Abstract
Although gamma interferon (IFN-γ) and interleukin-10 (IL-10) have been shown to be critically involved in the pathogenesis of African trypanosomiasis, the contributions to this disease of CD4+ and CD8+ T cells, the major potential producers of the two cytokines, are incompletely understood. Here we show that, in contrast to previous findings, IFN-γ was produced by CD4+, but not CD8+, T cells in mice infected with Trypanosoma brucei. Without any impairment in the secretion of IFN-γ, infected CD8−/− mice survived significantly longer than infected wild-type mice, suggesting that CD8+ T cells mediated mortality in an IFN-γ-independent manner. The increased survival of infected CD8−/− mice was significantly reduced in the absence of IL-10 signaling. Interestingly, IL-10 was also secreted mainly by CD4+ T cells. Strikingly, depletion of CD4+ T cells abrogated the prolonged survival of infected CD8−/− mice, demonstrating that CD4+ T cells mediated protection. Infected wild-type mice and CD8−/− mice depleted of CD4+ T cells had equal survival times, suggesting that the protection mediated by CD4+ T cells was counteracted by the detrimental effects of CD8+ T cells in infected wild-type mice. Interestingly, CD4+ T cells also mediated the mortality of infected mice in the absence of IL-10 signaling, probably via excessive secretion of IFN-γ. Finally, CD4+, but not CD8+, T cells were critically involved in the synthesis of IgG antibodies during T. brucei infections. Collectively, these results highlight distinct roles of CD4+ and CD8+ T cells in the context of IFN-γ and IL-10 during T. brucei infections.
INTRODUCTION
Trypanosoma brucei species are protozoan parasites that cause severe disease and death to humans and animals in Africa (1–4). The parasites have developed highly sophisticated mechanisms to escape host immune responses, including antigenic variation of the variant surface glycoprotein (VSG) (3, 5), immunosuppression (4, 6, 7), and splenic B cell depletion (8, 9). For practical and ethical reasons, mouse models have become an alternative and have proven to be a cornerstone for studying African trypanosomiasis of humans and domestic animals (2). BALB/c mice are highly susceptible to T. brucei and Trypanosoma congolense infections, whereas C57BL/6 mice are relatively resistant, as measured by levels of parasitemia, immunosuppression, and survival time (10–12). Immunological experiments are often performed using C57BL/6 mice, because most of the gene-deficient mice available have the C57BL/6 background.
Early studies showed that clearance of the parasites takes place mainly in the liver (13, 14). Further studies demonstrated that the parasites are cleared by Kupffer cells via phagocytosis (15), which is mediated by IgM as well as IgG antibodies (Abs) specific for VSG (16, 17). More recently, using IgM-deficient and B cell-deficient mice, it has been shown that IgG, but not IgM, Abs play a dominant role in the clearance of the parasites (18, 19). Gamma interferon (IFN-γ), produced by VSG-specific T cell receptor αβ-positive (TCRαβ+) CD4+ T cells (20), is critical for host resistance to African trypanosomes (18, 21–24). It is likely that IFN-γ exerts its protective effect through macrophage activation, resulting in secretion of tumor necrosis factor alpha (TNF-α) and nitric oxide, which mediate parasite lysis or death (18, 25–27). However, overactivation of macrophages driven by excessive production of IFN-γ, particularly in the absence of interleukin-10 (IL-10) signaling, induces liver pathology, which kills the infected mice (15, 28, 29). As a regulatory cytokine, IL-10 is required to downregulate macrophage activation (15, 23, 28). Thus, IFN-γ and IL-10 play crucial roles in protective as well as pathological immune responses during African trypanosomiasis (1, 4).
CD4+ and CD8+ T cells are the major potential producers of IFN-γ and IL-10. Although the important roles of IFN-γ and IL-10 in the pathogenesis of African trypanosomiasis have been documented, the roles of CD4+ and CD8+ T cells in the development of the disease are not fully understood. In this study, we evaluated the contributions of CD4+ and CD8+ T cells to the pathogenesis of this disease. In particular, we focused on how their contributions were related to IFN-γ and IL-10.
MATERIALS AND METHODS
Mice.
Female 8- to 10-week-old BALB/c AnNCrlBR (BALB/c) mice and 5- to 6-week-old female outbred Swiss white mice (CD1) were purchased from the National Cancer Institute (Frederick, MD). CD4−/− and CD8−/− BALB/c mice (30, 31) were bred in-house. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee and Institutional Bio-safety Committee of the University of Maryland, College Park.
Parasites.
T. brucei variable antigen type (VAT) 10-26 was obtained from Terry Pearson, University of Victoria, Victoria, Canada. Frozen stabilates of parasites were used for infecting CD1 mice immunosuppressed with cyclophosphamide, and passages were made every third day as described previously (32). Parasites were purified from the blood of infected CD1 mice by DEAE-cellulose chromatography (33) and were used for infecting BALB/c mice.
Hybridomas and Abs.
The rat hybridoma 1B1.3a (blocking mouse IL-10 receptor [IL-10R]), antibody GK1.5 (specific for mouse CD4), and antibody 53-6.72 (specific for mouse CD8) were purchased from the American Type Culture Collection (ATCC), Manassas, VA. A purified antibody (clone 2.4G2) against mouse CD16/CD32 (FcγIII/II receptors), biotin-conjugated rat anti-mouse CD4 (clone RM4-5), and biotin-conjugated rat anti-mouse IFN-γ (clone XMG1.2) were purchased from BD Biosciences. Biotin-conjugated rat anti-mouse CD3 (clone 17A2), biotin-conjugated rat IgG2b, phycoerythrin (PE)-conjugated anti-mouse IFN-γ (clone XMG1.2), PE-Cy7-conjugated anti-mouse IL-10 (clone JES5-16E3), peridinin chlorophyll protein (PerCP)-Cy5.5-conjugated anti-mouse CD3 (clone 145-2C11), fluorescein isothiocyanate (FITC)-conjugated anti-mouse CD4 (clone GK1.5), allophycocyanin (APC)-conjugated anti-mouse CD8 (clone H35-17.2), PE-conjugated rat-IgG1, FITC-conjugated rat-IgG2b, and APC-conjugated rat-IgG2b were purchased from eBioscience. Biotin-conjugated rat IgG2a and biotin-conjugated rat IgG1 were obtained from Cedarlane.
Infections, treatment of mice with MAbs, estimation of parasitemia, and survival times of mice.
Mice were infected intraperitoneally (i.p.) with 103 T. brucei VAT 10-26 parasites. Some groups of infected mice were injected i.p. with a rat anti-mouse CD4 monoclonal antibody (MAb) (clone GK1.5), a rat anti-mouse CD8 MAb (clone 53-6.72), a rat anti-mouse IL-10R MAb (clone 1B1.3a), or rat IgG (as a control). A drop of blood was taken from the tail of each infected mouse. Parasitemia was estimated by counting the number of parasites present in at least 10 fields at ×400 magnification by phase-contrast microscopy. The survival time was defined as the number of days after infection that the infected mice remained alive.
Flow cytometry.
Spleen cells were collected from T. brucei-infected BALB/c mice at various time points after infection or from uninfected BALB/c mice (as a negative control). The cells were diluted to 5 × 106/ml and were cultured (200 μl/well) in a 96-well plate for 48 h. After 44 h of incubation, 2 μM monensin (GolgiStop; BD Biosciences) was added to the cultures. Four hours later, cells were harvested and were washed twice in staining buffer (BD Biosciences). Alternatively, the spleen cells were cultured in the presence of 1× Cell Stimulation Cocktail (plus protein transport inhibitors; eBioscience) for 12 h. The cells were incubated (15 min, 4°C) with a purified antibody (clone 2.4G2) against mouse CD16/CD32 (FcγIII/II receptors) to block nonspecific binding of Abs to Fc receptors, washed with staining buffer, resuspended in staining buffer, and stained with MAbs specific for cell surface markers or with the relevant isotype-matched control Abs. Cells were washed twice with staining buffer. Cells were fixed with formaldehyde, and cell membranes were permeabilized in Cytofix/Cytoperm solution (BD Biosciences). Intracellular staining was then performed using MAbs specific for IFN-γ or IL-10, or using isotype-matched control Abs. Samples were resuspended in phosphate-buffered saline (PBS) containing 1% formaldehyde, tested by fluorescence-activated cell sorting (FACS), and analyzed using FlowJo software.
Immunocytochemistry.
Spleen cells were isolated from T. brucei-infected BALB/c mice on day 6 after infection or from uninfected BALB/c mice (as a control). The cells were diluted at 2.5 × 106/ml. Four hundred twenty-five microliters of the cell solution was put in each chamber of eight-chamber Lab-Tek slides (Miles Scientific) and was cultured for 48 h at 37°C under a 5% CO2 atmosphere. Then the chamber slides were disassembled. Immunofluorescent double staining for cell surface markers and intracellular IFN-γ was performed on ice as described previously (34). In brief, the cells were rinsed with PBS, blocked with Fc block (purified MAb 2.4G2) and 2% bovine serum albumin (BSA), and then incubated with biotin-conjugated anti-mouse CD3 or anti-mouse CD4 MAbs, or with biotin-conjugated isotype controls, for 30 min. After three washes in PBS, the slides were incubated with Alexa Fluor 488-conjugated streptavidin (Molecular Probes) for 30 min, rinsed with PBS, and then fixed with 5% formalin. The cells were incubated with avidin D solution for 15 min, rinsed with PBS, and then incubated with a biotin solution (Avidin/Biotin Blocking kit; Vector Laboratories) for 15 min. The slides were rinsed with PBS, incubated with biotinylated rat anti-mouse IFN-γ (clone XMG1.2) or rat IgG1 conjugated with biotin (isotype control) in PBS containing 0.1% saponin (for cell permeabilization) for 30 min, rinsed with PBS, and then stained with Alexa Fluor 546-conjugated streptavidin.
Splenocyte cultures for measurement of cytokine synthesis.
Splenocytes were collected on day 6 or 21 from T. brucei-infected mice as well as from naïve mice. Cells were cultured at a concentration of 5 × 106/ml (200 μl/well) in 96-well tissue culture plates with or without lysates of T. brucei VAT 10-26 parasites in a humidified incubator containing 5% CO2. The parasite lysates were made by repeated freezing and thawing, and sonication, as described previously (35). The culture supernatant fluids were collected after 48 h, centrifuged at 1,500 × g for 10 min, and stored for cytokine assays at −20°C until use.
Liver leukocyte cultures for measurement of cytokine synthesis.
Intrahepatic leukocytes were isolated as described previously (36). Briefly, the liver was perfused with PBS until it became pale. Thereafter, the gallbladder was removed and the liver excised carefully from the abdomen. The liver was minced with surgical scissors and was forced gently through a 70-μm cell strainer using a sterile syringe plunger. The preparation obtained was suspended in 50 ml RPMI 1640 medium containing 10% fetal calf serum (FCS). The cell suspension was centrifuged at 30 × g with the off-brake setting for 10 min at 4°C. The supernatant obtained was centrifuged at 300 × g with the high-brake setting for 10 min at 4°C. The pellet was resuspended in 10 ml 37.5% Percoll in Hanks balanced salt solution (HBSS) containing 100 U/ml heparin and was then centrifuged at 850 × g with the off-brake setting for 30 min at 23°C. This new pellet was resuspended in 2 ml ACK (ammonium-chloride-potassium) buffer for erythrocyte lysis, incubated at room temperature for 5 min, and then supplemented with 8 ml RPMI 1640 medium containing 10% FCS, followed by centrifugation at 300 × g with the high-brake setting for 10 min at 8°C. Cells were collected and were cultured at a concentration of 5 × 106/ml (200 μl/well) in 96-well tissue culture plates in a humidified incubator containing 5% CO2. The culture supernatant fluids were collected after 48 h, centrifuged at 1,500 × g for 10 min, and stored for cytokine assays at −20°C until use.
Cytokine assays.
Recombinant murine cytokines (IFN-γ and IL-10) and Abs specific to these cytokines for use in enzyme-linked immunosorbent assays (ELISAs) were purchased from BD Biosciences. The levels of cytokines in culture supernatant fluids or plasma were determined by routine sandwich ELISAs using Immuno-4 plates (Dynex Technologies), according to the manufacturer's protocols. Each sample was tested for each cytokine in triplicate.
ELISAs for trypanosome-specific Abs.
The whole-trypanosome lysate was prepared by repeated freezing and thawing of freshly isolated T. brucei VAT 10-26 or VAT x (purified from the blood of BALB/c mice on day 20 after infection with T. brucei VAT 10-26) in the presence of 5 mM N-tosyl-l-lysine chloromethyl ketone (TLCK; Sigma), and the total protein content was determined by the Bio-Rad Protein Assay kit (Bio-Rad Laboratories) as described previously (34, 37). ELISA plates were coated overnight at 4°C with 50 μl of the lysate containing 25 μg/ml total protein. The plates were washed twice with PBS-Tween, and nonspecific binding sites were blocked for 2 h at room temperature with 200 μl of PBS containing 10% heat-inactivated fetal bovine serum (PBS-FBS). Serum samples (100 μl), diluted 1:50 in PBS-FBS, were added to each well and were incubated for 2 h at 37°C. After washing (four times), 100 μl of previously determined dilutions of peroxidase-conjugated goat anti-mouse isotype-specific antibodies (Southern Biotechnology Associates) in PBS-FBS was added to each well, and the mixture was incubated for 2 h at room temperature. The plates were washed eight times, and color development was achieved by adding 100 μl of 2,2′-azino-di-(3-ethylbenzthiazoline sulfonate) (ABTS; Kirkegaard & Perry) and incubating for 15 to 30 min at room temperature. Optical densities were read in a microtiter plate reader at a wavelength of 405 nm.
Statistical analysis.
Data are represented as means ± standard errors of the means (SEM). The significance of differences was determined by two-way analysis of variance (ANOVA), the Student t test, or a log rank test using GraphPad Prism software, version 4.0.
RESULTS
CD8+ T cells mediate the mortality of BALB/c mice infected with T. brucei.
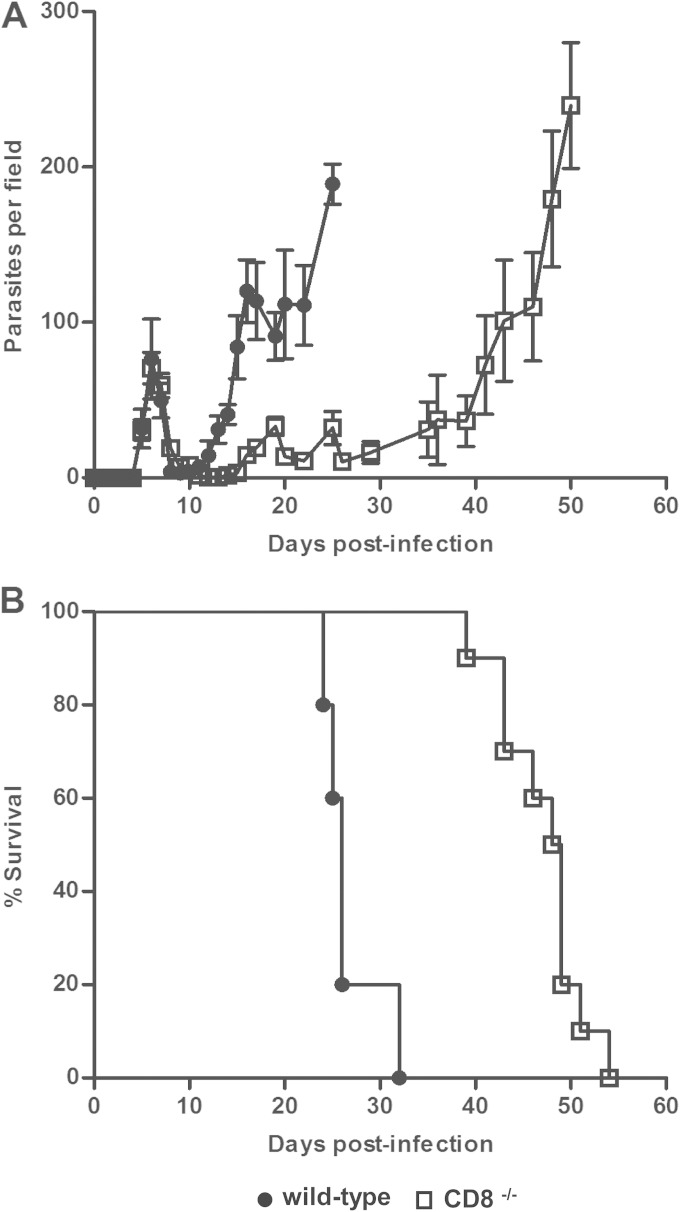
We infected wild-type and CD8−/− BALB/c mice with 103 T. brucei VAT 10-26 parasites. As shown in Fig. 1A, all infected mice could control the first wave of parasitemia. Infected wild-type mice could not control the second wave of parasitemia and succumbed to the infection on day 24 to 32 after infection, with a mean survival time of 26.6 ± 1.4 days (Fig. 1B). In contrast, infected CD8−/− mice succumbed to infection on day 39 to 54, with an average survival time of 47.1 ± 1.4 days (Fig. 1B). Thus, infected CD8−/− mice survived significantly longer (P < 0.01) than infected wild-type mice, demonstrating that CD8+ T cells mediated the mortality of BALB/c mice infected with T. brucei VAT 10-26, as reported previously for relatively resistant C57BL/6 mice (23, 38).
FIG 1.
CD8−/− mice infected with T. brucei have lower levels of parasitemia and survive significantly longer than infected wild-type mice. Groups of CD8−/− (□) (n = 10) or wild-type (●) (n = 5) BALB/c mice were infected with 103 T. brucei VAT 10-26 parasites. Mice were monitored for parasitemia (A) and survival (B). Data are presented as means ± SEM and are representative of results of two separate experiments.
Mortality mediated by CD8+ T cells is not associated with IFN-γ secretion.
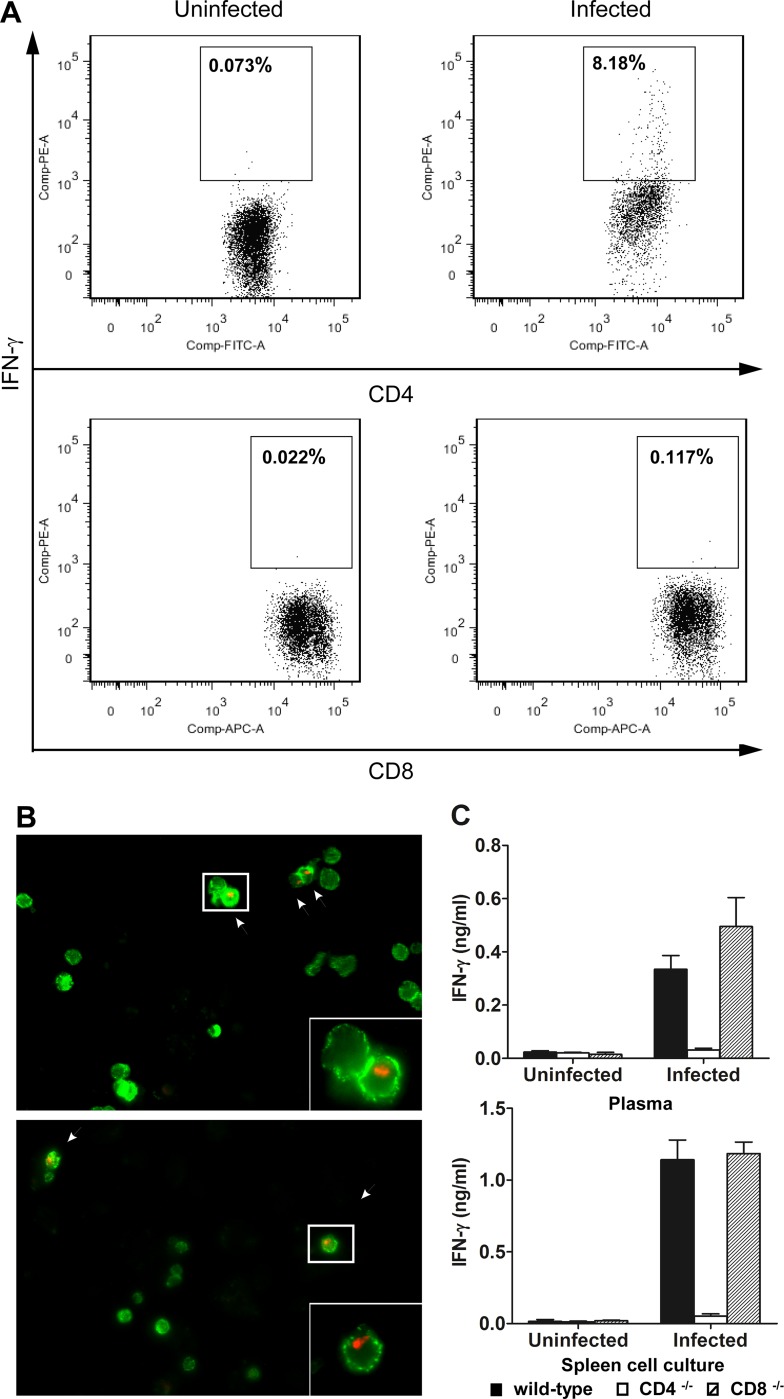
Early results suggested that CD8+ T cells mediated the mortality of C57BL/6 mice infected with T. brucei AnTat1.1E through their secretion of IFN-γ, which promoted parasite growth (38, 39). Thus, we first determined the cellular source of IFN-γ in wild-type BALB/c mice infected with T. brucei VAT 10-26. Spleen cells obtained from infected mice and naïve mice were cultured in vitro. The cells were collected for staining of cell surface markers and intracellular IFN-γ, which were detected by flow cytometry. IFN-γ-producing cells were detected in the spleen cultures of mice infected with T. brucei VAT 10-26 (Fig. 2A). No IFN-γ-secreting cells were detected in the spleen cultures of naïve mice (Fig. 2A). IFN-γ was produced by CD4+ T cells rather than CD8+ T cells in BALB/c mice infected with T. brucei VAT 10-26 (Fig. 2A). To confirm the flow cytometry data, we also performed immunocytochemical double staining for cell surface makers and intracellular IFN-γ. We found IFN-γ-producing cells in the spleen cultures of infected mice (Fig. 2B) but not in those of naïve mice (data not shown). All IFN-γ-producing cells expressed the CD3 as well as the CD4 marker (Fig. 2B).
FIG 2.
IFN-γ is secreted by CD4+ but not CD8+ T cells in mice infected with T. brucei. (A) Spleen cells collected from T. brucei-infected BALB/c mice on day 6 after infection or from naïve mice were cultured at a concentration of 5 × 106/ml in 96-well plates (200 μl/well) for 48 h, and 2 μM monensin (GolgiStop; BD Biosciences) was added to the cultures at 44 h. Staining for CD4 or CD8 and intracellular IFN-γ was performed as described in Materials and Methods. No IFN-γ-producing cells were detected in naïve mice (left). IFN-γ-producing cells were detected in infected mice, and they were CD4+ and CD8− (right). (B) Spleen cells collected from T. brucei-infected BALB/c mice on day 6 after infection were cultured at a concentration of 5 × 106/ml for 48 h in eight-chamber Lab-Tek slides. The chamber slides were disassembled, and immunofluorescent double staining for CD3 or CD4 and intracellular IFN-γ was performed on ice. IFN-γ+ (red, arrows) cells were CD3+ (green, top) and CD4+ (green, bottom). Original magnifications, ×400 for panels and ×1,000 for insets. (C) Groups of 5 CD4−/− (open bars), CD8−/− (hatched bars), or wild-type (filled bars) BALB/c mice were either infected with 103 T. brucei VAT 10-26 parasites or left uninfected. Mice were killed on day 6 after infection, and plasma samples were collected. Spleen cells were collected and were cultured for 48 h. The amounts of IFN-γ in plasma or supernatant fluids of spleen cultures were determined by ELISA. Data are presented as means ± SEM and are representative of results of two separate experiments.
To further characterize the link between IFN-γ secretion and CD8+ T cell-mediated mortality, we next measured IFN-γ levels in the plasma and the supernatant fluids of spleen cultures of wild-type, CD4−/−, and CD8−/− BALB/c mice infected with T. brucei VAT 10-26. A large amount of IFN-γ was synthesized in vivo or in vitro by spleen cells in infected wild-type mice (Fig. 2C). No significant difference in IFN-γ levels was noted between CD8−/− and wild-type mice infected with T. brucei VAT 10-26 (Fig. 2C), demonstrating that CD8 deficiency did not affect the secretion of IFN-γ. In contrast, IFN-γ production was completely abrogated (under the detection limit) in CD4−/− mice infected with T. brucei VAT 10-26 (Fig. 2C). In addition, we compared the secretion of IFN-γ by spleen cells and liver leukocytes purified from wild-type mice with that for mice depleted of CD4+ or CD8+ T cells at the acute stage (day 6) and the chronic stage (day 21) of T. brucei infection. Depletion of CD4+, but not CD8+, T cells abolished IFN-γ secretion by spleen cells on day 6 after infection (P < 0.01) (see Fig. S1 in the supplemental material). IFN-γ was undetectable in all three groups on day 21 after infection. Importantly, depletion of CD4+, but not CD8+, T cells abrogated the production of IFN-γ by liver leukocytes in both the acute and chronic stages of the infection (P < 0.01) (see Fig. S1).
To confirm the antigen specificity of IFN-γ production by CD4+ T cells, we measured the secretion of IFN-γ by spleen cells from naïve mice and infected mice in the absence or presence of various concentrations of T. brucei VAT 10-26 lysate antigens as described previously (35). Although there was no significant difference in IFN-γ secretion between the absence and the presence of the parasite lysates for infected mice, spleen cells purified from infected wild-type mice or infected mice depleted of CD8+ T cells, but not from infected mice depleted of CD4+ T cells, secreted significantly larger amounts (P < 0.01) of IFN-γ than spleen cells from naïve mice (undetectable) when cultured with trypanosome lysates (see Table S1 in the supplemental material). We suggest that the failure of trypanosome lysates to enhance IFN-γ secretion by spleen cells is due to the fact that the spleen cultures already contained considerable numbers of live trypanosomes, since we could observe the live parasites in the spleen culture under microscopy (data not shown). These parasites may generate sufficient trypanosome VSG in the cultures. Taken together, these data strongly suggest that IFN-γ was produced by CD4+ T cells rather than CD8+ T cells in BALB/c mice infected with T. brucei VAT 10-26. Neither CD8 deficiency nor depletion of CD8+ T cells affected the production of IFN-γ. Thus, the mortality mediated by CD8+ T cells in BALB/c mice infected with T. brucei was not due to IFN-γ. This is in striking contrast to previous observations for C57BL/6 mice infected with T. brucei AnTat1.1E (38).
The increased survival time of infected CD8−/− mice is significantly reduced in the absence of IL-10 signaling.
Since IL-10 is crucial for the survival of mice infected with African trypanosomes (15, 23), we next examined the role of IL-10 in the prolonged survival of CD8−/− mice infected with T. brucei VAT 10-26. We first examined the cellular source of IL-10. As shown in Fig. 3A, infected CD8−/− and wild-type mice had equal amounts of IL-10 in their plasma and supernatant fluids of spleen cultures. In contrast, infected CD4−/− mice produced significantly less IL-10 than infected wild-type and CD8−/− mice (P < 0.01), suggesting that IL-10 was secreted mainly by CD4+ T cells. In addition, depletion of CD4+, but not CD8+, T cells significantly reduced the production of IL-10 by spleen cells as well as liver leukocytes on days 6 and 21 after T. brucei infection (see Fig. S1 in the supplemental material). To directly detect the IL-10-producing cells, spleen cells were stained with an anti-IL-10 MAb. IL-10-producing cells were not detectable in spleen cultures with or without parasite lysates by flow cytometry (data not shown). However, IL-10-producing cells were detected when a cell stimulation cocktail was added to the cultures (see Fig. S2 in the supplemental material). As shown in Fig. S2, the majority of IL-10-producing cells were CD4+ cells. Taken together, these data demonstrated that IL-10 was produced mainly by CD4+ T cells in BALB/c mice infected with T. brucei VAT 10-26.
FIG 3.
The increased survival of CD8−/− mice infected with T. brucei is abolished by anti-IL-10R treatment. (A) Groups of 5 CD4−/− (open bars), CD8−/− (hatched bars), or wild-type (filled bars) BALB/c mice were either infected with 103 T. brucei VAT 10-26 parasites or left uninfected. Mice were killed on day 6 after infection, and plasma samples were collected. Spleen cells were collected and were cultured for 48 h. The amounts of IL-10 in plasma or supernatant fluids of spleen cultures were determined by ELISA. (B) Groups of 5 CD8−/− (squares) or wild-type (circles) BALB/c mice were infected with 103 T. brucei VAT 10-26 parasites and were treated with 200 μg of an anti-IL-10R MAb (open symbols) or rat IgG as a control (filled symbols) on days 3 and 5 after infection, respectively. Mice were monitored for parasitemia and survival. (C) Groups of 5 CD8−/− (open bars) or wild-type (filled bars) BALB/c mice were infected with 103 T. brucei VAT 10-26 parasites and were treated with 200 μg of an anti-IL-10R MAb or rat IgG as a control on days 3 and 5 after infection, respectively. Plasma samples were collected from the mice on day 6 after infection. Levels of IFN-γ and IL-10 in plasma were determined by ELISA. Data are presented as means ± SEM and are representative of results from two separate experiments.
Next, we infected wild-type and CD8−/− mice with T. brucei VAT 10-26 and treated the mice with an anti-IL-10R MAb or with rat IgG as a control. As expected, infected wild-type mice treated with an anti-IL-10R MAb died significantly earlier (9.6 ± 0.2 days) (P < 0.01) than infected wild-type mice treated with rat IgG (27.6 ± 1.4 days) (Fig. 3B). Importantly, infected CD8−/− mice treated with an anti-IL-10R MAb succumbed to the infection on day 9 to 12 after infection and had significantly shorter survival times (11 ± 0.5 days) (P < 0.01) than infected CD8−/− mice treated with rat IgG (48.2 ± 2.6 days) (Fig. 3B). There was no significant difference in parasitemia or survival between infected CD8−/− mice treated with an anti-IL-10R MAb and infected wild-type mice treated with an anti-IL-10R MAb (Fig. 3B). Interestingly, the blockade of IL-10R dramatically enhanced the levels of IFN-γ and IL-10 in the plasma of both CD8−/− and wild-type mice infected with T. brucei (P < 0.01) (Fig. 3C). However, there was no significant difference in the plasma IFN-γ and IL-10 levels between infected CD8−/− and wild-type mice following administration of the anti-IL-10R MAb (Fig. 3C). Taken together, these results demonstrated that the increased survival of infected CD8−/− mice was abolished in the absence of IL-10 signaling, probably due to a spike in the production of IFN-γ by CD4+ T cells.
CD4+ T cells mediate protection, which is counteracted by the detrimental effects of CD8+ T cells in wild-type mice infected with T. brucei.
We next characterized the contributions of CD4+ T cells to the pathogenesis of T. brucei infection in BALB/c mice. In agreement with previous observations with infected C57BL/6 mice (23), there was no significant difference in parasitemia or survival between CD4−/− and wild-type BALB/c mice infected with T. brucei VAT 10-26 (Fig. 4A). These results were interesting and raised the possibility that CD4+ T cells played a negligible role in the course of T. brucei infection, which seemed unlikely, because splenic dendritic cells appeared to activate VSG-specific CD4+ Th1 cells to mediate protection (20, 40, 41). Alternatively, the CD4+ T cells could have been protective, but their protective functions could have been overwhelmed or counteracted by the detrimental effects mediated by CD8+ T cells. To address this issue, we made a double-negative model by in vivo depletion of CD4+ T cells (99% depletion) in CD8−/− mice using an anti-CD4 MAb (clone GK1.5). Interestingly, infected CD8−/− mice treated with the anti-CD4 MAb developed high levels of parasitemia at earlier time points, and survived for significantly shorter times (28 ± 0.7 days versus 50 ± 1.3 days) (P < 0.01), than infected CD8−/− mice treated with rat IgG, providing direct evidence that CD4+ T cells were required for protection. Although infected wild-type mice treated with rat IgG and infected CD8−/− mice treated with an anti-CD4 MAb could control the first wave of parasitemia, both groups of mice succumbed to the second wave of parasitemia, with equal survival times (Fig. 4B). Taken together, these results suggested that although CD4+ T cells mediated protection, the protective role was counteracted by the detrimental effects of CD8+ T cells during T. brucei infections, resulting in equal survival of infected wild-type mice and infected CD8−/− mice depleted of CD4+ T cells.
FIG 4.
CD4+ T cells mediate protection, which is counteracted by the detrimental effects of CD8+ T cells. (A) Groups of CD4−/− (▲) (n = 10) or wild-type (●) (n = 5) BALB/c mice were infected with 103 T. brucei VAT 10-26 parasites. Mice were monitored for parasitemia and survival. (B) Groups of 4 CD8−/− BALB/c mice (○) were infected with 103 T. brucei VAT 10-26 parasites and were treated with 1, 0.5, 0.5, 0.5, and 0.5 mg of a rat anti-mouse CD4 MAb on days −2, 1, 8, 15, and 22 after infection, respectively. As controls, groups of 4 CD8−/− (□) or wild-type (●) BALB/c mice were infected with 103 T. brucei VAT 10-26 parasites and were treated with 1, 0.5, 0.5, 0.5, and 0.5 mg of rat IgG on days −2, 1, 8, 15, and 22 after infection, respectively. Mice were monitored for parasitemia and survival. Data are presented as means ± SEM and are representative of results from three separate experiments.
CD4+ T cells mediate the early mortality of infected mice in the absence of IL-10 signaling.
We next examined the role of CD4+ T cells in the absence of IL-10 signaling. Blockade of IL-10R significantly shortened the survival times of wild-type mice infected with T. brucei VAT 10-26 (P < 0.01) but did not affect the parasitemia or survival of infected CD4−/− BALB/c mice (Fig. 5A), demonstrating that CD4+ T cells mediated the early mortality of infected mice in the absence of IL-10 signaling. Since we have shown previously that blockade of IL-10R led to excessive IFN-γ secretion, which killed mice infected with T. congolense (15), we measured the levels of IFN-γ and IL-10 in the plasma of CD4−/− and wild-type mice infected with T. brucei VAT 10-26 following administration of an anti-IL-10R MAb. Although administration of an anti-IL-10R MAb significantly enhanced plasma IFN-γ and IL-10 levels in both infected wild-type and infected CD4−/− mice (P < 0.01), the plasma IFN-γ levels of infected wild-type mice reached 1.9 ng/ml, 3.6-fold that of infected CD4−/− mice, after blockade of IL-10R (Fig. 5B). Thus, CD4+ T cells mediated the early mortality of infected mice in the absence of IL-10 signaling, probably via their secretion of IFN-γ.
FIG 5.
CD4+ T cells mediate the early mortality of infected mice in the absence of IL-10 function. (A) Groups of 5 CD4−/− (triangles) or wild-type (circles) BALB/c mice were infected with 103 T. brucei VAT 10-26 parasites and were treated with 200 μg of an anti-IL-10R MAb (open symbols) or rat IgG (filled symbols) on days 3 and 5 after infection, respectively. Mice were monitored for parasitemia and survival. (B) Groups of 5 CD4−/− (open bars) or wild-type (filled bars) BALB/c mice were infected with 103 T. brucei VAT 10-26 parasites and were treated with 200 μg of an anti-IL-10R MAb or rat IgG as a control on day 3 or 5 postinfection, respectively. Plasma samples were collected from the mice on day 6 after infection. Levels of IFN-γ and IL-10 in plasma were determined by ELISA. Data are presented as means ± SEM and are representative of results from two separate experiments.
CD4+, but not CD8+, T cells contribute to the synthesis of IgG Abs specific for T. brucei VAT 10-26 or T. brucei VAT x.
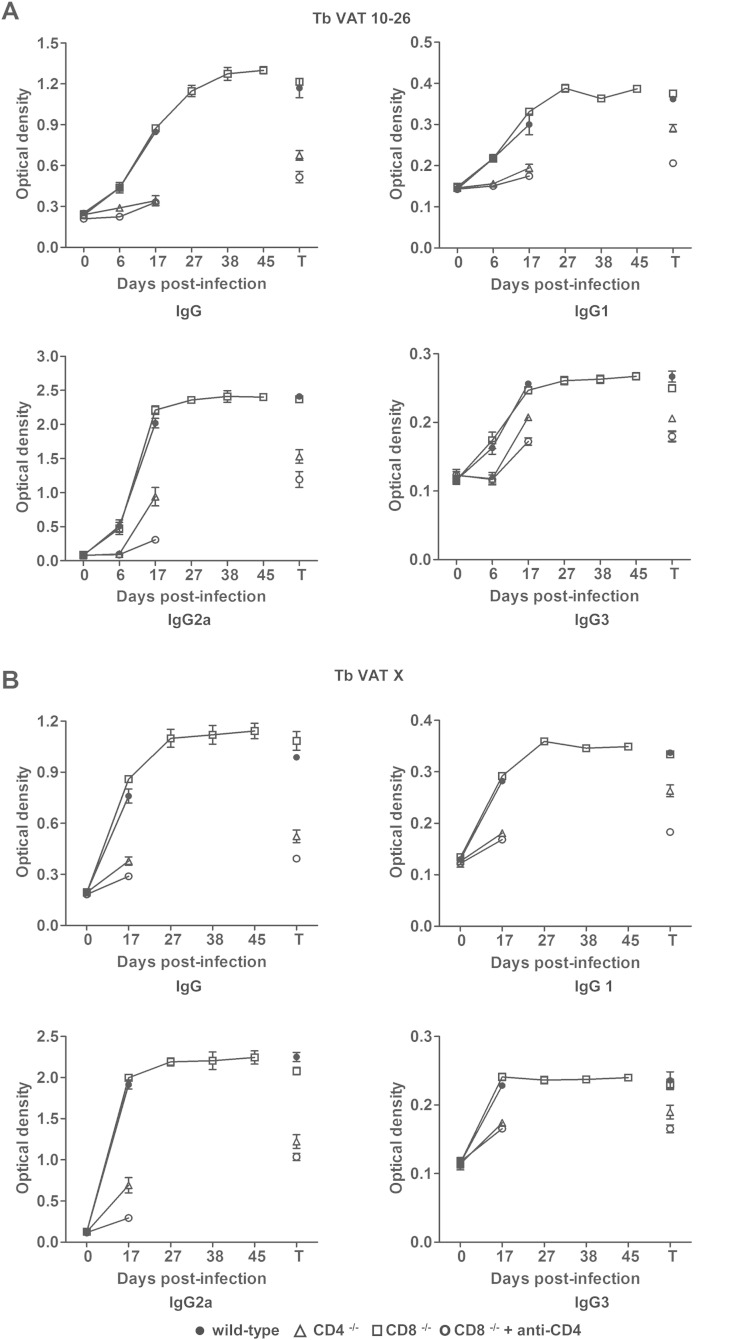
We next set out to address whether parasite-specific IgG Abs were affected by the absence of CD4+ or CD8+ T cells. For this purpose, wild-type, CD4−/−, or CD8−/− mice, or CD8−/− mice depleted of CD4+ T cells, were infected with T. brucei VAT 10-26. We measured the levels of IgG Abs specific for T. brucei VAT 10-26 or VAT x (parasites isolated on day 20 after infection) in plasma at various time points after infection. Significantly larger amounts of total IgG, IgG1, IgG2a, or IgG3 Abs specific for T. brucei VAT 10-26 were noted in infected wild-type or CD8−/− mice on day 6 after infection than on day 0 after infection (P < 0.01) (Fig. 6A). In contrast, levels of those Abs were very low in CD4−/− mice or CD8−/− mice depleted of CD4+ T cells on day 6 after infection (Fig. 6A). On day 17 after infection, although the levels of total IgG, IgG1, IgG2a, or IgG3 Abs specific for T. brucei VAT 10-26 or VAT x in plasma were moderately enhanced in infected CD4−/− mice or infected CD8−/− mice depleted of CD4+ T cells as well, the levels of those Abs in plasma from these animals were still significantly lower (P < 0.01) than those in infected wild-type or CD8−/− mice (Fig. 6A and B). The total IgG, IgG1, IgG2a, or IgG3 Abs specific for T. brucei VAT 10-26 or VAT x reached the highest levels in the plasma of infected CD8−/− mice on day 27 after infection, and these high Ab levels were maintained until the death of the mice. At the terminal stage, there was no significant difference in the levels of all measured Abs specific for T. brucei VAT 10-26 or VAT x in plasma between infected CD8−/− mice and infected wild-type mice (Fig. 6A and B). However, the amounts of those Abs in wild-type or CD8−/− mice were significantly higher than those in infected CD4−/− mice or in infected CD8−/− mice depleted of CD4+ T cells at the terminal stage (P < 0.01) (Fig. 6A and B). Overall, these data demonstrated that CD4+, but not CD8+, T cells were crucial for the synthesis of parasite-specific Abs. Since IgG Abs are associated with protection in African trypanosomiasis (18, 19, 37), we suggest that CD4+ T cells mediate protection, at least in part, via their contribution to the generation of IgG Abs.
FIG 6.
The synthesis of IgG Abs specific for T. brucei VAT 10-26 or VAT x is affected by a deficiency of CD4+ T cells but not by a deficiency of CD8+ T cells. Groups of 4 CD4−/− (△), CD8−/− (□), or wild-type (●) BALB/c mice were infected with 103 T. brucei VAT 10-26 parasites. Another group of 4 CD8−/− mice (○) was infected with 103 T. brucei VAT 10-26 parasites and was treated with 1, 0.5, 0.5, 0.5, and 0.5 mg of a rat anti-mouse CD4 MAb (clone GK1.5) on days −2, 1, 8, 15, and 22 after infection, respectively. Plasma samples were collected from the infected mice on days 0, 6, 17, 27, 38, and 45 after infection or at the terminal stages (T). The levels of Abs specific for T. brucei VAT 10-26 (A) or VAT x (B) in plasma were measured by ELISA. Data are presented as means ± SEM and are representative of results from two separate experiments.
DISCUSSION
We have made four original observations, as follows. (i) Without any impairment in the secretion of IFN-γ, infected CD8−/− BALB/c mice still survived significantly longer than infected wild-type mice, suggesting that the mortality mediated by CD8+ T cells was not due to IFN-γ. (ii) The enhanced survival of infected CD8−/− mice was significantly reduced in the absence of IL-10 signaling. (iii) Depletion of CD4+ T cells abrogated the prolonged survival of infected CD8−/− mice, demonstrating that CD4+ T cells mediated protection. However, the protection was counteracted by the detrimental effects of CD8+ T cells in infected wild-type mice. (iv) Blockade of IL-10R shortened the survival of infected wild-type mice, but not that of infected CD4−/− mice, demonstrating that CD4+ T cells mediate mortality in the absence of IL-10 signaling.
CD8−/− BALB/c mice infected with T. brucei VAT 10-26 survived significantly longer than infected wild-type mice, indicating that CD8+ T cells mediated mortality. This finding is consistent with previous data obtained from C57BL/6 mice infected with T. brucei AnTat1.1E (23, 38). It has been reported that T. brucei produces a T lymphocyte-triggering factor, which stimulates CD8+ T cells to secrete IFN-γ (38, 39). CD8+ T cell-released IFN-γ, as a growth-stimulating factor, supported parasite growth (38, 39). Thus, previous data suggested that CD8+ T cells killed mice via their secretion of IFN-γ during T. brucei infections. In our model, both FACS and immunohistochemical analyses confirmed that IFN-γ was produced by CD4+ but not CD8+ T cells in BALB/c mice infected with T. brucei VAT 10-26. Furthermore, analysis of IFN-γ secretion revealed complete abrogation of IFN-γ production (under the detection limit) in plasma and spleen cultures of infected CD4−/− mice, while CD8 deficiency did not affect IFN-γ synthesis at all. The reason why the cellular source of IFN-γ in our model was inconsistent with that in previous reports remains unclear. One possible explanation for these discrepancies may lie with the strains of infected mice and the parasites. Nevertheless, it is unlikely that CD8+ T cells mediated mortality through IFN-γ secretion to stimulate parasite growth in our model, because CD4+, not CD8+, T cells were the major producers of IFN-γ. On the other hand, CD4 T cells produced IFN-γ; even so, they did not mediate early death. Thus, we concluded that the mortality mediated by CD8+ T cells was not related to IFN-γ, at least in BALB/c mice infected with T. brucei VAT 10-26. However, our results did not exclude the possibility that IFN-γ-mediated death occurred in C57BL/6 mice infected with T. brucei AnTat1.1E (38, 39).
Early studies have shown a correlation between high IFN-γ levels in serum, low levels of parasitemia, and host resistance during infection with African trypanosomes (21). Subsequent studies by Hertz et al. demonstrated, for the first time, that IFN-γ is a critical factor in determining host resistance to the parasites (22). The protective role of IFN-γ in African trypanosomiasis was confirmed later by other groups (18, 23, 42). However, excessive secretion of IFN-γ also kills susceptible BALB/c mice infected with T. congolense (29), as well as relatively resistant C57BL/6 mice infected with T. brucei or T. congolense in the absence of IL-10 function (15, 23). It is noteworthy that IFN-γ mediates mortality via excessive activation of monocytic cells, including Kupffer cells (15) and TNF/inducible nitric oxide synthase-producing dendritic cells (TIP-DCs) (43, 44), resulting in liver pathology (15, 28). This is in contrast to the mechanism proposed earlier for IFN-γ-mediated mortality, i.e., stimulation of parasite growth by IFN-γ (38, 39). Recent data have suggested that IL-10, probably produced by Foxp3+ regulatory T cells (28), is crucial for the survival of infected mice via downregulation of IFN-γ production (15, 23). Interestingly, the enhanced survival of CD8−/− mice was abrogated by administration of an anti-IL-10R MAb, suggesting that IL-10 is crucial for the prolonged survival of infected CD8−/− mice. The abolishment of the increased survival of CD8−/− mice is likely due to the spike in IFN-γ production by CD4+ T cells following the blockade of IL-10R. The level of parasitemia was very low when infected CD8−/− mice treated with the anti-IL-10R MAb died, suggesting that the mice may have died from immunopathology rather than from parasite growth stimulated by IFN-γ as previously proposed (38, 39).
A VSG-specific CD4+ T cell-response with secretion of IFN-γ has been characterized during infection with African trypanosomes (20). More recently, it has been shown that splenic dendritic cells are the primary cells responsible for activating naïve VSG-specific CD4+ T cell responses (41). Interestingly, during infection, VSG-specific CD4+ T cells recognize peptides distributed throughout the N-terminal domain sequence of the VSG molecules but do not recognize peptide sequences from the conserved C-terminal domain (40). These results suggested that the VSG-specific CD4+ T cell response to African trypanosomes is linked to host resistance during infection. Paradoxically, there is no significant difference in parasitemia or survival between relatively resistant wild-type C57BL/6 and CD4 knockout mice during T. brucei AnTat1.1E infection (23). In agreement with this observation with C57BL/6 mice (23), CD4 deficiency did not significantly affect the parasitemia or survival of BALB/c mice infected with T. brucei VAT 10-26 in our model. These results suggested that CD4+ T cells play a limited role during T. brucei infections. Alternatively, CD4+ T cells may play a protective role, which is, however, overwhelmed or counteracted by the detrimental role of CD8+ T cells in mice infected with T. brucei. If the latter is the case, it would be not realistic to judge CD4+ T cell function by simply comparing infected CD4−/− mice with wild-type mice, because of the interference of CD8+ T cells in wild-type mice. To better address this question, we constructed a double-negative model by in vivo depletion of CD4+ T cells to exclude the interference of CD8+ T cells (i.e., the detrimental effects of CD8+ T cells). Strikingly, the survival of CD8−/− mice infected with T. brucei was dramatically reduced by depletion of CD4+ T cells, providing direct evidence that CD4+ T cells mediated protection. The fact that infected wild-type mice and CD8−/− mice depleted of CD4+ T cells had equal survival times further suggested that the protective role of CD4+ T cells was counteracted by the deleterious role of CD8+ T cells during T. brucei infection in wild-type mice. These results highlight the complicated interactions of CD8+ T cells with CD4+ T cells during the course of African trypanosome infection.
Another important finding of this study is that CD4+ T cells also mediate the mortality of mice infected with T. brucei if IL-10 signaling is lacking. Although we have shown previously that IFN-γ mediated the early mortality of mice infected with T. congolense in the absence of IL-10 signaling (15), it was not known which subset of cells mediated the mortality. In the current study, blockade of IL-10R significantly reduced the survival times of infected wild-type mice and enhanced the production of IFN-γ but did not affect the survival of infected CD4−/− mice, demonstrating that CD4+ T cells were the killers of the infected wild-type mice. Considering the fact that CD4+ T cells were the major producers of IFN-γ in mice infected with T. brucei in our model, we suggest that CD4+ T cells mediated mortality via their excessive secretion of IFN-γ in the absence of IL-10 signaling.
In summary, we have provided evidence that CD4+ T cells and CD8+ T cells play distinct roles in the pathogenesis of mice infected with T. brucei. These roles are closely related to IFN-γ and IL-10, the cytokines that have recently proven essential for the regulation of immune responses to African trypanosomiasis. However, the complicated interactions between CD4+ and CD8+ T cells, which have been poorly addressed, deserve further investigation.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by a start-up fund from the University of Maryland (grant to M.S.).
We thank Yunsheng Wang for assistance with FACS analyses.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00357-15.
REFERENCES
- 1.Bosschaerts T, Guilliams M, Stijlemans B, De Baetselier P, Beschin A. 2009. Understanding the role of monocytic cells in liver inflammation using parasite infection as a model. Immunobiology 214:737–747. doi: 10.1016/j.imbio.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Magez S, Caljon G. 2011. Mouse models for pathogenic African trypanosomes: unravelling the immunology of host-parasite-vector interactions. Parasite Immunol 33:423–429. doi: 10.1111/j.1365-3024.2011.01293.x. [DOI] [PubMed] [Google Scholar]
- 3.Mansfield JM, Paulnock DM. 2005. Regulation of innate and acquired immunity in African trypanosomiasis. Parasite Immunol 27:361–371. doi: 10.1111/j.1365-3024.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- 4.Tabel H, Wei G, Shi M. 2008. T cells and immunopathogenesis of experimental African trypanosomiasis. Immunol Rev 225:128–139. doi: 10.1111/j.1600-065X.2008.00675.x. [DOI] [PubMed] [Google Scholar]
- 5.Cross GA. 1990. Cellular and genetic aspects of antigenic variation in trypanosomes. Annu Rev Immunol 8:83–110. doi: 10.1146/annurev.iy.08.040190.000503. [DOI] [PubMed] [Google Scholar]
- 6.Hudson KM, Byner C, Freeman J, Terry RJ. 1976. Immunodepression, high IgM levels and evasion of the immune response in murine trypanosomiasis. Nature 264:256–258. doi: 10.1038/264256a0. [DOI] [PubMed] [Google Scholar]
- 7.Uzonna JE, Kaushik RS, Gordon JR, Tabel H. 1998. Immunoregulation in experimental murine Trypanosoma congolense infection: anti-IL-10 antibodies reverse trypanosome-mediated suppression of lymphocyte proliferation in vitro and moderately prolong the lifespan of genetically susceptible BALB/c mice. Parasite Immunol 20:293–302. [DOI] [PubMed] [Google Scholar]
- 8.Bockstal V, Guirnalda P, Caljon G, Goenka R, Telfer JC, Frenkel D, Radwanska M, Magez S, Black SJ. 2011. T. brucei infection reduces B lymphopoiesis in bone marrow and truncates compensatory splenic lymphopoiesis through transitional B-cell apoptosis. PLoS Pathog 7:e1002089. doi: 10.1371/journal.ppat.1002089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Radwanska M, Guirnalda P, De Trez C, Ryffel B, Black S, Magez S. 2008. Trypanosomiasis-induced B cell apoptosis results in loss of protective anti-parasite antibody responses and abolishment of vaccine-induced memory responses. PLoS Pathog 4:e1000078. doi: 10.1371/journal.ppat.1000078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenblatt HC, Diggs CL, Rosenstreich DL. 1984. Trypanosoma rhodesiense: analysis of the genetic control of resistance among mice. Infect Immun 44:107–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogunremi O, Tabel H. 1995. Genetics of resistance to Trypanosoma congolense in inbred mice: efficiency of apparent clearance of parasites correlates with long-term survival. J Parasitol 81:876–881. doi: 10.2307/3284033. [DOI] [PubMed] [Google Scholar]
- 12.Tabel H, Kaushik RS, Uzonna JE. 2000. Susceptibility and resistance to Trypanosoma congolense infections. Microbes Infect 2:1619–1629. doi: 10.1016/S1286-4579(00)01318-6. [DOI] [PubMed] [Google Scholar]
- 13.Dempsey WL, Mansfield JM. 1983. Lymphocyte function in experimental African trypanosomiasis. V. Role of antibody and the mononuclear phagocyte system in variant-specific immunity. J Immunol 130:405–411. [PubMed] [Google Scholar]
- 14.Macaskill JA, Holmes PH, Whitelaw DD, McConnell I, Jennings FW, Urquhart GM. 1980. Immunological clearance of 75Se-labelled Trypanosoma brucei in mice. II. Mechanisms in immune animals. Immunology 40:629–635. [PMC free article] [PubMed] [Google Scholar]
- 15.Shi M, Pan W, Tabel H. 2003. Experimental African trypanosomiasis: IFN-γ mediates early mortality. Eur J Immunol 33:108–118. doi: 10.1002/immu.200390013. [DOI] [PubMed] [Google Scholar]
- 16.Kaushik RS, Uzonna JE, Gordon JR, Tabel H. 1999. Innate resistance to Trypanosoma congolense infections: differential production of nitric oxide by macrophages from susceptible BALB/c and resistant C57BL/6 mice. Exp Parasitol 92:131–143. doi: 10.1006/expr.1999.4408. [DOI] [PubMed] [Google Scholar]
- 17.Shi M, Wei G, Pan W, Tabel H. 2004. Trypanosoma congolense infections: antibody-mediated phagocytosis by Kupffer cells. J Leukoc Biol 76:399–405. doi: 10.1189/jlb.1003500. [DOI] [PubMed] [Google Scholar]
- 18.Magez S, Radwanska M, Drennan M, Fick L, Baral TN, Brombacher F, De Baetselier P. 2006. Interferon-gamma and nitric oxide in combination with antibodies are key protective host immune factors during Trypanosoma congolense Tc13 infections. J Infect Dis 193:1575–1583. doi: 10.1086/503808. [DOI] [PubMed] [Google Scholar]
- 19.Magez S, Schwegmann A, Atkinson R, Claes F, Drennan M, De Baetselier P, Brombacher F. 2008. The role of B-cells and IgM antibodies in parasitemia, anemia, and VSG switching in Trypanosoma brucei-infected mice. PLoS Pathog 4:e1000122. doi: 10.1371/journal.ppat.1000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schleifer KW, Filutowicz H, Schopf LR, Mansfield JM. 1993. Characterization of T helper cell responses to the trypanosome variant surface glycoprotein. J Immunol 150:2910–2919. [PubMed] [Google Scholar]
- 21.de Gee AL, Sonnenfeld G, Mansfield JM. 1985. Genetics of resistance to the African trypanosomes. V. Qualitative and quantitative differences in interferon production among susceptible and resistant mouse strains. J Immunol 134:2723–2726. [PubMed] [Google Scholar]
- 22.Hertz CJ, Filutowicz H, Mansfield JM. 1998. Resistance to the African trypanosomes is IFN-γ dependent. J Immunol 161:6775–6783. [PubMed] [Google Scholar]
- 23.Namangala B, Noel W, De Baetselier P, Brys L, Beschin A. 2001. Relative contribution of interferon-gamma and interleukin-10 to resistance to murine African trypanosomosis. J Infect Dis 183:1794–1800. doi: 10.1086/320731. [DOI] [PubMed] [Google Scholar]
- 24.Schopf LR, Filutowicz H, Bi XJ, Mansfield JM. 1998. Interleukin-4-dependent immunoglobulin G1 isotype switch in the presence of a polarized antigen-specific Th1-cell response to the trypanosome variant surface glycoprotein. Infect Immun 66:451–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lucas R, Magez S, De Leys R, Fransen L, Scheerlinck JP, Rampelberg M, Sablon E, De Baetselier P. 1994. Mapping the lectin-like activity of tumor necrosis factor. Science 263:814–817. doi: 10.1126/science.8303299. [DOI] [PubMed] [Google Scholar]
- 26.Magez S, Geuskens M, Beschin A, del Favero H, Verschueren H, Lucas R, Pays E, de Baetselier P. 1997. Specific uptake of tumor necrosis factor-alpha is involved in growth control of Trypanosoma brucei. J Cell Biol 137:715–727. doi: 10.1083/jcb.137.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magez S, Radwanska M, Drennan M, Fick L, Baral TN, Allie N, Jacobs M, Nedospasov S, Brombacher F, Ryffel B, De Baetselier P. 2007. Tumor necrosis factor (TNF) receptor-1 (TNFp55) signal transduction and macrophage-derived soluble TNF are crucial for nitric oxide-mediated Trypanosoma congolense parasite killing. J Infect Dis 196:954–962. doi: 10.1086/520815. [DOI] [PubMed] [Google Scholar]
- 28.Guilliams M, Oldenhove G, Noel W, Herin M, Brys L, Loi P, Flamand V, Moser M, De Baetselier P, Beschin A. 2007. African trypanosomiasis: naturally occurring regulatory T cells favor trypanotolerance by limiting pathology associated with sustained type 1 inflammation. J Immunol 179:2748–2757. doi: 10.4049/jimmunol.179.5.2748. [DOI] [PubMed] [Google Scholar]
- 29.Uzonna JE, Kaushik RS, Gordon JR, Tabel H. 1998. Experimental murine Trypanosoma congolense infections. I. Administration of anti-IFN-γ antibodies alters trypanosome-susceptible mice to a resistant-like phenotype. J Immunol 161:5507–5515. [PubMed] [Google Scholar]
- 30.Rahemtulla A, Fung-Leung WP, Schilham MW, Kundig TM, Sambhara SR, Narendran A, Arabian A, Wakeham A, Paige CJ, Zinkernagel RM, Miller RG, Mak TW. 1991. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature 353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 31.Fung-Leung WP, Schilham MW, Rahemtulla A, Kundig TM, Vollenweider M, Potter J, van Ewijk W, Mak TW. 1991. CD8 is needed for development of cytotoxic T cells but not helper T cells. Cell 65:443–449. doi: 10.1016/0092-8674(91)90462-8. [DOI] [PubMed] [Google Scholar]
- 32.Tabel H. 1982. Activation of the alternative pathway of bovine complement by Trypanosoma congolense. Parasite Immunol 4:329–335. doi: 10.1111/j.1365-3024.1982.tb00444.x. [DOI] [PubMed] [Google Scholar]
- 33.Lanham SM, Godfrey DG. 1970. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol 28:521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- 34.Shi M, Wei G, Pan W, Tabel H. 2006. Experimental African trypanosomiasis: a subset of pathogenic, IFN-γ-producing, MHC class II-restricted CD4+ T cells mediates early mortality in highly susceptible mice. J Immunol 176:1724–1732. doi: 10.4049/jimmunol.176.3.1724. [DOI] [PubMed] [Google Scholar]
- 35.Wei G, Bull H, Zhou X, Tabel H. 2011. Intradermal infections of mice by low numbers of African trypanosomes are controlled by innate resistance but enhance susceptibility to reinfection. J Infect Dis 203:418–429. doi: 10.1093/infdis/jiq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blom KG, Qazi MR, Matos JB, Nelson BD, DePierre JW, Abedi-Valugerdi M. 2009. Isolation of murine intrahepatic immune cells employing a modified procedure for mechanical disruption and functional characterization of the B, T and natural killer T cells obtained. Clin Exp Immunol 155:320–329. doi: 10.1111/j.1365-2249.2008.03815.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Uzonna JE, Kaushik RS, Gordon JR, Tabel H. 1999. Cytokines and antibody responses during Trypanosoma congolense infections in two inbred mouse strains that differ in resistance. Parasite Immunol 21:57–71. doi: 10.1046/j.1365-3024.1999.00202.x. [DOI] [PubMed] [Google Scholar]
- 38.Olsson T, Bakhiet M, Hojeberg B, Ljungdahl A, Edlund C, Andersson G, Ekre HP, Fung-Leung WP, Mak T, Wigzell H, Fiszer U, Kristensson K. 1993. CD8 is critically involved in lymphocyte activation by a T. brucei brucei-released molecule. Cell 72:715–727. doi: 10.1016/0092-8674(93)90400-K. [DOI] [PubMed] [Google Scholar]
- 39.Olsson T, Bakhiet M, Edlund C, Hojeberg B, Van der Meide PH, Kristensson K. 1991. Bidirectional activating signals between Trypanosoma brucei and CD8+ T cells: a trypanosome-released factor triggers interferon-gamma production that stimulates parasite growth. Eur J Immunol 21:2447–2454. doi: 10.1002/eji.1830211022. [DOI] [PubMed] [Google Scholar]
- 40.Dagenais TR, Demick KP, Bangs JD, Forest KT, Paulnock DM, Mansfield JM. 2009. T-cell responses to the trypanosome variant surface glycoprotein are not limited to hypervariable subregions. Infect Immun 77:141–151. doi: 10.1128/IAI.00729-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dagenais TR, Freeman BE, Demick KP, Paulnock DM, Mansfield JM. 2009. Processing and presentation of variant surface glycoprotein molecules to T cells in African trypanosomiasis. J Immunol 183:3344–3355. doi: 10.4049/jimmunol.0802005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drennan MB, Stijlemans B, Van den Abbeele J, Quesniaux VJ, Barkhuizen M, Brombacher F, De Baetselier P, Ryffel B, Magez S. 2005. The induction of a type 1 immune response following a Trypanosoma brucei infection is MyD88 dependent. J Immunol 175:2501–2509. doi: 10.4049/jimmunol.175.4.2501. [DOI] [PubMed] [Google Scholar]
- 43.Bosschaerts T, Guilliams M, Stijlemans B, Morias Y, Engel D, Tacke F, Herin M, De Baetselier P, Beschin A. 2010. Tip-DC development during parasitic infection is regulated by IL-10 and requires CCL2/CCR2, IFN-γ and MyD88 signaling. PLoS Pathog 6:e1001045. doi: 10.1371/journal.ppat.1001045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guilliams M, Movahedi K, Bosschaerts T, VandenDriessche T, Chuah MK, Herin M, Acosta-Sanchez A, Ma L, Moser M, Van Ginderachter JA, Brys L, De Baetselier P, Beschin A. 2009. IL-10 dampens TNF/inducible nitric oxide synthase-producing dendritic cell-mediated pathogenicity during parasitic infection. J Immunol 182:1107–1118. doi: 10.4049/jimmunol.182.2.1107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.