ABSTRACT
Dendritic cells (DCs) and macrophages are present in the tissues of the anogenital tract, where HIV-1 transmission occurs in almost all cases. These cells are both target cells for HIV-1 and represent the first opportunity for the virus to interfere with innate recognition. Previously we have shown that both cell types fail to produce type I interferons (IFNs) in response to HIV-1 but that, unlike T cells, the virus does not block IFN induction by targeting IFN regulatory factor 3 (IRF3) for cellular degradation. Thus, either HIV-1 inhibits IFN induction by an alternate mechanism or, less likely, these cells fail to sense HIV-1. Here we show that HIV-1 (but not herpes simplex virus 2 [HSV-2] or Sendai virus)-exposed DCs and macrophages fail to induce the expression of all known type I and III IFN genes. These cells do sense the virus, and pattern recognition receptor (PRR)-induced signaling pathways are triggered. The precise stage in the IFN-inducing signaling pathway that HIV-1 targets to block IFN induction was identified; phosphorylation but not K63 polyubiquitination of TANK-binding kinase 1 (TBK1) was completely inhibited. Two HIV-1 accessory proteins, Vpr and Vif, were shown to bind to TBK1, and their individual deletion partly restored IFN-β expression. Thus, the inhibition of TBK1 autophosphorylation by binding of these proteins appears to be the principal mechanism by which HIV-1 blocks type I and III IFN induction in myeloid cells.
IMPORTANCE Dendritic cells (DCs) and macrophages are key HIV target cells. Therefore, definition of how HIV impairs innate immune responses to initially establish infection is essential to design preventative interventions, especially by restoring initial interferon production. Here we demonstrate how HIV-1 blocks interferon induction by inhibiting the function of a key kinase in the interferon signaling pathway, TBK1, via two different viral accessory proteins. Other viral proteins have been shown to target the general effects of TBK1, but this precise targeting between ubiquitination and phosphorylation of TBK1 is novel.
INTRODUCTION
Dendritic cells (DCs) and macrophages are key target cells for HIV-1, and are both found in all the tissues of the anogenital tract that make up the portals of virus entry (1, 2). Langerhans cells (LCs) represent the first line of contact between HIV-1 and the immune system in tissues containing a stratified squamous epithelium and can efficiently transfer the virus to T cells (3). They have recently been shown to take up HIV-1 within 15 to 60 min of exposure in vagina (4) or foreskin (5). Similarly, lamina propria DCs have recently been shown to transport HIV across the colonic mucosa (6, 7). Similarly, rectal and anal macrophages are also susceptible to HIV-1 infection (8). These cells also represent the first opportunity for the virus to interfere with innate recognition, and we have previously shown that human DCs and macrophages both fail to produce type I IFNs in response to HIV-1 (9, 10).
A key function of the innate immune system is the secretion of IFNs in response to viral infection. These antiviral cytokines consist of three families: type I (IFN-α, -β, -ε, -κ, and -ω), type II (IFN-γ), and type III (IFN-λ1 to λ3). Type I and III IFNs are secreted by a variety of cell types at the sites of pathogen entry, whereas type II IFNs are secreted by T cells and NK cells. IFNs bind receptors on surrounding cells, inducing hundreds of IFN-stimulated genes (ISGs), which establishes an antiviral state. Thus, most successful viruses have evolved strategies to evade the induction of these cytokines (11, 12). IFN-inducing signaling pathways are triggered when pathogens are detected by one of a variety of pattern recognition receptors (PRRs), consisting of Toll-like receptors (TLRs) on the cell surface and in endosomes and RNA-binding RIGI-like receptors (RLRs) or one of a growing number of DNA sensors, both in the cytosol (13, 14). Binding of these receptors to pathogen associated molecular patterns (PAMPs) triggers the association of one of various adaptor proteins, which then induce the formation of a signaling complex consisting of TNF receptor-associated factor 3 (TRAF3), TANK-binding kinase 1 (TBK1), and IFN regulatory factor 3 (IRF3). TRAF3 then mediates K63-linked polyubiquitination both of itself and of TBK1, which triggers TBK1 autophosphorylation (15). TBK1 phosphorylates IRF3, which then dimerizes, dissociates from the signaling complex, and translocates to the nucleus, where it binds to specific promoters and induces the induction of type I and III IFNs. Thus, the formation of the TRAF3-TBK1-IRF3 signaling complex is key to the induction of IFNs, and many viruses interfere with it through targeting any of these three proteins, disrupting complex formation or functionality (16–20).
A unique feature of lentiviruses such as HIV and simian immunodeficiency virus (SIV) is that they encode a number of multifunctional accessory proteins which have enabled them to evolve strategies to evade the host immune system (21). HIV-1 encodes four of these proteins, i.e., Vpr, Vif, Vpu, and Nef, while HIV-2 and SIV also encode Vpx. They help overcome inhibition of replication by host restriction factors in myeloid cells (especially DCs and macrophages) and include SAM domain- and HD domain-containing protein 1 (SAMHD1) via Vpx, apolipoprotein B mRNA-editing enzyme and catalytic polypeptide-like 3G (APOBEC3G) via Vif, and tetherin via Vpu. Vpr, Vif, and Vpu also play a key role in interfering with the induction of type I IFNs in T cells (22–25) by targeting IRF3 for degradation by either the proteasome (Vpr and Vif) (24, 25) or perhaps lysosomes (Vpu) (23, 26). However, we have previously shown that although DCs and macrophages also fail to produce type I IFNs in response to HIV-1, IRF3 is not degraded in these cells (9, 10). Thus, the virus must be inhibiting IFN induction via an alternative mechanism, or the cells fail to detect HIV-1. In this study, we present clear evidence that these cells do sense HIV-1 and trigger PRR-activated signal transduction pathways leading to the formation of IFN-specific signaling complexes consisting of TRAF3, TBK1, and IRF3. The exact stage in the signaling pathway that HIV-1 targets to block IFN induction was then identified: blocking the phosphorylation of TBK1. Furthermore, we show that both Vpr and Vif physically interact with TBK1, indicating that HIV-1 blocks IFN induction in myeloid cells via a physical interaction between TBK1 and the accessory proteins Vpr and Vif, which blocks the autophosphorylation of TBK1.
MATERIALS AND METHODS
Preparation of MMDCs and MDMs.
Monocyte-derived DCs (MDDC) and monocyte-derived macrophages (MDMs) were generated from CD14+ monocytes isolated from peripheral blood mononuclear cells (PBMCs) using CD14 magnetic beads (Miltenyi Biotech) as described previously (9, 10, 27).
Ethics statement.
Human whole blood was obtained from the Sydney Red Cross. All samples were from anonymous donors, and IRB approval was obtained to process the samples.
Viral stock preparation.
Purified high-titer HIV-1BaL stocks on the order of 2 × 109 50% tissue culture infective doses (TCID50/ml) were produced using tangential filter concentration and titrated as described previously (28–30). Briefly, SUPT1.CCR5-CL.30 cells (human non-Hodgkin's T lymphocyte lymphoma, contributed by James Hoxie at the University of Pennsylvania) were infected with vesicular stomatitis virus G (VSV-G)-pseudotyped pBaL and then passaged for approximately 8 weeks. Twice a week, virus-containing supernatants were harvested and stored at 4°C and fresh feeder cells added. HIV-1-infected supernatants were concentrated using tangential filter concentration using the Millipore Lab scale system (Millipore, Billerica, MA, USA) and 2× Pellicon filters connected in parallel (300 kDa) (Millipore). CD45+ microvesicles were depleted from supernatant using CD45 magnetic beads (Miltenyi Biotech). Virus (18 ml) was incubated at room temperature with 2 ml microbeads for 2 h before addition to the top of a LS column. CD45-depleted virus that flowed through the column were concentrated further by ultracentrifugation with 1 ml underlaid 20% sucrose cushion and centrifuged at 100,000 × g (Beckman Optima XL-100K ultracentrifuge with 70Ti rotor) at 4°C for 90 min. The 50% tissue culture infectious dose (TCID50) values were generated in TZM-BL cells (NIH AIDS Research and Reference Reagent Program, contributed by John Kappes and Xiaoyun Wu) and measured by long terminal repeat (LTR) β-galactosidase reporter gene expression after a single round of infection. The endotoxin levels of these virus stocks were below the detectable limit of 0.005 U/ml or 0.0005 ng/ml (Limulus amebocyte lysate assay; Sigma), and testing for residual tumor necrosis factor alpha (TNF-α), IFN-α, IFN-β, and IFN-γ by enzyme-linked immunosorbent assay (ELISA) (R&D Systems) was negative. Plasmids encoding wild-type HIV-1NLAD8 or virus with the accessory proteins Vpr, Vif, and Vpu deleted were a kind gift from Jonson Mak (Monash University, Melbourne, Australia) and were used to make pseudotyped HIV-1 virus stocks by cotransfection into HEK293T cells with a plasmid encoding the VSV-G protein.
Treatment of cultured cells with HIV-1, HSV-2, and Sendai virus.
Day 5 MDDCs or MDMs were seeded at 1 × 106 cells/ml and treated with HIV-1BaL at a multiplicity of infection (MOI) of 3 or 1, herpes simplex virus 2 (HSV-2186) at an MOI of 3, or Sendai virusCantell at 150 hemagglutinin (HA) units/ml.
qPCR.
Quantitative PCR (QPCR) was carried out at as described previously (9, 10) using a SYBR green master mix (Agilent Technologies) and an Mx3005P thermocycler (Stratagene). The relative quantitation method (ΔΔCT) was used to evaluate gene expression and normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression.
Immunoprecipitation assays.
MDDCs and MDMs were treated with HIV-1BaL, HSV-2, or Sendai virus or were mock treated. Alternately, HEK293T cells were transfected with mammalian expression vectors encoding TBK1, pBaL, or Vpr using polyethyleneimine (PEI) as previously described (10). Cells were washed in ice cold phosphate-buffered saline (PBS) and lysed with 30 μl/1 × 106 cells Phosphosafe lysis buffer (Merck Millipore) containing protease inhibitors. The lysates were incubated for 60 min at 4°C and then centrifuged at 18,407 × g for 10 min to pellet cell debris. Supernatants were transferred to fresh Eppendorf tubes and precleared by incubation with 50 μl protein G-agarose beads (50% slurry) (GE Healthcare) for 1.5 h, while an additional 50 μl beads (50% slurry) was also incubated with antibody to the protein of interest (anti-TBK1; Cell Signaling) at 4°C for 90 min. Both solutions were centrifuged for 2 min at 1,000 × g to pellet nonspecifically bound lysate and remove excess antibody. Precleared lysates were added to bead-bound antibody, incubated for 3 h or overnight at 4°C, and then washed five times with PBS. The immunocomplexes/lysates were boiled (95°C) for 8 min in 10 μl 4× SDS loading buffer with dithiothreitol (DTT) (0.62 mg/10 μl loading buffer) per 30 μl lysate and then processed for Western blotting.
Western blotting.
Western blotting was carried out using lysates derived from MDDCs, MDMs, or transfected HEK293T cells or immunocomplexes derived from immunoprecipitation assays as described previously (9, 10) using a the Turboblotter system (Bio-Rad) and polyvinylidene difluoride (PVDF) membranes. Blots were probed with polyclonal rabbit antibodies directed against K63 ubiquitin, phospo-IRF3, TBK1, phospho-TBK1, IKKε, STING, TRAF3, and GAPDH (Cell Signaling) and mouse monoclonal antibodies directed against IRF3 (provided by Michael Gale, University of Washington), HIV-1 Vpr (Cosmobio), HIV-1 Vif (Abcam), and GAPDH (Abd Serotec).
Immunofluorescence microscopy.
Immunofluorescence microscopy was carried out using a DeltaVision microscope as described previously (2, 10).
PLA.
The Duolink proximity ligation assay (PLA) (Sigma) was performed according to the manufacturer's instructions. Cells were permeabilized and then incubated with one of the following pairs of primary antibodies; mouse anti-TBK1 (Abcam) and rabbit anti-TRAF3 (Cell Signaling), rabbit anti-TBK1 (Cell Signaling) and mouse anti-IRF3 (Michael Gale), or mouse anti-IRF3 (Michael Gale) and rabbit anti-TRAF3 (Cell Signaling). The plus and minus (anti-rabbit and anti-mouse) PLA probes were then added to the cells and incubated in a preheated humidity chamber for an hour at 37°C. The antibodies were washed off, and a 1:5 dilution of the ligation stock (containing oligonucleotides that hybridize the PLA probes) with a 1:40 dilution of the ligase was added to the samples and left at 37°C for 30 min. This was washed off and the cells incubated with a 1:5 dilution of amplification stock and a 1:80 dilution of the polymerase for 100 min at 37°C. Cells were then mounted onto slides with Duolink mounting medium with DAPI (4′,6′-diamidino-2-phenylindole). Slides were visualized through a 60× or 100× 1.4-numerical-aperture (NA) oil immersion lens with an inverted Olympus IXM70 microscope and a Photometrics CoolSnap HQ2 camera. Serial optical sections (0.4 μm; 7M18 sections) were acquired for all labelings, and images were then deconvolved using DeltaVision SoftWoRx software version 5.5. Three-dimensional analyses were performed after deconvolution, also using SoftWoRx software, where Z stacks were rebuilt and orthogonal views generated. The number of fluorescent puncta in three sets of 100 cell profiles were counted and expressed as mean ± standard deviation.
RESULTS
Human DCs and macrophages do not produce type I or type III interferons in response to HIV-1 due to failure in IRF3 activation.
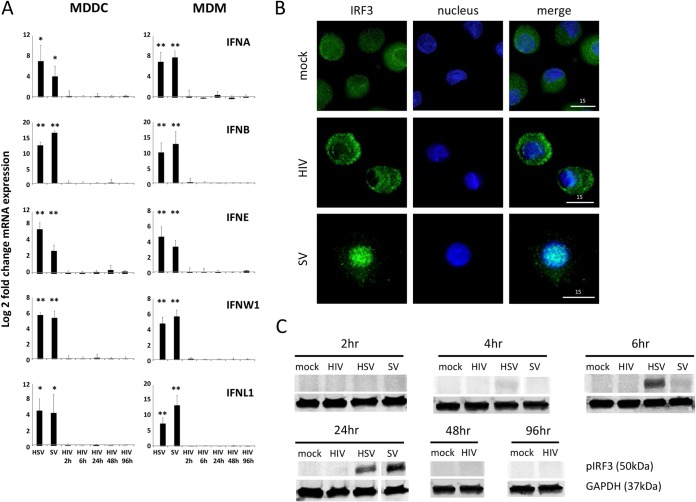
Previously we have shown that both DCs and macrophages fail to produce IFN-α or -β in response to HIV-1 treatment but do express a subset of ISGs (9, 10), which was mediated by a direct effect of the virus via IRF1 and/or -7. However, it is important to exclude the possibility that novel type I IFNs or type III IFNs are involved, especially for type III IFNs, as they act predominantly at epithelial surfaces such as portals of HIV entry (31). Therefore, MDDCs and MDMs were treated with either HIV-1 for 2 to 96 h or HSV-2 or Sendai virus for 2 to 24 h, and gene expression levels of the full complement of type I (IFN-α, -β, -ε, -κ, and -ω) and type III (IFN-λ1 to -λ3) IFNs were measured by qPCR (Fig. 1A). MDDCs and MDMs did not express even basal levels of the genes encoding IFN-κ, -λ2, and -λ3 in response to any treatment. Both cell types expressed basal levels of the genes encoding IFN-α, -β, -ε, -ω, and -λ1 in mock-treated cells, which were all upregulated in response to both Sendai virus and HSV-2. However, no type I or III IFN gene upregulation was observed in response to HIV-1 in either cell type. We also confirmed our previous observation (9) that in contrast to the case for Sendai virus, IRF3 does not translocate to the nucleus in HIV-1-treated MDDCs (Fig. 1B). Furthermore, Western blotting was used to demonstrate that IRF3 does not become phosphorylated in HIV-1-treated MDDCs (Fig. 1C), as we have previously shown in MDMs (10). In contrast, IRF3 does become phosphorylated in MDDCs treated with HSV-2 (within 4 to 6 h) and Sendai virus (within 24 h).
FIG 1.
Human dendritic cells and macrophages do not produce type I or type III interferons in response to HIV-1 due to a failure of IRF3 activation. MDDCs and MDMs were treated with purified HIV-1BaL at an MOI of 3 (MDDCs) or 1 (MDMs) for 2 to 96 h or with HSV-2186 at an MOI of 1 or Sendai virusCantell (SV) for 2 to 24 h. (A) Type I and III IFN gene expression was determined by qPCR, and data for Sendai virus and HSV-1 at 18 h only are shown as a positive control for IFN induction. Data were normalized to GAPDH expression and are represented as log2 fold change in the ratio of mean treatment values to mean mock control values (mean ± standard error of the mean [SEM], n = 5) (P values were calculated using a paired two-tailed t test: *, P < 0.05; **, P < 0.01). No upregulation of IFN-κ, -λ2, or -λ3 mRNA was detected in either cell type in response to any treatment. (B) Nuclear translocation of IRF3 in MDDCs was visualized by immunofluorescence microscopy, with IRF3 shown in green and the nucleus (DAPI staining) shown in blue. Images were taken using a DeltaVision deconvolution microscope at a magnification of ×60. Representative images from four experiments at 14 h after virus treatment are shown. (C) Phospho-IRF3 and GAPDH protein expression levels in MDDCs were determined by Western blotting. Representative data from five independent experiments are shown.
HIV-1 is sensed by human dendritic cells and macrophages, resulting in the recruitment of TBK1 signaling complexes associated with type I IFN induction.
The lack of type I and III IFN induction in HIV-1-treated MDDCs and MDMs could be due to either a lack of sensing by PRRs or a direct inhibitory effect of the virus on PRR-induced signaling pathways. In these signaling pathways, TBK1 is recruited to a signaling complex which contains an E3 ubiquitin ligase (e.g., TRAF3 or -6) and a transcription factor (e.g., IRF3 or -7) (32). Activation of such pathways can be visualized by fluorescence microscopy as TBK1 staining shifts from diffuse cytoplasmic to punctate. Thus, in order to determine if HIV-1 is sensed by MDDCs and MDMs we visualized the TBK1 distribution in response to HIV-1 and lipopolysaccharide (LPS) as a positive control (Fig. 2A). TBK1 staining was diffusely cytoplasmic in mock-treated DCs and macrophages and became punctate within 2 h of LPS treatment and within 6 h of HIV-1 treatment, indicating that HIV-1 is sensed by these cells. Such punctate staining was observed in approximately 60% of cells (data not shown). TBK1 can recruit to a number of different signaling complexes associated with many different outcomes (33). We therefore next used a proximity ligation assay to determine whether TBK1 became associated with a signaling complex associated with IFN induction which contains TRAF3 and IRF3 (Fig. 2B and C) in MDDCs in response to HIV-1 or Sendai virus treatment. This assay identifies proteins that are within 40 nm of each other, indicative of a physical interaction (34). TBK1/IRF3, TBK1/TRAF3, and IRF3/TRAF3 were clearly shown to have a physical interaction after 6 h with both viruses. To confirm that direct interactions were occurring between these proteins, we also carried out a coimmunoprecipitation assay for the two most abundant proteins in the signaling complex, TBK1 and TRAF3 (Fig. 2D). TRAF3 was clearly shown to coimmunoprecipitate with TBK1 at much greater levels in cells treated with both HSV-2 and HIV-1 than in mock-treated cells. Thus, not only is HIV-1 sensed by DCs and macrophages, but TBK1 is also recruited to signaling complexes that lead to type I IFN induction.
FIG 2.
HIV-1 is sensed by human dendritic cells and macrophages, resulting in the recruitment of TBK1 signaling complexes associated with type I interferon induction. (A) Day 6 MDDCs and MDMs were treated with LPS (10 μg/ml) for 2 h or HIV-1BaL (MOI of 1) for 6 h or mock treated, and the TBK1 distribution was determined by immunofluorescence deconvolution microscopy at a magnification of ×60. TBK1 is shown in green and the nucleus (DAPI staining) in blue. (B and C) Day 6 MDDCs were treated with HIV-1BaL or Sendai virus or mock treated for 6 h, and proximity ligation assays (PLA) were carried out for the following pairs of antibodies: mouse anti-TBK1 and rabbit anti-TRAF3, rabbit anti-TBK1 and mouse anti-IRF3, and rabbit anti-TRAF3 and mouse anti-IRF3. After nucleic acid ligation, rolling-circle amplification, and incubation with an FITC-conjugated HIV-1 p24 antibody and DAPI, red fluorescent dots, indicative of an interaction between two proteins of interest, were counted. Green dots are HIV-1 particles. (B) Representative images are shown for TBK1/TRAF3, TRAF3/IRF3, and TBK1/IRF3. (C) The graphs represent quantitative analysis of the number of PLA fluorescent red puncta counted within a mean of 100 cell profiles ± one SE. Positive cells counted are representative of three donors. Outlier cells containing large numbers of puncta are shown with an X. P values were calculated using a paired two-tailed t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001. (D) MDDCs were treated with HIV-1BaL (MOI of 1) or Sendai virus or mock treated for 4 h. A coimmunoprecipitation assay was then carried out using an antibody to TBK1. Western blotting was then conducted on the immunoprecipitated TBK1 complex and the blots probed for TRAF3 (upper band) and TBK1 (lower band).
HIV-1 does not block IFN induction by targeting signaling proteins for proteasomal degradation.
A common feature of many viruses is their ability to block IFN induction by targeting key components of IFN signaling complexes for proteasomal degradation, especially TRAF3, IRF3, TBK1, and STING (11, 35, 36). Indeed, in T cells HIV-1 is able to target IRF3 for proteasomal degradation (24, 25). We therefore next determined if any of these three signaling proteins were targeted for proteasomal degradation in MDDCs and MDMs in response to HIV-1 (11, 37). As shown in Fig. 3, all proteins were present in similar amounts in both mock- and HIV-1-treated MDDCs (Fig. 3A) and MDMs (Fig. 3B) at all time points ranging from 6 h to 96 h posttreatment, indicating that HIV infection does not induce degradation.
FIG 3.

Interferon-inducing signal transduction proteins are not degraded in HIV-1-treated dendritic cells and macrophages. MDDCs (A) and MDMs (B) were mock treated or treated with HIV-1BaL at an MOI of 1 (MDMs) or 3 (MDDCs) for 6 to 96 h, and the expression levels of TBK1, TRAF3, IRF3, and STING were determined by Western blotting (results are representative of three experiments).
TBK1 is ubiquitinated but not phosphorylated in human DCs and macrophages in response to HIV-1.
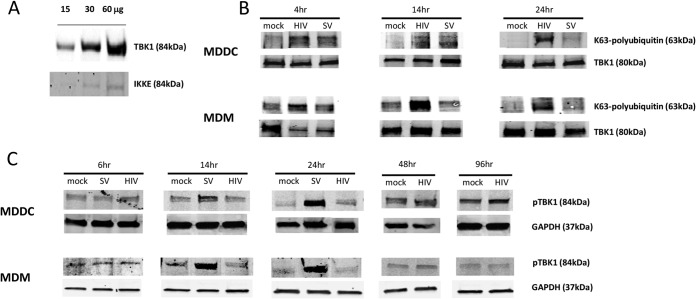
IRF3 can be phosphorylated by either TBK1 or IKKε. TBK1 has been shown to be the predominant kinase in immune cells as it is expressed at much higher levels (38–40). TBK1 must first undergo K63-linked polyubiquitination by TRAF3 followed by transautophosphorylation before phosphorylating IRF3, which then dimerizes and translocates to the nucleus. We confirmed that TBK1 was expressed at much higher levels than IKKε in MDDCs by Western blotting (Fig. 4A). We next determined if TBK1 underwent K63-linked polyubiquitination and phosphorylation in response to HIV-1 and Sendai virus treatment in both MDDCs and MDMs. We employed an immunoprecipitation assay and showed that TBK1 underwent K63-linked polyubiquitination in response to HIV-1 in greater amounts than the Sendai virus positive control in both cell types. This was detectable by 4 h posttreatment and also at the later time points of 14 and 24 h, where greater ubiquitination was observed than in response to the Sendai virus positive control (Fig. 4B). As a phospho-TBK1 antibody was commercially available, we next performed Western blotting and showed that there was no increase in TBK1 phosphorylation (beyond basal phosphorylation levels) in response to HIV-1 in both MDDCs and MDMs between 6 and 96 h posttreatment. In contrast, Sendai virus induced TBK1 phosphorylation by 14 h postinfection (Fig. 4C). Thus, we identify TBK1 as an HIV-1 target in order to block IFN induction; i.e., HIV-1 blocks TBK1 phosphorylation, rendering it incapable of phosphorylating IRF3.
FIG 4.
TBK1 is ubiquitinated but not phosphorylated in HIV-1-treated MDDCs and MDMs. (A) TBK1 and IKKε levels were determined in increasing amounts (15, 30, and 60 μg) of mock MDDC cell lysates. The IKKε image was acquired with a much longer exposure time than that for TBK1 in order to visualize the very faint bands (results are representative of three experiments). (B and C) MDDCs and MDMs were mock treated or treated with HIV-1BaL at an MOI of 1 (MDMs) or 3 (MDDCs) for 6 to 96 h or with Sendai virus for 4 to 24 h (results are representative of five experiments). (B) A TBK1 pulldown assay was conducted on all cell lysates and K63 ubiquitination and TBK1 expression levels determined by Western blotting using anti-K63 ubiquitin and anti-TBK1 antibodies. (C) Phospho-TBK1 expression levels were determined by Western blotting using a phospho-TBK1 antibody.
The HIV-1 accessory proteins Vpr and Vif form a physical interaction with TBK1.
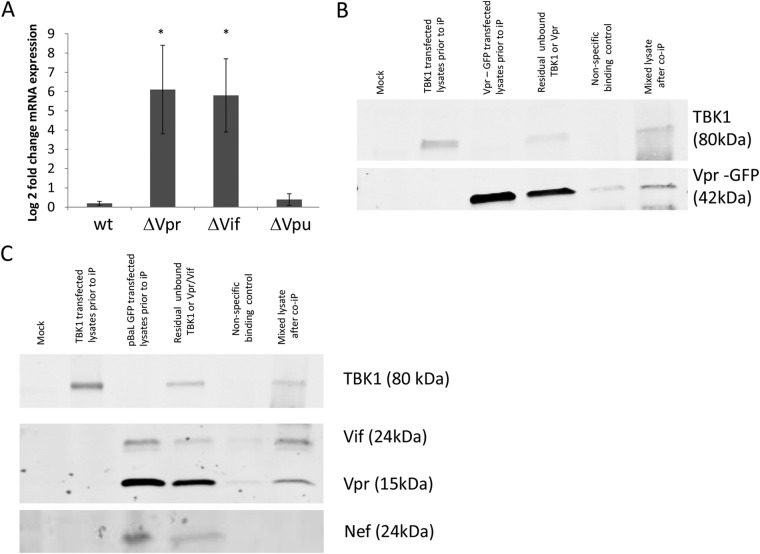
In order to identify potential HIV-1 proteins responsible for blocking IFN induction, we treated MDDCs with VSV-G-pseudotyped HIV-1 with the Vpr, Vif, and Vpu accessory proteins deleted and then measured IFN-β gene induction in these cells. Vpr, Vif, and Vpu have previously been shown to play a role in inhibiting IFN induction in T cells (9, 23–25). MDDCs treated with the Vpr and Vif (but not Vpu) deletion mutants all responded by inducing some IFN-β gene expression (Fig. 5A), implying that both of these HIV-1 accessory proteins may play a role in inhibiting type I IFN induction. We hypothesized that the most likely mechanism by which this occurs is via an interaction between one or both of these proteins with TBK1, inhibiting autophosphorylation in the presence of ubiquitination. The most likely candidate was Vpr, as it is present at higher levels in the HIV-1 virion than the other accessory proteins. We therefore carried out coimmunoprecipitation assays in HEK293T cells transfected with plasmids encoding TBK1 and green fluorescent protein (GFP)-tagged Vpr. When we mixed the lysates and immunoprecipitated TBK1, we found that Vpr coimmunoprecipitated with TBK1 (Fig. 5B), suggesting an interaction between TBK1 and Vpr. We next carried out this experiment in a more physiological setting by using the HIV-1BaL genome rather than a Vpr expression vector. Critically, we again observed Vpr coimmunoprecipitation with TBK1 from these mixed cell lysates. Furthermore, we observed that the Vif protein also coimmunoprecipitated with TBK1 but that the Nef protein did not (Fig. 5C).
FIG 5.
Defining HIV-1 accessory proteins that bind TBK1. (A) Day 2 MDDCs were treated with VSV-G-pseudotyped HIV-1 accessory protein deletion mutants for 24 h and IFN-β gene expression levels determined by qPCR. Data were normalized to GAPDH expression and are represented as the log2 fold change of the ratio of mean HIV-1 treatment values to mean mock control values (mean ± SEM, n = 5) (P values were calculated using a paired two-tailed t test: *, P < 0.05 **). (B and C) HEK293T cells were mock transfected or transfected with mammalian expression vectors encoding TBK1 or Vpr-GFP (B) or TBK1 or HIV-1BaL provirus (pBaL) (C). The TBK1 and Vpr-GFP (B) or TBK1 and pBaL (C) cell lysates were then mixed, and a coimmunoprecipitation assay was then carried out using an antibody to TBK1, which was left overnight. Western blotting was then conducted on the immunoprecipitated TBK1 complex, and the blots probed for TBK1 and Vpr (B) and TBK1, Vpr, Vif, and Nef (C). The data presented are representative of three experiments.
DISCUSSION
This study followed from our previous studies with human DCs (9) and macrophages (10) and investigated the mechanism by which HIV-1 blocks type I and III IFN induction in these cells. Despite demonstrating that both cells do detect the virus and initiate PRR-induced IFN-stimulating signal transduction pathways, we confirmed that both cells types fail to produce type I and III IFNs basally or in response to HIV-1. This inhibitory effect is not dependent on strain differences or on envelope, as we previously showed similar responses with HIVBaL and VSV-G-pseudotyped NL43 strains (9). The precise stage in the type I and III IFN signaling pathway targeted by HIV-1 to block the induction of these IFNs was then defined; i.e., HIV-1 blocks the phosphorylation of TBK1 after its polyubiquitination. Finally, we showed that deletion of two viral accessory proteins (Vpr and Vif) from the viral genome results in a virus that stimulates detectable type I IFN induction. Importantly, both Vpr and Vif interact with TBK1, providing a mechanism for immunomodulation of IFN induction. We propose that Vpr and Vif binding of TBK1 disrupts TBK1 transautophosphorylation (15) and subsequent IRF3 phosphorylation, nuclear translocation, and induction of type I/III IFN gene expression. This study therefore shows for the first time the mechanism by which HIV directly blocks the IFN induction pathway in myeloid cells.
IFN-κ, -λ1, and -λ2 were also not induced in DCs or macrophages in response to HSV or Sendai virus. Lack of expression of IFN-κ is consistent with the fact that it is expressed and secreted predominantly by keratinocytes (41). Although one study did detect very low basal expression of IFN-κ mRNA in MDDCs, IFN-γ (a known inducer of IFN-κ) did not upregulate its expression (42). Similarly, IFN-λs are reported to be expressed by epithelial cells of the anogenital and respiratory tracts rather than by immune cells (43). For example, murine macrophages express high levels of type I IFNs but not type III IFNs in response to HSV, consistent with our findings (44). Apart from IFN-κ, we found that all other type I IFN genes were upregulated in response to HSV-2 and Sendai virus and also, interestingly, IFN-λ1. Upregulation of the IFN-α, -β, and -ω genes is not surprising, as they are well characterized to be released during innate immune activation and promote antiviral responses (45). The finding that IFN-ε was upregulated in both MDMs and MDDCs in response to HSV-2 and Sendai virus is of more interest, however, as in mice this IFN cannot be induced through any known pathogen-induced PRR pathway and is instead hormonally regulated (46). To our knowledge, this is the first description of IFN-ε being induced in immune cells in response to pathogens.
TBK1 is a node protein involved in both IFN and NF-κB signaling pathways and is recruited to a number of signaling complexes, resulting in a variety of outcomes other than IFN induction (32). Therefore, we used a proximity ligation assay and a coimmunoprecipitation assay to confirm that TBK1 formed an IFN-inducing signaling complex with TRAF3 and IRF3. These observations using natural HIV-1 infection are important to confirm studies with nonphysiologically large inputs of purified genomic HIV-1 RNA, which has been previously shown to induce RIG-I signaling pathways in macrophages (47).
We next examined whether HIV-1 proteins block the induction of type I and III IFNs. In HIV-1-treated T cells, which also fail to produce type I IFNs, Vpr, Vif, and Vpu have all been implicated in the process by targeting IRF3 to either the proteasome (Vpr and Vif) or possibly the lysosome (Vpu) for degradation (22–26). We have previously shown that IRF3 is not targeted for degradation in either DCs or macrophages in response to HIV-1 but that it does fail to translocate to the nucleus (9, 10). We therefore wondered if HIV-1 targets other components of IFN signaling complexes for degradation, namely, TBK1, TRAF3, and STING (Fig. 3), as all of these proteins are known to be degraded in response to viral infections (11). However, none of these proteins were degraded in response to HIV-1 infection at any time point ranging from 6 to 96 h.
Following the formation of the TRAF3-TBK1-IRF3 IFN-inducing signaling complex, TBK1 undergoes K63-linked polyubiquitination on lysine residues 30 and 401 by TRAF3, which is a prerequisite for its subsequent transautophosphorylation (15). The leader proteinase of foot-and-mouth disease virus (35) and the PLP2 protein of murine hepatitis virus (48) are both known to block IFN induction by deubiquitinating TBK1, so we next investigated whether TBK1 is ubiquitinated in HIV-1-treated DCs and macrophages (Fig. 4B). In both cell types, ubiquitinated TBK1 was detected by 4 h after HIV-1 treatment continuing to 24 h, so we next determined if TBK1 became phosphorylated. Strikingly, in contrast to the Sendai virus positive control, TBK1 failed to be phosphorylated in both cell types in response to HIV-1, even as late as 96 h posttreatment. Thus, the precise point in the signaling pathway at which HIV-1 blocks IFN induction was defined.
The most likely explanation for HIV-1 blocking the phosphorylation but not ubiquitination of TBK1 was a physical interaction between an HIV-1 protein(s) and TBK1. These were first identified by infecting DCs with mutant HIV-1 viruses with the likely candidates Vpr, Vif, and Vpu accessory protein deleted. MDDCs infected with Vpr and Vif deletion mutants were able to partly restore IFN-β expression, but not those infected with the Vpu deletion mutant. The inhibitory effects of Vpr and Vif were similar to their effects in T cells (24, 25) but were mediated by a completely different mechanism. In contrast to one study in T cells (23), but in agreement with another (26), the Vpu deletion mutant was not able to induce IFN induction in DCs. As shown by immunoprecipitation, both Vpr and Vif formed physical interactions with TBK1. This strongly suggests that these proteins are able to bind TBK1 and block its phosphorylation. Several viruses have evolved to block IFN induction by interfering with the TRAF3-TBK1-IRF3 signaling complex via a physical interaction with a virally encoded protein. Examples include the porcine epidemic diarrhea virus N protein (49), the severe acute respiratory syndrome (SARS) coronavirus M protein (16, 19), the hepatitis C virus NS2 protein (17), the Ebola virus VP35 protein (50), and the rabies virus phosphoprotein P (20). However, none of these studies have reported a specific interaction of the viral protein with TBK1 but rather have reported that the formation or function of the signaling complex is prevented. Thus, it appears that HIV-1 has evolved a novel mechanism to block IFN induction in myeloid cells which differs from that in T cells. While these studies represent, to our knowledge, the first mechanistic definition of immunomodulation of innate immune responses in myeloid cells by HIV-1 proteins Vpr and Vif via targeting of TBK1, future studies are required to precisely define the molecular regions interacting between TBK1 and Vpr or Vif and the reasons for the differences in HIV-1 blocking of IFNs in myeloid and lymphoid cells.
Our results may appear to be in conflict with those showing that reversal of inhibition of HIV reverse transcription by SAMHD1 in MDDCs by Vpx restores interferon production (51, 52), overwhelming any potential viral inhibitory mechanisms. However, increasing the MOI of the HIV inoculum to obtain infection of up to 30% of DCs in the cell culture does not induce interferon (A. N. Harman et al., unpublished data). This apparent paradox can be explained by a balance of increased delivery of HIV RNA and then DNA, which increases interferon stimulation (through DNA sensors like cGAS) while at the same time increasing the virion delivery of Vpr/Vif for inhibition of TBK activation. This is quite different from increasing the DNA/RNA sensor stimulation without increased Vpr/Vif delivery through knockdown of SAMHD1.
These results may appear paradoxical in view of recently reported studies in SIV-macaque models which show an important role of IFN-α in controlling SIV at early stages of infection and of the relative interferon resistance of transmitted founder strains in humans (53, 54). However, there is evidence that this IFN-α is produced by locally infiltrating cells, especially plasmacytoid DCs (55, 56), probably compensating for inhibition of IFN-β production by initially HIV-infected myeloid DCs and/or macrophages during penetration of anogenital mucosa. The initial expansion of HIV in these interferon-deficient cells may actually assist in development of IFN-α-resistant mutants. Thus, our studies may have practical significance for HIV-1 infection of DCs and macrophages in the mucosa or where macrophages are the major reservoir of infection, such as brain and bone marrow. In both settings, reversing the inhibition of IFN-β induction by HIV-1 may reduce spread. In mucosal DCs this may restore an early IFN barrier to mucosal HIV-1 penetration by creating a tighter bottleneck to prevent the development of IFN-α-resistant HIV-1 mutants.
The MDDCs used here most closely resemble CD14+ lamina propria/dermal DCs in vivo (2). Resident tissue macrophages, including microglial cells, are acquired prenatally, whereas infiltrating macrophages are derived from blood monocytes (such as the MDMs used here). In addition, alteration in macrophage phenotype by the cytokine environment toward M1 and then M2 polarization has been postulated to occur during HIV disease progression (57). Indeed, the plasticity of macrophages is even greater than this (58). M1/M2a polarization leads to decreased HIV replication (59, 60). However, there is substantial productive infection with HIV in (M0) MDMs cultured in human serum in vitro, and this is similar to that in ex vivo “resting” microglial cells (61), so these MDMs do provide a reasonable model. Furthermore, the mechanism of IFN inhibition was similar in both MDMs and MDDCs (derived by granulocyte-macrophage colony-stimulating factor [GM-CSF]/interleukin-4 [IL-4] stimulation), so it is likely to be common in myeloid cells. Nevertheless, future studies will aim to confirm our findings in authentic tissue DCs and macrophages.
ACKNOWLEDGMENTS
We thank Stuart Turville (The Kirby Institute, University of New South Wales, Sydney, New South Wales, Australia) for providing us with GFP-Vpr and The Westmead Research HUB Cell Imaging Facility for the usage of the DeltaVision microscope.
Use of the DeltaVision microscope is funded by Westmead Millennium Institute Infrastructure funding, a University of Sydney NHMRC major equipment grant (K9626N6257), and a University of Sydney, Faculty of Medicine, major equipment grant.
REFERENCES
- 1.Harman AN, Kim M, Nasr N, Sandgren KJ, Cameron PU. 2013. Tissue dendritic cells as portals for HIV entry. Rev Med Virol 23:319–333. doi: 10.1002/rmv.1753. [DOI] [PubMed] [Google Scholar]
- 2.Harman AN, Bye CR, Nasr N, Sandgren KJ, Kim M, Mercier SK, Botting RA, Lewin SR, Cunningham AL, Cameron PU. 2013. Identification of lineage relationships and novel markers of blood and skin human dendritic cells. J Immunol 190:66–79. doi: 10.4049/jimmunol.1200779. [DOI] [PubMed] [Google Scholar]
- 3.Nasr N, Lai J, Botting RA, Mercier SK, Harman AN, Kim M, Turville S, Center RJ, Domagala T, Gorry PR, Olbourne N, Cunningham AL. 2014. Inhibition of two temporal phases of HIV-1 transfer from primary Langerhans cells to T cells: the role of langerin. J Immunol 193:2554–2564. doi: 10.4049/jimmunol.1400630. [DOI] [PubMed] [Google Scholar]
- 4.Shen R, Kappes JC, Smythies LE, Richter HE, Novak L, Smith PD. 2014. Vaginal myeloid dendritic cells transmit founder HIV-1. J Virol 88:7683–7688. doi: 10.1128/JVI.00766-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ganor Y, Zhou Z, Tudor D, Schmitt A, Vacher-Lavenu MC, Gibault L, Thiounn N, Tomasini J, Wolf JP, Bomsel M. 2010. Within 1 h, HIV-1 uses viral synapses to enter efficiently the inner, but not outer, foreskin mucosa and engages Langerhans-T cell conjugates. Mucosal Immunol 3:506–522. doi: 10.1038/mi.2010.32. [DOI] [PubMed] [Google Scholar]
- 6.Cavarelli M, Foglieni C, Rescigno M, Scarlatti G. 2013. R5 HIV-1 envelope attracts dendritic cells to cross the human intestinal epithelium and sample luminal virions via engagement of the CCR5. EMBO Mol Med 5:776–794. doi: 10.1002/emmm.201202232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cunningham AL, Harman A, Nasr N. 2013. Initial HIV mucosal infection and dendritic cells. EMBO Mol Med 5:658–660. doi: 10.1002/emmm.201202763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McElrath MJ, Smythe K, Randolph-Habecker J, Melton KR, Goodpaster TA, Hughes SM, Mack M, Sato A, Diaz G, Steinbach G, Novak RM, Curlin ME, Lord JD, Maenza J, Duerr A, Frahm N, Hladik F, Network NHVT. 2013. Comprehensive assessment of HIV target cells in the distal human gut suggests increasing HIV susceptibility toward the anus. J Acquir Immune Defic Syndr 63:263–271. doi: 10.1097/QAI.0b013e3182898392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harman AN, Lai J, Turville S, Samarajiwa S, Gray L, Marsden V, Mercier SK, Jones K, Nasr N, Rustagi A, Cumming H, Donaghy H, Mak J, Gale M Jr, Churchill M, Hertzog P, Cunningham AL. 2011. HIV infection of dendritic cells subverts the IFN induction pathway via IRF-1 and inhibits type 1 IFN production. Blood 118:298–308. doi: 10.1182/blood-2010-07-297721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nasr N, Maddocks S, Turville SG, Harman AN, Woolger N, Helbig KJ, Wilkinson J, Bye CR, Wright TK, Rambukwelle D, Donaghy H, Beard MR, Cunningham AL. 2012. HIV-1 infection of human macrophages directly induces viperin which inhibits viral production. Blood 120:778–788. doi: 10.1182/blood-2012-01-407395. [DOI] [PubMed] [Google Scholar]
- 11.Cunningham AL, Donaghy H, Harman AN, Kim M, Turville SG. 2010. Manipulation of dendritic cell function by viruses. Curr Opin Microbiol 13:524–529. doi: 10.1016/j.mib.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 12.Devasthanam AS. 2014. Mechanisms underlying the inhibition of interferon signaling by viruses. Virulence 5:270–277. doi: 10.4161/viru.27902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kawai T, Akira S. 2011. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity 34:637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Barber GN. 2014. STING-dependent cytosolic DNA sensing pathways. Trends Immunol 35:88–93. doi: 10.1016/j.it.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Ma X, Helgason E, Phung QT, Quan CL, Iyer RS, Lee MW, Bowman KK, Starovasnik MA, Dueber EC. 2012. Molecular basis of Tank-binding kinase 1 activation by transautophosphorylation. Proc Natl Acad Sci U S A 109:9378–9383. doi: 10.1073/pnas.1121552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siu KL, Kok KH, Ng MH, Poon VK, Yuen KY, Zheng BJ, Jin DY. 2009. Severe acute respiratory syndrome coronavirus M protein inhibits type I interferon production by impeding the formation of TRAF3.TANK.TBK1/IKKepsilon complex. J Biol Chem 284:16202–16209. doi: 10.1074/jbc.M109.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaukinen P, Sillanpaa M, Nousiainen L, Melen K, Julkunen I. 2013. Hepatitis C virus NS2 protease inhibits host cell antiviral response by inhibiting IKKepsilon and TBK1 functions. J Med Virol 85:71–82. doi: 10.1002/jmv.23442. [DOI] [PubMed] [Google Scholar]
- 18.Yu S, Chen J, Wu M, Chen H, Kato N, Yuan Z. 2010. Hepatitis B virus polymerase inhibits RIG-I- and Toll-like receptor 3-mediated beta interferon induction in human hepatocytes through interference with interferon regulatory factor 3 activation and dampening of the interaction between TBK1/IKKepsilon and DDX3. J Gen Virol 91:2080–2090. doi: 10.1099/vir.0.020552-0. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Yang X, Zheng Y, Yang Y, Xing Y, Chen Z. 2014. SARS coronavirus papain-like protease inhibits the type I interferon signaling pathway through interaction with the STING-TRAF3-TBK1 complex. Protein Cell 5:369–381. doi: 10.1007/s13238-014-0026-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brzozka K, Finke S, Conzelmann KK. 2005. Identification of the rabies virus alpha/beta interferon antagonist: phosphoprotein P interferes with phosphorylation of interferon regulatory factor 3. J Virol 79:7673–7681. doi: 10.1128/JVI.79.12.7673-7681.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duggal NK, Emerman M. 2012. Evolutionary conflicts between viruses and restriction factors shape immunity. Nat Rev Immunol 12:687–695. doi: 10.1038/nri3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doehle BP, Chang K, Fleming L, McNevin J, Hladik F, McElrath MJ, Gale M Jr. 2012. Vpu-deficient HIV strains stimulate innate immune signaling responses in target cells. J Virol 86:8499–8506. doi: 10.1128/JVI.00424-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Doehle BP, Chang K, Rustagi A, McNevin J, McElrath MJ, Gale M Jr. 2012. Vpu mediates depletion of interferon regulatory factor 3 during HIV infection by a lysosome-dependent mechanism. J Virol 86:8367–8374. doi: 10.1128/JVI.00423-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doehle BP, Hladik F, McNevin JP, McElrath MJ, Gale M Jr. 2009. Human immunodeficiency virus type 1 mediates global disruption of innate antiviral signaling and immune defenses within infected cells. J Virol 83:10395–10405. doi: 10.1128/JVI.00849-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okumura A, Alce T, Lubyova B, Ezelle H, Strebel K, Pitha PM. 2008. HIV-1 accessory proteins VPR and Vif modulate antiviral response by targeting IRF-3 for degradation. Virology 373:85–97. doi: 10.1016/j.virol.2007.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hotter D, Kirchhoff F, Sauter D. 2013. HIV-1 Vpu does not degrade interferon regulatory factor 3. J Virol 87:7160–7165. doi: 10.1128/JVI.00526-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harman AN, Wilkinson J, Bye CR, Bosnjak L, Stern JL, Nicholle M, Lai J, Cunningham AL. 2006. HIV induces maturation of monocyte-derived dendritic cells and Langerhans cells. J Immunol 177:7103–7113. doi: 10.4049/jimmunol.177.10.7103. [DOI] [PubMed] [Google Scholar]
- 28.Mercier SK, Donaghy H, Botting RA, Turville SG, Harman AN, Nasr N, Ji H, Kusebauch U, Mendoza L, Shteynberg D, Sandgren K, Simpson RJ, Moritz RL, Cunningham AL. 2013. The microvesicle component of HIV-1 inocula modulates dendritic cell infection and maturation and enhances adhesion to and activation of T lymphocytes. PLoS Pathog 9:e1003700. doi: 10.1371/journal.ppat.1003700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harman AN, Kraus M, Bye CR, Byth K, Turville SG, Tang O, Mercier SK, Nasr N, Stern JL, Slobedman B, Driessen C, Cunningham AL. 2009. HIV-1-infected dendritic cells show 2 phases of gene expression changes, with lysosomal enzyme activity decreased during the second phase. Blood 114:85–94. doi: 10.1182/blood-2008-12-194845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nasr N, Harman A, Turville S, Cunningham AL. 2014. HIV infection of dendritic cells. Methods Mol Biol 1087:221–232. doi: 10.1007/978-1-62703-670-2_18. [DOI] [PubMed] [Google Scholar]
- 31.Uze G, Monneron D. 2007. IL-28 and IL-29: newcomers to the interferon family. Biochimie 89:729–734. doi: 10.1016/j.biochi.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 32.Helgason E, Phung QT, Dueber EC. 2013. Recent insights into the complexity of Tank-binding kinase 1 signaling networks: the emerging role of cellular localization in the activation and substrate specificity of TBK1. FEBS Lett 587:1230–1237. doi: 10.1016/j.febslet.2013.01.059. [DOI] [PubMed] [Google Scholar]
- 33.Pilli M, Arko-Mensah J, Ponpuak M, Roberts E, Master S, Mandell MA, Dupont N, Ornatowski W, Jiang S, Bradfute SB, Bruun JA, Hansen TE, Johansen T, Deretic V. 2012. TBK-1 promotes autophagy-mediated antimicrobial defense by controlling autophagosome maturation. Immunity 37:223–234. doi: 10.1016/j.immuni.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soderberg O, Gullberg M, Jarvius M, Ridderstrale K, Leuchowius KJ, Jarvius J, Wester K, Hydbring P, Bahram F, Larsson LG, Landegren U. 2006. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature methods 3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, Fang L, Li P, Sun L, Fan J, Zhang Q, Luo R, Liu X, Li K, Chen H, Chen Z, Xiao S. 2011. The leader proteinase of foot-and-mouth disease virus negatively regulates the type I interferon pathway by acting as a viral deubiquitinase. J Virol 85:3758–3766. doi: 10.1128/JVI.02589-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H, Zheng C, Xing J, Wang S, Li S, Lin R, Mossman KL. 2011. Varicella-zoster virus immediate-early protein ORF61 abrogates the IRF3-mediated innate immune response through degradation of activated IRF3. J Virol 85:11079–11089. doi: 10.1128/JVI.05098-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bonjardim CA, Ferreira PC, Kroon EG. 2009. Interferons: signaling, antiviral and viral evasion. Immunol Lett 122:1–11. doi: 10.1016/j.imlet.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liang Q, Fu B, Wu F, Li X, Yuan Y, Zhu F. 2012. ORF45 of Kaposi's sarcoma-associated herpesvirus inhibits phosphorylation of interferon regulatory factor 7 by IKKepsilon and TBK1 as an alternative substrate. J Virol 86:10162–10172. doi: 10.1128/JVI.05224-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tojima Y, Fujimoto A, Delhase M, Chen Y, Hatakeyama S, Nakayama K, Kaneko Y, Nimura Y, Motoyama N, Ikeda K, Karin M, Nakanishi M. 2000. NAK is an IkappaB kinase-activating kinase. Nature 404:778–782. doi: 10.1038/35008109. [DOI] [PubMed] [Google Scholar]
- 40.Yu T, Yi YS, Yang Y, Oh J, Jeong D, Cho JY. 2012. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediators Inflamm 2012:979105. doi: 10.1155/2012/979105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.LaFleur DW, Nardelli B, Tsareva T, Mather D, Feng P, Semenuk M, Taylor K, Buergin M, Chinchilla D, Roshke V, Chen G, Ruben SM, Pitha PM, Coleman TA, Moore PA. 2001. Interferon-kappa, a novel type I interferon expressed in human keratinocytes. J Biol Chem 276:39765–39771. doi: 10.1074/jbc.M102502200. [DOI] [PubMed] [Google Scholar]
- 42.Nardelli B, Zaritskaya L, Semenuk M, Cho YH, LaFleur DW, Shah D, Ullrich S, Girolomoni G, Albanesi C, Moore PA. 2002. Regulatory effect of IFN-kappa, a novel type I IFN, on cytokine production by cells of the innate immune system. J Immunol 169:4822–4830. doi: 10.4049/jimmunol.169.9.4822. [DOI] [PubMed] [Google Scholar]
- 43.Kotenko SV. 2011. IFN-lambdas. Curr Opin Immunol 23:583–590. doi: 10.1016/j.coi.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ank N, Iversen MB, Bartholdy C, Staeheli P, Hartmann R, Jensen UB, Dagnaes-Hansen F, Thomsen AR, Chen Z, Haugen H, Klucher K, Paludan SR. 2008. An important role for type III interferon (IFN-lambda/IL-28) in TLR-induced antiviral activity. J Immunol 180:2474–2485. doi: 10.4049/jimmunol.180.4.2474. [DOI] [PubMed] [Google Scholar]
- 45.Seo Y, Kim M, Choi M, Kim S, Park K, Oh I, Chung S, Suh H, Hong S, Park S. 2011. Possible role of phosphoinositide-3-kinase in Mx1 protein translation and antiviral activity of interferon-omega-stimulated HeLa cells. Pharmacology 87:224–231. doi: 10.1159/000324536. [DOI] [PubMed] [Google Scholar]
- 46.Fung KY, Mangan NE, Cumming H, Horvat JC, Mayall JR, Stifter SA, De Weerd N, Roisman LC, Rossjohn J, Robertson SA, Schjenken JE, Parker B, Gargett CE, Nguyen HP, Carr DJ, Hansbro PM, Hertzog PJ. 2013. Interferon-epsilon protects the female reproductive tract from viral and bacterial infection. Science 339:1088–1092. doi: 10.1126/science.1233321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Solis M, Nakhaei P, Jalalirad M, Lacoste J, Douville R, Arguello M, Zhao T, Laughrea M, Wainberg MA, Hiscott J. 2011. RIG-I-mediated antiviral signaling is inhibited in HIV-1 infection by a protease-mediated sequestration of RIG-I. J Virol 85:1224–1236. doi: 10.1128/JVI.01635-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zheng D, Chen G, Guo B, Cheng G, Tang H. 2008. PLP2, a potent deubiquitinase from murine hepatitis virus, strongly inhibits cellular type I interferon production. Cell Res 18:1105–1113. doi: 10.1038/cr.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ding Z, Fang L, Jing H, Zeng S, Wang D, Liu L, Zhang H, Luo R, Chen H, Xiao S. 2014. Porcine epidemic diarrhea virus nucleocapsid protein antagonizes beta interferon production by sequestering the interaction between IRF3 and TBK1. J Virol 88:8936–8945. doi: 10.1128/JVI.00700-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Prins KC, Cardenas WB, Basler CF. 2009. Ebola virus protein VP35 impairs the function of interferon regulatory factor-activating kinases IKKepsilon and TBK-1. J Virol 83:3069–3077. doi: 10.1128/JVI.01875-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. 2010. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 467:214–217. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Laguette N, Sobhian B, Casartelli N, Ringeard M, Chable-Bessia C, Segeral E, Yatim A, Emiliani S, Schwartz O, Benkirane M. 2011. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 474:654–657. doi: 10.1038/nature10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fenton-May AE, Dibben O, Emmerich T, Ding H, Pfafferott K, Aasa-Chapman MM, Pellegrino P, Williams I, Cohen MS, Gao F, Shaw GM, Hahn BH, Ochsenbauer C, Kappes JC, Borrow P. 2013. Relative resistance of HIV-1 founder viruses to control by interferon-alpha. Retrovirology 10:146. doi: 10.1186/1742-4690-10-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sandler NG, Bosinger SE, Estes JD, Zhu RT, Tharp GK, Boritz E, Levin D, Wijeyesinghe S, Makamdop KN, del Prete GQ, Hill BJ, Timmer JK, Reiss E, Yarden G, Darko S, Contijoch E, Todd JP, Silvestri G, Nason M, Norgren RB Jr, Keele BF, Rao S, Langer JA, Lifson JD, Schreiber G, Douek DC. 2014. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature 511:601–605. doi: 10.1038/nature13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Li G, Cheng M, Nunoya J, Cheng L, Guo H, Yu H, Liu YJ, Su L, Zhang L. 2014. Plasmacytoid dendritic cells suppress HIV-1 replication but contribute to HIV-1 induced immunopathogenesis in humanized mice. PLoS Pathog 10:e1004291. doi: 10.1371/journal.ppat.1004291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. 2009. Glycerol monolaurate prevents mucosal SIV transmission. Nature 458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herbein G, Varin A. 2010. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology 7:33. doi: 10.1186/1742-4690-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xue J, Schmidt SV, Sander J, Draffehn A, Krebs W, Quester I, De Nardo D, Gohel TD, Emde M, Schmidleithner L, Ganesan H, Nino-Castro A, Mallmann MR, Labzin L, Theis H, Kraut M, Beyer M, Latz E, Freeman TC, Ulas T, Schultze JL. 2014. Transcriptome-based network analysis reveals a spectrum model of human macrophage activation. Immunity 40:274–288. doi: 10.1016/j.immuni.2014.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schlaepfer E, Rochat MA, Duo L, Speck RF. 2014. Triggering TLR2, -3, -4, -5, and -8 reinforces the restrictive nature of M1- and M2-polarized macrophages to HIV. J Virol 88:9769–9781. doi: 10.1128/JVI.01053-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cassol E, Cassetta L, Rizzi C, Alfano M, Poli G. 2009. M1 and M2a polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J Immunol 182:6237–6246. doi: 10.4049/jimmunol.0803447. [DOI] [PubMed] [Google Scholar]
- 61.Albright AV, Shieh JT, O'Connor MJ, Gonzalez-Scarano F. 2000. Characterization of cultured microglia that can be infected by HIV-1. J Neurovirol 6(Suppl 1):S53–S60. [PubMed] [Google Scholar]