Abstract
Polymorphonuclear neutrophils (PMNs) are essential cellular constituents in the innate host response, and their recruitment to the lungs and subsequent ubiquitous phagocytosis controls primary respiratory infection. Cystic fibrosis pulmonary disease is characterized by progressive pulmonary decline governed by a persistent, exaggerated inflammatory response dominated by PMNs. The principal contributor is chronic Pseudomonas aeruginosa biofilm infection, which attracts and activates PMNs and thereby is responsible for the continuing inflammation. Strategies to prevent initial airway colonization with P. aeruginosa by augmenting the phagocytic competence of PMNs may postpone the deteriorating chronic biofilm infection. Anti-P. aeruginosa IgY antibodies significantly increase the PMN-mediated respiratory burst and subsequent bacterial killing of P. aeruginosa in vitro. The mode of action is attributed to IgY-facilitated formation of immobilized bacteria in aggregates, as visualized by fluorescence microscopy and the induction of increased bacterial hydrophobicity. Thus, the present study demonstrates that avian egg yolk immunoglobulins (IgY) targeting P. aeruginosa modify bacterial fitness, which enhances bacterial killing by PMN-mediated phagocytosis and thereby may facilitate a rapid bacterial clearance in airways of people with cystic fibrosis.
INTRODUCTION
Innate immunity is vital for controlling primary infection in the respiratory tract. Activation of cellular constituents in the innate host response promotes development and differentiation of adaptive host mechanisms, and the subsequent synergistic interplay eliminates encountered pathogens and establishes long-lasting protective immunity (1). Polymorphonuclear neutrophils (PMNs) are essential determinants in the innate host response and are readily recruited to the site of infection. Their immune response is activated in part by bacterial shedding of immunostimulatory pathogen-associated molecular patterns (PAMPs), like lipopolysaccharide (LPS), DNA, cell wall components, and flagella, which are recognized by epithelial pattern recognition receptors (PRRs), such as Toll-like receptors (TLRs), C-type lectin receptors, and the cytoplasmic NOD-like receptors (NLRs) (2, 3, 4). The stimulation of PRRs activates downstream pathway signaling through an adaptor molecule, MyD88, and this leads to nuclear translocation of the transcription factor nuclear factor κB (NF-κB) (5). NF-κB activates gene promoters controlling a broad range of cytokines and initiates the expression of proinflammatory effectors. The subsequent expression of tumor necrosis factor alpha (TNF-α) upregulates the cellular adhesion molecule ICAM-1 on epithelial cells, which is the ligand for β2-integrin on PMNs, priming the extravasation of PMNs (6) to the alveolar lumen, where the cells eventually commence their bactericidal task of phagocytizing and killing pathogens.
Phagocytosis is a sequential process involving recognition of damaging pathogens, followed by attachment, engulfment, and degradation. The phagocytic process is greatly enhanced by bacterial opsonization, especially with IgG and fragments of complement effector C3 (7). The engagement of phagocyte receptors and opsonized bacteria activates cytoskeletal contractile components, causing invagination of the membrane and extension of pseudopods around the microbe. The consecutive interplay of receptor-opsonin pairs conducts the engulfment of bacteria within a phagosome, leading to formation of the phagolysosome by fusion of the phagosome and lysosomal compartments containing bactericidal products. The bactericidal mechanisms of PMNs are thus characterized by the production of antimicrobial metabolites, such as peptides, proteases, and reactive oxygen species (ROS), during phagocytosis (8). Phagocytosis terminates with the degradation of microbes and the apoptotic consequences for PMNs and subsequent engulfment by macrophages, initiating the resolution of inflammation (9).
Cystic fibrosis (CF) pulmonary disease is characterized by prominent airway inflammation, as evidenced by PMN accumulation and excessive concentrations of the neutrophil chemokine interleukin-8 (IL-8) (10, 11, 12). The sustained PMN activation produces tissue-destructive components, like neutrophil elastase (13), proteases (14), and ROS, which contribute to the pulmonary disease via tissue degradation (15). The deterioration with chronic airway inflammation is attributed to recurring bacterial colonization, which eventually progresses into chronic infection due to failure of eradication of bacteria, e.g., due to biofilm formation. The normal cessation of inflammation is annulled, and the PMNs are arrested in an accelerated state, aggravating the destruction of lung tissue and further reinforcing inflammatory responses. P. aeruginosa is the predominant bacterial pathogen in CF, and the opportunistic pathogen readily adapts to the mucus-rich environment in the CF lung (16). Chronic infection with P. aeruginosa is associated with a decline in lung function and frequent exacerbations (17), and early colonization with P. aeruginosa is a predictor of a poor prognosis (18). The initial colonization of planktonic P. aeruginosa is eradicated efficiently by competent PMNs (19). However, recurrent colonization triggers bacterial adaptation to the airway milieu, causing a shift from the planktonic state to the biofilm mode of growth and the selection for bacterial mutants with abundant production of the exopolysaccharide alginate (20), thereby establishing mucoid phenotypes which are resilient to phagocytosis (21, 22). Thus, approaches to moderate the innate host response at early stages of CF disease, prior to development of chronic infection, by improving the phagocytic character of PMNs may assist current antibiotic treatment regimens in reducing P. aeruginosa colonization in non-chronically infected patients.
Passive immunotherapy is a potent and promising adjuvant to standard therapy against infectious diseases (23). Egg yolk immunoglobulins (IgY) have been used successfully to eradicate infectious diseases in animals (24), and prophylaxis with egg yolk immunoglobulins (IgY) targeting P. aeruginosa reduces colonization in CF patients (25). The encouraging results from clinical studies on the efficacy of IgY immunotherapy to prevent gastrointestinal infections (26) and animal models showing a favorable impact of IgY therapy on influenza virus infection (27, 28) suggest the potential benefit of anti-P. aeruginosa IgY prophylaxis in non-chronically infected CF patients. Thus, pathogens entering the respiratory airways of CF patients may be readily killed by PMNs due to moderation of the phagocytic activity mediated by IgY immunotherapy. In the present study, we report that IgY opsonization induces increased hydrophobicity and aggregation of P. aeruginosa, both of which facilitate augmented phagocytic activity of PMNs and subsequent bacterial killing.
MATERIALS AND METHODS
Anti-Pseudomonas aeruginosa IgY.
Solutions containing specific anti-P. aeruginosa (S-IgY) or nonspecific (C-IgY) IgY antibodies were obtained from ImmunSystem I.M.S. (Uppsala, Sweden). The anti-P. aeruginosa IgY antibodies employed in these experiments were harvested from egg yolks of White leghorn chickens immunized with six different P. aeruginosa O-antigen strains (O1, O3, O5, O6, O9, and O11).
P. aeruginosa PAO1 vaccine strain was grown in LB broth. From a frozen stock, an overnight culture was prepared and recultured in LB to obtain an optical density at 600 nm (OD600) of 0.5. The log-phase bacteria were washed twice in phosphate-buffered saline (PBS) before application.
Neutrophil isolation.
Human PMNs were harvested from healthy donors and separated via density gradient centrifugation. Erythrocytes were allowed to sediment in 5% dextran, and the leukocyte-enriched plasma was layered on Lymphoprep (Axis-Shield, Norway) and centrifuged at 2,200 × g for 15 min at room temperature. The supernatants were discarded, and the remaining erythrocytes within the pelleted PMNs were removed by hypertonic lysis. The purified PMNs were resuspended in Krebs-Ringer buffer supplemented with 10 mM glucose (KRB).
Respiratory burst assay.
The production of ROS during the oxidative burst was measured by luminol-enhanced chemiluminescence. PAO1 was opsonized by S-IgY or C-IgY for 60 min at 37°C on a platform rocker to allow aggregation prior to application. For control experiments, PAO1 was opsonized by human AB positive serum and treated similarly to IgY-opsonized organisms. Isolated PMNs at a density of 5 × 106 cells/ml were mixed with preopsonized bacteria (5 × 107 CFU/ml) in a 96-well microtiter plate (Nunc). Luminol was finally added to the wells, and the luminol-enhanced chemiluminescence detecting phagocyte-derived ROS was recorded every second minute by using a luminometer (Wallac 1420 Victor2; PerkinElmer) at 37°C for 1 h.
Bactericidal assay.
The killing of preopsonized bacteria by PMNs was performed in polypropylene tubes with a final volume of 600 μl/tube according to the protocol described by Green and colleagues (29). Briefly, isolated PMNs (5 × 106 cells/ml) were mixed with PAO1 (5 × 107 CFU/ml), resulting in a bacterium-to-PMN ratio of 10:1. The bacteria were opsonized prior to application by using 10% S-IgY, C-IgY, or human AB positive serum for 60 min at 37°C on a platform rocker. At time points 0 and 30 min postmixing of PMNs and PAO1, 50 μl of the reaction mixture was removed and added to 2.45 ml H2O (pH 11), initiating cellular lysis for 5 min before vigorously vortexing and plating onto modified Conradi-Drigalski plates (Statens Serum Institut, Copenhagen, Denmark) and incubation overnight at 37°C. Colony counting the next day determined the PMN-mediated bacterial killing based on the loss of bacterial viability during 30 min of phagocytosis compared to that in S-IgY-opsonized controls.
Neutrophil receptor blockage.
To examine the role of Fc receptor-mediated phagocytosis, human Fc receptors (FcγRI, FcγRII, and FcγRIII) were blocked by FcγRI monoclonal antibodies (MAbs; anti-CD64), FcγRII MAbs (anti-CD16), and FcγRIII MAbs (anti-CD32) (R&D Systems, Minneapolis, MN, USA). Freshly isolated PMNs (5 × 106/ml) were pretreated with 0.5 μg of anti-CD16, anti-CD32, or anti-CD64 for 15 min at 37°C followed by the detection of ROS production in a luminol chemiluminescence assay as previously described, where PMNs were allowed to phagocytize PAO1 preopsonized with S-IgY, C-IgY, or IgG.
Bacterial hydrophobicity.
The partitioning of the aqueous and hydrocarbon phases via the microbial adhesion to hydrocarbons (MATH) test was utilized to determine hydrophobicity. The affinity of bacteria to hexane in a water-hexane two-phase system, as described by Rosenberg et al. (30), was applied. Washed PAO1 cells were preopsonized with S-IgY, C-IgY, or IgG and suspended in 3 ml PBS to an OD600 of 1.0. A volume of 0.5 ml n-hexane was carefully layered on top of the bacterial suspension in disposable glass tubes. The tube contents were vortexed for 60 s, and after phase separation occurred, the OD of the aqueous phase was measured. The relative bacterial hydrophobicity was expressed as the hydrophobicity index (HI), calculated as follows: HI = (initial absorbance – absorbance after phase separation)/(initial absorbance). Accordingly, hydrophobic bacteria have lower HIs than hydrophilic organisms.
Flow cytometry.
Fluorescent PAO1 cells (tagged with green fluorescent protein [GFP]) were mixed with either S-IgY or C-IgY and allowed to aggregate at room temperature for 60 min before estimation of size according to the forward light scatter (FSC-A), measured using a FACScanto apparatus (BD Biosciences). Bacteria were discriminated according to green fluorescence intensity collected in the FL-1 channel, and at least 10,000 events were recorded for each sample. Cytometer setup and tracking beads (BD Biosciences) were used for instrument calibration, and flow data were processed and analyzed by using Diva (BD Biosciences).
Indirect immunofluorescence microscopy.
PAO1 vaccine strain cells cultured in LB medium were washed twice in PBS and mixed with 10% S-IgY at 37°C for 1 h on a platform rocker. After washing twice with PBS, Texas Red-conjugated rabbit anti-chicken IgG secondary antibody (Abcam plc, Cambridge, United Kingdom), diluted 1:500 with PBS containing 1% bovine albumin serum (BSA), was added and the mixture was incubated at 37°C for 1 h on a platform rocker. After washing twice with PBS, aliquots were smeared on a microscope slide and air dried, followed by addition of a drop of 4′,6-diamidino-2-phenylindole (DAPI) stain and mounting on a coverslip. Immunofluorescent micrographs of specimens were obtained using a fluorescence microscope.
Bacterial internalization by PMNs.
The uptake of bacteria in PMNs was examined by confocal scanning laser microscopy (CSLM; Zeiss Imager.Z2, LSM 710; Zeiss, Germany) and the accompanying software (Zeiss Zen 2010 v. 6.0). Overnight cultures of fluorescent PAO1 cells (GFP) were adjusted to a density of 108 CFU/ml in PBS. Aliquots of PAO1 cells (50 μl) were opsonized with 10% S-IgY in a 96-well microtiter plate and kept at room temperature for 2 h to allow aggregation. Phagocytosis was initiated by addition of freshly isolated PMNs (107 cells/ml) in KRB to the bacterial suspension, and bacterial internalization by PMNs was visualized after incubation for 30 min at 37°C and 200 rpm.
RESULTS
The respiratory burst produced by PMNs phagocytizing P. aeruginosa (PAO1) is significantly increased by S-IgY.
ROS are produced by PMN phagocytosis of bacteria during the respiratory burst. Via a luminol-enhanced chemiluminescence assay, we quantified ROS production based on luminescence when activated by oxidants produced during the respiratory burst. Figure 1 shows the chemiluminescence detected during the phagocytosis of P. aeruginosa PAO1 by PMNs. The bacteria were preopsonized with serum and increasing concentrations of S-IgY or C-IgY, or with PBS (non-IgY) as control. S-IgY augments the chemiluminescence in a concentration-dependent manner, and the lowest concentration of S-IgY tested (0.1%) significantly increased the chemiluminescence compared to non-IgY (P < 0.003). Although the chemiluminescence was increased by C-IgY opsonization compared to that with non-IgY, the effects of the non-P. aeruginosa-specific C-IgY remained inferior to those of S-IgY at all concentrations tested. Blocking of Fcγ receptors did not alter the respiratory burst from PMNs phagocytizing P. aeruginosa (Fig. 2).
FIG 1.
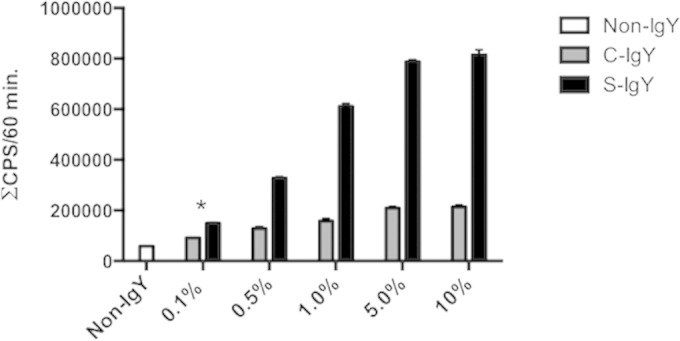
Respiratory burst assay results. The production of reactive oxygen was detected by luminol-enhanced chemiluminescence during phagocytosis of PAO1 cells by PMNs. The chemiluminescence, given in counts per second (CPS), was recorded using luminol and a microplate fluorescence reader (Wallac 1420 Victor2; PerkinElmer) during 60 min of phagocytosis. Each panel shows the cumulative CPS ± the standard error of the mean of experiments run in duplicate. *, lowest concentration of S-IgY to give a significant increase in respiratory burst, compared to the non-IgY control (P < 0.003).
FIG 2.
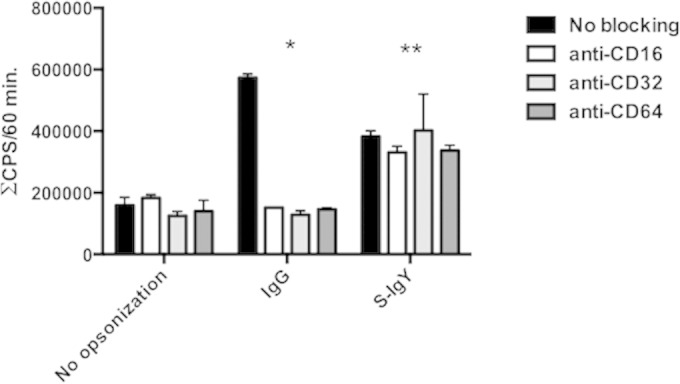
The consequence of Fc receptor blocking on the cumulative burst of PMNs phagocytizing S-IgY-, C-IgY-, or IgG-opsonized PAO1 cells. Fc receptors of PMNs were blocked with FcγRI MAb (anti-CD64), FcγRII MAb (anti-CD16), and FcγRIII MAb (anti-CD32) prior to phagocytosis. The chemiluminescence, given in counts per second (CPS), was determined by using luminol in a microplate fluorescence reader (Wallac 1420 Victor2; PerkinElmer) during 60 min of phagocytosis. Each panel shows the cumulative CPS ± the standard error of the mean of experiments run in duplicate from three different donors. *, blocking Fc receptors (CD16, CD32, and CD64) significantly reduced the cumulative chemiluminescence detected when PMNs phagocytized IgG-opsonized bacteria (P < 0.001). **, no significant difference in chemiluminescence was detected when PMNs phagocytized S-IgY-opsonized PAO1 cells.
The PMN-facilitated bacterial killing was enhanced by IgY.
The loss of bacterial viability during PMN phagocytosis was estimated by mixing non-IgY-opsonized bacteria and PMNs and plating diluted samples overnight, followed by colony counting. Figure 3 shows the reduction in bacterial viability after 30 min of phagocytosis of P. aeruginosa PAO1 with respect to the initial S-IgY-opsonized inoculum. The phagocytic killing of S-IgY-opsonized bacteria was augmented compared to that of C-IgY (P < 0.03) or non-IgY-opsonized bacteria. Thus, the proportion of viable S-IgY-opsonized PAO1 cells was reduced by 87% compared to non-IgY bacteria and by 79% in comparison to C-IgY-opsonized bacteria. As observed in Fig. 1, the opsonization of PAO1 with C-IgY seemed to have an effect on the phagocytic activity of PMNs, and the bacterial viability of C-IgY-opsonized PAO1 was subsequently reduced by 39% compared to the non-IgY control (P < 0.05). Thus, it seems that a nonspecific effect of IgY exists.
FIG 3.
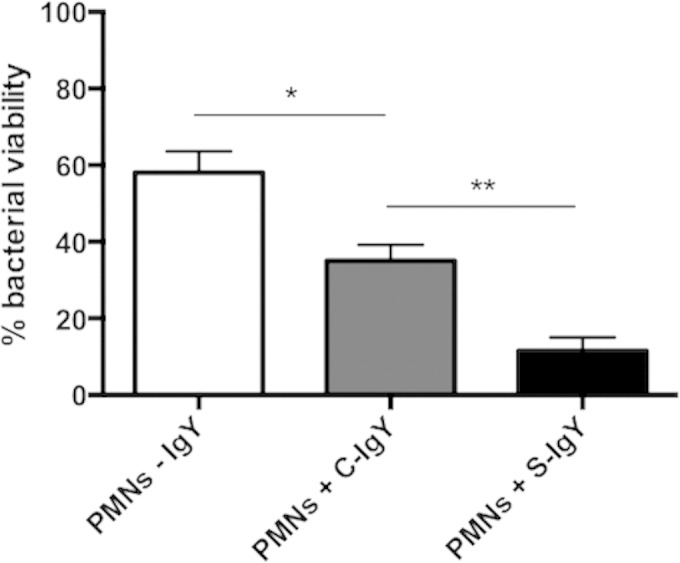
PMN-mediated bacterial killing, defined as the proportion of viable bacteria after 30 min of phagocytosis compared to S-IgY-opsonized controls. Isolated PMNs were challenged with S-IgY- or C-IgY-opsonized or non-IgY (-IgY) PAO1 cells, and the number of viable bacteria was determined by colony counting the next day. Each bar shows the percent survival ± the standard error of the mean of experiments run in duplicates. *, P < 0.05; **, P < 0.03.
Addition of S-IgY facilitates the formation of bacterial aggregates.
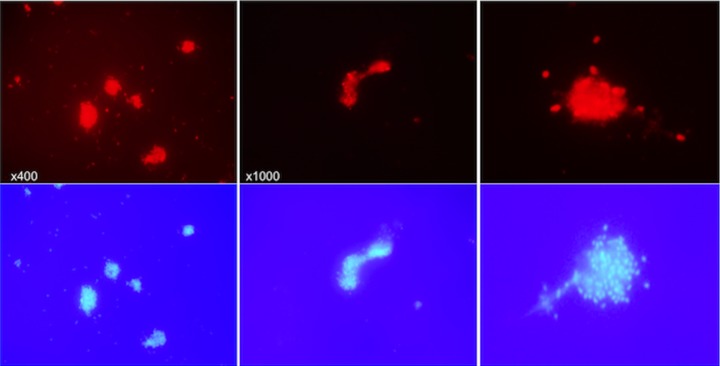
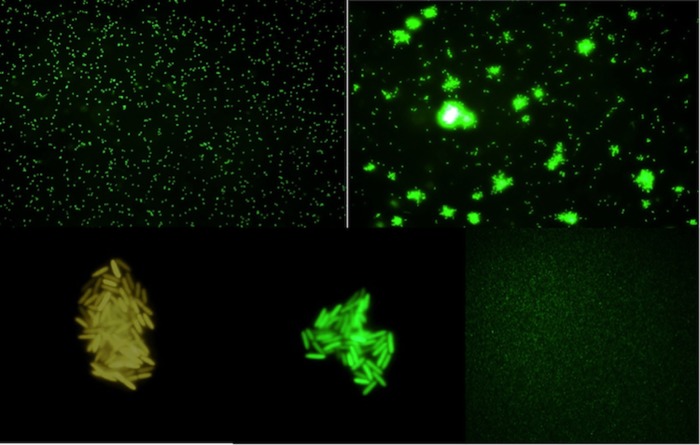
The visualization of S-IgY-opsonized bacteria by indirect immunofluorescence microscopy showed the formation of bacterial aggregates (Fig. 4). Prior to addition of the Texas Red fluorescent anti-chicken secondary antibody, PAO1 cells suspended in PBS were opsonized by S-IgY for 1 h. No aggregates are formed when the bacteria were fixed on a microscope slide prior to the addition of S-IgY and secondary antibody, because the fixation restricted bacterial movement and clumping. In order to exclude any implication of the secondary antibody on the formation of aggregates, a GFP-tagged PAO1 strain was added S-IgY (Fig. 5). The images illustrate that dispersed bacteria seem to accumulate in nodular aggregates after the addition of S-IgY, suggesting that the opsonization of PAO1 causes the bacteria to clump to each other.
FIG 4.
Different images from indirect immunofluorescence microscopy of PAO1 opsonized with S-IgY. PAO1 was opsonized with 10% S-IgY and allowed to aggregate for 60 min at 37°C prior to addition of Texas Red-conjugated rabbit anti-chicken IgG secondary antibody. The top images show the Texas Red-fluorescent anti-chicken IgG-detecting S-IgY antibodies. The bottom images display the corresponding DAPI-stained images.
FIG 5.
The time-dependent development of S-IgY-mediated aggregates of PAO1 (GFP). (Upper left) Dispersed bacteria prior to addition of S-IgY. (Upper right) Formation of bacterial aggregates 30 min after S-IgY was added. (Bottom) Close-ups of bacterial aggregates 2 h after S-IgY addition. The bottom right image shows the non-IgY control after 2 h.
In order to assess the difference in sizes of bacterial aggregates, we employed flow cytometry, which provides the opportunity to determine the size distribution of constituents in a bacterial solution by recording the FSC-A of PAO1 cells selected according to the fluorescent intensity from GFP tagging. Thus, bacteria were opsonized by S-IgY or C-IgY and allowed to aggregate for 60 min before the fluorescent intensity or forward scatter (FSC-A) equivalent versus mean size was determined (Fig. 6). As illustrated in Fig. 6, the mean size of S-IgY-opsonized bacteria was greater than that for C-IgY-opsonized (P < 0.05) or non-IgY-opsonized bacteria due to the formation of aggregates. Additionally, the estimated size of C-IgY-opsonized bacteria was significantly greater than that of non-IgY controls (P < 0.01).
FIG 6.
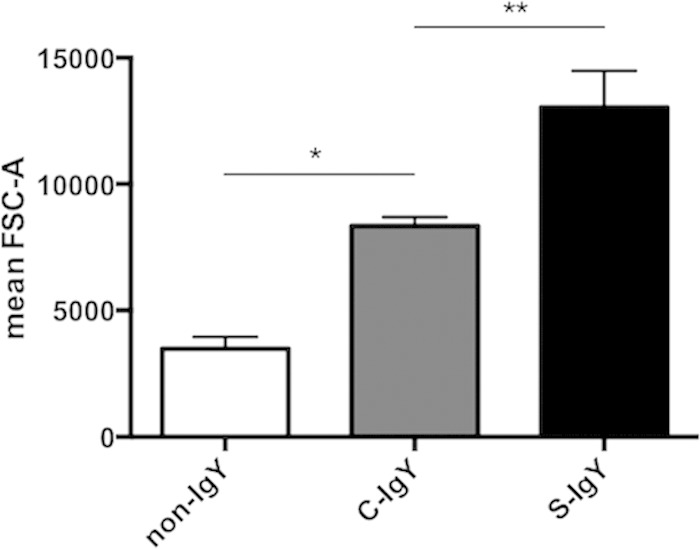
The distribution of mean particle size, measured by fluorescence-activated cell sorting. PAO1 was opsonized with S-IgY or C-IgY and allowed to aggregate for 60 min prior to determining the particle size distribution (based on FSC-A). *, P < 0.01; **, P < 0.05.
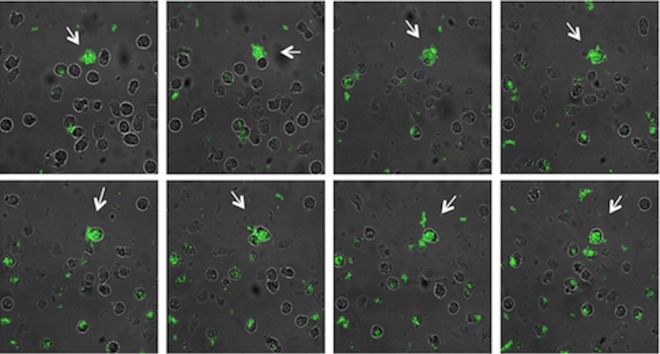
The internalization of bacterial aggregates in PMNs was examined by CLSM, enabling real-time visualization of phagocytosis (Fig. 7). The consecutive images show the clumping of S-IgY-opsonized GFP-tagged PAO1 cells and their internalization in PMNs. The ingestion of PAO1 cells was not hampered by bacterial accumulation; thus, the phagocytic competence remained sufficient in spite of sizeable aggregates (see Movie S1 in the supplemental material).
FIG 7.
Still images from time-lapse microscopy of PMNs phagocytizing PAO1 cells during a time course of 20 min. S-IgY-opsonized PAO1 cells (GFP) were allowed to aggregate for 1 h at 37°C prior to addition of PMNs. Arrows display bacterial aggregates becoming internalized by PMNs.
The aggregation of PAO1 cells mediated by S-IgY increases the bacterial hydrophobicity.
The impact of IgY opsonization on bacterial hydrophobicity was examined with the MATH test (Fig. 8). Demonstrated by differences in the HI, the affinity of PAO1 cells to n-hexane varied according to opsonization. Nonopsonized bacteria had low affinity to the hydrocarbon n-hexane; however, alteration of the bacterial surface may moderate the hydrophilic character of the bacteria, and immunoglobulin opsonization increases the hydrophobicity of PAO1. The mean HI of nonopsonized PAO1 cells was 2.33, which was increased to 17.26 by IgG opsonization (P < 0.004). The HI score was further increased to 31.17 by C-IgY opsonization and was most prominent with S-IgY, increasing the mean HI score to 46.35. The bacterial affinity to n-hexane, and hence the hydrophobic character of PAO1, was augmented nearly 20-fold when bacteria were opsonized by S-IgY.
FIG 8.
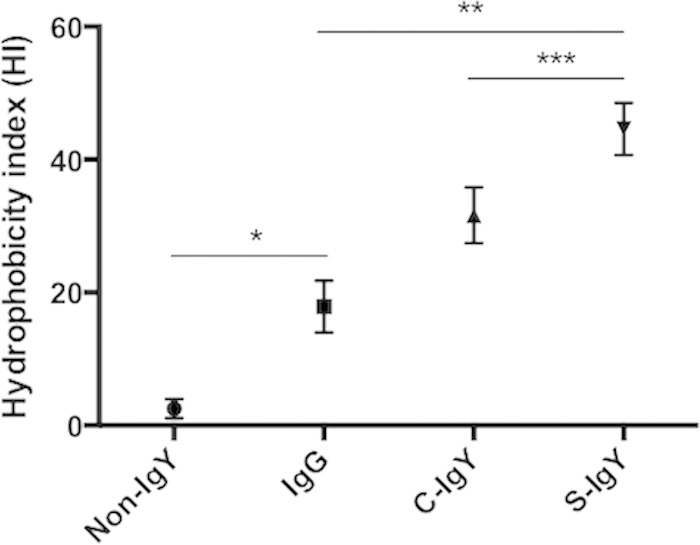
The MATH test was applied to evaluate bacterial hydrophobicity. After thoroughly mixing PAO1 and the hydrocarbon n-hexane, a two-phase separation was established and the difference in the OD pre- and postmixing is expressed as the hydrophobicity index. Thus, a reduction of the OD in the aqueous phase after mixing, e.g., bacteria were localized in the n-hexane phase, increased the HI. PAO1 prior to mixing with n-hexane was opsonized by S-IgY, C-IgY, or IgG. Results are depicted as the HI ± the standard error of the mean from experiments run in duplicate. *, P < 0.004; **, P < 0.002; ***, P < 0.02.
DISCUSSION
The initial colonization with nonmucoid P. aeruginosa in CF patients can possibly be eradicated by early aggressive antipseudomonal antibiotic therapy (31, 32). Antibiotic eradication therapy (AET) is dogmatic in the current management of CF infections and has delayed the onset of chronic infection (33). Antibiotic maintenance therapy is employed to control chronic infections in CF patients and amends lung function, reduces pulmonary exacerbations, and improves the quality of life of CF patients (34). Although the current antibiotic regimens have reduced morbidity and mortality of CF patients, novel therapeutic approaches are needed. Interventions targeting the initial colonization of P. aeruginosa are favorable, as reducing early bacterial infections hampers progression into the vicious chronic course of infection. Oral prophylaxis with anti-P. aeruginosa IgY may represent a potent adjuvant to AET in reducing initial colonization with P. aeruginosa in CF patients (26). The mode of action may be a prompt bacterial clearance by PMNs as IgY opsonization of P. aeruginosa augments the PMN-mediated respiratory burst and subsequent bacterial killing in current in vitro studies. Thus, viable bacteria are reduced by 87% when anti-P. aeruginosa IgY (S-IgY) is allowed to opsonize the bacteria prior to phagocytosis (Fig. 3). PMNs identify either endogenous components of the bacterial surface or serum elements (opsonins) attached to it. The opsonins bind to and tag the bacterial pathogens for enhanced neutralization by PMNs. The most important opsonic receptors are the receptors for the Fc portions of immunoglobulins (FcγRI, FcγRII, and FcγRIII) (35) and the receptors for complement components (complement receptor 3 [CR3]) (36). Nonopsonic receptors, such as scavenger receptors, play a role in pathogen recognition and phagocytosis (37), and phagocytosis of fungi is facilitated by the nonopsonic receptor dectin-1 (38). Blocking Fcγ receptors does not alter the respiratory burst from PMNs that phagocytize P. aeruginosa (Fig. 2), and IgY is reported not to activate the complement system. Thus, phagocytosis of IgY-opsonized bacteria is probably not mediated through conventional receptor-mediated phagocytosis. Altering the physical state of bacteria can influence the phagocytic efficiency. Parameters such as size and shape affect the amount of engaged opsonin receptors, the level opsonin elements on surfaces, and the capacity of pseudopods to surround the bacteria; thus, target geometry is an important parameter in phagocytosis (39). By employing microscopy, we observed that addition of IgY antibodies to a bacterial suspension mediated the formation of bacterial aggregates (Fig. 4). The indirect immunofluorescence images showed the immobilization and clumping of bacteria that formed within minutes of opsonization. Prior to opsonization with IgY antibodies, GFP-tagged P. aeruginosa (PAO1) cells were dispersed in a single-cell fashion (Fig. 5). However, addition of anti-P. aeruginosa IgY (S-IgY) readily resulted in bacterial aggregates consisting of immobilized organisms attached to each other. The enlarged target geometry induced by IgY-mediated bacterial aggregation may augment the phagocytic efficiency, as the correlation between bacterial aggregation and enhanced phagocytosis has been observed previously. Increasing the chain length of Streptococcus pneumoniae by the agglutinating effect of F(ab′)2 antibody enhances bacterial clearance by PMNs, probably due to larger amounts of opsonin deposits (40). Extracellular vesicles are reported to interact with bacterial surfaces, thereby promoting bacterial aggregation and enhanced phagocytosis of Bifidobacterium breve (41). Although bacterial killing is not observed, the induction of Enterococcus faecalis (42) and Escherichia coli (43) aggregates is associated with increased bacterial uptake in PMNs. The present observation that an augmented phagocytic capacity of PMNs is facilitated by bacterial aggregation may be plausible, and by employing CLSM we were able to visualize the uptake of IgY-mediated bacterial aggregates in PMNs (Fig. 7).
In addition, IgY opsonization may induce further physiochemical alterations of bacteria, affecting phagocytosis due to the structure of avian immunoglobulin. Human immunoglobulins consist of two identical light chains and two identical heavy chains overall containing 1,320 to 1,540 amino acid residues (44). Similar to its mammalian counterpart IgG, avian IgY is comprised of two identical light chains and two identical heavy chains (45). Likewise, IgY is structurally polarized in the fragment antigen-binding (Fab) region, containing antigen-binding sites, and the fragment crystallizable (Fc) region responsible for opsonization. Although structurally similar, IgY is a larger immunoglobulin than its mammalian counterpart due to elongated heavy chains. The subsequent larger IgY Fc region comprised by additional amino acid residues renders the IgY more hydrophobic than equivalent mammalian IgG. Indeed, IgY-coated latex microspheres are more hydrophobic than corresponding IgG-coated microspheres (46), and based on comparisons of the molecular stabilities of different immunoglobulins by using a fluorescence technique, IgY was observed to be more hydrophobic than mammalian IgG (47). When immunoglobulins interact with recognized antigens, the hydrophobic Fc portion is orientated opposite the antigen and is exposed to an Fc-receptor interaction; thus, IgY opsonization may confer increased surface hydrophobicity. Bacterial hydrophobicity is an important virulence factor of various pathogens, because it facilitates adherence to epithelial cells and can be induced by agents that increase hydrophobicity. Adhesion of Streptococcus pyogenes to pharyngeal epithelial cells is determined by strain hydrophobicity, and subinhibitory concentrations of rifampin lower the hydrophobicity and reduce cell adhesion (48). The postantibiotic effect of penicillin decreases the adherence of Lancefield group B streptococci to human buccal cells by reducing the bacterium's hydrophobicity (49), and diminishing the hydrophobicity of uropathogenic E. coli by subinhibitory concentrations of amikacin and ciprofloxacin reduces the adherence to uroepithelial cells (50). Additionally, surface hydrophobicity plays a role in bacterial adhesion to phagocytic cells (51). Decades ago it was recognized that opsonization affecting bacterial hydrophobicity alters the phagocytic capacity, and thus the extent of complement and IgG opsonization of Salmonella enterica serovar Typhimurium modifies surface hydrophobicity and bacterial phagocytosis (52). Strain-specific variations in surface hydrophobicity may influence phagocytosis. Hydrophobic strains of Streptococcus dysgalactiae are readily phagocytosed by macrophages compared to hydrophilic counterparts (53), and Staphylococcus aureus fast-growing phenotypes exhibit increased surface hydrophobicity and phagocytic killing compared to slow-growing hydrophilic phenotypes (54). Various antibiotics can alter phagocytic killing by modifying the bacterial surface and the subsequent hydrophobicity. Prior exposure of a subinhibitory aztreonam concentration increased the hydrophobicity and phagocytic killing of P. aeruginosa, while brief exposures to suprainhibitory concentrations had similar effects on other Gram negative bacteria (E. coli, Serratia marcescens, Klebsiella pneumoniae and Salmonella Typhimurium) (55). Subinhibitory cefodizime treatment of K. pneumoniae cells rendered the cells more hydrophobic and more susceptible to phagocytic killing than seen with untreated bacteria (56). Interestingly, bacterial hydrophobicity seems to counteract a lack of opsonization and ensures competent phagocytosis without the presence of complement or immunoglobulins. Thus, nonopsonized hydrophobic strains of Bacteroides buccae, Porphyromonas gingivalis, and Fusobacterium nucleatum were readily phagocytosed by PMNs, whereas hydrophilic strains needed human serum opsonization to ensure equivalent phagocytic killing (57). In the absence of opsonins, only cells of a hydrophobic strain of Staphylococcus saprophyticus were ingested and killed by PMNs, while human serum or IgG was required for efficient phagocytosis of hydrophilic strains (58). By employing the MATH test, we demonstrated that IgY increases the hydrophobicity of P. aeruginosa (Fig. 8). The extended hydrophobic Fc fragment of IgY renders the immunoglobulin more hydrophobic than mammalian IgG; accordingly, opsonization with IgY antibodies modifies the bacterial surface by enhancing hydrophobicity.
IgY induces alterations of bacteria that augment the respiratory burst and subsequent bacterial killing by PMNs. The IgY-induced formation of immobilized bacteria in aggregates and the improved adherence to phagocytic cells by increased surface hydrophobicity are possible modifications to facilitate a more rapid and effortless phagocytic clearance. The impact of IgY on phagocytosis is not enabled by conventional receptor-mediated phagocytosis, but more likely attributable to non-receptor-mediated mechanisms via increased hydrophobicity and aggregation of the bacteria.
The efficacy of IgY immunotherapy to manage infection has been evaluated in clinical studies and animal models with encouraging results. Various pathogens can be targeted, and the low-cost, simple production method renders IgY a promising therapeutic agent to control infectious diseases. Oral prophylaxis (gargling) with anti-P. aeruginosa IgY antibodies is beneficial in CF (25). The presence of anti-P. aeruginosa IgY in the respiratory tract may facilitate rapid and effortless bacterial clearance due to the IgY-induced effects on phagocytosis. Interestingly, the nonopsonic phagocytosis of nonmucoid P. aeruginosa clinical strains correlates with bacterial hydrophobicity (59), and the essential IgG receptor Fcγ RIII (CD16) is observed to be downregulated in PMNs isolated from CF sputum (60), implying that non-receptor-mediated phagocytosis may be important in the CF setting. Thus, the observed IgY-induced hydrophobicity and aggregation of bacteria augment the phagocytic capability of PMNs and may facilitate a rapid and competent host response in the airways of CF patients. The observation that IgY antibodies have nonspecific impacts, as demonstrated by the effects of C-IgY on the respiratory burst and bacterial killing, may be rationalized by the polyclonal nature of IgY encompassing cross-reactivity. Thus, oral prophylaxis with IgY antibodies may assist in controlling colonization with non-P. aeruginosa pathogens.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by the European Union FP7 support for the IMPACTT Project (grant agreement number 261095).
We report no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02970-14.
REFERENCES
- 1.Serbina NV, Pamer EG. 2008. Coordinating innate immune cells to optimize microbial killing. Immunity 29:672–674. doi: 10.1016/j.immuni.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akira S, Uematsu S, Takeuchi O. 2006. Pathogen recognition and innate immunity. Cell 124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Diacovich L, Gorvel JP. 2010. Bacterial manipulation of innate immunity to promote infection. Nat Rev Microbiol 8:117–128. doi: 10.1038/nrmicro2295. [DOI] [PubMed] [Google Scholar]
- 4.Drummond RA, Brown GD. 2013. Signalling C-type lectins in antimicrobial immunity. PLoS Pathog 9:e1003417. doi: 10.1371/journal.ppat.1003417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cohen J. 2002. The immunopathogenesis of sepsis. Nature 420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 6.Mizgerd JP. 2002. Molecular mechanisms of neutrophil recruitment elicited by bacteria in the lungs. Semin Immunol 14:123–132. doi: 10.1006/smim.2001.0349. [DOI] [PubMed] [Google Scholar]
- 7.Joiner KA, Brown EJ, Frank MM. 1984. Complement and bacteria: chemistry and biology in host defense. Annu Rev Immunol 2:461–491. [DOI] [PubMed] [Google Scholar]
- 8.Nauseef WM. 2007. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev 219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x. [DOI] [PubMed] [Google Scholar]
- 9.Serhan CN, Savill J. 2005. Resolution of inflammation: the beginning programs the end. Nat Immunol 6:1191–1197. doi: 10.1038/ni1276. [DOI] [PubMed] [Google Scholar]
- 10.Hoiby N, Giwercman B, Jensen T, Johansen H. K., Kronborg G, Pressler T, Kharazmi A. 1993. Immune response in cystic fibrosis: helpful or harmful?, p 133–141. In Scobar H, Baquero F, Suarez L (ed.), Clinical ecology of cystic fibrosis. Excerpta Medica, Amsterdam, Netherlands. [Google Scholar]
- 11.Kharazmi A, Schiøtz PO, Høiby N, Baek L, Döring G. 1986. Demonstration of neutrophil chemotactic activity in the sputum of cystic fibrosis patients with Pseudomonas aeruginosa infection. Eur J Clin Invest 16:143–148. [DOI] [PubMed] [Google Scholar]
- 12.Tabary O, Corvol H, Boncoeur E, Chadelat K, Fitting C, Cavaillon JM, Clément A, Jacquot J. 2006. Adherence of airway neutrophils and inflammatory response are increased in CF airway epithelial cell-neutrophil interactions. Am J Physiol Lung Cell Mol Physiol 290:L588–L596. doi: 10.1152/ajplung.00013.2005. [DOI] [PubMed] [Google Scholar]
- 13.Döring G, Goldstein W, Botzenhart K, Kharazmi A, Schiøtz PO, Høiby N, Dasgupta M. 1986. Elastase from polymorphonuclear leucocytes: a regulatory enzyme in immune complex disease. Clin Exp Immunol 64:597–605. [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein W, Döring G. 1986. Lysosomal enzymes from polymorphonuclear leukocytes and proteinase inhibitors in patients with cystic fibrosis. Am Rev Respir Dis 134:49–56. [DOI] [PubMed] [Google Scholar]
- 15.Hull J, Vervaart P, Grimwood K, Phelan P. 1997. Pulmonary oxidative stress response in young children with cystic fibrosis. Thorax 52:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoiby N. 1982. Microbiology of lung infections in cystic fibrosis patients. Acta Paediatr Scand Suppl 301:33–54. [PubMed] [Google Scholar]
- 17.Schaedel C, de Monestrol I, Hjelte L, Johannesson M, Kornfält R, Lindblad A, Strandvik B, Wahlgren L, Holmberg L. 2002. Predictors of deterioration of lung function in cystic fibrosis. Pediatr Pulmonol 33:483–491. doi: 10.1002/ppul.10100. [DOI] [PubMed] [Google Scholar]
- 18.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. 2002. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol 34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 19.Jensen ET, Kharazmi A, Lam K, Costerton JW, Høiby N. 1990. Human polymorphonuclear leukocyte response to Pseudomonas aeruginosa grown in biofilms. Infect Immun 58:2383–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pedersen SS, Høiby N, Espersen F, Koch C. 1992. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax 47:6–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjarnsholt T, Jensen PØ, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Høiby N. 2009. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol 44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 22.Pier GB, Coleman F, Grout M, Franklin M, Ohman DE. 2001. Role of alginate O acetylation in resistance of mucoid Pseudomonas aeruginosa to opsonic phagocytosis. Infect Immun 69:1895–1901. doi: 10.1128/IAI.69.3.1895-1901.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Keller MA, Stiehm ER. 2000. Passive immunity in prevention and treatment of infectious diseases. Clin Microbiol Rev 13:602–614. doi: 10.1128/CMR.13.4.602-614.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu Y, Li X, Jin L, Zhen Y, Lu Y, Li S, You J, Wang L. 2011. Application of chicken egg yolk immunoglobulins in the control of terrestrial and aquatic animal diseases: a review. Biotechnol Adv 29:860–868. doi: 10.1016/j.biotechadv.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson E, Larsson A, Olesen HV, Wejåker PE, Kollberg H. 2008. Good effect of IgY against Pseudomonas aeruginosa infections in cystic fibrosis patients. Pediatr Pulmonol 43:892–899. doi: 10.1002/ppul.20875. [DOI] [PubMed] [Google Scholar]
- 26.Rahman S, Higo-Moriguchi K, Htun KW, Taniguchi K, Icatlo FC Jr, Tsuji T, Kodama Y, Van Nguyen S, Umeda K, Oo HN, Myint YY, Htut T, Myint SS, Thura K, Thu HM, Fatmawati NN, Oguma K. 2012. Randomized placebo-controlled clinical trial of immunoglobulin Y as adjunct to standard supportive therapy for rotavirus-associated diarrhea among pediatric patients. Vaccine 30:4661–4669. doi: 10.1016/j.vaccine.2012.04.091. [DOI] [PubMed] [Google Scholar]
- 27.Wallach MG, Webby RJ, Islam F, Walkden-Brown S, Emmoth E, Feinstein R, Gronvik KO. 2011. Cross-protection of chicken immunoglobulin Y antibodies against H5N1 and H1N1 viruses passively administered in mice. Clin Vaccine Immunol 18:1083–1090. doi: 10.1128/CVI.05075-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen HH, Tumpey TM, Park HJ, Byun YH, Tran LD, Nguyen VD, Kilgore PE, Czerkinsky C, Katz JM, Seong BL, Song JM, Kim YB, Do HT, Nguyen T, Nguyen CV. 2010. Prophylactic and therapeutic efficacy of avian antibodies against influenza virus H5N1 and H1N1 in mice. PLoS One 5:e10152. doi: 10.1371/journal.pone.0010152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Green JN, Winterbourn CC, Hampton MB. 2007. Analysis of neutrophil bactericidal activity. Methods Mol Biol 412:319–332. doi: 10.1007/978-1-59745-467-4_21. [DOI] [PubMed] [Google Scholar]
- 30.Rosenberg M. 2006. Microbial adhesion to hydrocarbons: twenty-five years of doing MATH. FEMS Microbiol Lett 262:129–134. doi: 10.1111/j.1574-6968.2006.00291.x. [DOI] [PubMed] [Google Scholar]
- 31.Frederiksen B, Koch C, Høiby N. 1997. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr Pulmonol 23:330–335. [DOI] [PubMed] [Google Scholar]
- 32.Taccetti G, Campana S, Festini F, Mascherini M, Döring G. 2005. Early eradication therapy against Pseudomonas aeruginosa in cystic fibrosis patients. Eur Respir J 26:458–461. doi: 10.1183/09031936.05.00009605. [DOI] [PubMed] [Google Scholar]
- 33.Döring G, Flume P, Heijerman H, Elborn JS, Consensus Study Group . 2012. Treatment of lung infection in patients with cystic fibrosis: current and future strategies. J Cyst Fibros 11:461–479. doi: 10.1016/j.jcf.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Høiby N, Koch C. 2000. Maintenance treatment of chronic Pseudomonas aeruginosa infection in cystic fibrosis. Thorax 55:349–350. doi: 10.1136/thorax.55.5.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nimmerjahn F, Ravetch JV. 2008. Fcγ receptors as regulators of immune responses. Nat Rev Immunol 8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 36.Berger M, Gaither IA, Frank MM. 1981. Complement receptors. Clin Immunol Rev 1:471–545. [PubMed] [Google Scholar]
- 37.Areschoug T, Gordon S. 2009. Scavenger receptors: role in innate immunity and microbial pathogenesis. Cell Microbiol 11:1160–1169. doi: 10.1111/j.1462-5822.2009.01326.x. [DOI] [PubMed] [Google Scholar]
- 38.Drummond RA, Brown GD. 2011. The role of Dectin-1 in the host defence against fungal infections. Curr Opin Microbiol 14:392–399. doi: 10.1016/j.mib.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 39.Champion JA, Mitragotri S. 2006. Role of target geometry in phagocytosis. Proc Natl Acad Sci U S A 103:4930–4934. doi: 10.1073/pnas.0600997103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dalia AB, Weiser JN. 2011. Minimization of bacterial size allows for complement evasion and is overcome by the agglutinating effect of antibody. Cell Host Microbe 10:486–496. doi: 10.1016/j.chom.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Bergenhenegouwen J, Kraneveld AD, Rutten L, Kettelarij N, Garssen J, Vos AP. 2104. Extracellular vesicles modulate host-microbe responses by altering TLR2 activity and phagocytosis. PLoS One 9:e89121. doi: 10.1371/journal.pone.0089121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rakita RM, Vanek NN, Jacques-Palaz K, Mee M, Mariscalco MM, Dunny GM, Snuggs M, Van Winkle WB, Simon SI. 1999. Enterococcus faecalis bearing aggregation substance is resistant to killing by human neutrophils despite phagocytosis and neutrophil activation. Infect Immun 67:6067–6075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fexby S, Bjarnsholt T, Jensen PØ, Roos V, Høiby N, Givskov M, Klemm P. 2007. Biological Trojan horse: antigen 43 provides specific bacterial uptake and survival in human neutrophils. Infect Immun 75:30–34. doi: 10.1128/IAI.01117-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Edelman GM. 1991. Antibody structure and molecular immunology. Scand J Immunol 34:1–22. [DOI] [PubMed] [Google Scholar]
- 45.Warr GW, Magor KE, Higgins DA. 1995. IgY: clues to the origins of modern antibodies. Immunol Today 16:392–398. [DOI] [PubMed] [Google Scholar]
- 46.Dávalos-Pantoja L, Ortega-Vinuesa JL, Bastos-González D, Hidalgo-Álvarez R. 2001. Colloidal stability of IgG- and IgY-coated latex microspheres. Colloids Surf B Biointerfaces 20:165–175. doi: 10.1016/S0927-7765(00)00189-2. [DOI] [PubMed] [Google Scholar]
- 47.Shimizu M, Nagashima H, Sano K, Hashimoto K, Ozeki M, Tsuda K, Hatta H. 1992. Molecular stability of chicken and rabbit immunoglobulin G. Biosci Biotechnol Biochem 56:270–274. [DOI] [PubMed] [Google Scholar]
- 48.Tylewska S, Hjertén S, Wadström T. 1981. Effect of subinhibitory concentrations of antibiotics on the adhesion of Streptococcus pyogenes to pharyngeal epithelial cells. Antimicrob Agents Chemother 20:563–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Araújo AM, Oliveira IC, Mattos MC, Benchetrit LC. 2008. Cell surface hydrophobicity and adherence of a strain of group B streptococci during the post-antibiotic effect of penicillin. Rev Inst Med Trop Sao Paulo 50:203–207. doi: 10.1590/S0036-46652008000400003. [DOI] [PubMed] [Google Scholar]
- 50.Wojnicz D, Jankowski S. 2007. Effects of subinhibitory concentrations of amikacin and ciprofloxacin on the hydrophobicity and adherence to epithelial cells of uropathogenic Escherichia coli strains. Int J Antimicrob Agents 29:700–704. doi: 10.1016/j.ijantimicag.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 51.van Oss CJ. 1978. Phagocytosis as a surface phenomenon. Annu Rev Microbiol 32:19–39. [DOI] [PubMed] [Google Scholar]
- 52.Stendahl O, Tagesson C, Magnusson KE, Edebo L. 1977. Physiochemical consequences of opsonization of Salmonella typhimurium with hyperimmune IgG and complement. Immunology 32:11–18. [PMC free article] [PubMed] [Google Scholar]
- 53.Calvinho LF, Almeida RA, Oliver SP. 1996. Influence of Streptococcus dysgalactiae surface hydrophobicity on adherence to mammary epithelial cells and phagocytosis by mammary macrophages. Zentralbl Veterinarmed B 43:257–266. [DOI] [PubMed] [Google Scholar]
- 54.Domingue G, Costerton JW, Brown MR. 1996. Bacterial doubling time modulates the effects of opsonisation and available iron upon interactions between Staphylococcus aureus and human neutrophils. FEMS Immunol Med Microbiol 16:223–228. [DOI] [PubMed] [Google Scholar]
- 55.Pruul H, Lewis G, McDonald PJ. 1988. Enhanced susceptibility of gram-negative bacteria to phagocytic killing by human polymorphonuclear leucocytes after brief exposure to aztreonam. J Antimicrob Chemother 22:675–686. [DOI] [PubMed] [Google Scholar]
- 56.Nomura S, Nagayama A. 1995. Mechanism of enhancement of bactericidal activity of phagocytes against Klebsiella pneumoniae treated with subminimal inhibitory concentrations of cefodizime. Chemotherapy 41:267–275. [DOI] [PubMed] [Google Scholar]
- 57.Kerosuo E, Haapasalo M, Alli K, Lounatmaa K. 1990. Ingestion of Bacteroides buccae, Bacteroides oris, Porphyromonas gingivalis, and Fusobacterium nucleatum by human polymorphonuclear leukocytes in vitro. Oral Microbiol Immunol 5:202–207. [DOI] [PubMed] [Google Scholar]
- 58.Maródi L, Burján P, Rozgonyi F. 1990. Opsonic requirements and surface hydrophobicity of novobiocin-resistant, coagulase-negative staphylococci. J Med Microbiol 32:19–24. [DOI] [PubMed] [Google Scholar]
- 59.Speert DP, Loh BA, Cabral DA, Salit IE. 1986. Nonopsonic phagocytosis of nonmucoid Pseudomonas aeruginosa by human neutrophils and monocyte-derived macrophages is correlated with bacterial piliation and hydrophobicity. Infect Immun 53:207–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Voglis S, Quinn K, Tullis E, Liu M, Henriques M, Zubrinich C, Peñuelas O, Chan H, Silverman F, Cherepanov V, Orzech N, Khine AA, Cantin A, Slutsky AS, Downey GP, Zhang H. 2009. Human neutrophil peptides and phagocytic deficiency in bronchiectatic lungs. Am J Respir Crit Care Med 180:159–166. doi: 10.1164/rccm.200808-1250OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.