 | 30–2.57 | 46.6–1.8 | 30–2.8 | 47.11–2.94 |
| Space group | P41212 | P3221 | P41212 | P3221 | P41212 |
| Cell dimensions | |||||
| a, b, c (Å) | 91.6, 91.6, 117.8 | 94.6, 94.6, 101.0 | 91.7, 91.7, 118.1 | 95.2, 95.2, 101.3 | 94.2, 94.2, 121.5 |
| α, β, γ (°) | 90, 90, 90 | 90, 90, 120 | 90, 90, 90 | 90, 90, 120 | 90, 90, 90 |
| Rmerge | 6.1 | 6.5 | 5.9 | 10.6 | 9.8 |
| I/sI | 25.6 | 9.3 | 24.15 | 10.7 | 12.8 |
| Completeness (%) | 99.9 | 99.0 | 99.8 | 100 | 99.6 |
| Multiplicity | 6.5 | 2.8 | 8.7 | 5.4 | 6.7 |
| Refinement | |||||
| Resolution (Å) | 30.0–2.00 | 30.0–2.57 | 46.6–1.8 | 30–2.8 | 47.11–2.94 |
| No. of reflections | |||||
| Total | 225,734 | 89,958 | 412,193 | 72,396 | 82,046 |
| Unique | 34,625 | 19,374 | 47,356 | 13,477 | 12,228 |
| Rwork/Rfreea | 21.47/24.76 | 23.33/25.67 | 20.29/22.66 | 24.61/26.60 | 20.79/25.82 |
| No. of residues | |||||
| Protein | 476 | 473 | 477 | 473 | 475 |
| RNA | 15 | 15 | 13 | ||
| Water | 147 | 31 | 280 | 12 | 14 |
| Ligands (no.) | 5 | 3 | 6 | 1 | 1 |
| B factors (Å2) | 17.34 | 38.761 | 26.58 | 46.92 | 52.64 |
| Protein | 17.22 | 33.642 | 25.90 | 42.48 | 49.22 |
| RNA | 95.400 | 99.25 | 98.45 | ||
| Water + ligands + ions | 19.99 | 54.824 | 34.64 | 58.95 | 53.65 |
| RMSD | |||||
| Bond lengths (Å) | 0.004 | 0.004 | 0.004 | 0.004 | 0.004 |
| Bond angles (°) | 0.810 | 0.738 | 0.791 | 0.723 | 0.763 |
| Ramachandran plot (% [no. of residues in the region/total no. of residues) | |||||
| Preferred regions | 98.7 (470/476) | 97 (459/473) | 99.2 (473/477) | 97 (459/473) | 98.1 (467/475) |
| Allowed regions | 1.3 (6/476) | 3 (14/473) | 0.8 (4/477) | 3 (14/473) | 1.7 (8/475) |
Rwork = ∑hkl ||Fobs(hkl)| − |Fcalc(hkl)||/∑hkl | Fobs(hkl)|, where Fobs and Fcalc are the structure factors, deduced from measured intensities and calculated from the model, respectively. Rfree is equivalent to Rwork but for 5% of the total reflections chosen at random and omitted from refinement. hkl, Miller index.
Swiss Light Source, Paul Scherrer Institut, Switzerland.
Soleil Synchrotron, Gif sur Yvette, France.
Structure determination and refinement.
Two different space groups were obtained; the two unliganded 3D mutant enzymes (K18E and K20E) and the 3D(K20A)-GCAUGGGCCC complex crystallized in the space group P41212, while 3D(K18E)-GCAUGGGCCC and 3D(K20E)-GCAUGGGCCC cocrystals belonged to the trigonal space group P3221 (Table 1). The initial maps for the tetragonal crystal structures were obtained after rigid-body fitting of the coordinates of the isolated wild-type polymerase (crystallized in the tetragonal P41212 [Protein Data Bank (PDB) accession number 1U09]) to the new unit cells, using the program REFMAC5 (CCP4i) (22, 23). Initial maps for the trigonal crystal forms were obtained following the same procedure but using the P3221 coordinates of 3D (PDB 1WNE) (12) as a starting model (Table 1). In the five structures, the weighted 2|Fo| − |Fc| and |Fo| − |Fc| difference maps (where Fo and Fc are the observed and calculated structure factor amplitudes, respectively) clearly allowed the rebuilding of the mutated residues and other regions presenting conformational changes as a consequence of the mutations. The difference density maps were also clear to allow tracing of the RNA template-primer decanucleotides in the polymerase-RNA complexes. Several cycles of automatic refinement, performed with REFMAC5, were alternated with manual model rebuilding using Coot (24). The refinement statistics are summarized in Table 1.
Protein structure accession numbers.
The refined structures were deposited in the Protein Data Bank under accession numbers 4WYL for 3D(K18E), 4WZM for 3D(K18E)-RNA, 4WYW for 3D(K20E), 4WZQ for 3D(K20E)-RNA, and 4X2B for 3D(K20A)-RNA.
RESULTS
Effect of substitutions at residues K18 and K20 on RNA binding, processivity, and polymerization activities.
Ribavirin-resistant mutant FMDV 3D(SSI) displayed structural alterations at the amino-terminal region of the polymerase (10), affecting the same residues that have been recognized as an NLS (Fig. 1A) (13). Since substitutions K18E and K20E within the NLS impaired nuclear localization and virus infectivity (13), we examined the effect of these substitutions on polymerase function. The wild-type (termed 3Dwt) and mutant [3D(K18E) and 3D(K20E)] 3D polymerases were purified and characterized for their RNA-binding affinity and polymerase activity. Binding of 3D(K18E) and 3D(K20E) to RNA was severely impaired compared to that of 3Dwt (Fig. 1B), with binding values slightly above background level, marked by a triple mutant with substitutions G118D, V239M, and G373D [3D(DMD)] that was previously characterized as displaying undetectable RNA binding (data not shown) (14). Replacing residues K18 and K20 with alanines in 3D(K18A), 3D(K20A), and the 3D(KAKA) double mutant produced a less dramatic loss of RNA binding (Fig. 1B). A partial recovery in RNA binding by all mutant polymerases was observed when UTP was present, but impaired binding to RNA was still observed in the polymerases with substitutions in the NLS region (Fig. 1C).
3D processivity was measured with a heteropolymeric template-primer, with ATP, UTP, and CTP as substrates (Fig. 1D). Extension to the maximum-length product of 18 nucleotides was 4- to 5-fold lower for 3D(K18E), 3D(K20E), and 3D(KAKA) than for 3Dwt, while 3D(K18A) and 3D(K20A) displayed intermediate processivity levels (Fig. 1D, bottom right panel). Full extension of the primer using all nucleotides (ATP, GTP, UTP, and CTP) failed to give comparable quantitative data due to the poor processivity of the mutant polymerases (data not shown). Therefore, substitutions at the FMDV 3D N terminus affect polymerase processivity.
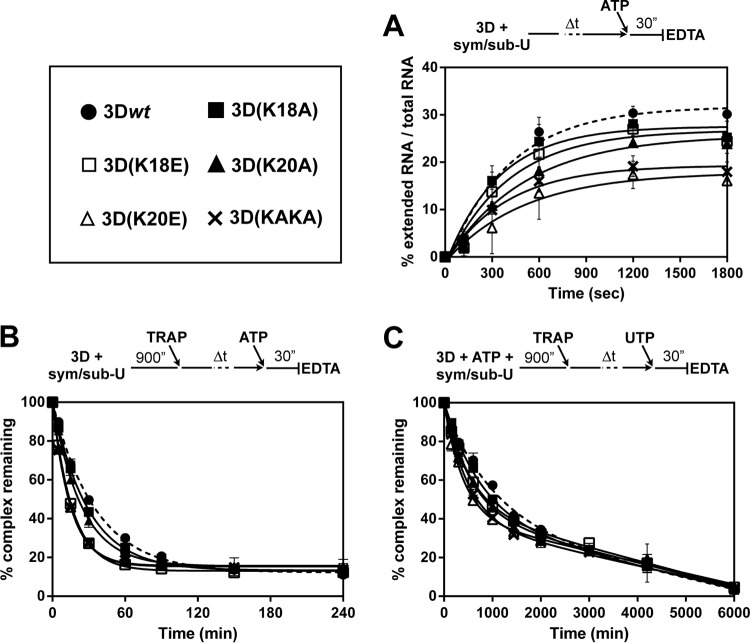
To determine how efficiently the different polymerases assemble with the RNA, an association assay was carried out in which the 3D-RNA binding step was followed by the incorporation of ATP (9) (Fig. 2A). The mutant polymerases with substitutions in residue 20 showed a lower rate of assembly (kassembly) to sym/sub-U than the rest of the enzymes tested, and K18A displayed a higher kassembly than 3Dwt (Table 2).
FIG 2.
Association and dissociation of FMDV 3Dpol-sym/sub-U complexes. (A) Kinetics of assembly of FMDV 3Dpol-sym/sub-U complexes. 3D proteins were incubated with labeled sym/sub-U, and ATP (50 μM) was added after 0, 2, 5, 10, 20, and 30 min; the reaction was allowed to proceed for 30 s and then was quenched by the addition of EDTA (83 mM). The percent RNA elongated at the indicated preincubation times was fit to a single exponential equation. Association of 3Dwt to RNA is represented as a dashed line. The values are the averages of three determinations, and standard deviations are given. (B) Kinetics of dissociation of FMDV 3Dpol-sym/sub-U substrate and 3Dpol-sym/sub-U product complexes. FMDV 3Dpol (3 μM) was incubated with stoichiometrically 32P-labeled sym/sub-AU (0.5 μM duplex) for 900 s at 37°C, at which point a trap (25 μM unlabeled sym/sub-U to trap polymerase molecules not bound to the labeled sym/sub) was added to the reaction mixture. At times 0, 5, 15, 30, 90, 150, 240, and 360 min after the addition of the trap, ATP (50 μM) was added, and the reaction was allowed to proceed for 30 s and was then quenched by the addition of EDTA (83 mM). The graph shows the percentage of complexes remaining at the indicated preincubation times of RNA with the different 3D polymerases. Data were fit to a one-phase exponential decay equation ♣CHK♣[A = (A0 − B) · exp(−koff · t) + B, where A is the amount of 3D-RNA complexes, B is the total amount of remaining complexes, and t is time)♣CHK♣. Dissociation of 3Dwt from RNA is represented as a dashed line. The values are the averages of three determinations, and standard deviations are given. (C) Kinetics of dissociation of FMDV 3Dpol-sym/sub-U substrate and 3Dpol-sym/sub-U product complexes. Reactions were performed as described for panel B but contained ATP (10 μM) during the assembly reaction. At times 0, 150, 300, 600, 1,000, 1,440, 2,000, 3,000, 4,200, and 6,000 min after the addition of the trap, UTP (50 μM) was added. The graph shows the percentage of complexes remaining at the different preincubation times of RNA with the different 3D polymerases. Data were fit to a biphasic exponential function, where the fast phase represented the dissociation [Fast = (A0 − B) · %Fast · 0.01, where A is the amount of 3D-RNA complexes and B is the total amount of remaining complexes)♣CHK♣. Dissociation of 3Dwt from RNA is represented as a dashed line. The values are the averages of three determinations, and standard deviations are given.
TABLE 2.
Kinetic parameters of 3D-RNA complex assembly and stability for FMDV 3Dwt, 3D(K18E), 3D(K20E), 3D(K18A), 3D(K20A), and 3D(KAKA)a
| Enzyme | kassembly (10−4 s−1)b |
koffc |
|
|---|---|---|---|
| ERn (10−3 s−1) | ERn+1 (10−4 s−1) | ||
| 3Dwt | 25 ± 4 | 28 ± 1 | 10 ± 4 |
| 3D(K18E) | 25 ± 5 | 60 ± 1 | 19 ± 4 |
| 3D(K20E) | 18 ± 7 | 67 ± 2 | 25 ± 3 |
| 3D(K18A) | 30 ± 5 | 34 ± 1 | 13 ± 2 |
| 3D(K20A) | 20 ± 3 | 42 ± 2 | 18 ± 2 |
| 3D(KAKA) | 26 ± 6 | 67 ± 2 | 20 ± 2 |
Kinetic parameters were obtained from the data shown in Fig. 2 according to the procedures detailed in Materials and Methods.
Rate of assembly of the different polymerases to sym/sub-U.
Rate of dissociation of the complex 3D-sym/sub-U either with no nucleotide incorporated (ERn) or with incorporation of AMP (ERn+1). ERn, enzyme-RNA complex, where n is the length of the primer.
The rate at which the different polymerases dissociate (koff) from the RNA was determined in the absence and presence of ATP. (i) The dissociation of the 3D-RNA complex incubated without ATP is faster for the mutant polymerases than for 3Dwt (Fig. 2B), leading to a 2.4-fold higher koff for 3D(K20E) and 3D(KAKA) than for 3Dwt (Table 2). Consistently, the koff values inversely correlate with the processivity of the different 3Ds tested. To prove that the faster decay of incorporation of the mutant polymerases due to complex dissociation was not masked by a faster thermal inactivation of these enzymes, an inactivation assay was carried out in parallel following a similar approach, resulting in no significant differences between the enzymes (see Fig. S1 posted at http://www2.cbm.uam.es:8080/cv-303/SupplMatFerrer-Orta.pdf). (ii) Incubation of FMDV 3Ds, sym/sub-U, and ATP resulted in a more stable complex than the one formed in the absence of ATP (9) (Fig. 2C). The results showed a much slower dissociation of 3D from the RNA than in the assay in the absence of ATP. The decay can be fit to a biphasic, exponential function. A first nonlinear and fast phase represents the dissociation of the complex, and a linear and slow component may represent the inactivation of the polymerase. Based on the fast component of the kinetics, we determined the koff for the different polymerases. Once again, the mutant polymerases displayed faster complex dissociation than 3Dwt (Fig. 2C; Table 2).
RNA synthesis was measured using poly(A)/oligo(dT) as the template-primer, whereas the initiation of RNA synthesis was measured by uridylylation of VPg. The mutant 3D RdRps displayed impaired activity in the two assays (see Fig. S1B, C, and D posted at http://www2.cbm.uam.es:8080/cv-303/SupplMatFerrer-Orta.pdf). Again, the decrease was more dramatic for the E mutants and double-A-substituted mutant than for the single-A-substituted mutants.
Nucleotide-binding affinities and polymerization rates for AMP and for ribavirin monophosphate incorporation.
The RNA-binding and polymerase activity assays indicated a significant negative effect of substitutions at positions 18 and 20 of 3D. To study the consequences of these substitutions in AMP incorporation with heteropolymeric template-primers, incorporation under single-turnover conditions using pre-steady-state kinetics was measured. The catalytic efficiencies (kpol/Kd,ATP) of the mutant polymerases were at least 3.6-fold lower than the efficiency of 3Dwt (Fig. 3A; Table 3).
FIG 3.
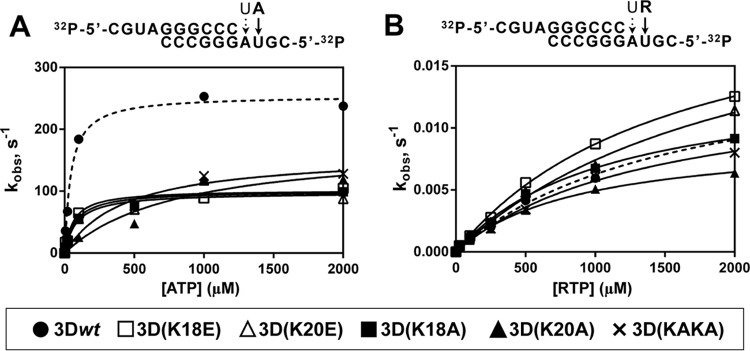
Pre-steady-state kinetics of nucleotide incorporation by 3Dwt and mutants. Above each panel, the nucleotide sequence of 5′-end-labeled and annealed sym/sub used and the nucleotides to be incorporated are depicted. (A) 3D enzymes were preincubated at 37°C with sym/sub-AU and UTP for 900 s at 37°C to allow the formation of 3D-RNA product complex, with UMP incorporated at the first position (discontinuous arrow on sym/sub RNA). The 3D-RNA product was then mixed with ATP using a rapid chemical quench-flow apparatus, and reactions were quenched by addition of EDTA (0.5 M). Time courses at fixed nucleotide concentrations were fit to an exponential curve to obtain the observed rate constant (kobs) for nucleotide incorporation at the second position. The observed rate constants were then plotted as a function of nucleotide concentration, and the data were fit to a hyperbola to obtain the maximal observed rate constant for nucleotide incorporation (kpol) and the apparent dissociation constant (Kd,ATP). From these constants the efficiency of incorporation of the NTP substrate tested (kpol/Kd,ATP) was obtained (Table 3). Values for AMP incorporation by 3Dwt are represented with a dashed line. Procedures are further detailed in Materials and Methods. (B) 3D was preincubated at 37°C with sym/sub-AU and UTP for 900 s to allow the formation of 3D-RNA product complex, with UMP incorporated at the first position (discontinuous arrow on sym/sub RNA). The 3D-RNA product was then mixed with RTP, and reactions were quenched by addition of EDTA (83 mM). Time courses at fixed nucleotide concentrations were fit to an exponential curve to obtain the observed rate constant (kobs) for nucleotide incorporation at the second position. The observed rate constants were then plotted as a function of nucleotide concentration, and the data were fit to a hyperbola to obtain the maximal observed rate constant for nucleotide incorporation (kpol) and the apparent dissociation constant (Kd,RTP). From these constants the efficiency of incorporation of the NTP substrate tested (kpol/Kd,RTP) was obtained (Table 3). Values for RMP incorporation by 3Dwt are represented with a dashed line. Procedures are further detailed in Materials and Methods.
TABLE 3.
Kinetic constants for nucleotide incorporation by FMDV 3Dwt, 3D(K18E), 3D(K20E), 3D(K18A), 3D(K20A), and 3D(KAKA)
| Enzyme | A on sym/sub-AUa |
R on sym/sub-AUa |
(kpol/Kd,ATP)ATP/(kpol/Kd,RTP)RTP (104)e | ||||
|---|---|---|---|---|---|---|---|
| Kd,ATP (μM)b | kpol (s−1)c | kpol/Kd,ATP (μM−1s−1)d | Kd,RTP (μM)b | kpol (10−3 s−1)c | kpol/Kd,RTP (10−6 μM−1s−1)d | ||
| 3Dwt | 44 ± 7 | 255 ± 9 | 5.8 ± 0.9 | 1604 ± 271 | 16 ± 2 | 10 ± 2 | 58 ± 15 |
| 3D(K18E) | 62 ± 17 | 102 ± 6 | 1.6 ± 0.5 | 1587 ± 125 | 23 ± 1 | 14 ± 1 | 11 ± 4 |
| 3D(K20E) | 76 ± 43 | 101 ± 11 | 1.3 ± 0.8 | 2144 ± 409 | 23 ± 3 | 11 ± 2 | 12 ± 8 |
| 3D(K18A) | 85 ± 20 | 98 ± 5 | 1.2 ± 0.3 | 1087 ± 110 | 14 ± 1 | 13 ± 2 | 9 ± 3 |
| 3D(K20A) | 814 ± 547 | 177 ± 50 | 0.2 ± 0.2 | 883 ± 99 | 9 ± 1 | 10 ± 2 | 2 ± 2 |
| 3D(KAKA) | 434 ± 227 | 161 ± 25 | 0.4 ± 0.2 | 1421 ± 203 | 14 ± 1 | 10 ± 2 | 4 ± 2 |
Kinetic parameters were obtained from the data shown in Fig. 3 according to the procedures detailed in Materials and Methods. A, adenosine; R, ribavirin.
Kd,NTP, dissociation constant for NTP binding.
kpol, optimal polymerization rate constant.
kpol/Kd,NTP, catalytic efficiency.
(kpol/Kd,ATP)ATP/(kpol/Kd,RTP)RTP, selectivity for discrimination in favor of incorporating the cognate nucleotide (AMP) instead of the nucleotide analogue (RMP).
Since alterations in the NLS region were associated with ribavirin resistance (10), it was relevant to compare the effect of substitutions at the NLS region on the incorporation of ribavirin monophosphate (RMP). To determine the catalytic efficiency for RMP incorporation by the different polymerases, pre-steady-state kinetics at various RTP concentrations were carried out. Despite evidencing a decrease in polymerase activity, the mutant polymerases exhibited similar or even higher rates for RMP incorporation than 3Dwt (Fig. 3B and Table 3). When the catalytic efficiencies for incorporation of the cognate nucleotide versus RMP were compared, much lower selectivity values were obtained for the mutant polymerases (Table 3). A higher incorporation of RMP by the mutant polymerases was confirmed by testing incorporation at a single concentration and either base pairing a templating U or base pairing a templating C (see Fig. S2, S3, S4, and S5 posted at http://www2.cbm.uam.es:8080/cv-303/SupplMatFerrer-Orta.pdf). These results suggest that changes in residues located in the NLS of FMDV 3D have an effect on template copying fidelity.
Overall structure of the 3D(K18E) and 3D(K20E) mutant polymerases.
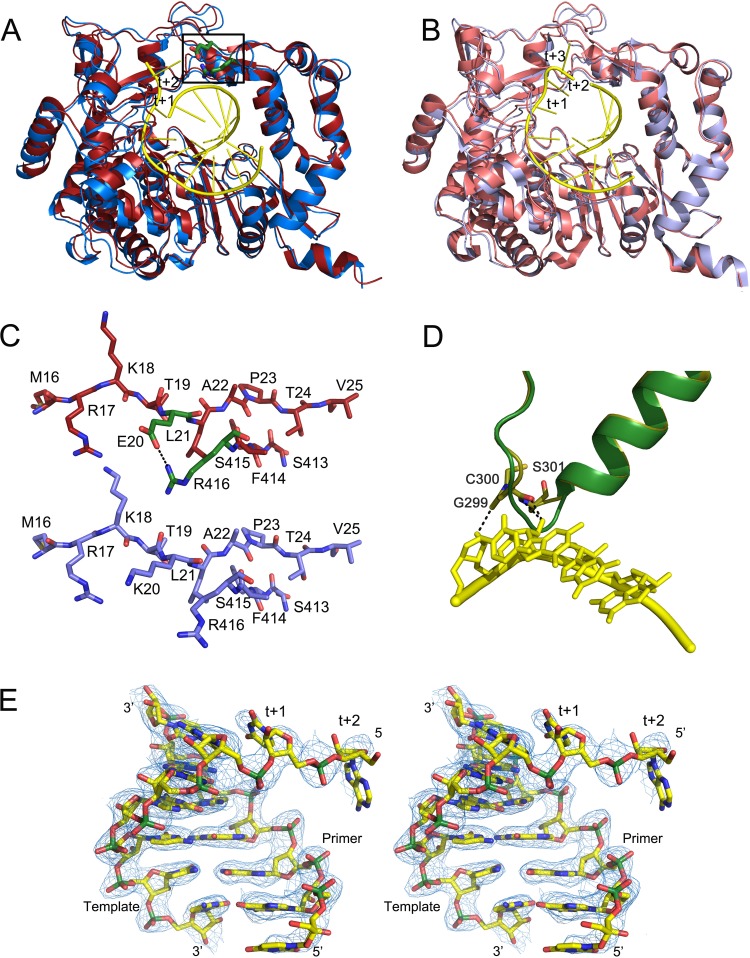
The X-ray structures of the unliganded 3D(K18E) and 3D(K20E) were solved to 2.0-Å and 1.8-Å resolution, respectively. In both structures, the initial difference maps allowed unequivocal tracing of the substituted sites and surrounding residues that were omitted from the initial models to eliminate model bias. Structural comparisons showed that mutant polymerases were similar to 3Dwt. Superimpositions of all 476 amino acids of the two mutant polymerases onto 3Dwt yielded root mean square deviation (RMSD) values of 0.31 Å and 0.29 Å for 3D(K18E) and 3D(K20E), respectively. Despite the overall similarities, individual domain superimpositions revealed that in 3D(K20E) the thumb domain is displaced toward the fingers domain, resulting in a more closed conformation of the enzyme central cavity that is stabilized by a well-defined salt bridge between the substituted amino acid E20 and the positively charged residue R416 in the thumb domain (Fig. 4A and B). This markedly closed conformation of an unbound form of 3D has not been previously observed with either FMDV or other picornaviruses. A substantial interdomain rearrangement would be necessary in this polymerase to allow RNA binding (Fig. 4A).
FIG 4.
Conformational changes in the 3D(K20E) and 3D(K18E) mutants on RNA binding. The figure shows structural superimpositions of the unbound (red) and RNA-bound (slate blue) structures of the 3D(K20E) mutant, showing the overall interdomain movements (A), compared with those of the 3Dwt structures (wt unbound, PDB accession number 1U09; wt RNA-bound, PDB 1WNE) (B). In the two panels, the bound RNA molecules are shown as sticks in yellow, with the downstream templating nucleotides at positions +1 and +2 explicitly labeled. Residues E20 and R416 that participate in the salt bridge linking fingers and thumb subdomains are shown as green sticks within the squared region. (C) Close-up of the squared region, showing the conformation and interactions around the E20-R416 salt bridge in the 3D(K20E) mutant (top), compared to the equivalent region in the 3Dwt. The RNA model is placed inside in atom-type sticks with carbons in yellow and phosphates in green. (D) Conformational rearrangement of the β9-α11 loop in the 3D(K18E) structure on RNA binding. The polymerase fragment is represented in green for the unbound state and in yellow sticks for the RNA-bound structure. The bound RNA template is also shown in yellow. (E) Stereo view of a σA-weighted 2|Fo| − |Fc| electron density map (1σ) around the RNA bound to the 3D(K20E) structure.
Detailed comparisons revealed that the main structural differences between unbound 3D(K18E) and 3Dwt lie at the β9-α11 loop where residues G299 to S301 are moved ∼3.1Å toward the active site in an orientation that disturbs the entrance of the RNA template (Fig. 4D). In contrast, the β9-α11 loop showed similar conformations in the 3Dwt and in the 3D(K20E) mutant. The closed conformation of 3D(K20E) and the displacement of loop β9-α11 in 3D(K18E) provide two different structural alterations compatible with the low RNA-binding activity of the two enzymes (Fig. 1B).
Structure of mutants 3D(K18E) and 3D(K20E) in complex with RNA.
We were able to obtain the cocrystals of 3D polymerase mutants K18E and K20E with the heteropolymeric RNA sym/sub-U despite the low RNA-binding activity exhibited by these mutants. The 3D(K18E)-RNA and 3D(K20E)-RNA complexes were solved at 2.57-Å and 2.8-Å resolution, respectively (Table 1). The structures indicate the presence of the duplex portion of the template-primer in the central cavity of the enzyme, and the RNA has similar interactions with the polymerase active-site residues as previously described in 3Dwt-RNA complexes (5, 12; reviewed in reference 3) (Fig. 4E). The major differences lie in the orientation and contacts involving the 5′ overhang RNA moiety that occupies the template channel. The template (t) nucleotide t+1 is fully stacked on the upstream RNA duplex, as observed in all 3Dwt-RNA complexes. However, the conformation and contacts of the RNA template at position t+2 (A3) differs considerably from those of 3Dwt in the two mutant polymerase complexes (Fig. 5). Unfortunately, the t+1 and t+2 nucleotides are the only ordered fragments of the template overhang moiety in the 3D mutant structures, and the base A3 shows poor electron density. Attempts aimed at obtaining ternary complexes with RTP failed, even when different concentrations of the nucleotide analogue and different soaking times were used.
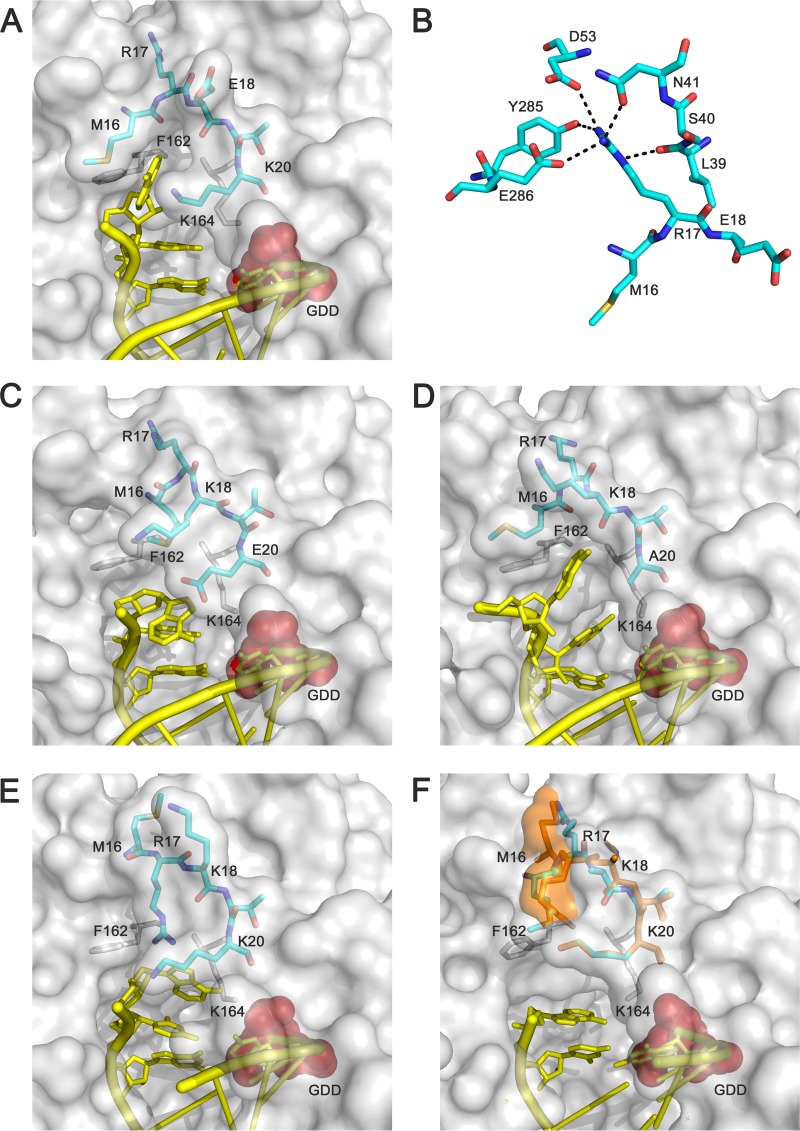
FIG 5.
Interactions in the FMDV 3D template channel at the entrance of the active site. Gallery of structures that show the conformational changes of the N-terminal region, residues 16 to 20, and the different interactions established with the RNA template. (A) The 3D(K18E)-RNA complex. Close-ups of the interactions involving the reoriented R17 are shown as follows: with residues S40-N41 of loop α1-α2, D53 of α3, and Y285-E286 of α9-β9 (B); the 3D(K20E)-RNA complex (C); the 3D(K20A)-RNA complex (D); wild-type 3D-RNA complex (PDB accession number 1WNE) (E); and the 3D(M296I)-RNA mutant complex with a misincorporated GMP (PDB 3KOA) (F). The polymerase is represented with its molecular surface in gray, with the acidic residues in the active site shown in red; the N-terminal residues 16 to 20 are depicted as sticks in cyan, the RNA is shown in yellow, and others residues involved in the binding RNA are represented as gray sticks. Residues 16 to 17 in the 3D(M296I)-RNA complex appear in two different conformations highlighted in cyan and orange.
The comparison between the unliganded 3D(K18E) and 3D(K20E) and the RNA-bound structures shows distinct conformational changes that are induced on RNA binding. (i) The structure of the 3D(K18E)-RNA complex indicates that the β9-α11 loop is moved toward the fingers domain, thereby regaining the open position that allows the entrance of the template nucleotide t+1 into the active site (Fig. 4D). (ii) The 3D(K20E)-RNA complex displays an overall interdomain reorganization, consisting of an ∼10° rotation of the fingers with respect to the thumb domain, which results in the opening of the polymerase central cavity, facilitating RNA entry (Fig. 4A). (iii) Upon RNA binding, the two substituted enzymes undergo important conformational changes in the N-terminal region; residues M16 to K18 of the template channel appear totally reorganized relative to 3Dwt bound to RNA (5, 12) (Fig. 5). In 3Dwt elongation complexes, the R17 side chain establishes various contacts with the t+2 nucleotide, which is oriented toward the central cavity, partially stacked to t+1 (5, 12). Small rearrangements of the R17 side chain were also observed when one incoming nucleotide was bonded to the active site and after chain translocation (Fig. 5). However, in the 3D(K18E)-RNA and 3D(K20E)-RNA complexes, the fully reoriented R17 points toward the polymerase interior, establishing new interactions with residues S40 and N41 (within the α1-α2 loop), with D53, and with Y285 and E286 (both within the β9-α11 loop) (Fig. 5). In these complexes, the main and side chains of M16 are also rotated, pointing toward the template channel. All together, these rearrangements result in the opening of new pockets in the template channel, which are surrounded by residues M16, K/E18, or K/E/A20 of the polymerase N terminus, R127 from motif G, and F162 and K164 from motif F. Comparisons of the different structures suggest that the pockets that exhibit different shapes and charge distributions are the new binding sites of the visible portion of the t+2 nucleotides (Fig. 5 and 6). Therefore, modifications of NLS residues K18 and K20 in FMDV 3D can give rise to local structural rearrangements that alter the RNA template-binding properties and the kinetics of nucleotide incorporation.
FIG 6.
The templating t+2 binding pockets in the different FMDV 3D-RNA complexes. Conformational changes around the pocket, comparing the wild-type 3D-RNA (PDB accession number 1WNE) (A) and 3D(K20E)-RNA (B) structures, are shown in ball-and-stick representation. In panels C to F, the template channel pockets are shown as molecular surfaces, with the electrostatic potential shown in blue and red for the positive and negative charges, respectively, for the following: wild-type 3D-RNA complex (C), 3D(K20E)-RNA (D), 3D(K20A)-RNA (E), and 3D(K18E)-RNA (F). The template nucleotide t+2, occupying the pocket, is shown as atom type sticks.
DISCUSSION
Picornaviruses have compact genomes, and many of their encoded processed proteins or processing intermediates are multifunctional (1). In the present report we have probed the molecular mechanisms by which the N-terminal region of FMDV 3D is involved in RNA template binding and nucleotide incorporation, including incorporation of ribavirin, presently under investigation in antiviral protocols based on lethal mutagenesis (25). The results establish a dual function for a polymerase domain in nuclear localization (13) and RNA synthesis. The results may be relevant also for other picornaviruses since amino acid sequences related to the NLS of FMDV are present in their polymerase sequences (1).
The dual function of the 3D NLS of FMDV has been studied by combining biochemical and structural analyses, and the latter have revealed that changes at 3D residues 16 to 20 can have unpredictable consequences in the recognition of standard nucleotides and nucleotide analogues. The pre-steady-state kinetics indicated decreased efficiency for incorporating AMP by all mutant polymerases tested but not for incorporation of RMP (Fig. 3). These differences are reflected in selectivity values (Table 3) which suggest a lower potential of discrimination between the incorporation of AMP and RMP by the mutant polymerases than by 3Dwt. The changes in selectivity observed in 3D(K18E) and 3D(K20E) were mainly due to altered rates of incorporation rather than to significant changes in nucleotide recognition, suggesting that changes in the nucleotide entry channel would not be linked to this altered nucleotide recognition. Both substituted enzymes displayed increased RMP incorporation, probably facilitated by the alterations of the template channels of the enzymes bound to RNA (Fig. 4 and 5). K18E and K20E are the first substitutions described in FMDV 3D that enhance incorporation of a nucleotide analogue, suggesting the possibility that drugs might be designed that alter the template channel to render the polymerase more sensitive to mutagenic analogues.
The three-dimensional structures of 3D(K18E) and 3D(K20E) have provided new insights into the structural flexibility of FMDV 3D. Changes include global interdomain rearrangements and large movements in the template channel and in the β9-α11 loop. The structural alterations have to be interpreted in the context of an increasing number of picornavirus RdRp structures trapped into multiple stages of RNA polymerization (3, 7, 26). Such structures have demonstrated a remarkable flexibility of the polymerase regions implicated in driving the template nucleotides toward the catalytic cavity. The N-terminal residues (from 17 to 20) lining the channel in FMDV 3D interact with the RNA template near the single-strand/double-strand junction (5, 11, 12). In 3D(K18E) and 3D(K20E) bound to RNA, the rearrangements affect residues 16 to 18. The basic side chain of R17 is involved in different interactions with the template nucleotide t+2, which points toward the active-site cavity, stacked with the t+1 nucleotide that is located in the opening of the central cavity, in close contact with the β9-α11 loop (Fig. 5). The equivalent residue of R17 in the 3D polymerase of enteroviruses is P20 (poliovirus numbering). The structures of various enterovirus elongation complexes show that P20 and surrounding residues form a conserved pocket where the t+2 nucleotide binds (6, 7) (Fig. 5). This pocket in the enteroviral polymerases seems to be a preformed structure that is also present in the unbound enzymes. In contrast, the FMDV wild-type enzyme lacks a preformed pocket in the template channel, and the t+2 nucleotide is oriented toward the active-site cavity (Fig. 5), which constitutes an important structural difference between the enterovirus and FMDV catalytic complexes. The regions forming the template and ribonucleotide triphosphate (rNTP) binding channels and those that participate in RNA translocation are involved in both polymerase activity and fidelity (3, 5–7, 10–12, 26–30). Interestingly, the rearrangements observed in the template channel of 3D(K18E) and 3D(K20E) are very similar to those in the ribavirin-resistant mutant 3D(SSI) (10), suggesting that subtle changes may produce either an increase or decrease in nucleotide analogue incorporation. Moreover, the structure of the single mutant 3D(M296I) catalytic complex, trapped after the misincorporation of G in front of U, showed a disorder in the same polymerase region consisting of two alternative conformations of residues M16 and R17 (11).
The unliganded 3D(K18E) shows the β9-α11 loop in a closed conformation, resembling the conformations found for the equivalent loops in the structures of the GTP- and Br-UTP-bound enterovirus 71 3D (29) and, to a lesser extent, the RdRp VP1 of the birnavirus infectious bursal disease virus (IBDV) in its unbound form (30). In the RNA-bound form of 3D(K18E), the loop appears rearranged toward a complete open form, allowing the positioning of the template nucleotide t+1 in the active site (Fig. 4). These data suggest that the β9-α11 loop dynamics is directly involved in the regulation of the template binding activity and that the K18E mutation in the template channel would interfere with the delicate equilibrium between the different conformations of this loop via long-distance interactions.
3D(K20E), but not 3D(K18E), shows a tightly closed conformation of the central cavity that was stabilized by the salt bridge established between E20 and the R416 side chain of the thumb domain (Fig. 4A and C). This electrostatic interaction seems to be the responsible for the lower RNA-binding activity exhibited by the E20 mutant because the structural modification is expected to increase the energy barrier of the rearrangement required to adopt a conformation compatible with RNA binding. This also explains why 3D(K20E) displayed the lowest kassembly value of all polymerases tested (Table 2). Once the RNA template-primer is bound to the enzyme, the local conformational change of residues M16-R17 would facilitate the reorientation of the E20 side chain apart from the thumb residue R416, favoring the opening of the central cavity for RNA template-primer binding (Fig. 4).
In conclusion, the structural and functional data presented here show that substitutions at residue K18 or K20 of the FMDV 3D polymerase, which belongs to an NLS present in the enzyme, result in two conformational alterations that diminish RNA binding. Consistently, binding of RNA results in two different structural rearrangements in the mutant enzymes. Once the RNA is bound, both enzymes display the opening of new pockets in the template channel that are associated with enhanced incorporation of RMP.
ACKNOWLEDGMENTS
We thank M. Álvarez for many valuable suggestions and discussions and A. I. de Ávila and I. Gallego for expert technical assistance.
Research in Barcelona was supported by grant BIO2011-24333 from the Spanish MINECO and by SILVER Cooperation project GA number 260644 of the European Union, Seventh Framework Program. Work in Madrid was supported by grants BFU2011-23604 and BIO2011-24351 from MINECO, PLATESA-S2013/ABI-2906 from CAM, and Fundación Ramón Areces. Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd) is funded by Instituto de Salud Carlos III. X-ray data were collected at the European Synchrotron Radiation Facility (ESRF) beamlines ID23.1 and ID29 (Grenoble, France) within a block allocation group (BAG Barcelona) and the XALOC beamline at the Alba Synchrotron (Cerdanyola de Valles, Spain) with the collaboration of Alba staff. Financial support was provided by the ESRF and Alba. C.F.-O. is a recipient of a JAE postdoctoral contract supported by the Fondo Social Europeo.
REFERENCES
- 1.Ehrenfeld E, Domingo E, Roos RP. 2010. The picornaviruses. ASM Press, Washington, DC. [Google Scholar]
- 2.Newman JF, Cartwright B, Doel TR, Brown F. 1979. Purification and identification of the RNA-dependent RNA polymerase of foot-and-mouth disease virus. J Gen Virol 45:497–507. doi: 10.1099/0022-1317-45-2-497. [DOI] [PubMed] [Google Scholar]
- 3.Ferrer-Orta C, Agudo R, Domingo E, Verdaguer N. 2009. Structural insights into replication initiation and elongation processes by the FMDV RNA-dependent RNA polymerase. Curr Opin Struct Biol 19:752–758. doi: 10.1016/j.sbi.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Ferrer-Orta C, Arias A, Agudo R, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. 2006. The structure of a protein primer-polymerase complex in the initiation of genome replication. EMBO J 25:880–888. doi: 10.1038/sj.emboj.7600971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrer-Orta C, Arias A, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. 2007. Sequential structures provide insights into the fidelity of RNA replication. Proc Natl Acad Sci U S A 104:9463–9468. doi: 10.1073/pnas.0700518104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gong P, Peersen OB. 2010. Structural basis for active site closure by the poliovirus RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A 107:22505–22510. doi: 10.1073/pnas.1007626107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gong P, Kortus MG, Nix JC, Davis RE, Peersen OB. 2013. Structures of coxsackievirus, rhinovirus, and poliovirus polymerase elongation complexes solved by engineering RNA mediated crystal contacts. PLoS One 8:e60272. doi: 10.1371/journal.pone.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sierra M, Airaksinen A, Gonzalez-Lopez C, Agudo R, Arias A, Domingo E. 2007. Foot-and-mouth disease virus mutant with decreased sensitivity to ribavirin: implications for error catastrophe. J Virol 81:2012–2024. doi: 10.1128/JVI.01606-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arias A, Arnold JJ, Sierra M, Smidansky ED, Domingo E, Cameron CE. 2008. Determinants of RNA-dependent RNA polymerase (in)fidelity revealed by kinetic analysis of the polymerase encoded by a foot-and-mouth disease virus mutant with reduced sensitivity to ribavirin. J Virol 82:12346–12355. doi: 10.1128/JVI.01297-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agudo R, Ferrer-Orta C, Arias A, de la Higuera I, Perales C, Perez-Luque R, Verdaguer N, Domingo E. 2010. A multi-step process of viral adaptation to a mutagenic nucleoside analogue by modulation of transition types leads to extinction-escape. PLoS Pathog 6:e1001072. doi: 10.1371/journal.ppat.1001072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrer-Orta C, Sierra M, Agudo R, de la Higuera I, Arias A, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. 2010. Structure of foot-and-mouth disease virus mutant polymerases with reduced sensitivity to ribavirin. J Virol 84:6188–6199. doi: 10.1128/JVI.02420-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferrer-Orta C, Arias A, Perez-Luque R, Escarmis C, Domingo E, Verdaguer N. 2004. Structure of foot-and-mouth disease virus RNA-dependent RNA polymerase and its complex with a template-primer RNA. J Biol Chem 279:47212–47221. doi: 10.1074/jbc.M405465200. [DOI] [PubMed] [Google Scholar]
- 13.Sanchez-Aparicio MT, Rosas MF, Sobrino F. 2013. Characterization of a nuclear localization signal in the foot-and-mouth disease virus polymerase. Virology 444:203–210. doi: 10.1016/j.virol.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 14.Arias A, Agudo R, Ferrer-Orta C, Perez-Luque R, Airaksinen A, Brocchi E, Domingo E, Verdaguer N, Escarmis C. 2005. Mutant viral polymerase in the transition of virus to error catastrophe identifies a critical site for RNA binding. J Mol Biol 353:1021–1032. doi: 10.1016/j.jmb.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 15.Arnold JJ, Cameron CE. 2000. Poliovirus RNA-dependent RNA polymerase (3Dpol). Assembly of stable, elongation-competent complexes by using a symmetrical primer-template substrate (sym/sub). J Biol Chem 275:5329–5336. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook J, Russell DW. 2001. Molecular cloning: a laboratory manual, 3rd ed Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. [Google Scholar]
- 17.Agudo R, Arias A, Pariente N, Perales C, Escarmis C, Jorge A, Marina A, Domingo E. 2008. Molecular characterization of a dual inhibitory and mutagenic activity of 5-fluorouridine triphosphate on viral RNA synthesis. Implications for lethal mutagenesis. J Mol Biol 382:652–666. doi: 10.1016/j.jmb.2008.07.033. [DOI] [PubMed] [Google Scholar]
- 18.Leslie AGW. 1991. Macromolecular data processing, p 27–38. In Moras D, Podjarny AD, Thiery JC (ed), Crystallographic computing, vol 5 Oxford University Press, Oxford, United Kingdom. [Google Scholar]
- 19.Kabsch W. 2010. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr D Biol Crystallogr 66:133–144. doi: 10.1107/S0907444909047374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kabsch W. 2010. XDS. Acta Crystallogr D Biol Crystallogr 66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Potterton E, Briggs P, Turkenburg M, Dodson E. 2003. A graphical user interface to the CCP4 program suite. Acta Crystallogr D Biol Crystallogr 59:1131–1137. doi: 10.1107/S0907444903008126. [DOI] [PubMed] [Google Scholar]
- 22.Murshudov GN, Skubak P, Lebedev AA, Pannu NS, Steiner RA, Nicholls RA, Winn MD, Long F, Vagin AA. 2011. REFMAC5 for the refinement of macromolecular crystal structures. Acta Crystallogr D Biol Crystallogr 67:355–367. doi: 10.1107/S0907444911001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murshudov GN, Vagin AA, Dodson EJ. 1997. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr D Biol Crystallogr 53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- 24.Emsley P, Cowtan K. 2004. Coot: model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr 60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 25.Domingo E, Sheldon J, Perales C. 2012. Viral quasispecies evolution. Microbiol Mol Biol Rev 76:159–216. doi: 10.1128/MMBR.05023-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garriga D, Ferrer-Orta C, Querol-Audi J, Oliva B, Verdaguer N. 2013. Role of motif B loop in allosteric regulation of RNA-dependent RNA polymerization activity. J Mol Biol 425:2279–2287. doi: 10.1016/j.jmb.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 27.Moustafa IM, Shen H, Morton B, Colina CM, Cameron CE. 2011. Molecular dynamics simulations of viral RNA polymerases link conserved and correlated motions of functional elements to fidelity. J Mol Biol 410:159–181. doi: 10.1016/j.jmb.2011.04.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sholders AJ, Peersen OB. 2014. Distinct conformations of a putative translocation element in poliovirus polymerase. J Mol Biol 426:1407–1419. doi: 10.1016/j.jmb.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu Y, Lou Z, Miao Y, Yu Y, Dong H, Peng W, Bartlam M, Li X, Rao Z. 2010. Structures of EV71 RNA-dependent RNA polymerase in complex with substrate and analogue provide a drug target against the hand-foot-and-mouth disease pandemic in China. Protein Cell 1:491–500. doi: 10.1007/s13238-010-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garriga D, Navarro A, Querol-Audi J, Abaitua F, Rodriguez JF, Verdaguer N. 2007. Activation mechanism of a noncanonical RNA-dependent RNA polymerase. Proc Natl Acad Sci U S A 104:20540–20545. doi: 10.1073/pnas.0704447104. [DOI] [PMC free article] [PubMed] [Google Scholar]