ABSTRACT
Recent evidence suggests that even in treated infections, human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) replication may continue in lymph nodes (LN), serving as a potential virus reservoir. Here we investigated the effects of lentivirus infection on natural killer (NK) cell frequencies, phenotypes, and functions in naive and acutely or chronically SIVmac239-infected rhesus macaques. Compared to that in naive animals, we observed a 3-fold-greater frequency of cytotoxic CD16+ CD56− NK cells in LN of chronically infected macaques. However, NK cells did not appear to be trafficking to LN, as homing markers CD62L and CCR7 did not increase on circulating NK cells during infection. LN NK cells demonstrated enhanced cytotoxicity in acute infection, with 2-fold increases in perforin expression and 3-fold increases in CD107a expression following mitogen stimulation. Lysis of K562 cells by LN NK cells from acutely infected animals was greater than lysis by preinfection samples from the same animals. LN NK cells from chronically infected animals lysed K562 cells more efficiently than LN NK cells from uninfected animals, but importantly, surrogate markers of cytotoxicity in infected macaques were disproportionately greater than ex vivo killing. Furthermore, Tim-3, an indicator of activation and/or exhaustion, was upregulated 3-fold on LN NK cells in chronically infected animals. Collectively, these data suggest that LN NK cells are skewed toward a cytotoxic phenotype during SIV infection but may become dysfunctional and exhausted in chronic disease.
IMPORTANCE The accumulation of CD16+ CD56− NK cells in the SIV-infected lymph node without changes in NK homing to the LN could suggest that these cells are differentiating in situ. Surprisingly, this increase in frequency of the cytotoxic subset of NK cells is not accompanied by an increase of similar magnitude in the cytolytic function of LN lymphocytes. This functional modulation, together with the higher Tim-3 expression observed on LN NK cells isolated from chronically infected animals than on those from naive macaques, is indicative of an exhausted phenotype. This exhaustion could contribute to the robust replication of HIV and SIV in the LN during acute and chronic stages of infection, allowing the survival of infected cells and maintenance of a viral reservoir.
INTRODUCTION
Natural killer (NK) cells are cytotoxic effector cells that can lyse virus-infected and malignant cells without prior exposure to antigen. Genetic evidence suggests that these cells are essential for the control of immunodeficiency virus infections, as some NK cell receptors coexpressed with their ligands are associated with delayed progression to AIDS in human immunodeficiency virus (HIV) infection (1, 2). Furthermore, the immune pressure exerted by NK cells upon this virus is demonstrated by mutations in HIV that are associated with expression of certain NK cell receptors (3). NK cell activation by HIV-infected cells is dependent on Nef-mediated downregulation of major histocompatibility complex (MHC) class I surface expression, which decreases availability of these ligands for inhibitory NK cell receptors (4, 5). In addition to this lessening of inhibition, Vpr-mediated upregulation of UL16-binding proteins (ULBPs) 1, 2, and 3 on HIV-infected cells can trigger lysis through the activating NKG2D receptor (6, 7). NK cells activated by immunodeficiency virus-infected cells can inhibit viral replication by lysis of infected cells (8) and secretion of chemokines CCL3, CCL4, and CCL5, which inhibit HIV entry (9).
Infection with HIV or simian immunodeficiency virus (SIV) in turn affects NK cell distribution and function. During acute HIV infection, the cytotoxic CD56dim CD16+ NK cell subset expands in peripheral blood mononuclear cells (PBMC), whereas cytokine-producing CD56bright NK cells contract in this compartment (10). Likewise, in SIV infection of rhesus macaques, CD16+ NK cells become more prevalent in the periphery, while CD56+ NK cells accumulate in the gut and acquire a more cytotoxic phenotype (11, 12). In HIV infection, further viral replication causes the rise of abnormal CD56− CD16+ NK cells that are anergic, exhibiting low CD107a degranulation responses (10, 13). Functional impairment of NK cells during progressive HIV or SIV infection also includes diminished antibody-dependent cellular cytotoxic (ADCC) function (14, 15) and cytokine secretion (16, 17), whereas chemokine secretion is unaffected (9, 18). Lymph node (LN) NK cells, conversely, become more activated during HIV infection (19).
Secondary lymphoid tissues (LTs), including LN, are important sites of viral replication during HIV and SIV infection (20, 21). Resting CD4+ T cells (22, 23), T follicular helper cells (24), and extracellular trapped virus on follicular dendritic cells (25, 26) all represent potential reservoirs for immunodeficiency virus infection in the LTs. Compartmentalization of infected cells within LTs has been demonstrated in SIV-infected macaques, in which most SIV-producing cells are located in the follicular zone during chronic infection prior to the onset of AIDS and in the extrafollicular zone away from SIV-specific cytotoxic T lymphocytes (CTLs) during AIDS (27). Replication of HIV within LTs continues even under antiretroviral therapy in HIV-infected individuals (28). The importance of LTs in disease pathogenesis has also been demonstrated by the prevalence of LN-specific gene expression changes, beginning in acute infection and sustained to high levels in chronic infection, that are unique to pathogenic hosts of SIV infection (29).
In this study, we sought to examine NK cell subset distribution, phenotype, and function in the LN compartment. We find that accumulation of CD16+ CD56− NK cells of the cytotoxic subset in LNs of chronically infected rhesus macaques are not accompanied by a similar increase in cytolytic LN lymphocyte function, perhaps due to exhaustion of these NK cells. These findings have implications for the persistence of HIV and SIV replication in LTs during all stages of infection and under antiretroviral therapy.
MATERIALS AND METHODS
Animals and SIV infections.
Animals were housed at the New England Primate Research Center or at the National Cancer Institute, National Institutes of Health, according to the standards of the American Association for Accreditation of Laboratory Animal Care. Protocols, including all procedures performed, were approved by the Harvard Medical School Animal Care and Use Committee and the National Institute of Allergy and Infectious Diseases, National Institutes of Health. Animals were monitored daily by veterinary staff, and those showing signs of significant weight loss, disease, or distress were provided dietary supplementation and medication as necessary. Euthanization with an overdose of barbiturates was carried out in accordance with the guidelines of the American Veterinary Medical Association.
Tissue samples from 26 Indian rhesus macaques were analyzed in this study, including 12 SIV-naive animals, 6 acutely infected animals, and 8 animals chronically infected with SIVmac239. PBMC data from additional naive and SIV-infected macaques on which we have previously published (12) are included in Fig. 2. For pooled LN and PBMC samples, some preinfection samples are included in the naive group. Animals were infected intravenously, and acutely infected animals were sacrificed and tissue samples harvested at day 14 postinfection. Chronically infected animals were sacrificed between 162 and 707 days postinfection, with a median duration of infection of 372 days. Chronic viral loads at time of necropsy were between 1.7 and 6.4 log10 copies of viral RNA/ml plasma, with a median of 5.1 log10 copies of viral RNA/ml plasma. CD4+ T cell frequencies in blood were between 54% and 22% of T cells (median, 39%), and absolute CD4+ T cell counts were between 23 and 445 cells/μl (median, 225 cells/μl). The acutely SIV-infected animal cohort is described elsewhere (30, 31). All animals were free of simian retrovirus type D or simian T-lymphotropic virus type 1.
FIG 2.
Comparison of LN homing markers on blood NK cells from naive and SIV-infected macaques. (A and B) Frequencies of CCR7+ (A) and CD62L+ (B) cells among NK cells in PBMC. (C and D) Frequencies of CCR7+ (C) and CD62L+ (D) expression on NK cell subpopulations as defined by CD16 and CD56 expression from naive (green), acutely infected (purple), and chronically infected (orange) animals. Horizontal bars indicate median values, and asterisks indicate significant differences by the Mann-Whitney U test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
Tissue collection and processing.
Axillary and inguinal lymph node (PLN), mesenteric lymph node (MLN), and pararectal/paracolonic lymph node (PaLN) tissues were collected from humanely euthanized macaques at the indicated time points. Preinfection PLN biopsy specimens were taken from animals in the acute infection group at day 14 before infection. PBMC were isolated by density gradient centrifugation of EDTA-treated blood over lymphocyte separation medium (MP Biomedicals, Solon, OH), followed by lysis of red blood cells using a hypotonic ammonium chloride solution.
Antibodies and flow cytometry.
Antibodies to the following antigens were used in this study and were obtained from BD Biosciences unless otherwise specified: active-caspase-3-Alexa 647 (clone C92-605), CCR7-Alexa 700 (clone 150503; R&D Systems), CD3-allophycocyanin (APC)-Cy7 (clone SP34.2), CD8α-Qdot605 (clone T8/7Pt-3F9; NIH Nonhuman Primate Reagent Resource Program), CD8α-APC-Cy7 (clone SK1), CD16-Alexa 700 (clone 3G8), CD45-fluorescein isothiocyanate (FITC) (clone D058-1283), CD45-peridinin chlorophyll protein (PerCP)-Cy5.5 (clone Tu116), CD56-phycoerythrin (PE)-Cy7 (clone NCAM16.2), CD62L-FITC (clone SK11), NKG2A-PE (clone Z199; Beckman-Coulter), NKG2A-Pacific Blue (clone Z199, in-house custom conjugate; Beckman-Coulter), Ki67-FITC (clone B56), and perforin-Pacific Blue (in-house custom conjugate, clone Pf-344; Mabtech). Samples were acquired on an LSR II instrument (BD Biosciences, La Jolla, CA), and analysis was conducted with FlowJo (version 9.6.4; Tree Star Inc., Ashland, OR).
NK stimulation assay.
Mononuclear cells were incubated with phorbol myristate acetate (PMA) (50 ng/ml) and ionomycin (1 μg/ml) or cultured in RPMI 1640 medium containing 10% fetal bovine serum (FBS) (R10) alone. For all samples, anti-CD107a (PerCP-Cy5, clone H4A3) was included at a concentration of 20 μl/ml, and GolgiPlug (brefeldin A) and GolgiStop (monensin) were included at 6 μg/ml. Samples were incubated for 12 h at 37°C in 5% CO2 and then permeabilized using Fix & Perm reagents (Invitrogen) and stained intracellularly with anti-MIP-1β (FITC conjugate, clone 24006; R&D Systems), anti-gamma interferon (anti-IFN-γ) (PE-Cy7 conjugate, clone B27; Invitrogen), and anti-tumor necrosis factor alpha (anti-TNF-α) (Alexa 700 conjugate, clone Mab11).
Cytotoxicity assay.
Lymphocytes isolated from LNs were incubated overnight in R10 at 37°C in 5% CO2 with equal numbers of carboxyfluorescein succinimidyl ester (CFSE)-stained RAJI and PKH26-stained K562 target cells at total effector-to-target cell (E:T) ratios ranging from 5:1 to 40:1. Cells were stained with Aqua vital dye, and lysis of K562 cells was quantified by the frequency of Aqua+ cells among PKH26+ cells. NK-resistant RAJI cells were included as controls to determine the frequency of lysed K562 cells. Specific lysis was calculated as (percent sample lysis − percent background lysis)/(100 − percent background lysis).
Statistical analyses.
Prism 6.0 software (GraphPad Software Inc., La Jolla, CA) was used for all graphical and statistical analyses. Two-tailed Mann-Whitney U tests were used where indicated, and a P value of <0.05 was considered statistically significant.
RESULTS AND DISCUSSION
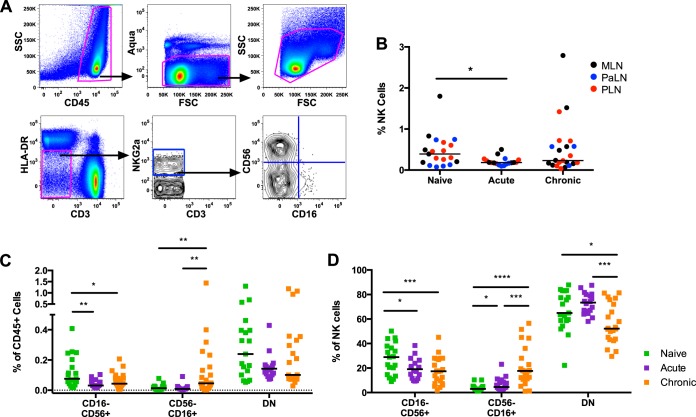
Lymphocytes were isolated from LNs of naive and acutely or chronically SIV-infected rhesus macaques, and live NK cells were identified as Aqua dye-negative CD45+ CD3− HLA-DR− NKG2a+ as shown in Fig. 1A. First we examined the frequency of NK cells among CD45+ cells and found a significantly lower frequency during acute infection (Fig. 1B) (P = 0.01, Mann-Whitney U test). These data are consistent with the overall decline in NK cells during acute SIV disease (31). We then evaluated the frequencies of the three main subsets of rhesus NK cells defined by expression of CD16 and CD56: CD16− CD56+ (CD56+), CD16+ CD56− (CD16+), and CD16− CD56− (DN). Whereas the frequency of CD56+ NK cells among CD45+ cells was lower in acute and chronic SIV infection than in naive animals, the frequency of CD16+ NK cells was higher in chronically SIV-infected animals than in naive or acutely infected macaques (Fig. 1C). Similar changes in NK cell subpopulations were observed when analyzed as a fraction of the bulk NK cell population (Fig. 1D). Together these data suggest that the transient decrease in bulk NK cell frequency during acute infection may be a result of the loss of CD56+ NK cells, whereas the rebound of bulk NK cells in chronic infection may be due largely to the increased frequency of CD16+ NK cells.
FIG 1.
Frequency of NK cell subsets in LNs from naive and SIV-infected macaques. (A) Representative gating for CD16 and CD56 subsets of CD45+ HLA-DR− CD3− NKG2a+ NK cells. (B) The frequency of NK cells among CD45+ lymphocytes is shown for lymphocytes isolated from the MLNs (black), PaLNs (blue), and PLNs (red). (C and D) Frequencies of three NK cell subsets among total CD45+ lymphocytes (C) and as a fraction of bulk NK cells (D) as defined by CD16 and CD56 expression from LN of naive (green), acutely infected (purple), and chronically infected (orange) animals. Horizontal bars indicate median values, and asterisks indicate significant differences by the Mann-Whitney U test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
We next sought to determine whether the changes observed in LN NK cell frequency were a consequence of differences in NK cell trafficking during infection. We examined the frequency of surface expression of LN homing markers CCR7 and CD62L on NK cells in peripheral blood of naive and acutely or chronically SIV-infected macaques. Both CCR7 and CD62L were downregulated on NK cells in circulation and generally on all NK cell subpopulations, most notably during acute SIV infection (Fig. 2A to D). This result suggests that decreased trafficking to the LNs may in part account for the low frequency of LN NK cells during acute infection but does not inform the high frequency of CD16+ NK cells observed in chronic infection. This agrees with previous findings that peripheral NK cells increase trafficking to the gut, not the LNs, during SIV infection (11).
We then evaluated phenotypic properties and turnover of LN NK cells in uninfected and SIV-infected animals. Intracellular staining for perforin demonstrated that expression is higher in LN NK cells isolated from acutely infected macaques than in those from naive macaques and is higher still in those from chronically infected animals (Fig. 3A). Caspase-3 expression was also higher in LN NK cells from infected animals of both the acute and chronic groups than in those isolated from naive animals (Fig. 3B). Finally, the frequency of Ki-67+ NK cells in LNs was higher in acutely infected animals than in naive or chronically infected animals (Fig. 3C). In general, the increases in perforin were found among all subsets of NK cells, but increases in Ki67 and caspase-3 were most pronounced in CD56+ and DN NK cells, which could be indicative of turnover in these precursor populations (Fig. 3D to F). The correlation of this turnover with increasing cytotoxic arming of these NK cells with progressive infection, as suggested by perforin staining, points to a highly activated NK cell compartment.
FIG 3.

Comparison of perforin, caspase-3, and Ki-67 expression in LN NK cells in naive and SIV-infected macaques. (A to C) Mean fluorescence intensity (MFI) of perforin (A) and caspase-3 (B) staining and frequency of Ki-67+ cells (C) among NK cells isolated from the MLNs (black), PaLNs (blue), and PLNs (red). (D to F) MFI of perforin (D) and caspase-3 (E) staining and frequency of Ki-67+ cells (F) among NK cell subpopulations. Horizontal bars indicate median values, and asterisks indicate significant differences by the Mann-Whitney U test (*, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001).
To investigate the function of the LN NK cells isolated from animals with different SIV infection statuses, we then conducted an NK cell stimulation assay. Lymphocytes isolated from LNs were mitogen stimulated to determine their cytokine expression and activation potential ex vivo. These cells were stained for the degranulation marker CD107a and cytokines IFN-γ, TNF-α, and MIP-1β. Although cytokine expression was equivalent among LN NK cells isolated from naive and SIV-infected macaques (Fig. 4A to C), the frequency of CD107a+ LN NK cells was significantly higher in acutely and chronically SIV-infected animals than in uninfected animals (Fig. 4D). This increase in frequency is consistent with the higher perforin expression in LN NK cells of infected animals and again suggests a more active NK cell population with cytolytic potential in these LNs.
FIG 4.
Functional activity of LN NK cells from naive and SIV-infected macaques. Lymphocytes were isolated from the MLNs (black), PaLNs (blue), and PLNs (red) and were stimulated by treatment with PMA and ionomycin. IFN-γ (A), TNF-α (B), and MIP-1β (C) production and CD107a expression (D) by NK cells are shown. Horizontal bars indicate median values, and asterisks indicate significant differences by the Mann-Whitney U test (**, P < 0.01).
To directly evaluate the cytolytic activity of LN NK cells, we used a flow cytometry-based cytotoxicity assay. Briefly, lymphocytes isolated from the LNs of macaques were coincubated with red fluorescent-labeled (PKH26) MHC class I-deficient K562 cells. Using this functional assay, we found that cells from chronically SIV-infected animals were more lytically active at all E:T ratios than those from naive animals, reaching a 50% increase at the 40:1 ratio (Fig. 5A). Although these data did not reach statistical significance, there was a clear trend in increasing cytotoxicity. It should be noted that due to the construction of the assay, which measures cell loss by Aqua dye, we could in fact be underestimating the number of lysed cells that have become necrotic and are no longer detectable. The availability of both pre- and postinfection samples from the six acutely infected animals in this study enabled animal-matched analysis of the effect of acute infection on LN NK cell lytic function. We observed higher lytic activity in all samples isolated in acute infection than in those isolated from the same animals preinfection (Fig. 5B). However, the higher cytolytic activity of LN NK cells in acute infection than in preinfection was much less dramatic than the difference in perforin and CD107a staining observed between LN NK cells from naive and acutely infected macaques. Furthermore, the greater cytolytic activity of LN NK cells from chronically infected animals compared to naive animals is less than would be expected based on the magnitude of the increase in frequency of the cytolytic subset of CD16+ NK cells in the LNs of chronically infected animals. Collectively, these data indicate that the cytotoxic potential of accumulating LN NK cells does not necessarily correlate with actual target cell killing (Table 1). Dysregulation of NK cell cytotoxicity begins early in HIV infection and eventually results in accumulation of dysfunctional CD16+ CD56− NK cells (10), which could be analogous to the CD16+ LN NK cells observed in our chronic studies.
FIG 5.
LN NK cell cytotoxicity is enhanced during SIV infection. (A) Lymphocytes were isolated from naive or chronically SIV-infected macaque PLNs and incubated with PKH26-stained K562 cells and CFSE-stained RAJI cells overnight at the indicated total E:T ratios. Flow cytometry analysis of the frequency of Aqua dye-positive cells among PKH26+ cells was used to identify lysed K562 cells. Mean percent specific lysis with error bars indicating standard errors of the means (SEM) is shown for experiments using cells from at least eight different animals per group. (B) Comparison of paired pre- and postinfection PLN samples from animals sacrificed during acute infection. The P values shown were determined by the Wilcoxon matched-pairs test; P values of <0.05 are considered significant.
TABLE 1.
Correlation of cytotoxic NK cell parameters
| Group | Parameter | Correlation with: |
|
|---|---|---|---|
| Perforin | CD107a | ||
| Naive | CD107a | r = 0.538, P = 0.050 | |
| Lysisa | r = 0.894, P < 0.001 | r = 0.894, P < 0.001 | |
| Acute infection | CD107a | r = −0.270, P = NSb | |
| Lysis | r = −0.042, P = NS | r = −0.036, P = NS | |
| Chronic infection | CD107a | r = −0.257, P = NS | |
| Lysis | r = 0.138, P = NS | r = 0.271, P = NS | |
| Total | CD107a | r = 0.461, P = 0.003 | |
| Lysis | r = 0.280, P = NS | r = −0.505, P = NS | |
Specific lysis from the 40:1 killing assay.
NS, not significant.
Since our cytotoxicity assays suggested that the accumulating CD16+ NK cells might not be fully functional, we next examined Tim-3 expression on LN NK cells as a potential marker for differentiation, exhaustion, and/or anergy. We observed that the frequency of Tim-3+ cells among bulk NK cells was higher in chronically infected animals than in uninfected macaques, with a similar trend toward higher expression on the individual NK cell subsets analyzed (Fig. 6). These data are consistent with recent findings by Amancha et al. showing that Tim-3 is also increased on circulating NK cells during SIV infection (32). The increase in LN NK Tim-3 expression indicates that the functional differences observed between naive and SIV-infected animals may in part be due to the development of anergy, such that an increased frequency of CD16+ NK cells and enhanced perforin expression do not result in similar increases in cytolytic function. Although Tim-3 expression can actually delineate mature cytotoxic NK cells, its engagement results in suppression of cytotoxicity (33). Indeed, the ligand for Tim-3, Gal-9, is increased in HIV patients, and the continued exposure of Tim-3+ NK cells to ligands leads to exhaustion and/or anergy (34), which could explain our observations. Recent reports specific to SIV-infected macaques indicate that Tim-3 also delineates exhausted T cells in SIV infection and that they accumulate in lymph nodes during disease (35). Together these data could suggest that, like T cells, NK cells could accumulate and become functionally exhausted in SIV-infected LNs, likely driven by low-level virus replication. Alternatively, some reports contest that Tim-3 expression does not necessarily delineate lymphocyte exhaustion, but rather delineates cell activation, particularly on antigen-specific cells (32, 36). Therefore, while these data are compelling, additional experiments will be needed to fully ascertain the role of Tim-3 on NK cells during HIV and SIV infection.
FIG 6.

Tim-3 expression is upregulated on PLN NK cells during chronic SIV infection. The frequencies of Tim-3+ cells among bulk NK cells and NK cell subsets are shown. Bars indicate mean + SEM from experiments using cells from at least three different animals per group. Asterisks indicate significant differences by the Mann-Whitney U test (*, P < 0.05).
This study of LN NK cells in naive and SIV-infected rhesus macaques reveals that despite a higher prevalence of CD16+ NK cells in the LNs of chronically infected macaques, the cytolytic function of LN NK cells is not similarly enhanced in chronic SIV infection. Although initial activation of NK cells in acute disease may limit virus replication, low-level virus replication in the LNs could be associated with eventual NK cell anergy, resulting in a loss of control of replicating virus. These findings could have broad implications for persistence of HIV and SIV replication in the LNs of infected individuals and the virus reservoir. Interestingly, Tim-3 blockade has already been shown to reverse NK cell exhaustion and promote tumor lysis in anticancer studies (37, 38). Prevention of the development of NK cell anergy or stimulation of function during chronic infection could similarly be of interest in developing strategies to eradicate HIV and SIV reservoirs.
ACKNOWLEDGMENTS
We thank Elaine Roberts and Angela Carville for dedicated animal care, Jackie Gillis and Michelle Connole for expert technical assistance, and R. Paul Johnson for contribution of macaque samples.
This work was supported by NIH (R21 AI101170), CHAVI/HVTN Early Career Investigator (U19 AI067854), amFAR (108547-53-RGRL), and CFAR Developmental (P30 AI060354) grants to R.K.R. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract no. HHSN261200800001E.
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
REFERENCES
- 1.Martin MP, Gao X, Lee JH, Nelson GW, Detels R, Goedert JJ, Buchbinder S, Hoots K, Vlahov D, Trowsdale J, Wilson M, O'Brien SJ, Carrington M. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat Genet 31:429–434. doi: 10.1038/ng934. [DOI] [PubMed] [Google Scholar]
- 2.Martin MP, Qi Y, Gao X, Yamada E, Martin JN, Pereyra F, Colombo S, Brown EE, Shupert WL, Phair J, Goedert JJ, Buchbinder S, Kirk GD, Telenti A, Connors M, O'Brien SJ, Walker BD, Parham P, Deeks SG, McVicar DW, Carrington M. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet 39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alter G, Heckerman D, Schneidewind A, Fadda L, Kadie CM, Carlson JM, Oniangue-Ndza C, Martin M, Li B, Khakoo SI, Carrington M, Allen TM, Altfeld M. 2011. HIV-1 adaptation to NK-cell-mediated immune pressure. Nature 476:96–100. doi: 10.1038/nature10237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonaparte MI, Barker E. 2004. Killing of human immunodeficiency virus-infected primary T-cell blasts by autologous natural killer cells is dependent on the ability of the virus to alter the expression of major histocompatibility complex class I molecules. Blood 104:2087–2094. doi: 10.1182/blood-2004-02-0696. [DOI] [PubMed] [Google Scholar]
- 5.Cohen GB, Gandhi RT, Davis DM, Mandelboim O, Chen BK, Strominger JL, Baltimore D. 1999. The selective downregulation of class I major histocompatibility complex proteins by HIV-1 protects HIV-infected cells from NK cells. Immunity 10:661–671. doi: 10.1016/S1074-7613(00)80065-5. [DOI] [PubMed] [Google Scholar]
- 6.Ward J, Bonaparte M, Sacks J, Guterman J, Fogli M, Mavilio D, Barker E. 2007. HIV modulates the expression of ligands important in triggering natural killer cell cytotoxic responses on infected primary T-cell blasts. Blood 110:1207–1214. doi: 10.1182/blood-2006-06-028175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ward J, Davis Z, DeHart J, Zimmerman E, Bosque A, Brunetta E, Mavilio D, Planelles V, Barker E. 2009. HIV-1 Vpr triggers natural killer cell-mediated lysis of infected cells through activation of the ATR-mediated DNA damage response. PLoS Pathog 5:e1000613. doi: 10.1371/journal.ppat.1000613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fogli M, Mavilio D, Brunetta E, Varchetta S, Ata K, Roby G, Kovacs C, Follmann D, Pende D, Ward J, Barker E, Marcenaro E, Moretta A, Fauci AS. 2008. Lysis of endogenously infected CD4+ T cell blasts by rIL-2 activated autologous natural killer cells from HIV-infected viremic individuals. PLoS Pathog 4:e1000101. doi: 10.1371/journal.ppat.1000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fehniger TA, Herbein G, Yu H, Para MI, Bernstein ZP, O'Brien WA, Caligiuri MA. 1998. Natural killer cells from HIV-1+ patients produce C-C chemokines and inhibit HIV-1 infection. J Immunol 161:6433–6438. [PubMed] [Google Scholar]
- 10.Alter G, Teigen N, Davis BT, Addo MM, Suscovich TJ, Waring MT, Streeck H, Johnston MN, Staller KD, Zaman MT, Yu XG, Lichterfeld M, Basgoz N, Rosenberg ES, Altfeld M. 2005. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood 106:3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 11.Reeves RK, Evans TI, Gillis J, Johnson RP. 2010. Simian immunodeficiency virus infection induces expansion of alpha4beta7+ and cytotoxic CD56+ NK cells. J Virol 84:8959–8963. doi: 10.1128/JVI.01126-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reeves RK, Gillis J, Wong FE, Yu Y, Connole M, Johnson RP. 2010. CD16− natural killer cells: enrichment in mucosal and secondary lymphoid tissues and altered function during chronic SIV infection. Blood 115:4439–4446. doi: 10.1182/blood-2010-01-265595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mavilio D, Lombardo G, Benjamin J, Kim D, Follman D, Marcenaro E, O'Shea MA, Kinter A, Kovacs C, Moretta A, Fauci AS. 2005. Characterization of CD56−/CD16+ natural killer (NK) cells: a highly dysfunctional NK subset expanded in HIV-infected viremic individuals. Proc Natl Acad Sci U S A 102:2886–2891. doi: 10.1073/pnas.0409872102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He X, Li D, Luo Z, Liang H, Peng H, Zhao Y, Wang N, Liu D, Qin C, Wei Q, Yan H, Shao Y. 2013. Compromised NK cell-mediated antibody-dependent cellular cytotoxicity in chronic SIV/SHIV infection. PLoS One 8:e56309. doi: 10.1371/journal.pone.0056309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmad A, Morisset R, Thomas R, Menezes J. 1994. Evidence for a defect of antibody-dependent cellular cytotoxic (ADCC) effector function and anti-HIV gp120/41-specific ADCC-mediating antibody titres in HIV-infected individuals. J Acquir Immune Defic Syndr 7:428–437. [PubMed] [Google Scholar]
- 16.Azzoni L, Papasavvas E, Chehimi J, Kostman JR, Mounzer K, Ondercin J, Perussia B, Montaner LJ. 2002. Sustained impairment of IFN-gamma secretion in suppressed HIV-infected patients despite mature NK cell recovery: evidence for a defective reconstitution of innate immunity. J Immunol 168:5764–5770. doi: 10.4049/jimmunol.168.11.5764. [DOI] [PubMed] [Google Scholar]
- 17.LaBonte ML, McKay PF, Letvin NL. 2006. Evidence of NK cell dysfunction in SIV-infected rhesus monkeys: impairment of cytokine secretion and NKG2C/C2 expression. Eur J Immunol 36:2424–2433. doi: 10.1002/eji.200635901. [DOI] [PubMed] [Google Scholar]
- 18.Li H, Reeves RK. 2012. Functional perturbation of classical natural killer and innate lymphoid cells in the oral mucosa during SIV infection. Front Immunol 3:417. doi: 10.3389/fimmu.2012.00417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luteijn R, Sciaranghella G, van Lunzen J, Nolting A, Dugast AS, Ghebremichael MS, Altfeld M, Alter G. 2011. Early viral replication in lymph nodes provides HIV with a means by which to escape NK-cell-mediated control. Eur J Immunol 41:2729–2740. doi: 10.1002/eji.201040886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pantaleo G, Graziosi C, Butini L, Pizzo PA, Schnittman SM, Kotler DP, Fauci AS. 1991. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A 88:9838–9842. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderford TH, Bleckwehl C, Engram JC, Dunham RM, Klatt NR, Feinberg MB, Garber DA, Betts MR, Silvestri G. 2011. Viral CTL escape mutants are generated in lymph nodes and subsequently become fixed in plasma and rectal mucosa during acute SIV infection of macaques. PLoS Pathog 7:e1002048. doi: 10.1371/journal.ppat.1002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Siliciano JD, Kajdas J, Finzi D, Quinn TC, Chadwick K, Margolick JB, Kovacs C, Gange SJ, Siliciano RF. 2003. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med 9:727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 23.Shen A, Zink MC, Mankowski JL, Chadwick K, Margolick JB, Carruth LM, Li M, Clements JE, Siliciano RF. 2003. Resting CD4+ T lymphocytes but not thymocytes provide a latent viral reservoir in a simian immunodeficiency virus-Macaca nemestrina model of human immunodeficiency virus type 1-infected patients on highly active antiretroviral therapy. J Virol 77:4938–4949. doi: 10.1128/JVI.77.8.4938-4949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Weatherall C, Bailey M, Alcantara S, De Rose R, Estaquier J, Wilson K, Suzuki K, Corbeil J, Cooper DA, Kent SJ, Kelleher AD, Zaunders J. 2013. Simian immunodeficiency virus infects follicular helper CD4 T cells in lymphoid tissues during pathogenic infection of pigtail macaques. J Virol 87:3760–3773. doi: 10.1128/JVI.02497-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith BA, Gartner S, Liu Y, Perelson AS, Stilianakis NI, Keele BF, Kerkering TM, Ferreira-Gonzalez A, Szakal AK, Tew JG, Burton GF. 2001. Persistence of infectious HIV on follicular dendritic cells. J Immunol 166:690–696. doi: 10.4049/jimmunol.166.1.690. [DOI] [PubMed] [Google Scholar]
- 26.Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, Lifson JD, Silvestri G, Estes JD. 2012. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood 120:4172–4181. doi: 10.1182/blood-2012-06-437608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Connick E, Folkvord JM, Lind KT, Rakasz EG, Miles B, Wilson NA, Santiago ML, Schmitt K, Stephens EB, Kim HO, Wagstaff R, Li S, Abdelaal HM, Kemp N, Watkins DI, MaWhinney S, Skinner PJ. 2014. Compartmentalization of simian immunodeficiency virus replication within secondary lymphoid tissues of rhesus macaques is linked to disease stage and inversely related to localization of virus-specific CTL. J Immunol 193:5613–5625. doi: 10.4049/jimmunol.1401161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fletcher CV, Staskus K, Wietgrefe SW, Rothenberger M, Reilly C, Chipman JG, Beilman GJ, Khoruts A, Thorkelson A, Schmidt TE, Anderson J, Perkey K, Stevenson M, Perelson AS, Douek DC, Haase AT, Schacker TW. 2014. Persistent HIV-1 replication is associated with lower antiretroviral drug concentrations in lymphatic tissues. Proc Natl Acad Sci U S A 111:2307–2312. doi: 10.1073/pnas.1318249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lederer S, Favre D, Walters KA, Proll S, Kanwar B, Kasakow Z, Baskin CR, Palermo R, McCune JM, Katze MG. 2009. Transcriptional profiling in pathogenic and non-pathogenic SIV infections reveals significant distinctions in kinetics and tissue compartmentalization. PLoS Pathog 5:e1000296. doi: 10.1371/journal.ppat.1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li H, Evans TI, Gillis J, Connole M, Reeves RK. 8 December 2014. Bone marrow-imprinted gut-homing of plasmacytoid dendritic cells (pDCs) in acute simian immunodeficiency virus infection results in massive accumulation of hyperfunctional CD4+ pDCs in the mucosae. J Infect Dis doi: 10.1093/infdis/jiu671. [DOI] [PubMed] [Google Scholar]
- 31.Li H, Richert-Spuhler LE, Evans TI, Gillis J, Connole M, Estes JD, Keele BF, Klatt NR, Reeves RK. 2014. Hypercytotoxicity and rapid loss of NKp44+ innate lymphoid cells during acute SIV infection. PLoS Pathog 10:e1004551. doi: 10.1371/journal.ppat.1004551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amancha PK, Hong JJ, Ansari AA, Villinger F. 2015. Up-regulation of Tim-3 on T cells during acute simian immunodeficiency virus infection and on antigen specific responders. AIDS 29:531–536. doi: 10.1097/QAD.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ndhlovu LC, Lopez-Verges S, Barbour JD, Jones RB, Jha AR, Long BR, Schoeffler EC, Fujita T, Nixon DF, Lanier LL. 2012. Tim-3 marks human natural killer cell maturation and suppresses cell-mediated cytotoxicity. Blood 119:3734–3743. doi: 10.1182/blood-2011-11-392951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jost S, Moreno-Nieves UY, Garcia-Beltran WF, Rands K, Reardon J, Toth I, Piechocka-Trocha A, Altfeld M, Addo MM. 2013. Dysregulated Tim-3 expression on natural killer cells is associated with increased Galectin-9 levels in HIV-1 infection. Retrovirology 10:74. doi: 10.1186/1742-4690-10-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fujita T, Burwitz BJ, Chew GM, Reed JS, Pathak R, Seger E, Clayton KL, Rini JM, Ostrowski MA, Ishii N, Kuroda MJ, Hansen SG, Sacha JB, Ndhlovu LC. 2014. Expansion of dysfunctional Tim-3-expressing effector memory CD8+ T cells during simian immunodeficiency virus infection in rhesus macaques. J Immunol 193:5576–5583. doi: 10.4049/jimmunol.1400961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qiu Y, Chen J, Liao H, Zhang Y, Wang H, Li S, Luo Y, Fang D, Li G, Zhou B, Shen L, Chen CY, Huang D, Cai J, Cao K, Jian L, Zeng G, Chen ZW. 2012. Tim-3-expressing CD4+ and CD8+ T cells in human tuberculosis (TB) exhibit polarized effector memory phenotypes and stronger anti-TB effector functions. PLoS Pathog 8:e1002984. doi: 10.1371/journal.ppat.1002984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.da Silva IP, Gallois A, Jimenez-Baranda S, Khan S, Anderson AC, Kuchroo VK, Osman I, Bhardwaj N. 2014. Reversal of NK-cell exhaustion in advanced melanoma by Tim-3 blockade. Cancer Immunol Res 2:410–422. doi: 10.1158/2326-6066.CIR-13-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang YH, Zhu C, Kondo Y, Anderson AC, Gandhi A, Russell A, Dougan SK, Petersen BS, Melum E, Pertel T, Clayton KL, Raab M, Chen Q, Beauchemin N, Yazaki PJ, Pyzik M, Ostrowski MA, Glickman JN, Rudd CE, Ploegh HL, Franke A, Petsko GA, Kuchroo VK, Blumberg RS. 2015. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 517:386–390. [DOI] [PMC free article] [PubMed] [Google Scholar]