ABSTRACT
Adeno-associated virus (AAV) is a helper-dependent parvovirus that requires coinfection with adenovirus (AdV) or herpes simplex virus 1 (HSV-1) to replicate. In the absence of the helper virus, AAV can persist in an episomal or integrated form. Previous studies have analyzed the DNA damage response (DDR) induced upon AAV replication to understand how it controls AAV replication. In particular, it was shown that the Mre11-Rad50-Nbs1 (MRN) complex, a major player of the DDR induced by double-stranded DNA breaks and stalled replication forks, could negatively regulate AdV and AAV replication during coinfection. In contrast, MRN favors HSV-1 replication and is recruited to AAV replication compartments that are induced in the presence of HSV-1. In this study, we examined the role of MRN during AAV replication induced by HSV-1. Our results indicated that knockdown of MRN significantly reduced AAV DNA replication after coinfection with wild-type (wt) HSV-1 or HSV-1 with the polymerase deleted. This effect was specific to wt AAV, since it did not occur with recombinant AAV vectors. Positive regulation of AAV replication by MRN was dependent on its DNA tethering activity but did not require its nuclease activities. Importantly, knockdown of MRN also negatively regulated AAV integration within the human AAVS1 site, both in the presence and in the absence of HSV-1. Altogether, this work identifies a new function of MRN during integration of the AAV genome and demonstrates that this DNA repair complex positively regulates AAV replication in the presence of HSV-1.
IMPORTANCE Viral DNA genomes trigger a DNA damage response (DDR), which can be either detrimental or beneficial for virus replication. Adeno-associated virus (AAV) is a defective parvovirus that requires the help of an unrelated virus such as adenovirus (AdV) or herpes simplex virus 1 (HSV-1) for productive replication. Previous studies have demonstrated that the cellular Mre11-Rad50-Nbs1 (MRN) complex, a sensor and regulator of the DDR, negatively regulates AAV replication during coinfection with AdV, which counteracts this effect by inactivating the complex. Here, we demonstrate that MRN positively regulates AAV replication during coinfection with HSV-1. Importantly, our study also indicates that MRN also favors integration of AAV genomes within the human AAVS1 site. Altogether, this work indicates that MRN differentially regulates AAV replication depending on the helper virus which is present and identifies a new function of this DNA repair complex during AAV integration.
INTRODUCTION
Adeno-associated virus (AAV) is a defective parvovirus (dependovirus) that is commonly used as a vector for in vivo gene therapy. Wild-type (wt) AAV is defined as a nonpathogenic virus which can infect humans and several other animal species (1). The AAV particle is composed of a nonenveloped capsid and a single-stranded DNA (ssDNA) molecule of approximately 4.7 kb. The AAV genome contains two open reading frames, rep and cap, flanked by inverted terminal repeats (ITRs) which serve as origins of DNA replication. Four Rep proteins are produced from the rep gene by two different promoters and splicing patterns. The major Rep proteins, Rep78 and Rep68, display DNA-binding, endonuclease, and helicase activities that are essential for AAV genome replication. Three structural proteins (VP1, -2, and -3) and one assembly-activating protein are produced from the cap gene. Several AAV serotypes and variants have been identified and classified on the basis of amino acid variations of the capsid proteins (2).
AAV is classified as a dependovirus because it can replicate only in the presence of an unrelated helper virus and enters a latent phase in its absence. During the latent phase, the AAV genome delivered into the nucleus is rapidly converted into a double-stranded DNA (dsDNA) molecule and persists in a silent form that is either maintained as an episome or integrated within cellular chromosomes. Unique among mammalian viruses is its preference to integrate within a specific locus of human chromosome 19, designed AAVS1 (3–9).
During the productive phase, replication of AAV takes place almost exclusively in the nucleus within viral replication compartments. Several viruses were identified as being able to complement AAV's replication deficiency, including adenovirus (AdV) and herpes simplex virus 1 (HSV-1) (10). The identification of AdV helper functions previously indicated that five AdV genes, E1a, E1b-55K, E2a, and E4Orf6 genes and virus-associated (VA) RNAs, were sufficient to efficiently help AAV. Besides the AdV DNA-binding protein (E2a), whose role is presently unclear, the helper activity of AdV did not include any viral proteins involved in viral DNA replication. In contrast, we and others have shown that at least 10 HSV-1 genes are required for AAV replication (11–14). These include genes for transcriptional and posttranscriptional regulatory proteins, as well as several replication enzymes, notably the HSV-1 polymerase complex UL30/UL42. Altogether, these results strongly suggested a higher and stronger dependence of AAV on HSV-1 for replication than AdV. A proteomic analysis, performed to identify the cellular factors recruited by Rep proteins during AAV replication, further indicated that, in addition to the HSV-1 helper factors, several cellular proteins involved in DNA metabolism were also recruited to the AAV viral replication compartments (14). Among these factors were some belonging to the family of DNA repair proteins and, notably, Rad50, which is part of the Mre11-Rad50-Nbs1 (MRN) DNA repair complex.
MRN is a key player in the cellular responses initiated on dsDNA breaks (DSB) or in general on detection of abnormal DNA structures such as stalled DNA replication forks (15). After detection of the DNA lesion, MRN can initiate the DNA damage response (DDR) by recruiting and activating the signaling kinase ataxia telangiectasia mutated (ATM), which is involved in phosphorylation of a multitude of downstream substrates and in the control of the checkpoint response (16–18). The MRN complex is also involved in ATM and Rad3-related (ATR) activation (19, 20). Depending on the nature of the DNA lesion and on the cell status, the DDR can finally result in either cell apoptosis or repair via two main pathways: homologous recombination (HR) or nonhomologous-end joining (NHEJ) (21). MRN is essential for repair by HR, which involves the 5′-to-3′ resection of the DNA at the break and then repair by strand invasion of a homologous DNA template. Therefore, HR requires the presence of homologous sequences which are generated during the S phase of the cell cycle. In contrast, in terminally differentiated cells, repair occurs via NHEJ by ligation of the broken DNA ends via two main pathways. The first, designated canonical NHEJ (C-NHEJ), results in the direct joining of the DNA ends without any modification (22). This pathway takes place in the presence of the Ku proteins, which avidly bind to the DSB and the XRCC4/ligase IV activities. The second, named alternative NHEJ (A-NHEJ), includes several different mechanisms which are also referred as backup NHEJ or microhomology-mediated end joining. Despite this heterogeneity, A-NHEJ, like HR, involves a resection of DNA sequences at the junction and repair by exploiting short homologies (22). Importantly, MRN was shown to be involved in A-NHEJ by inducing the resection of the DNA junctions (23, 24). The essential role of MRN in DNA repair is linked to its wide spectrum of biochemical functions, which include binding to DNA, DNA tethering, ATP hydrolysis, and endo- and exonuclease activities (15, 25).
Not surprisingly, MRN was shown to control the replication of several viruses (26). In particular, MRN could inhibit AdV replication by inducing the concatemerization of its genome (27, 28). Three AdV early proteins, E4(orf3), E4(orf6), and E1b55KDa, can counteract this effect by delocalizing MRN and by inducing its degradation (27–30). Interestingly, two of these proteins, E4(orf6) and E1b55KDa, are essential for the AdV helper effect on AAV (10), and a further study has shown that their helper activity was linked to their capacity to degrade MRN, indicating that this complex could also inhibit AAV replication (31). In addition, MRN could also inhibit transduction by recombinant AAV (rAAV) vectors, most likely by binding to the viral ITR (31, 32). In contrast, MRN was recruited within viral replication compartments and exerted a positive effect on HSV-1 lytic replication (33, 34). Altogether, these observations suggested that MRN could play different roles in AAV replication depending on the helper virus that is present.
The aim of this study was to investigate the effect of MRN on AAV replication during coinfection with HSV-1. We showed that knockdown of MRN significantly reduced the replication of the AAV genome after coinfection with HSV-1. This effect was specific for wt AAV, since it was dependent on cis-acting sequences present within the rep gene. Importantly, the positive regulation of AAV replication by MRN required its DNA tethering but not its exonuclease activity. Finally, our data showed that knockdown of MRN could also negatively regulate AAV integration within the human AAVS1 site, an event that was greatly enhanced by coinfection with HSV-1. Altogether, this work demonstrates that MRN can differentially regulate AAV replication depending on the helper virus which is present and identifies a new function of this DNA repair complex during integration of the AAV genome.
MATERIALS AND METHODS
Cells and viruses.
HeLa cells (kindly provided by A. Recchia, University of Modena, Italy) and derived cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; Life Technologies) supplemented with 10% fetal bovine serum (FCS; HyClone) and 1% penicillin-streptomycin (5,000 U/ml; Invitrogen). The previously described HA-16 and 7374 cells are derived from HeLa and Detroit cells latently infected with wt AAV-2 (35, 36).
The HSV-1 viruses used in the study were wt HSV-1-1 (17 syn+) and HSV-1ΔPol (HP66) (provided by D. Coen, Harvard University, Boston, MA, USA). Wild-type and mutant HSV-1 stocks were produced and titrated on Vero or polB3 cells expressing the HSV-1 UL30 gene (provided by C. Hwang, SUNY Health Science Center, Syracuse, NY, USA) by standard procedures. Titers were expressed as particle-forming units (PFU)/ml. Wild-type AdV type 5 (ATCC VR-5) was produced and titrated as previously described (37). Titers are expressed as infectious particles (IP) per milliliter.
Stocks of wt and recombinant AAV-2 particles were generated by calcium phosphate transfection of HEK-293 cells as described previously using the pXX6 or pDG helper plasmid (38, 39). The vector particles were purified on cesium chloride or iodixanol gradients as previously described (40, 41) and the numbers of genome particles (GP) per milliliter were determined by quantitative PCR (qPCR).
Plasmids.
Plasmids encoding wt Rad50, mutated Rad50 (S1202R) (42, 43), wt Mre11, mutated Mre11 (pMre11-3;HD120/130LV) (44), and mutated Rad51 (SmRad51) (45) were kindly provided by B. Lopez. The plasmid coding for Nbs1 (pcDNa3-p95FLmyc) was kindly provided by J. Petrini. The plasmid encoding short-hairpin RNA (shRNA)-resistant Rad50 protein was obtained by introducing four conservative mutations in the seven-amino-acid region targeted by the shRNA using the QuikChange Lightning directed mutagenesis kit (Stratagene) following the manufacturer's instructions. Plasmid pRep, used to transiently express Rep proteins, contains the AAV-2 genome with the ITR deleted (40).
Lentivirus-mediated shRNA silencing.
A mixture of three lentiviral vectors expressing short-hairpin microRNA 30 (sh-miR30) targeting three different regions of Rad50 mRNA were used to transduce target cells. Target sequences were as follows: ShRNA#1, 5′TGCTGTTGACAGTGAGCGCCGACCATCATTGAATGTCTAATAGTGAAGCCACAGATGTATTAGACATTCAATGATGGTCGTTGCCTACTGCCTCGGA3′; ShRNA#2, 5′TGCTGTTGACAGTGAGCGACTGGGATTCAATGTTCATTAATAGTGAAGCCACAGATGTA3′; ShRNA#3, 5′TGCTGTTGACAGTGAGCGCCTGCGACTTGCTCCAGATAAATAGTGAAGCCACAGATGTA3′.
As a control, shRNA sequences targeting the luciferase mRNA (5′AGCTCCCGTGAATTGGAATCC3′) were used (46). Lentiviral vectors carrying specific sh-miR30s were produced by transient DNA transfection of HEK293T cells with DNAs encoding HIV-1 Gag-Pol and the pantropic VSVg envelope and a miniviral genome carrying the sh-miR30 expression cassette, as well as a puromycin resistance gene (at a DNA ratio of 8:4:8). Two days after transfection, supernatants were filtered through a 0.45-μm syringe filter and then purified by ultracentrifugation at 25,000 rpm through a 25% sucrose cushion for 2 h. Virions were then resuspended and quantified by exogenous-reverse transcription assay against standards of known infectivity, as described in detail before (46).
For the generation of cells knocked down for Rad50 or control cells, HeLa cells were plated at 2 × 105 cells/well in a 6-well plate and consecutively infected three times with concentrated lentiviral vector at a multiplicity of infection (MOI) of 3 particles/cells. After 3 to 4 days, cells were plated in a 10-cm dish and incubated in the presence of puromycin (1.5 μg/ml final). After 3 days of selection, knockdown was checked by Western blotting, and the cells were immediately used for the desired experiments. Importantly, the growth and the viability of the cells were monitored using the alamarBlue assay (Pierce) (data not shown).
Detection of AAV-AAVS1 junctions by TaqMan PCR.
Quantification of AAV integration within AAVS1 was performed as previously described, with some modifications (4, 47). Briefly, 1 μg of sample DNA was applied to a preamplification step. In a 50-μl reaction mixture, including a 200 nM concentration of each primer, pAAV-S1 (5′-TCAGAGGACATCACGTG-3′), and pITR (5′-GTTAATCATTAACTACAAGGAACCC-3′), the AAVS1-AAV junctions were preamplified in 18 cycles using the multiplex Taq polymerase (Qiagen, Hilden, Germany) according to the manufacturer′s instructions. Annealing temperature was 56°C. A 2-μl aliquot of the first PCR was then applied to real-time PCR using a 500 nM concentration of pAAV-S1, the pITR primer set, and a 200 nM concentration (each) of the 3′ fluorescein end-labeled donor probe (5′-TGTTGCTGCCCAAGGATGCT-3′) and a 5′ LC Red640 end-labeled acceptor probe (5′-TTTCCGGAGCACTTCCTTCTCG-34). All samples were analyzed in triplicates using a standard curve of genomic DNA from HA-16 cells (36) and β-globin cDNA for normalization.
Quantitative PCR.
Primers used for the qPCRs were as follows: rep-F (5′-GCAAGACCGGATGTTCAAAT-3′) and rep-R (5′-CCTCAACCACGTGATCCTTT-3′); gfp-F (5′-GAACGGCATCAAGGTGAACT-3′) and gfp-R (5′-TGCTCAGGTAGTGGTTGTCG-3′); β-globin-F (5′-CCCTTGGACCCAGAGGTTCT-3′) and β-globin-R (5′-CGAGCACTTTCTTGCCATGA-3′); and UL19-F (5′-GTTGTCGGGCTGCATAAACT-3′) and UL19-R (5′-TGCAGCAGCTGTTTTTGAAC-3′). The qPCRs were conducted on 10 ng of DNA using the FastStart universal SYBR green master reagent (Roche Diagnostics) according to the manufacturer's guidelines. The qPCR was run on the Step One Plus real-time PCR system (Applied Biosystems). All samples were run in duplicate, and the results were analyzed using ABI StepOne software v2.1. Absolute amounts of rep, gfp, and β-globin amplicons, in arbitrary units, were determined using dilutions of rep- and gfp-containing plasmids and genomic DNA from uninfected cells, respectively. Results are expressed as mean ratios of rep or gfp to β-globin DNA and standard deviations (SD) from at least three independent experiments. Statistical analyses were performed using the two-tailed Student's t test. Differences were considered significant when P was <0.05.
Western blot analysis.
The cells were collected, washed in phosphate-buffered saline (PBS), and lysed with RIPA buffer (20 mM Tris-HCl [pH 7.4], 50 mM NaCl, 1 mM EDTA, 0.5% Nonidet P-40, 0.5% deoxycholate, and 0.5% SDS) in the presence of a cocktail of protease inhibitors (Roche). Proteins were loaded on precast 5 to 15% SDS polyacrylamide gels and then transferred to nitrocellulose membranes using a Trans-blot Turbo transfer system (Roche). After saturation, the membranes were incubated overnight at 4°C with the appropriate antibody diluted in blocking buffer: anti-Rad50 (Abcam ab89, 1/500), anti-Mre11 (Abcam ab214, 1/500), anti-Nbs1(Abcam ab18178; 1/500), anti-ICP0 (Abcam ab6513, 1/1,000), anti-ICP4 (Abcam ab6514, 1/2,500), anti-ICP8 (Abcam ab20194, 1/1,000), anti-UL12 (rabbit polyclonal antibody bwp12, 1/15,000; kindly provided by S. Weller), anti-Rep (303.2, 1/20), anti-VP (B1, 1/100), anti-actin (Sigma A2228, 1/5,000 dilution). After PBS washes, a horseradish peroxidase-conjugated anti-mouse antibody (Sigma) was applied to the membranes at a 1/10,000 dilution for 1 h at room temperature. Finally, the membranes were incubated for 5 min with an enhanced chemiluminescence reagent (West Dura; Pierce) and exposed to an autoradiography film.
RESULTS
Knockdown of MRN reduces AAV replication in the presence of HSV-1.
To investigate the role of MRN during HSV-1-induced AAV replication, we used HeLa cells in which synthesis of the Rad50 protein was knocked down by expressing short hairpin RNAs (shRNA) against the Rad50 mRNA (HeLa shRad50). HeLa cells expressing shRNA against the luciferase mRNA (HeLa shLuc) were used as a control. Western blot analysis confirmed that Rad50 was efficiently knocked down (Fig. 1A). In these cells, the levels of the two other MRN components, Mre11 and Nbs1, were also reduced, indicating that the whole complex was destabilized. Importantly, knockdown (KD) of Rad50 did not alter the viability and cell cycle progression of Rad50-negative HeLa cells compared to control cells (data not shown).
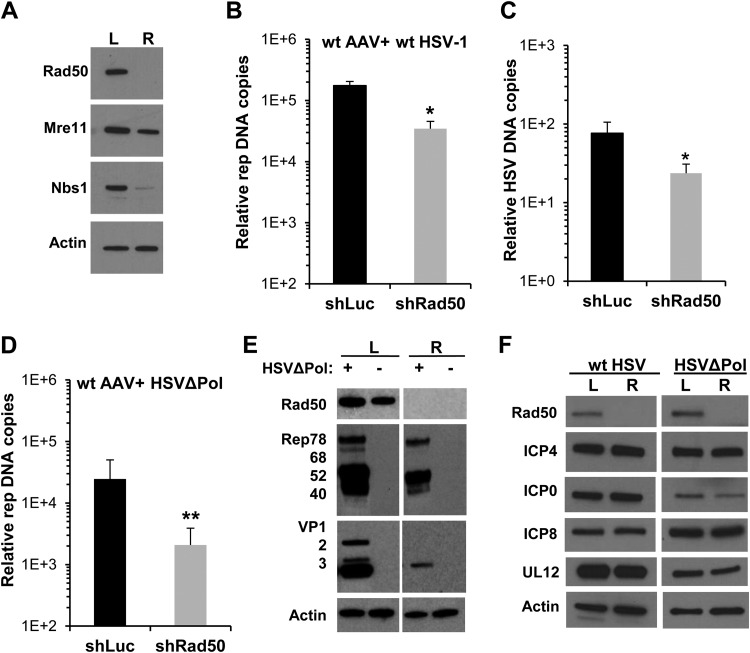
FIG 1.
Effect of Rad50 knockdown on AAV replication in the presence of HSV-1. (A) Western blot analysis of HeLa shRad50 (R) and HeLa shLuc (L) cells. (B and C) HeLa shRad50 or HeLa shLuc cells were coinfected with wt AAV (MOI, 103 GP/cell) and wt HSV-1 (MOI, 5 PFU/cell). Twenty hours postinfection (p.i.), DNA was extracted, and the amounts of AAV and HSV-1 genomes were quantified by qPCR. Results are expressed as the ratio of rep or UL19 to β-globin genes (mean and SD). (D) Quantification by qPCR of AAV DNA in cells coinfected with wt AAV and HSVΔPol (a replication-defective HSV-1 strain; MOI, 5 PFU/cell) for 20 h. (E) Western blot analysis of cells coinfected or not with wt AAV and HSVΔPol. (F) Synthesis of HSV-1 helper factors in HeLa shLuc and HeLa Rad50 cells infected with either wt HSV-1or HSVΔPol. *, P < 0.05; **, P < 0.01.
Rad50-negative cells and control cells were then infected with wt AAV and wt HSV-1. Analysis of AAV DNA levels by qPCR indicated that knockdown of Rad50 induced a significant decrease in AAV DNA replication (Fig. 1B). As expected from previous published studies, MRN KD also affected wt HSV-1 replication (Fig. 1C) (33). To verify if the decrease of AAV replication observed in the absence of MRN was independent of its effect on HSV-1, Rad50-negative and control cells were further coinfected with wt AAV and HSVΔPol, a replication-defective HSV-1 strain that was previously shown to sustain AAV replication (11, 48). In this setting, a significant reduction in AAV DNA was equally measured in HeLa shRad50 cells compared to HeLa shLuc cells, indicating that the effect of MRN on AAV replication was independent of its activity on HSV-1 genome replication (Fig. 1D). This reduction in AAV DNA was also confirmed by Southern blotting (data not shown) and was accompanied by a moderate decrease in the level of AAV Rep proteins, whereas the level of capsid proteins (VP1, VP2, and VP3) was most substantially affected (Fig. 1E). Importantly, the reduction in AAV DNA was not due to a decrease in the efficiency of infection with HSV-1, since the levels of the HSV-1 immediate early and early helper proteins ICP0, ICP4, ICP8, and UL12 were not significantly affected by the absence of Rad50 (Fig. 1F).
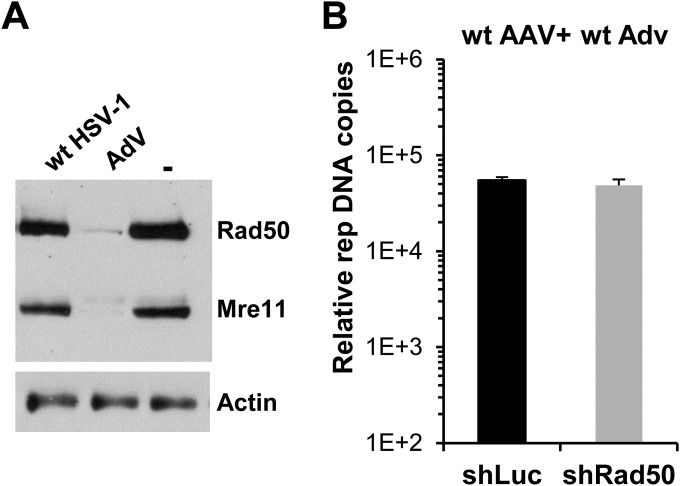
In contrast to HSV-1, wt AdV is able to specifically inhibit the MRN complex via the delocalization and degradation of Mre11 (27, 29). In agreement with these findings, Rad50 and Mre11 proteins were nearly undetectable in HeLa cells 48 h after infection with AdV (Fig. 2A). As expected, AAV replication was not reduced in shRad50 cells compared to shLuc cells when wt AdV was used as a helper (Fig. 2B). This result confirmed that Rad50-negative and -positive cells were equally susceptible to infection with AAV and were equally competent to induce its replication in the presence of AdV.
FIG 2.
Effect of Rad50 knockdown on AAV replication in the presence of AdV. (A) Level of Rad50 and Mre11 proteins in HeLa cells infected with either wt AdV (MOI, 50 IP/cell) or wt HSV-1 (MOI, 5 PFU/cell) at 48 h and 24 h p.i., respectively. (B) HeLa shRad50 and HeLa shLuc cells were coinfected with wt AAV (MOI, 103 GP/cell) and wt AdV for 48 h, and AAV DNA was quantified by qPCR. Results are expressed as the ratio of rep to β-globin genes (means and SD).
Altogether, these data indicated that the knockdown of Rad50 and consequently of MRN significantly reduced AAV DNA replication during coinfection with HSV-1.
The reduction of viral DNA replication in the absence of MRN is specific to wt AAV.
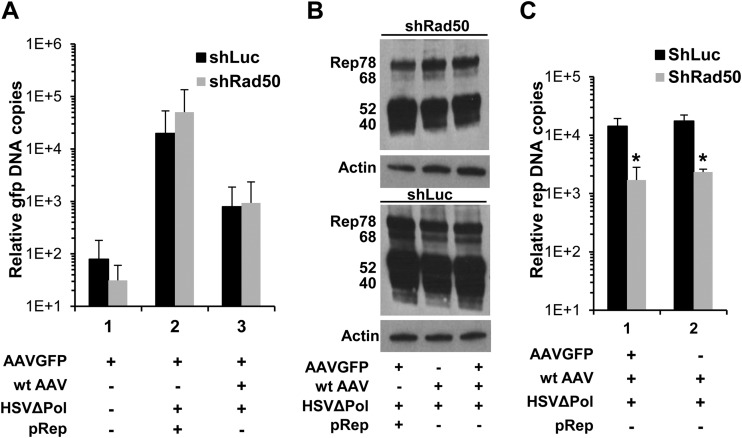
Previous studies indicated that MRN reduced transgene expression by rAAV vectors and suggested that this effect might be a consequence of MRN binding to the viral ITRs (31, 32, 49). MRN also inhibited replication of rAAV DNA in the presence of AdV helper activities (31). These studies strongly suggested that the viral ITRs were the main viral sequences targeted by MRN and responsible for its effects on AAV vector transduction and replication. Therefore, we asked if MRN could also affect replication of AAV vector in the presence of HSV-1. To answer this question, HeLa shRad50 and HeLa shLuc cells were infected with an AAV vector encoding GFP (AAVGFP) and HSVΔPol, and Rep proteins were provided either by transfecting cells with a Rep-expressing plasmid (pRep) or by coinfecting cells with wt AAV. As expected, AAVGFP genomes efficiently replicated in HeLa shLuc cells infected with HSV-1 (Fig. 3A and B). However, no decrease in AAV vector genome replication was detected in HeLa shRad50 cells under either of these conditions (Fig. 3A, lanes 2 and 3). In contrast, a significant decrease in wt AAV DNA levels was detected in HeLa shRad50 cells coinfected with AAVGFP or infected with wt AAV alone, thus confirming our previous observations (Fig. 3C). These results indicated that the reduction of viral replication was specific for wt AAV and further suggested that other viral sequences, besides the ITRs, were targeted by MRN. Importantly, the differential response of recombinant and wt AAV to Rad50 knockdown in coinfected cells also confirmed that the reduction in wt AAV replication was not due to a default in early steps of infection or in the level of either HSV-1 helper factors or Rep proteins.
FIG 3.
Effect of Rad50 knockdown on replication of recombinant and wt AAV. HeLa shRad50 and HeLa shLuc cells were coinfected with AAVGFP (MOI, 103 GP/cell) and HSVΔPol (MOI, 5 PFU/cell). Rep activity was provided either by transfection of a rep-expressing plasmid (pRep) or by infection with wt AAV (MOI, 103 GP/cell). Analysis of Rep protein levels by Western blotting (A) and quantification of either AAVGFP (B) or wt AAV (C) DNA were performed 48 h p.i. Results of qPCR experiments are expressed as the ratio of gfp or rep to β-globin genes (means and SD). *, P < 0.05.
MRN-responsive sequences lie within the rep gene.
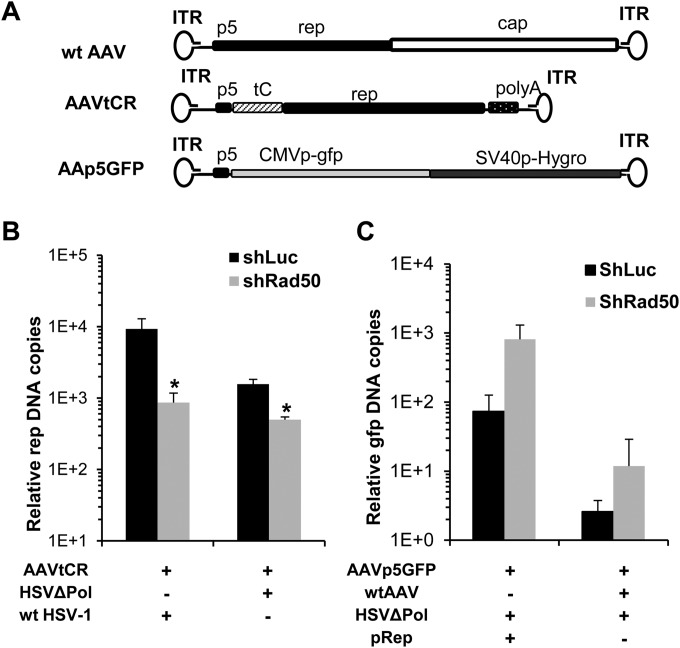
To identify the AAV sequences responsible for this effect, we first used AAV particles containing a modified genome (AAVtCR) with a deletion of the cap gene and in which the rep open reading frame (ORF) was fused to the tandem affinity purification tag (TAP)-Cherry sequences (Fig. 4A) (14). As previously observed for wt AAV, infection of HeLa shLuc and shRad50 cells with AAVtCR and either wt HSV-1 or HSVΔPol resulted in a significant decrease of AAVtCR DNA levels in the absence of MRN (Fig. 4B). Previous data from several groups have indicated that the AAV p5 promoter contained a cis-acting replication element (37, 50, 51) which was also responsible for inducing an ATR-mediated DNA damage response (52). We thus used a rAAV genome (AAVp5-GFP) containing the complete p5 promoter (Fig. 3A) and measured the level of AAV vector DNA generated in the presence or in the absence of Rad50 in HSV-1-coinfected cells. As previously, Rep activities were provided either by transfection of the pRep plasmid or by coinfection with wt AAV. Surprisingly, the absence of Rad50 did not affect replication of the AAVp5GFP genome under either set of conditions (Fig. 4C). In contrast, even if not statistically significant, an increase in rAAV genome replication was frequently observed. This effect may be linked to the previously reported ability of MRN to exert a negative effect on AAV genomes by binding to the ITRs. The same result was obtained using a minimal p5 replication element (data not shown) (37).
FIG 4.
Effect of the rep gene sequences on AAV genome replication in MRN-negative or -positive cells. (A) Structure of the wt and recombinant AAV genomes used. The AAVtCR genome is composed of the p5 promoter and the rep ORF fused at its N terminus to the TAP-Cherry sequences (14). The AAVp5GFP vector contains the entire p5 promoter (37). (B) HeLa shRad50 and HeLa shLuc cells were coinfected with AAVtCR (MOI, 103 GP/cell) and either HSVΔPol or wt HSV (MOI, 5 PFU/cell). DNA extracted 20 h p.i. was analyzed by qPCR using rep-specific primers. *, P < 0.05. (C) HeLa shRad50 and HeLa shLuc cells were coinfected with either AAVGFP or AAVp5GFP (MOI, 103 GP/cell) and HSVΔPol (MOI, 5 PFU/cell). Rep activity was provided either by transfection of pRep or coinfection with wt AAV. GFP DNA levels were measured by qPCR.
This result indicated that the presence of the rep gene in cis was sufficient to observe the decrease in AAV DNA replication in the absence of MRN and suggested that the MRN responsive sequences lay in the rep gene outside the p5 promoter region.
MRN activities involved in AAV replication.
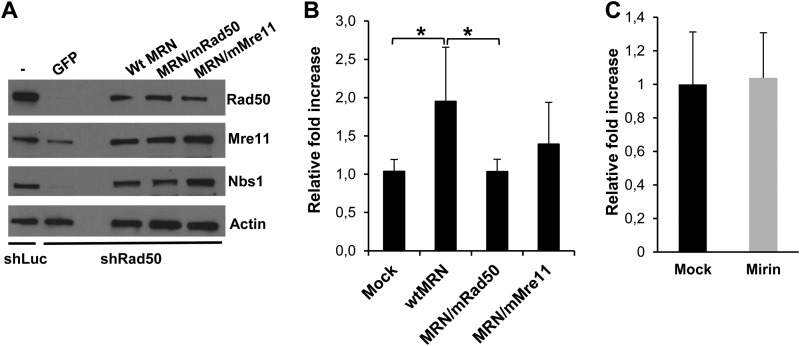
To determine which activity of MRN was involved in HSV-1-induced AAV genome replication, HeLa shRad50 cells were transiently complemented with a wt or mutated MRN complex and assayed for their ability to support AAV replication. To this end, HeLa shRad50 cells were either mock transfected or transfected with equimolar amounts of three plasmids expressing Nbs1 and either wt or mutated Mre11 and Rad50 proteins. Western blot analysis indicated that transfection of these plasmids resulted in a significant level of restoration of MRN proteins except for Rad50, which was still incompletely reconstituted despite having the target shRNA nucleotides mutated (Fig. 5A). Coinfection of MRN-transfected cells with wt AAV and HSVΔPol resulted in a 2-fold increase in AAV replication compared to that in mock-transfected cells, indicating that complementation with Nbs1, Mre11, and Rad50 proteins was able to partially increase AAV DNA levels (Fig. 5B, wtMRN). This result might be due to difficulties in efficiently reconstituting a functional MRN complex in all of the cells as well as to a lower stability of the shRNA-resistant Rad50 mRNA. In this setting, complementation with a plasmid expressing a Rad50 protein (S1202R; mRad50 in Fig. 5) unable to tether DNA (42, 43) reversed AAV levels to baseline. Similarly, expression of a mutated Mre11 protein (Mre11-3; mMre11 in Fig. 5), defective for 3′-5′ exonuclease activity (44) did not significantly increase AAV replication compared to that in mock-transfected HeLa shRad50 cells. The latter result suggested that Mre11 nuclease activity was not involved in AAV replication. To further confirm this result, wt AAV replication was similarly monitored in cells treated with Mirin, a drug which specifically inhibits Mre11 exonuclease activity (53). As for Mre11-3 mutant protein, Mirin inhibits Mre11 exonuclease activity but does not affect binding of Mre11 to DNA and to the other MRN components (44, 53). The dose of Mirin used in these assays (50 μM) was defined in preliminary experiments and validated by its ability to efficiently inhibit HR and induce a prevalent G2 cell arrest but in the absence of cell toxicity (data not shown). Use of Mirin did not reduce AAV genome levels compared to dimethyl sulfoxide (DMSO)-treated cells (Fig. 5C). Altogether, these analyses indicated that the MRN DNA tethering activity was important for AAV DNA replication in the presence of HSV-1, whereas Mre11 exonuclease activity was dispensable.
FIG 5.
MRN activities participating in AAV replication. (A) HeLa shRad50 cells were transfected with a combination of three plasmids (25 nmol each) expressing Rad50, Mre11, and Nbs1 or with an equivalent amount of a control plasmid (GFP). Where indicated, plasmids encoding a mutated Rad50 protein (mRad50 for S1202R) or Mre11 (mMre11 for Mre11-3) protein were used. Forty-eight hours posttransfection, cells were coinfected with wt AAV and HSVΔPol as indicated previously. MRN proteins (A) and AAV DNA (B) were analyzed 24 h later. qPCR results are expressed as the relative increase in AAV replication over that in mock-transfected HeLa shRad50 cells. *, P < 0.05. (C) HeLa cells were pretreated with Mirin (50 μM) or DMSO (mock) for 4 h and then infected with wt AAV and HSVΔPol. AAV DNA levels were measured by qPCR as indicated above and expressed relative to those in DMSO-treated cells.
The MRN complex is critical for AAV site-specific integration both in the absence and in the presence of HSV-1.
Several studies have analyzed DDR-induced during AdV- or HSV-1-mediated wt AAV replication (54–56). However, only a few reports described how DNA repair pathways affect the wt AAV life cycle. In particular, some studies reported that proteins involved in NHEJ pathways could regulate the capacity of AAV to be integrated into the human AAVS1 site (57–59). Because MRN is involved in A-NHEJ pathways and, more generally, in the choice of the DNA repair pathway, we investigated if the presence of MRN could affect AAV integration into AAVS1 by quantifying the AAV-AAVS1 junctions within a 1.2-kb region upstream from the AAVS1 Rep binding site (RBS) (Fig. 6A) as described previously (4, 47).
FIG 6.
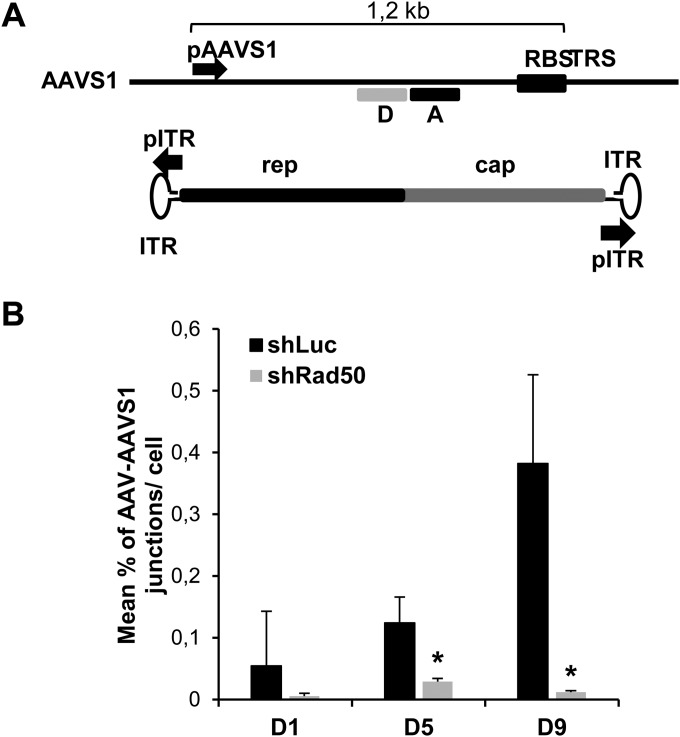
Quantification of AAV integration within AAVS1 in the absence of a helper virus. (A) Schematic view of the AAVS1 site and of the wt AAV genome. The black arrows indicate the primers used to amplify the AAV-AAVS1 junctions by TaqMan qPCR. The boxes labeled “D” and “A” indicate the positions of the donor and acceptor probes, respectively. RBS, Rep-binding site; TRS, terminal resolution site. (B) AAV junctions levels were measured in HeLa shRad50 and HeLa shLuc cells infected with wt AAV (MOI, 103 GP/cell) in the absence of a helper virus. Genomic DNA was extracted at the indicated days p.i. and analyzed by TaqMan qPCR as described above. Results are expressed as percent AAV-AAVS1 junctions per cell (means and SD). *, P < 0.05.
Detection of AAV integration was first performed in HeLa shLuc or HeLa shRad50 cells at different times after infection with wt AAV alone (Fig. 6B). At 24 h postinfection (p.i.), a low level of AAVS1-AAV junctions was detected in control HeLa shLuc cells which was moderately reduced in HeLa shRad50 cells. Upon passage of the infected HeLa shLuc cells the level of AAVS1-AAV junctions gradually increased, suggesting that AAV integration within the AAVS1 region was favored upon cell division. In striking contrast, the level of AAV-AAVS1 junctions remained low in HeLa shRad50 cells. These results strongly suggested that MRN was important to enhance AAV integration within the AAVS1 site.
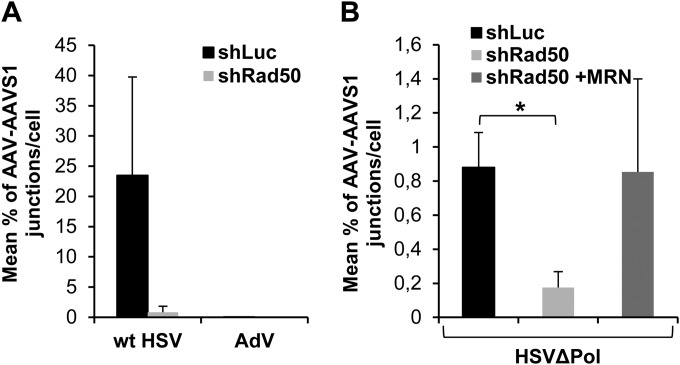
Our previous results indicated a positive role for MRN during AAV replication in the presence of HSV-1. Therefore, we further investigated whether AAV integration could be also detected under these conditions and what the effect of MRN was. Interestingly, AAV-AAVS1 junctions were detected 24 h after coinfection of HeLa shLuc cells with wt AAV and wt HSV-1, at a level which was significantly higher than that measured in the absence of the helper virus (Fig. 7A). Again, AAV integration in HeLa shRad50 cells coinfected with wt HSV-1 was lower than that measured in infected HeLa shLuc cells. Interestingly, under these conditions, AAV-AAVS1 junctions were not detected in cells coinfected with AAV and AdV even in HeLa shLuc cells (Fig. 7A).
FIG 7.
Quantification of AAV integration within AAVS1 in the presence of a helper virus. (A) Genomic DNA extracted 24 to 48 h p.i. from HeLa shLuc and HeLa shRad50 cells coinfected with wt AAV and either wtHSV-1 or AdV was analyzed as indicated in the legend to Fig. 5. (B) HeLa shRad50 cells were complemented with plasmids expressing wt Rad50 proteins or mock transfected and then infected with wt AAV and HSVΔPol. Results are expressed as percent AAV-AAVS1 junctions per cell (means and SD). *, P < 0.05.
To further confirm the role of MRN in AAV integration, a similar analysis was conducted on HeLa shRad50 cells complemented with a wt MRN complex and coinfected with wt AAV and HSVΔPol. Interestingly in the presence of HSVΔPol, the overall level of AAV-AAVS1 junctions in shLuc cells was significantly reduced compared to that in cells coinfected with wt HSV-1 (Fig. 7B). As observed before, depletion of MRN severely reduced the number of AAV-AAVS1 junctions. In this context, transient expression of MRN components in HeLa shRad50 cells was able to restore AAV integration to a level similar to that observed in HeLa shLuc cells (Fig. 7B).
Altogether, these results strongly suggested that the MRN complex could critically enhance AAV integration within AAVS1 in the absence and in the presence of HSV-1. In accordance with this conclusion, AAV integration within the AAVS1 1.2-kb region was not detected during coinfection of cells with AdV, probably because this helper virus can specifically inactivate and degrade the MRN complex.
DISCUSSION
The present study addressed the question of the impact of MRN on wt AAV replication during coinfection with HSV-1.
Our results first indicated that depletion of Rad50, which also affects the stability of the other MRN components, induced a reduction in wt AAV DNA replication during coinfection with HSV-1 (Fig. 1). Previous reports have shown that MRN can bind the viral ITRs and inhibit transduction with AAV vectors (31, 32, 49). Other studies also reported that during replication, the ITRs were targeted by the Ku proteins which could protect them against recognition by MRN (60). Indeed, the Ku proteins are the predominant DSB sensors that can protect DNA extremities against resection by other enzymes, such as MRN (21). Our results indicated that the positive effect of MRN was specific to wt AAV, since knockdown of MRN did not affect replication of AAV vectors. While these results do not exclude the possibility that MRN targets the ITRs, they strongly suggest that the effect of this complex on wt AAV genome replication is dependent on the presence of other internal viral sequences. Although the precise viral sequences targeted by MRN during replication remain to be identified, our studies indicate that they are located in the rep gene and that, interestingly, the p5 promoter sequence was not sufficient to render a rAAV genome responsive to the presence of MRN (Fig. 4). Even if these findings do not exclude the possibility that the p5 element may be required for the MRN effect to occur, this observation is in line with previous reports indicating that despite being able to induce a DDR response, the p5 element recruited factors of the ChK1/ATR pathway rather than MRN (52). Interestingly, a recent in silico study identified several strong secondary structures within the AAV genome that are conserved among the parvovirus family members, in particular, at the junctions between the rep and cap genes (61). The presence of such secondary sequences may attract MRN, as was recently hypothesized for the AAV ITRs (49).
The major question arising from these findings is that of how MRN controls AAV genome replication. One could argue that the role of MRN complex in DNA damage signaling, via activation of ATM and ATR, could be the cause of the observed decrease of AAV replication in the absence of this complex. However, even if previous studies have indicated that the ATM and ATR were activated during AAV replication in cells coinfected with AdV or HSV-1, inhibition of these kinases was not reported to significantly affect wt AAV replication (54–56). Our results indicating that Mirin, an inhibitor of MRN-dependent ATM activation (53), does not significantly affect AAV replication support this conclusion (Fig. 5B).
Upon detection of the DSB, MRN promotes DNA repair via either HR or NHEJ, and Mre11 plays an essential role through its 3′-5′ exonuclease and endonuclease activities (15). Among possible DNA repair pathways participating in AAV replication, several arguments were a priori in favor of NHEJ. Indeed, the DDR induced by coinfection with AAV and either AdV or HSV-1 was found to be mainly mediated by the activation of DNA-dependent protein kinase catalytic subunits (DNA-PKcs) (54–56). In addition, even if these studies did not demonstrate any significant effect on AAV replication when DNA-PKcs was inhibited, one additional report indicated that the depletion of Ku proteins or DNA-PKcs could reduce AAV replication in vitro and in vivo, respectively (60). Our results indicate that MRN exonuclease activity is not required for AAV DNA replication (Fig. 5). In accordance with these findings, some preliminary results obtained using mutant cells lines suggest that neither inactivation of either HR nor NHEJ reduces AAV replication (data not shown).
Our results rather indicated that the effect of MRN on AAV replication relied on the biochemical functions of Rad50 which, with Mre11, constitutes the catalytic component of the complex (15, 62). Rad50 is an ATPase which belongs to the structural maintenance of chromosome (SMC) family of proteins and displays the ability to tether DNA ends or chromosomal fragments over long distances via the formation of intermolecules bridges (63). Our complementation assay indicated that the Rad50 tethering activity is important to stimulate AAV replication (Fig. 5A). This observation suggests that MRN may contribute to AAV replication by bridging AAV DNA molecules, thus contributing to the structural organization of AAV viral replication compartments.
In addition to its effect on AAV genome replication our study also indicated that the absence of MRN also reduces AAV integration within the AAVS1 region proximal to the RBS both in the presence and in the absence of HSV-1 (Fig. 6 and 7). Interestingly, our results also indicate a much higher level of AAVS1-AAV junctions after coinfection with wt HSV-1 compared with HSVΔPol (Fig. 7). Even though we cannot exclude the possibility that part of the junctions measured by qPCR derive from virus-induced amplification of the AAVS1-AAV region (8), our result suggests that, as already suggested by other investigators, AAV integration may be enhanced by active replication.
Studies conducted to characterize AAV genomes integrated within AAVS1 have indicated that AAV integration events were localized in a region of 100 kb upstream of the RBS with a majority occurring in close proximity and that integration resulted in extensive rearrangements of the AAVS1 sequences (64, 65). In addition, genome-wide analysis of wt AAV integration events also confirmed the existence of several other integrations sites within the cell genome, which, even if targeted at lower frequencies than AAVS1, represented recurrent integration spots (65, 66). Our analyses clearly do not allow this conclusion, but it is tempting to speculate that MRN globally affects integration of AAV genomes not only in the entire AAVS1 site, at longer distances from the RBS, but also in other chromosomal spots. Future studies should be performed to confirm this hypothesis.
Proteins of the NHEJ pathway have been found to participate in AAV integration within AAVS1, although with contradictory results (57, 59). Interestingly, the analysis of the AAVS1-AAV junctions also revealed that many integration junctions corresponded to short regions of homology between AAV and AAVS1 sequences (65, 67, 68). These findings strongly suggest that AAV integration might be the result of a DNA repair pathway involving end-joining via microhomologies. Repair by using microhomologies takes places during A-NHEJ and single-strand annealing (SSA) (69). Both these pathways require the intervention of an exonuclease to initiate resection at the break. Interestingly, SSA was shown to be stimulated during infection by HSV-1 via the action of the viral UL12 exonuclease, a factor which has also been found to be associated with AAV Rep proteins in replication compartments (14, 70). The intervention of this repair pathway during AAV integration would be consistent with the fact that integration sites are generally distant from the AAVS1 terminal resolution site (TRS), which is the site where nicking by Rep occurs (9, 71). Additional studies should be performed to compare the effect of proteins involved in C-NHEJ, A-NHEJ, and SSA during AAV integration and to determine the relative importance of these pathways. Also, in vitro studies should be conducted to measure how MRN can contribute, together with Rep, to the tethering of AAV and AAVS1 DNA molecules (9).
Whatever the mechanism involved is, the requirement for MRN during AAV integration may explain why integrated wt AAV sequences were not detected in the majority of postmitotic tissues (72–75). Indeed, MRN constituents were shown to be downregulated in terminally differentiated muscle cells as well as in hepatocytes (76). Wild-type AAV integration may not be required in nondividing cells, since the genome can persist in an episomal form which is lost only upon cell division. In proliferating cells, an increased level of MRN might constitute a critical factor allowing integration of the viral genome and consequently its persistence during cell division.
AAV integration is generally considered an event occurring during the latent phase, but amplification and rearrangements of the AAVS1 site were also observed in the presence of Rep proteins alone or during AAV productive infection (8, 77). Our data indicated that AAV integration within AAVS1 occurred at a significant level during AAV replication induced by HSV-1. It is noteworthy that under these conditions, AAV integration was not detected in cells coinfected with AAV and AdV, thus confirming the requirement for MRN for efficient AAV integration within this AAVS1 region.
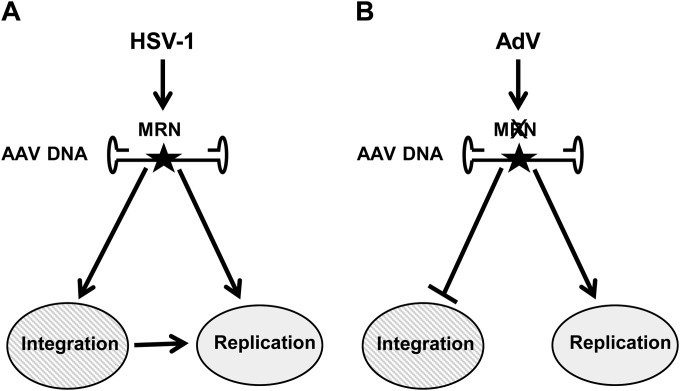
We propose the following hypothetical model to integrate these results with previous published data: in cells coinfected with wt AAV and HSV-1 (Fig. 8A), MRN is recruited within viral replication compartments where AAV genomes are synthetized. By binding to an internal AAV sequence, MRN contributes to AAV replication thanks to its DNA tethering activity. This complex can also favor AAV integration, which takes place through a yet-unknown repair pathway likely involving end-joining via microhomologies. The AAV genomes integrated within AAVS1 and potentially many other chromosomal sites may constitute optimal templates for rescue and replication by HSV-1 and thus contribute to the pool of newly replicated AAV genomes. In contrast, in the presence of AdV (Fig. 8B), inactivation/degradation of MRN may prevent AAV integration and also reduce replication. This hypothesis is consistent with our results showing that in cells coinfected with AdV, the amount of replicated AAV genomes is generally lower than that in cells coinfected with HSV-1 (Fig. 1B and 2). Importantly our model does not exclude the possibility that MRN may also participate in other steps of the AAV life cycle, such as rescue of integrated AAV genomes. Finally, this study shows that AAV is able to adapt its replication to the helper virus that is present and further confirms its capacity to behave as an opportunistic viral parasite.
FIG 8.
Hypothetical model to explain the effect of MRN on AAV replication during productive infection. (A) In the presence of HSV-1 the MRN complex is recruited within AAV replication compartments and favors both AAV genome replication and integration. AAV integrated genomes contribute to AAV replication, which is also directly performed using episomal AAV genomes as a template. (B) In the presence of AdV, the MRN complex is delocalized and then degraded. AAV integration is strongly reduced, resulting in a reduced level of AAV replication, and replicative forms arise solely from episomal genomes. The star indicates the presence of putative MRN binding sequences within the rep gene.
ACKNOWLEDGMENTS
We acknowledge the contribution of Isabelle Grosjean from the CelluloNet facility and all personnel of the platforms of SFR Biosciences Gerland-Lyon Sud (UMS344/US8) for their help.
This work was supported by INSERM, CNRS, Université Claude Bernard Lyon 1 and Ecole Normale Supérieure de Lyon. It was funded by grants from the Association Française contre les Myopathies (AFM) to A.S. and from the Center for Molecular Medicine Cologne (CMMC) to H.B.
REFERENCES
- 1.Flotte TR, Berns KI. 2005. Adeno-associated virus: a ubiquitous commensal of mammals. Hum Gene Ther 16:401–407. doi: 10.1089/hum.2005.16.401. [DOI] [PubMed] [Google Scholar]
- 2.Muzyczka N, Berns KI. 2001. Parvoviridae: the viruses and their replication, p 2327–2359. In Knipe DM, Howley PM (ed), Fields virology, vol 2 Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 3.Hüser D, Gogol-Doring A, Lutter T, Weger S, Winter K, Hammer EM, Cathomen T, Reinert K, Heilbronn R. 2010. Integration preferences of wildtype AAV-2 for consensus rep-binding sites at numerous loci in the human genome. PLoS Pathog 6:e1000985. doi: 10.1371/journal.ppat.1000985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hüser D, Weger S, Heilbronn R. 2002. Kinetics and frequency of adeno-associated virus site-specific integration into human chromosome 19 monitored by quantitative real-time PCR. J Virol 76:7554–7559. doi: 10.1128/JVI.76.15.7554-7559.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kotin RM, Linden RM, Berns KI. 1992. Characterization of a preferred site on human chromosome 19q for integration of adeno-associated virus DNA by non-homologous recombination. EMBO J 11:5071–5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linden MR, Ward P, Giraud C, Winocour E, Berns KI. 1996. Site-specific integration by adeno-associated virus. Proc Natl Acad Sci U S A 93:11288–11294. doi: 10.1073/pnas.93.21.11288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Linden RM, Winocour E, Berns KI. 1996. The recombination signals for adeno-associated virus site-specific integration. Proc Natl Acad Sci U S A 93:7966–7972. doi: 10.1073/pnas.93.15.7966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young SM Jr, McCarty DM, Degtyareva N, Samulski RJ. 2000. Roles of adeno-associated virus Rep protein and human chromosome 19 in site-specific recombination. J Virol 74:3953–3966. doi: 10.1128/JVI.74.9.3953-3966.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weitzman MD, Kyostio SR, Kotin RM, Owens RA. 1994. Adeno-associated virus (AAV) Rep proteins mediate complex formation between AAV DNA and its integration site in human DNA. Proc Natl Acad Sci U S A 91:5808–5812. doi: 10.1073/pnas.91.13.5808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geoffroy MC, Salvetti A. 2005. Helper functions required for wild type and recombinant adeno-associated virus growth. Curr Gene Ther 5:265–271. doi: 10.2174/1566523054064977. [DOI] [PubMed] [Google Scholar]
- 11.Alazard-Dany N, Nicolas A, Ploquin A, Strasser R, Greco A, Epstein AL, Fraefel C, Salvetti A. 2009. Definition of herpes simplex virus type 1 helper activities for adeno-associated virus early replication events. PLoS Pathog 5:e1000340. doi: 10.1371/journal.ppat.1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geoffroy MC, Epstein AL, Toublanc E, Moullier P, Salvetti A. 2004. Herpes simplex virus type 1 ICPO protein mediates activation of adeno-associated virus type 2 rep gene expression from a latent integrated form. J Virol 78:10977–10986. doi: 10.1128/JVI.78.20.10977-10986.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weindler FW, Heilbronn R. 1991. A subset of herpes simplex virus replication genes provides helper functions for productive adeno-associated virus replication. J Virol 65:2476–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicolas A, Alazard-Dany N, Biollay C, Arata L, Jolinon N, Kuhn L, Ferro M, Weller SK, Epstein AL, Salvetti A, Greco A. 2010. Identification of Rep-associated factors in herpes simplex virus type 1-induced adeno-associated virus type 2 replication compartments. J Virol 84:8871–8887. doi: 10.1128/JVI.00725-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stracker TH, Petrini JH. 2011. The MRE11 complex: starting from the ends. Nat Rev Mol Cell Biol 12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carson CT, Schwartz RA, Stracker TH, Lilley CE, Lee DV, Weitzman MD. 2003. The Mre11 complex is required for ATM activation and the G2/M checkpoint. EMBO J 22:6610–6620. doi: 10.1093/emboj/cdg630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JH, Paull TT. 2004. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 304:93–96. doi: 10.1126/science.1091496. [DOI] [PubMed] [Google Scholar]
- 18.Uziel T, Lerenthal Y, Moyal L, Andegeko Y, Mittelman L, Shiloh Y. 2003. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J 22:5612–5621. doi: 10.1093/emboj/cdg541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruhn C, Zhou ZW, Ai H, Wang ZQ. 2014. The essential function of the MRN complex in the resolution of endogenous replication intermediates. Cell Rep 6:182–195. doi: 10.1016/j.celrep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 20.Trenz K, Smith E, Smith S, Costanzo V. 2006. ATM and ATR promote Mre11 dependent restart of collapsed replication forks and prevent accumulation of DNA breaks. EMBO J 25:1764–1774. doi: 10.1038/sj.emboj.7601045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodarzi AA, Jeggo PA. 2013. The repair and signaling responses to DNA double-strand breaks. Adv Genet 82:1–45. doi: 10.1016/B978-0-12-407676-1.00001-9. [DOI] [PubMed] [Google Scholar]
- 22.Betermier M, Bertrand P, Lopez BS. 2014. Is non-homologous end-joining really an inherently error-prone process? PLoS Genet 10:e1004086. doi: 10.1371/journal.pgen.1004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rass E, Grabarz A, Plo I, Gautier J, Bertrand P, Lopez BS. 2009. Role of Mre11 in chromosomal nonhomologous end joining in mammalian cells. Nat Struct Mol Biol 16:819–824. doi: 10.1038/nsmb.1641. [DOI] [PubMed] [Google Scholar]
- 24.Xie A, Kwok A, Scully R. 2009. Role of mammalian Mre11 in classical and alternative nonhomologous end joining. Nat Struct Mol Biol 16:814–818. doi: 10.1038/nsmb.1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rupnik A, Lowndes NF, Grenon M. 2010. MRN and the race to the break. Chromosoma 119:115–135. doi: 10.1007/s00412-009-0242-4. [DOI] [PubMed] [Google Scholar]
- 26.Lilley CE, Chaurushiya MS, Weitzman MD. 2010. Chromatin at the intersection of viral infection and DNA damage. Biochim Biophys Acta 1799:319–327. doi: 10.1016/j.bbagrm.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stracker TH, Carson CT, Weitzman MD. 2002. Adenovirus oncoproteins inactivate the Mre11-Rad50-NBS1 DNA repair complex. Nature 418:348–352. doi: 10.1038/nature00863. [DOI] [PubMed] [Google Scholar]
- 28.Karen KA, Hoey PJ, Young CS, Hearing P. 2009. Temporal regulation of the Mre11-Rad50-Nbs1 complex during adenovirus infection. J Virol 83:4565–4573. doi: 10.1128/JVI.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Araujo FD, Stracker TH, Carson CT, Lee DV, Weitzman MD. 2005. Adenovirus type 5 E4orf3 protein targets the Mre11 complex to cytoplasmic aggresomes. J Virol 79:11382–11391. doi: 10.1128/JVI.79.17.11382-11391.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stracker TH, Lee DV, Carson CT, Araujo FD, Ornelles DA, Weitzman MD. 2005. Serotype-specific reorganization of the Mre11 complex by adenoviral E4orf3 proteins. J Virol 79:6664–6673. doi: 10.1128/JVI.79.11.6664-6673.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz RA, Palacios JA, Cassell GD, Adam S, Giacca M, Weitzman MD. 2007. The Mre11/Rad50/Nbs1 complex limits adeno-associated virus transduction and replication. J Virol 81:12936–12945. doi: 10.1128/JVI.01523-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cervelli T, Palacios JA, Zentilin L, Mano M, Schwartz RA, Weitzman MD, Giacca M. 2008. Processing of recombinant AAV genomes occurs in specific nuclear structures that overlap with foci of DNA-damage-response proteins. J Cell Sci 121:349–357. doi: 10.1242/jcs.003632. [DOI] [PubMed] [Google Scholar]
- 33.Lilley CE, Carson CT, Muotri AR, Gage FH, Weitzman MD. 2005. DNA repair proteins affect the lifecycle of herpes simplex virus 1. Proc Natl Acad Sci U S A 102:5844–5849. doi: 10.1073/pnas.0501916102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson DE, Weller SK. 2004. Recruitment of cellular recombination and repair proteins to sites of herpes simplex virus type 1 DNA replication is dependent on the composition of viral proteins within prereplicative sites and correlates with the induction of the DNA damage response. J Virol 78:4783–4796. doi: 10.1128/JVI.78.9.4783-4796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kotin RM, Berns KI. 1989. Organization of adeno-associated virus DNA in latently infected Detroit 6 cells. Virology 170:460–467. doi: 10.1016/0042-6822(89)90437-6. [DOI] [PubMed] [Google Scholar]
- 36.Walz C, Schlehofer JR. 1992. Modification of some biological properties of HeLa cells containing adeno-associated virus DNA integrated into chromosome 17. J Virol 66:2990–3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Francois A, Guilbaud M, Awedikian R, Chadeuf G, Moullier P, Salvetti A. 2005. The cellular TATA binding protein is required for Rep-dependent replication of a minimal adeno-associated virus type 2 p5 element. J Virol 79:11082–11094. doi: 10.1128/JVI.79.17.11082-11094.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grimm D, Kern A, Rittner K, Kleinschmidt J. 1998. Novel tools for production and purification of recombinant adeno-associated virus vectors. Hum Gene Ther 9:2745–2760. doi: 10.1089/hum.1998.9.18-2745. [DOI] [PubMed] [Google Scholar]
- 39.Xiao X, Li J, Samulski RJ. 1998. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol 72:2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salvetti A, Oreve S, Chadeuf G, Favre D, Cherel Y, Champion-Arnaud P, David-Ameline J, Moullier P. 1998. Factors influencing recombinant adeno-associated virus production. Hum Gene Ther 9:695–706. doi: 10.1089/hum.1998.9.5-695. [DOI] [PubMed] [Google Scholar]
- 41.Zolotukhin S, Byrne BJ, Mason E, Zolotukhin I, Summerford C, Samulski RJ, Muzyczka N. 1999. Recombinant adeno-associated virus purification using novel methods improves infectious titer and yield. Gene Ther 6:973–985. doi: 10.1038/sj.gt.3300938. [DOI] [PubMed] [Google Scholar]
- 42.Dupre A, Boyer-Chatenet L, Gautier J. 2006. Two-step activation of ATM by DNA and the Mre11-Rad50-Nbs1 complex. Nat Struct Mol Biol 13:451–457. doi: 10.1038/nsmb1090. [DOI] [PubMed] [Google Scholar]
- 43.Bhaskara V, Dupre A, Lengsfeld B, Hopkins BB, Chan A, Lee JH, Zhang X, Gautier J, Zakian V, Paull TT. 2007. Rad50 adenylate kinase activity regulates DNA tethering by Mre11/Rad50 complexes. Mol Cell 25:647–661. doi: 10.1016/j.molcel.2007.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arthur LM, Gustausson K, Hopfner KP, Carson CT, Stracker TH, Karcher A, Felton D, Weitzman MD, Tainer J, Carney JP. 2004. Structural and functional analysis of Mre11-3. Nucleic Acids Res 32:1886–1893. doi: 10.1093/nar/gkh343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lambert S, Lopez BS. 2000. Characterization of mammalian RAD51 double strand break repair using non-lethal dominant-negative forms. EMBO J 19:3090–3099. doi: 10.1093/emboj/19.12.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berger G, Durand S, Goujon C, Nguyen XN, Cordeil S, Darlix JL, Cimarelli A. 2011. A simple, versatile and efficient method to genetically modify human monocyte-derived dendritic cells with HIV-1-derived lentiviral vectors. Nat Protoc 6:806–816. doi: 10.1038/nprot.2011.327. [DOI] [PubMed] [Google Scholar]
- 47.Guilbaud M, Chadeuf G, Avolio F, Francois A, Moullier P, Recchia A, Salvetti A. 2008. Relative influence of the adeno-associated virus (AAV) type 2 p5 element for recombinant AAV vector site-specific integration. J Virol 82:2590–2593. doi: 10.1128/JVI.01956-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Toublanc E, Benraiss A, Bonnin D, Blouin W, Brument N, Cartier N, Epstein AL, Moullier P, Salvetti A. 2004. Identification of a replication-defective herpes simplex virus for recombinant adeno-associated virus type 2 (rAAV2) particle assembly using stable producer cell lines. J Gene Med 6:555–564. doi: 10.1002/jgm.542. [DOI] [PubMed] [Google Scholar]
- 49.Lentz TB, Samulski RJ. 2015. Insight into the mechanism of inhibition of adeno-associated virus by the mre11/rad50/nbs1 complex. J Virol 89:181–194. doi: 10.1128/JVI.01990-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nony P, Tessier J, Chadeuf G, Ward P, Giraud A, Dugast M, Linden RM, Moullier P, Salvetti A. 2001. Novel cis-acting replication element in the adeno-associated virus type 2 genome is involved in amplification of integrated rep-cap sequences. J Virol 75:9991–9994. doi: 10.1128/JVI.75.20.9991-9994.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tullis GE, Shenk T. 2000. Efficient replication of adeno-associated virus type 2 vectors: a cis-acting element outside of the terminal repeats and a minimal size. J Virol 74:11511–11521. doi: 10.1128/JVI.74.24.11511-11521.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fragkos M, Breuleux M, Clement N, Beard P. 2008. Recombinant adeno-associated viral vectors are deficient in provoking a DNA damage response. J Virol 82:7379–7387. doi: 10.1128/JVI.00358-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dupre A, Boyer-Chatenet L, Sattler RM, Modi AP, Lee JH, Nicolette ML, Kopelovich L, Jasin M, Baer R, Paull TT, Gautier J. 2008. A forward chemical genetic screen reveals an inhibitor of the Mre11-Rad50-Nbs1 complex. Nat Chem Biol 4:119–125. doi: 10.1038/nchembio.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Collaco RF, Bevington JM, Bhrigu V, Kalman-Maltese V, Trempe JP. 2009. Adeno-associated virus and adenovirus coinfection induces a cellular DNA damage and repair response via redundant phosphatidylinositol 3-like kinase pathways. Virology 392:24–33. doi: 10.1016/j.virol.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schwartz RA, Carson CT, Schuberth C, Weitzman MD. 2009. Adeno-associated virus replication induces a DNA damage response coordinated by DNA-dependent protein kinase. J Virol 83:6269–6278. doi: 10.1128/JVI.00318-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vogel R, Seyffert M, Strasser R, de Oliveira AP, Dresch C, Glauser DL, Jolinon N, Salvetti A, Weitzman MD, Ackermann M, Fraefel C. 2012. Adeno-associated virus type 2 modulates the host DNA damage response induced by herpes simplex virus 1 during coinfection. J Virol 86:143–155. doi: 10.1128/JVI.05694-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Daya S, Cortez N, Berns KI. 2009. Adeno-associated virus site-specific integration is mediated by proteins of the nonhomologous end-joining pathway. J Virol 83:11655–11664. doi: 10.1128/JVI.01040-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Romanova LG, Zacharias J, Cannon ML, Philpott NJ. 2011. Effect of poly(ADP-ribose) polymerase 1 on integration of the adeno-associated viral vector genome. J Gene Med 13:342–352. doi: 10.1002/jgm.1577. [DOI] [PubMed] [Google Scholar]
- 59.Song S, Lu Y, Choi YK, Han Y, Tang Q, Zhao G, Berns KI, Flotte TR. 2004. DNA-dependent PK inhibits adeno-associated virus DNA integration. Proc Natl Acad Sci U S A 101:2112–2116. doi: 10.1073/pnas.0307833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Choi YK, Nash K, Byrne BJ, Muzyczka N, Song S. 2010. The effect of DNA-dependent protein kinase on adeno-associated virus replication. PLoS One 5:e15073. doi: 10.1371/journal.pone.0015073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Muhire BM, Golden M, Murrell B, Lefeuvre P, Lett JM, Gray A, Poon AY, Ngandu NK, Semegni Y, Tanov EP, Monjane AL, Harkins GW, Varsani A, Shepherd DN, Martin DP. 2014. Evidence of pervasive biologically functional secondary structures within the genomes of eukaryotic single-stranded DNA viruses. J Virol 88:1972–1989. doi: 10.1128/JVI.03031-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van der Linden E, Sanchez H, Kinoshita E, Kanaar R, Wyman C. 2009. RAD50 and NBS1 form a stable complex functional in DNA binding and tethering. Nucleic Acids Res 37:1580–1588. doi: 10.1093/nar/gkn1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinoshita E, van der Linden E, Sanchez H, Wyman C. 2009. RAD50, an SMC family member with multiple roles in DNA break repair: how does ATP affect function? Chromosome Res 17:277–288. doi: 10.1007/s10577-008-9018-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Henckaerts E, Linden RM. 2010. Adeno-associated virus: a key to the human genome? Future Virol 5:555–574. doi: 10.2217/fvl.10.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Janovitz T, Klein IA, Oliveira T, Mukherjee P, Nussenzweig MC, Sadelain M, Falck-Pedersen E. 2013. High-throughput sequencing reveals principles of adeno-associated virus serotype 2 integration. J Virol 87:8559–8568. doi: 10.1128/JVI.01135-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huser D, Gogol-Doring A, Chen W, Heilbronn R. 2014. Adeno-associated virus type 2 wild-type and vector-mediated genomic integration profiles of human diploid fibroblasts analyzed by third-generation PacBio DNA sequencing. J Virol 88:11253–11263. doi: 10.1128/JVI.01356-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Drew HR, Lockett LJ, Both GW. 2007. Increased complexity of wild-type adeno-associated virus-chromosomal junctions as determined by analysis of unselected cellular genomes. J Gen Virol 88:1722–1732. doi: 10.1099/vir.0.82880-0. [DOI] [PubMed] [Google Scholar]
- 68.McAlister VJ, Owens RA. 2007. Preferential integration of adeno-associated virus type 2 into a polypyrimidine/polypurine-rich region within AAVS1. J Virol 81:9718–9726. doi: 10.1128/JVI.00746-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mehta A, Haber JE. 2014. Sources of DNA double-strand breaks and models of recombinational DNA repair. Cold Spring Harb Perspect Biol 6:a016428. doi: 10.1101/cshperspect.a016428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schumacher AJ, Mohni KN, Kan Y, Hendrickson EA, Stark JM, Weller SK. 2012. The HSV-1 exonuclease, UL12, stimulates recombination by a single strand annealing mechanism. PLoS Pathog 8:e1002862. doi: 10.1371/journal.ppat.1002862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lamartina S, Ciliberto S, Toniatti C. 2000. Selective cleavage of AAVS1 substrates by the adeno-associated virus type 2 Rep68 protein is dependent on topological and sequence constraints. J Virol 74:8831–8842. doi: 10.1128/JVI.74.19.8831-8842.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schnepp BC, Jensen RL, Chen CL, Johnson PR, Clark KR. 2005. Characterization of adeno-associated virus genomes isolated from human tissues. J Virol 79:14793–14803. doi: 10.1128/JVI.79.23.14793-14803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schnepp BC, Jensen RL, Clark KR, Johnson PR. 2009. Infectious molecular clones of adeno-associated virus isolated directly from human tissues. J Virol 83:1456–1464. doi: 10.1128/JVI.01686-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hernandez YJ, Wang J, Kearns WG, Loiler S, Poirier A, Flotte TR. 1999. Latent adeno-associated virus infection elicits humoral but not cell-mediated immune responses in a nonhuman primate model. J Virol 73:8549–8558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao G, Alvira MR, Somanathan S, Lu Y, Vandenberghe LH, Rux JJ, Calcedo R, Sanmiguel J, Abbas Z, Wilson JM. 2003. Adeno-associated viruses undergo substantial evolution in primates during natural infection. Proc Natl Acad Sci U S A 100:6081–6086. doi: 10.1073/pnas.0937739100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lovric J, Mano M, Zentilin L, Eulalio A, Zacchigna S, Giacca M. 2012. Terminal differentiation of cardiac and skeletal myocytes induces permissivity to AAV transduction by relieving inhibition imposed by DNA damage response proteins. Mol Ther 20:2087–2097. doi: 10.1038/mt.2012.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Huser D, Weger S, Heilbronn R. 2003. Packaging of human chromosome 19-specific adeno-associated virus (AAV) integration sites in AAV virions during AAV wild-type and recombinant AAV vector production. J Virol 77:4881–4887. doi: 10.1128/JVI.77.8.4881-4887.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]