ABSTRACT
Recent in planta studies have shown that strains Fny and LS of Cucumber mosaic virus (CMV) display differential genetic diversities, Fny and LS having higher and lower mutation frequencies, respectively (J. S. Pita and M. J. Roossinck, J Virol 87:790–797, 2012 http://dx.doi.org/10.1128/JVI.01891-12). In this article, we show that these virus strains have differential recombination frequencies as well. However, the high-diversity Fny strain is a low-recombination virus, whereas the very-low-diversity LS strain is instead a high-recombination virus. Unlike the mutation frequency that was determined by both RNAs 1 and 2, the control elements of recombination frequency reside predominantly within RNA 2, specifically within the 2a gene.
IMPORTANCE Recombination is an important mechanism in virus evolution that can lead to increased or decreased variation and is a major player in virus speciation events that can lead to emerging viruses. Although viral genomes show very frequent evidence of recombination, details of the mechanism involved in these events are still poorly understood. We show here that the reciprocal effects of high mutation frequency and low recombination frequency (and vice versa) involve the RNA-dependent RNA polymerase of the virus, and we speculate that these evolutionary events are related to differences in processivity for two strains of the same virus.
INTRODUCTION
Genetic recombination is the formation of chimeric molecules from segments previously separated on the same molecule or present on different parental molecules (1). RNA recombination is one of the major forces increasing diversity of RNA viruses. It has been well studied for many plant RNA viruses under natural or experimental conditions (for a recent review, see reference 2). In spite of extensive use of Cucumber mosaic virus (CMV) as a model system for virus evolution studies, not much about the role of recombination in the life cycle of CMV is known, other than a propensity for exchanges in the 3′ end in reassorted viruses (3, 4).
CMV (genus Cucumovirus; family Bromoviridae) is an extremely successful virus that infects plants all around the world. The variety of available strains of CMV and the divided genome of the virus provide a useful and convenient system to study many general principles of virus evolution and ecology. CMV RNA 1 encodes the methyltransferase and helicase domains (1a), and RNA 2 encodes the polymerase (2a), together forming the viral components of the replicase complex (5, 6). RNA 2 also encodes the 2b protein (7) involved in RNA silencing suppression, systemic spread, and symptom production, and RNA 3 encodes the 3a (movement) and coat proteins (8, 9). Previously, we used the CMV system to elucidate the forces behind RNA virus population diversity. We identified the genes that are related to the different population diversity levels in different strains of CMV, and we mapped the regions that are associated with high and low levels of diversity (10). We used the same system to understand the mechanisms underlying the fixation of newly generated variants in a viral population (3), a process that constitutes a key aspect of evolutionary dynamics. We found that the increase in relative fitness of such variants in CMV populations is associated with a better adaptation to the replicase complex. In this study, we investigate the contribution of recombination to the population dynamics of CMV by analyzing the progeny of pairs of RNA 3 variants carrying marker mutations in planta. We demonstrate the crucial role of the 2a gene in CMV RNA recombination, and we highlight differences between mutation and recombination from an evolutionary standpoint.
MATERIALS AND METHODS
Viruses and hosts.
The Fny and LS strains of CMV and the procedure to make infectious RNA transcripts from these CMV strains were previously described (11, 12). The intermolecular recombinant F2aLS2b, in which the Fny 2b gene was replaced with that of CMV LS, also was described previously (10). All studies were done with Nicotiana benthamiana.
Construction of CMV RNA 3 mutants bearing restriction enzyme markers.
We created a number of CMV Fny and LS RNA 3 restriction site mutants to use as reporters of recombination events. Wild-type cDNA clones of Fny and LS RNA 3 were used to conduct nucleotide substitution mutagenesis by PCR overlap extension and to introduce silent mutations that created a specific restriction site for each mutant (Table 1). The introduced restriction sites are located in the movement protein, the intergenic region, and the coat protein (Fig. 1). The mutations were confirmed by sequence analysis of cDNA clones. The BamHI mutant was created in a previous study (13).
TABLE 1.
RNA 3 restriction site mutants and mutagenesis/recombination assay primers
| Restriction site expressed by mutant | Primer useda |
Mutated nucleotide(s) | |||
|---|---|---|---|---|---|
| Forward |
Reverse |
||||
| Name | Sequence | Name | Sequence | ||
| BamHIb | Fny-1 | 5′-GACCGTGGaTCcTATTACGG-3′ | Fny-2 | 5′-CCGTAATAgGAtCCACGGTC-3′ | G1505A and T1508C |
| NcoI | Fny-3 | 5′-GTAACCCAtGGTCGTATTGCT-3′ | Fny-4 | 5′-TACGACCaTGGGTTACTTCG-3′ | T560C |
| SpeI | LS-1 | 5′-CCCCAATTAAcTAGTAACAATTTAT-3′ | LS-2 | 5′-GTTACTAgTTAATTGGGGAACGA-3′ | C285T |
| BglII | LS-3 | 5′-CTTCATCATCaGATCTTTCGG-3′ | LS-4 | 5′-CGGAAAGATCtGATGATGAAG-3′ | C1616A |
| NsiI | LS-5 | 5′-CTTTCTCATGcATGCTTCTCC-3′ | LS-6 | 5′-GGAGAAGCATgCATGAGAAAG-3′ | G1147C |
| SacII | LS-7 | 5′-GTCGCCCGCGgAGAGGTTCTC-3′ | LS-8 | 5′-GAGAACCTCTcCGCGGGCGAC-3′ | T1277G |
| 217-238Fc | 5′-AGAAAATGGCTACTGAGTGTGA-3′ | CN3nfc | 5′-GGCTGCAGTGGTCTCCTT-3′ | ||
| 48-70Fd | 5′-GTGTGTGTGTGTGTGTTAGTTAG-3′ | CN3nfd | 5′-GGCTGCAGTGGTCTCCTT-3′ | ||
Lowercase letters in the primer sequence indicate the mutated nucleotides.
For G1505A and T1508C, the nucleotides at 1505 and 1508 were changed from G to A and T to C, respectively, to generate the restriction enzyme site BamHI. The BamHI mutant used in this work was created in a previous study (11). The other mutants were constructed in a similar fashion.
Forward and reverse primers used for CMV strain Fny RNA 3 recombination assays. CN3nf is complementary to the final 18 nt of all CMV RNAs.
Forward and reverse primers used for CMV strain LS RNA 3 recombination assays. CN3nf is complementary to the final 18 nt of all CMV RNAs.
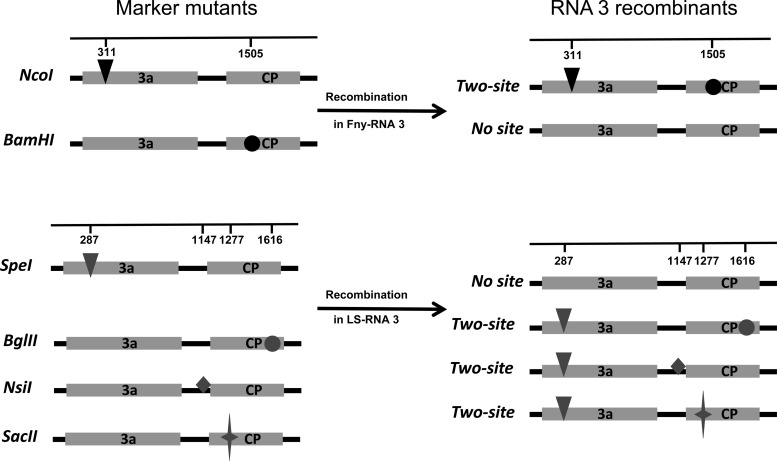
FIG 1.
Locations of marker mutations in CMV strain Fny and LS RNA 3 variants and in the projected homologous recombinants named “No site” and “Two-site.” The numbers on the scales give the positions of the marker mutations (mutated nucleotides) in the respective RNAs 3.
In planta recombination assay.
Equal amounts of pairs of CMV Fny or LS RNA 3 mutants (infectious RNA transcripts) were coinoculated into 3-week-old plants with infectious RNA transcripts of CMV RNAs 1 and 2, as previously described (10). At 15 days postinoculation (dpi), total RNA isolation from systemically infected leaves and high-fidelity reverse transcription (HiFi RT) were performed as previously described (10). For RT reactions, the first-strand primer CN3nf (Table 1) was used for both CMV Fny and LS RNA 3. The cDNAs produced were used as the templates for thermal-cycling reactions with the same first-strand primer and forward primer 217-238F or 48-70F (Table 1), specific for CMV Fny and LS RNA 3, respectively. The generated fragments were of 1,999 nucleotides (nt) for Fny RNA 3 and 2,150 nt for LS RNA 3. The thermal-cycling reactions were carried out as described previously (14).
The HiFi RT-PCR products of interest were purified by electrophoresis on 6% polyacrylamide gels followed by staining with methylene blue and eluted as follows. A piece of the gel containing the PCR band product of the expected size was cut out with a sterilized razor blade and placed into a 0.5-ml microcentrifuge tube containing a hole pierced with a needle. The tube was inserted into a 1.5-ml microcentrifuge tube, and both tubes were centrifuged at 13,000 rpm (16,000 × g) for 2 min to crush the excised acrylamide gel through the needle hole. The crushed product was recovered in the 1.5-ml microcentrifuge tube and resuspended in 250 μl of buffer (3 mM Tris-HCl, 0.2 mM EDTA) and 50 μl of 7.5 M NH4Oac and incubated at 65°C for 30 min. After the incubation, the resuspension was transferred to a 2-ml SpinX column containing a filter. The PCR product was separated from the polyacrylamide gel by centrifugation of the SpinX column at 13,000 rpm for 5 min, and the eluate was concentrated by ethanol precipitation.
The purifed PCR products were ligated into pGEM-T Easy vector (Promega). The ligation product was used to transform Escherichia coli DH5α competent cells that were later plated onto LB agar containing isopropyl-β-d-thiogalactopyranoside and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside. Positive clones were identified by colony PCR (at least 100 positive clones per virus tested). The PCR products were purified, digested with the appropriate restriction enzymes (BamHI and NcoI for strain Fny separately or SpeI and BglII/NsiI or SacII for strain LS) and analyzed by electrophoresis on a 1.3% agarose gel in Tris-borate-EDTA (TBE), and visualized with ethidium bromide.
Control RT-PCR assays.
Control experiments were conducted to eliminate the possibility of recombinant artifacts being generated by RT-PCR amplifications. For that purpose, total RNAs from two different plants infected, respectively, with the BamHI or NcoI mutant (for CMV Fny) or with the SpeI and BglII/NsiI or SacII mutant (for CMV LS) were mixed in equal concentrations, and the mixture was used to perform recombination assays following the protocol described above.
Sequence analyses.
The sequences of the deletion mutants were determined using the ABI 3730 DNA analyzer and analyzed using DNASTAR (Lasergene 11).
Statistical analysis.
Differences in recombination frequencies between viruses were tested for statistical significance using a one-way analysis of variance (ANOVA) test from StatPlus:mac LE.
RESULTS AND DISCUSSION
Infectivity and in planta stability of the constructed RNA 3 mutants.
The infectivity and the stability of each of the BamHI, NcoI, SpeI, BglII, NsiI, and SacII mutants were determined on N. benthamiana plants. Infectious RNA transcripts of each mutant were mixed with infectious transcripts of the appropriate RNAs 1 and 2 from CMV Fny or LS and inoculated on the leaves of 3-week-old plants. The time necessary for the appearance of the symptoms and their severity did not differ between plants inoculated with the mutants tested and plants infected with the wild-type CMV Fny or LS strain (data not shown). Restriction fragment length polymorphism (RFLP) analysis of the HiFi RT-PCR products (100 positive clones for each mutant) demonstrated that the introduced restriction sites were maintained in the RNA 3 mutant progenies at 15 dpi (data not shown), confirming the stability of the RNA 3 mutants during infection and the ability to recover the mutants from infected plants by the procedure described above.
Recombination events are rare in CMV Fny.
To be certain that we were detecting recombination in the virus infection, rather than artifactually in the RT-PCR, we mixed RNA templates from plants individually infected with each of two mutants and analyzed 325 clones obtained after amplification. No recombinants were identified in the clones derived from the control experiments, and BamHI and NcoI mutants could be recovered equally from infected plants (Table 2) (by ANOVA, P = 0.658 [with P > 0.05 considered significant]), so we used the following assay to calculate the recombination frequency in CMV Fny populations in N. benthamiana. In the absence of recombination between the coinoculated parental RNAs 3, the digestion pattern of each clone is identical to that of one of the parental clones. However, a recombination event between the parental RNAs 3 leads to the appearance of two kinds of recombinants, named “No site” and “Two-site” (Fig. 1), that can be detected by the restriction profile. For Fny, we obtained a low recombination frequency using this assay: <2% (Table 2). This is surprisingly low compared to the recombination frequencies published for other DNA and RNA plant viruses.
TABLE 2.
Host factor effects on recombination frequencies in CMV strain Fny
| Virus | Plant | Total no. of clones | No. of clones with restriction profile showna |
Recombination frequency (%) | |||
|---|---|---|---|---|---|---|---|
| BamHI | NcoI | No site | Two-site | ||||
| F1F2F3 control | 1 | 112 | 47 | 65 | 0 | 0 | 0 |
| 2 | 108 | 67 | 41 | 0 | 0 | 0 | |
| 3 | 105 | 55 | 50 | 0 | 0 | 0 | |
| Mean, 0 | |||||||
| F1F2F3 in N. benthamiana | 1 | 100 | 55 | 43 | 1 | 1 | 2.0 |
| 2 | 124 | 88 | 34 | 2 | 0 | 1.6 | |
| 3 | 102 | 52 | 48 | 1 | 1 | 1.9 | |
| Mean, 1.83 | |||||||
In the control experiments, individual infections with each mutant were used to create a mixture in vitro, prior to RT-PCR and RFLP analysis. In the experimental samples (N. benthamiana), the plants were inoculated with a mixture of transcripts from the two mutants.
Froissart et al. (15) showed that in Cauliflower mosaic virus populations, over 50% of progeny viral genomes were recombinants after a single host infection, indicating that recombination is very frequent in the populations of that DNA plant virus. The calculated recombination frequency in Brome mosaic virus (BMV), an RNA plant virus belonging to the same family as CMV, is 25% (16), about 12 times higher than that of CMV Fny. Therefore, the low recombination frequency obtained here indicates that recombination is not a very frequent phenomenon in the replication cycle of strain Fny.
Differential recombination dynamics in various Fny RNA 3 populations in N. benthamiana.
In an earlier study, RNA 3 progeny populations of the reassortant virus L1L2F3, consisting of RNAs 1 and 2 from CMV LS and RNA 3 from CMV Fny, contained mostly recombinants (3, 10). Here we measured the recombination frequency of L1L2F3 virus in an additional assay by coinoculating BamHI and NcoI mutants with infectious RNA transcripts of LS RNAs 1 and 2 and following the procedure described above to calculate the recombination frequency in L1L2F3 progeny populations at 15 dpi. We obtained a recombination frequency of 28% (Table 3), which is in the range of that of BMV (16).
TABLE 3.
Recombination frequencies in various CMV strain Fny RNA 3 populations in Nicotiana benthamiana
| Virus | Plant no. | Total no. of clones | No. of clones with restriction profile shown |
Recombination frequency (%)b | |||
|---|---|---|---|---|---|---|---|
| BamHI | NcoI | No site | Two-site | ||||
| F1F2F3a | 1 | 100 | 55 | 43 | 1 | 1 | 2.00 |
| 2 | 124 | 88 | 34 | 2 | 0 | 1.60 | |
| 3 | 102 | 52 | 48 | 1 | 1 | 1.90 | |
| Mean, 1.83 A | |||||||
| L1L2F3 | 1 | 131 | 56 | 37 | 23 | 15 | 29.00 |
| 2 | 104 | 45 | 29 | 17 | 13 | 28.80 | |
| 3 | 109 | 51 | 28 | 18 | 12 | 27.50 | |
| Mean, 28.43 B | |||||||
| F1L2F3 | 1 | 115 | 86 | 9 | 18 | 2 | 17.39 |
| 2 | 101 | 75 | 9 | 15 | 2 | 16.83 | |
| 3 | 122 | 92 | 7 | 20 | 3 | 18.85 | |
| 17.69 C | |||||||
| L1F2F3 | 1 | 135 | 07 | 122 | 4 | 2 | 4.44 |
| 2 | 107 | 05 | 97 | 3 | 2 | 4.67 | |
| 3 | 110 | 06 | 100 | 3 | 1 | 3.64 | |
| Mean, 4.25 D | |||||||
| F1F2aLS2bF3 | 1 | 107 | 76 | 30 | 1 | 0 | 0.93 |
| 2 | 120 | 85 | 33 | 2 | 0 | 1.66 | |
| 3 | 101 | 54 | 47 | 1 | 0 | 0.99 | |
| Mean, 1.19 A | |||||||
Data from Table 2.
Differences between recombination frequencies were determined by ANOVA (P < 0.05). Statistically significant differences are marked with different capital letters next to the recombination frequencies.
Recombination frequencies calculated in various F3 reassortant/recombinant viruses.
We measured the recombination frequencies in the F3 progeny supported by reassortant viruses between CMV strains Fny and LS: F1L2, L1F2, and F1F2aLS2bF3, in which the 2b gene of Fny was replaced with that of LS (Table 3). The recombination frequency for F1L2F3 was significantly higher than that for Fny (F1F2F3, P = 0.0000 and F = 666.97), indicating that the replacement of the RNA 2 of Fny with that of LS (L2) was enough to switch Fny to a high-recombination virus (Table 3) (P = 0.0019, F = 52.20). In our previous studies on mutation frequencies in these reassortant viruses, the opposite result was seen: the presence of F2 was sufficient to switch L1L2L3 from a low-diversity virus to a high-diversity virus (10).
The recombination frequency calculated for L1F2F3, although significantly higher than that of F1F2F3 (Table 3), is still not in the range of that of high-recombination viruses, indicating that RNA 1 may harbor a viral control element or elements of recombination frequency but not to the same level as RNA 2. The RNA 2b gene does not seem to contribute any effect on recombination frequency, since the calculated recombination frequency in the progeny of F1F2aLS2bF3 is not significantly different from that of F1F2F3 (Table 3) (P = 0.072, F = 5.92).
Recombination events are very frequent in CMV LS.
To be certain that the high recombination frequency of L1L2F3 was not due to the heterologous RNA 3, we constructed two CMV LS RNA 3 restriction site (SpeI and BglII) mutants to measure recombination frequency in LS populations (Table 1; Fig. 1). No recombinant was identified out of the 326 control clones (0%) derived from the control experiments (Table 4), and both SpeI and BglII mutants could be recovered equally from individually infected plants (by ANOVA, P = 0.289 [where P > 0.05 is significant]). Using this assay with LS, we obtained a recombination frequency of 28.2%, which is in the range of that of high-recombination viruses (Table 4) and essentially the same as what we found when the RNA 3 was derived from CMV Fny. Hence, the high recombination frequency calculated for L1L2F3 is not a result of its reassortant nature. Irrespective of the origin of the replicating RNA 3 (F3 or L3), the calculated recombination frequencies for L1L2 are in the higher-recombination range, while that of F1F2 (F1F2F3) is in the lower-recombination range (Tables 3 and 4), indicating that polymerases of different strains of the same virus generate very different recombination frequencies in plants.
TABLE 4.
CMV strain LS recombination frequencies calculated using various combinations of RNA 3 mutants
| Virus | Expt or plant | Total no. of clones | No. of clones with restriction profile showna | Recombination frequency (%)b | |||
|---|---|---|---|---|---|---|---|
| L1L2L3 control | Expt | SpeI | BglII | No site | Two-site | ||
| 1 | 115 | 50 | 65 | 0 | 0 | 0 | |
| 2 | 103 | 55 | 48 | 0 | 0 | 0 | |
| 3 | 108 | 48 | 60 | 0 | 0 | 0 | |
| Mean, 0 | |||||||
| L1L2L3 | Plant | SpeI | BglII | No site | Two-site | ||
| 1 | 104 | 74 | 0 | 30 | 0 | 28.80 | |
| 2 | 110 | 78 | 0 | 32 | 0 | 29.09 | |
| 3 | 101 | 74 | 0 | 27 | 0 | 26.73 | |
| Mean, 28.20 Ab | |||||||
| L1L2L3 | Plant | SpeI | NsiI | No site | Two-site | ||
| 1 | 105 | 86 | 0 | 19 | 0 | 18.00 | |
| 2 | 113 | 92 | 0 | 21 | 0 | 18.50 | |
| 3 | 102 | 86 | 0 | 16 | 0 | 15.68 | |
| Mean, 17.39 B | |||||||
| L1L2L3 | Plant | SpeI | SacII | No site | Two-site | ||
| 1 | 101 | 81 | 0 | 20 | 0 | 19.80 | |
| 2 | 112 | 89 | 0 | 23 | 0 | 20.53 | |
| 3 | 111 | 89 | 0 | 22 | 0 | 19.80 | |
| Mean, 20.04 C | |||||||
In the control experiments, individual infections with each mutant were used to create a mixture in vitro, prior to RT-PCR and RFLP analysis. In the experimental samples, the plants were inoculated with a mixture of transcripts from the two mutants.
Differences between recombination frequencies were determined by ANOVA (P < 0.05). Statistically significant differences are marked with different capital letters next to the recombination frequencies.
Although we could calculate recombination frequencies in L1L2L3 populations, we could not recover the BglII mutant from L1L2L3 progenies (Table 4) (i.e., the BglII mutant was recovered only in single infections), indicating that it might be under strong negative selection pressure in the context of a mixed infection. Hence, we used two additional CMV LS RNA 3 NsiI and SacII mutants (Table 1; Fig. 1) to calculate recombination frequencies in L1L2L3 populations. However, like the BglII mutant, we could not recover NsiI or SacII mutants from L1L2L3 progeny populations. All of the recombinants were of the wild-type sequence (No site). The recombination frequencies calculated with the NsiI and SacII mutants, although significantly different from the frequency calculated with the BglII mutant, were also in the high-recombination range (Table 4). The differences between the calculated recombination frequencies were most likely due to differences in the lengths of the segments provided for recombination (1,329 nt for the BglII mutant, 860 nt for the NsiI mutant, and 940 nt for the SacII mutant).
The control elements for recombination frequency reside in RNA 2.
The SpeI and BglII mutants were used to calculate recombination frequencies in the L3 progeny populations of various reassortant/recombinant viruses in N. benthamiana (Table 5). The calculated recombination frequency for L1F2L3 was in the low-recombination range (Table 5) compared to that of L1L2L3. Therefore, the replacement of CMV LS RNA 2 with that of CMV Fny was sufficient to switch LS to a low-recombination virus. This confirms that the control element or elements for recombination frequency reside in CMV RNA 2. Likewise, the recombination frequency of F1F2L3 was in the low-recombination range (Table 5), confirming that irrespective of the replicating RNA 3, F3 or L3, the calculated recombination frequencies were significantly lower when the polymerase is derived from Fny than when it is derived from LS (Tables 3, 5, and 6).
TABLE 5.
Recombination frequencies in various CMV strain LS RNA 3 populations in Nicotiana benthamiana
| Virus | Plant no. | Total no. of clones | No. of clones with restriction profile shown |
Recombination frequency (%)b | No. of deletion mutants | Deletion frequency (%) | |||
|---|---|---|---|---|---|---|---|---|---|
| SpeI | BglII | No site | Two-site | ||||||
| L1L2L3a | 1 | 104 | 74 | 0 | 30 | 0 | 28.80 | 0 | 0 |
| 2 | 110 | 78 | 0 | 32 | 0 | 29.09 | 0 | 0 | |
| 3 | 101 | 74 | 0 | 27 | 0 | 26.73 | 0 | 0 | |
| Mean, 28.20 A | Mean, 0 | ||||||||
| L1F2L3 | 1 | 112 | 0 | 101 | 3 | 0 | 2.67 | 8 | 7.14 |
| 2 | 102 | 0 | 93 | 2 | 0 | 1.96 | 7 | 6.86 | |
| 3 | 118 | 0 | 105 | 3 | 0 | 2.54 | 10 | 8.47 | |
| Mean, 2.39 B | Mean, 7.53 | ||||||||
| F1F2L3 | 1 | 101 | 0 | 99 | 2 | 0 | 1.98 | 0 | 0 |
| 2 | 105 | 0 | 103 | 2 | 0 | 1.90 | 0 | 0 | |
| 3 | 114 | 0 | 111 | 3 | 0 | 2.63 | 0 | 0 | |
| Mean, 2.17 B | Mean, 0 | ||||||||
| F1L2L3 | 1 | 113 | 96 | 0 | 17 | 0 | 15.04 | 0 | 0 |
| 2 | 100 | 86 | 0 | 14 | 0 | 14.00 | 0 | 0 | |
| 3 | 125 | 104 | 0 | 21 | 0 | 16.80 | 0 | 0 | |
| Mean, 15.28 C | Mean, 0 | ||||||||
| L1F2aLS2bL3 | 1 | 100 | 0 | 98 | 0 | 0 | 0 | 2 | 2 |
| 2 | 103 | 0 | 102 | 1 | 0 | 0.90 | 0 | 0 | |
| 3 | 106 | 0 | 106 | 0 | 0 | 0 | 0 | 0 | |
| Mean, 0.30 D | Mean, 0.65 | ||||||||
Data from Table 4.
Differences between recombination frequencies were determined by ANOVA (P < 0.05). Statistically significant differences are marked with different capital letters next to the recombination frequencies.
TABLE 6.
Relationship between CMV replicases and recombination frequencies based on the replicating RNA 3
| Viral replicase | Replicating RNA 3 | Recombination frequency (%) | P valuea |
|---|---|---|---|
| F1F2 | F3 | 1.83 | 0.265* |
| L3 | 2.17 | ||
| L1L2 | F3 | 28.43 | 0.809* |
| L3 | 28.20 | ||
| F1L2 | F3 | 17.69 | 0.076* |
| L3 | 15.28 | ||
| L1F2 | F3 | 4.25 | 0.008 |
| L3 | 2.39 |
Significance (P < 0.05) was determined by ANOVA. *, not significantly different.
The calculated recombination frequencies for F1L2L3 populations confirm that CMV control elements for recombination frequencies reside predominantly in RNA 2, since the replacement of F2 by L2 is enough to switch to a high-recombination virus (Table 5). Further mapping indicates that those control elements are located more precisely in the 2a gene. The replacement of CMV LS 2a with that of Fny 2a (L1F2aLS2bL3) is sufficient to switch LS (L1L2L3) to a low-recombination virus (Table 5).
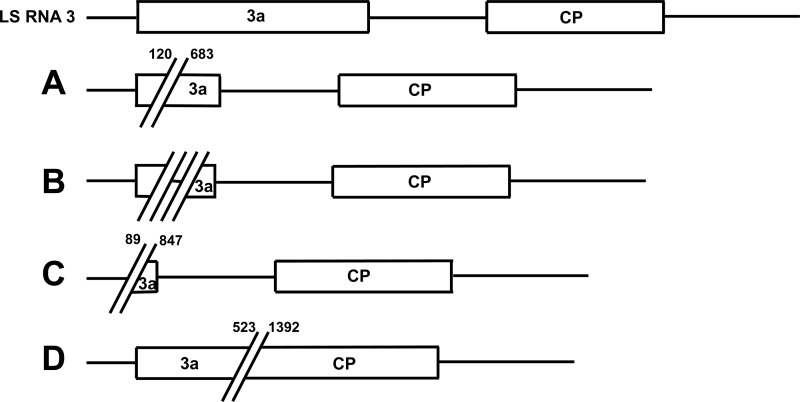
In addition to reducing the recombination frequency of CMV LS, Fny-2a also generated deletion mutants. Two deletion mutants were identified in the progeny populations of L1F2aLS2bL3. The same deletion mutants among others were found in the progeny of the reassortant virus L1F2L3 (Table 5). Based on the sizes of the deleted fragments and depending on the deletion sites, we could distinguish several different deletion mutants (Fig. 2). Similar deletion mutants were found associated with the Fny strain of CMV but not the Sny strain after serial passages in a tobacco host (17).
FIG 2.
Structures of RNA 3 deletion mutants. The horizontal lines represent noncoding regions, and the rectangles represent open reading frames (ORFs). Mutant A contains a 564-nucleotide (nt) in-frame deletion in the 3a ORF, spanning nt 120 to 683. Mutant B contains two deletions in the 3a ORF. The first one is located between nt 280 and 770, and the second one is an 81-nt in-frame deletion from nt 775. The two deletions are separated by 4 nt (AAAC). Mutant B was found in several clones. The deletion event in mutant C occurred between nt 89 and 847, almost resulting in the complete deletion of the 3a ORF. Mutant D is an in-frame deletion of 290 amino acids, stretching from the 3a ORF (nt 523) to the CP ORF (nt 1392) and encompassing the intergenic region. Different mutants were isolated from the same plant.
L1F2aLS2bL3 progeny populations present clear evidence that the CMV polymerase (2a) gene plays a critical role in the preferential selection of the replicating RNA 3 mutants. We found that L3 virus progeny populations always had only one mutant type, an SpeI or BglII mutant; both mutants were never found together. Interestingly, the SpeI mutant is selected in the presence of CMV LS RNA 2, whereas the BglII mutant is preferentially selected in the presence of either CMV Fny RNA 2 or Fny 2a (Table 5).
Recently, we showed that the Fny and LS strains of CMV have significantly different mutation frequencies. By using reassortant and intermolecular recombinant viruses between the two strains, we mapped the viral domains controlling mutation frequency in CMV (10). In this article, we used the same strains to map the viral control elements of recombination frequency in CMV. The results presented here provide insights into the different roles of recombination and point mutations in shaping population dynamics in CMV.
Serviene et al. (18) identified over 30 host genes suppressing or increasing RNA recombination of Tomato bushy stunt virus (TBSV). Among these genes, pmr1, which encodes an ion pump (Pmr1p) regulating Ca2+/Mn2+ influx into the Golgi apparatus from the cytosol, affects the frequency of viral recombination in yeast (19). The changes in frequency of recombination are likely due to TBSV replicase that switched to a superactive mode in pmr1-5′-deleted yeast (19). Interestingly, similarly to mutation frequency, we demonstrated here that the replicases of CMV Fny (F1F2) and LS (L1L2) generate different recombination frequencies in the same host, N. benthamiana, irrespective of the replicating RNA 3. Indeed, in the copy choice mechanism that is likely used in the recombination process described here, recombinant RNAs are formed due to switching of viral replicase between RNA templates. However, the switching properties depend in part on the replicase processivity (2), a measure of the average number of nucleotides copied per template association-disassociation cycle (20). In CMV, the active replicase consists of the 1a and 2a proteins as well as a host factor or factors.
Unlike mutation frequency, for which CMV control elements reside in both RNAs 1 and 2, we found that the recombination frequency control elements are located solely in RNA 2, more precisely in the 2a open reading frame (ORF). Mutational studies on BMV 2a polymerase also indicated the role of polymerase in recombination. A 2a mutation affected the precision as well as the location of RNA recombination sites (21). The authors speculated that the modified 2a forms a replicase complex that has limited RNA template-switching ability because of changes in its processivity that could lead to a decreased frequency of recombination. In Human immunodeficiency virus, a number of studies have suggested that processivity and fidelity are inversely linked, such that low-processivity polymerases have higher fidelity (22). If the processivity of CMV LS polymerase is low, we would expect higher recombination rates and higher fidelity, whereas if the CMV Fny polymerase is relatively higher in processivity, we would expect the opposite. Although we have not measured the polymerase fidelity, the differences in mutation frequency are likely a reflection of polymerase fidelity. Hence it is possible that due to differences in polymerase processivity, strain LS is highly recombinogenic, its polymerase promoting homologous recombination, whereas the polymerase of the nonrecombinogenic CMV strain Fny generates nonhomologous intramolecular recombination, leading to the formation of various defective RNAs (DRNAs) (Table 5 and Fig. 2).
From an evolutionary standpoint, recombination can either increase or decrease mutation frequency, as it can result in the addition of mutations when two mutated molecules recombine to maintain mutations, or the loss of mutations resulting in recombinants like the No-site recombinants seen in our assays. Since a lot of No-site progenies were observed with the LS CMV and no Two-site progeny could be detected, this may be a strategy to reduce mutation frequency in strain LS, where the mutants essentially revert to wild type through recombination. Of course, we cannot rule out the reversion by mutation and selection rather than by recombination, but since one of the mutants is very stable, this is seems less likely.
For the first time, we have provided a calculated recombination frequency for CMV and we have shown that the two strains, Fny and LS, in addition to having significantly different mutation frequencies also possess significantly different recombination frequencies. However, each strain presents an opposite character with respect to mutation and recombination frequencies. This results in an inner biological conflict in the replication cycle of these viruses. The strain with the lower recombination frequency (Fny) displays a higher mutation frequency, while the one displaying the lower mutation frequency (LS) has the higher recombination frequency. This could represent different evolutionary strategies by different strains of CMV to control population dynamics. Using the mutation frequency as the indicator of population variations, Schneider et al. (23) showed that Cowpea chlorotic mottle virus (CCMV) of the family Bromoviridae had no detectable variation through 10 serial passages on N. benthamiana, except for a single clone that underwent a recombination event, incorporating 24 nucleotides into the intercistronic region. Similarly, Allison et al. (24) showed that recombination was very frequent in CCMV populations, a strategy used by the virus to regenerate functional RNA genomes, and that recombination events occurred during the divergence of CCMV (25). With these limited examples, we can speculate that high mutation frequency and high recombination frequency may not coexist in virus replication cycles. Schneider et al. (23) showed that viral diversities always reach an equilibrium point that is characteristic of the virus-host interaction. If mutation frequency is a reflection of mutation rate, viruses with greater mutation rates may not tolerate high recombination rates as well as they could be pushed too close to extinction (26); alternatively, lower mutation frequencies may be reflective of higher recombination rates that resolve mutations. The difference may also be enzymatic: it is possible that higher processivity results in higher mutation rates, whereas lower processivity results in higher recombination rates.
ACKNOWLEDGMENTS
This work was supported by the Samuel Roberts Noble Foundation and by the Pennsylvania State University College of Agricultural Sciences.
REFERENCES
- 1.Nagy PD, Simon AE. 1997. New insights into the mechanisms of RNA recombination. Virology 235:1–9. doi: 10.1006/viro.1997.8681. [DOI] [PubMed] [Google Scholar]
- 2.Bujarski JJ. 2013. Genetic recombination in plant-infecting messenger-sense RNA viruses: overview and research perspectives. Front Plant Sci 4:68. doi: 10.3389/fpls.2013.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pita JS, Roossinck MJ. 2013. Fixation of emerging interviral recombinants in Cucumber mosaic virus populations. J Virol 87:1264–1269. doi: 10.1128/JVI.01892-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson JR, Tepfer M. 2009. The 3′ untranslated region of cucumber mosaic virus (CMV) subgroup II RNA3 arose by interspecific recombination between CMV and tomato aspermy virus. J Gen Virol 90:2293–2298. doi: 10.1099/vir.0.011452-0. [DOI] [PubMed] [Google Scholar]
- 5.Hayes RJ, Buck KW. 1990. Infectious cucumber mosaic virus RNA transcribed in vitro from clones obtained from cDNA amplified using the polymerase chain reaction. J Gen Virol 71:2503–2508. doi: 10.1099/0022-1317-71-11-2503. [DOI] [PubMed] [Google Scholar]
- 6.Nitta N, Takanami Y, Kuwata S, Kubo S. 1988. Inoculation with RNAs 1 and 2 of cucumber mosaic virus induces viral RNA replicase activity in tobacco mesophyll protoplasts. J Gen Virol 69:2695–2700. doi: 10.1099/0022-1317-69-10-2695. [DOI] [Google Scholar]
- 7.Ding S-W, Anderson BJ, Haase HR, Symons RH. 1994. New overlapping gene encoded by the cucumber mosaic virus genome. Virology 198:593–601. doi: 10.1006/viro.1994.1071. [DOI] [PubMed] [Google Scholar]
- 8.Boccard F, Baulcombe D. 1993. Mutational analysis of cis-acting sequences and gene function in RNA3 of cucumber mosaic virus. Virology 193:563–578. doi: 10.1006/viro.1993.1165. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki M, Kuwata S, Kataoka J, Masuta C, Nitta N, Takanami Y. 1991. Functional analysis of deletion mutants of cucumber mosaic virus RNA3 using an in vitro transcription system. Virology 183:106–113. doi: 10.1016/0042-6822(91)90123-S. [DOI] [PubMed] [Google Scholar]
- 10.Pita JS, Roossinck MJ. 2013. Mapping viral functional domains for genetic diversity in plants. J Virol 87:790–797. doi: 10.1128/JVI.01891-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzo TM, Palukaitis P. 1990. Construction of full-length cDNA clones of cucumber mosaic virus RNAs 1, 2 and 3: generation of infectious RNA transcripts. Mol Gen Genet 222:249–256. doi: 10.1007/BF00633825. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Hanada K, Palukaitis P. 1994. Mapping local and systemic symptom determinants of cucumber mosaic cucumovirus in tobacco. J Gen Virol 75:3185–3191. doi: 10.1099/0022-1317-75-11-3185. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Roossinck MJ. 2004. Genetic bottlenecks reduce population variation in an experimental RNA virus population. J Virol 78:10582–10587. doi: 10.1128/JVI.78.19.10582-10587.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pita JS, deMiranda JR, Schneider WL, Roossinck MJ. 2007. Environment determines fidelity for an RNA virus replicase. J Virol 81:9072–9077. doi: 10.1128/JVI.00587-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Froissart R, Roze D, Uzest M, Galibert L, Blanc S, Michalakis Y. 2005. Recombination every day: abundant recombination in a virus during a single multi-cellular host infection. PLoS Biol 3:e89. doi: 10.1371/journal.pbio.0030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruyere A, Wantroba M, Flasinski S, Dzianott A, Bujarski JJ. 2000. Frequent homologous recombination events between molecules of one RNA component in a multipartite RNA virus. J Virol 74:4214–4219. doi: 10.1128/JVI.74.9.4214-4219.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graves MV, Roossinck MJ. 1995. Characterization of defective RNAs derived from RNA 3 of the Fny strain of cucumber mosaic cucumovirus. J Virol 69:4746–4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Serviene E, Shapka N, Cheng C-P, Panavas T, Phuangrat B, Baker J, Nagy PD. 2005. Genome-wide screen identifies host genes affecting viral RNA recombination. Proc Natl Acad Sci U S A 102:10545–10550. doi: 10.1073/pnas.0504844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaag HM, Pogany J, Nagy PD. 2010. A host Ca2+/Mn2+ ion pump is a factor in the emergence of viral RNA recombinants. Cell Host Microbe 7:74–81. doi: 10.1016/j.chom.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 20.Breyer WA, Matthews BW. 2001. A structural basis for processivity. Protein Sci 10:1699–1711. doi: 10.1110/ps.10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figlerowicz M, Nagy PD, Bujarski JJ. 1997. A mutation in the putative RNA polymerase gene inhibits nonhomologous, but not homologous, genetic recombination in an RNA virus. Proc Natl Acad Sci U S A 94:2073–2078. doi: 10.1073/pnas.94.5.2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd SB, Kent SJ, Winnall WR. 2014. The high cost of fidelity. AIDS Res Hum Retroviruses 30:8–16. doi: 10.1089/aid.2013.0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider WL, Roossinck MJ. 2000. Evolutionarily related Sindbis-like plant viruses maintain different levels of population diversity in a common host. J Virol 74:3130–3134. doi: 10.1128/JVI.74.7.3130-3134.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Allison R, Thompson G, Ahlquist P. 1990. Regeneration of a functional RNA virus genome by recombination between deletion mutants and requirement for cowpea chlorotic mottle virus 3a and coat genes for systemic infection. Proc Natl Acad Sci U S A 87:1820–1824. doi: 10.1073/pnas.87.5.1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allison RF, Janda M, Ahlquist P. 1989. Sequence of cowpea chlorotic mottle virus RNAs 2 and 3 and evidence of a recombination event during bromovirus evolution. Virology 172:321–330. doi: 10.1016/0042-6822(89)90134-7. [DOI] [PubMed] [Google Scholar]
- 26.Bull JJ, Sanjuan R, Wilke CO. 2007. Theory of lethal mutagenesis for viruses. J Virol 81:2930–2939. doi: 10.1128/JVI.01624-06. [DOI] [PMC free article] [PubMed] [Google Scholar]