Abstract
Staphylococcus aureus is a Gram-positive pathogen that causes a wide range of infections in humans and animals. Bacterial ghosts are nonliving, empty cell envelopes and are well represented as novel vaccine candidates. In this study, we examined the immunogenicity and protective efficacy of S. aureus ghosts (SAGs) against a virulent challenge in rats. Nonliving SAGs were generated by using the MIC of sodium hydroxide. The formation of a transmembrane lysis tunnel structure in SAGs was visualized by scanning electron microscopy. To investigate these SAGs as a vaccine candidate, rats were divided into four groups, A (nonimmunized control), B (orally immunized), C (subcutaneously immunized), and D (intravenously immunized). The IgG antibody responses were significantly stronger in the SAG-immunized groups than in the nonimmunized control group (P < 0.05). Moreover, a significant increase in the populations of CD4+ and CD8+ T cells was observed in all three immunized groups (P < 0.05). We also found that serum bactericidal antibodies were significantly elicited in the SAG-immunized groups (P < 0.05). Most importantly, the bacterial loads in the immunized groups were significantly lower than those in the nonimmunized control group (P < 0.01). These results suggest that immunization with SAGs induces immune responses and provides protection against a virulent S. aureus challenge.
INTRODUCTION
Staphylococcus aureus is a Gram-positive bacterium that is responsible for a wide range of infections in both hospitals and communities (1). S. aureus pathogenicity can range from skin infections to life-threatening diseases such as osteomyelitis, endocarditis, pneumonia, bacteremia, and septicemia (2, 3). Moreover, it is a common food-borne pathogen that can cause gastroenteritis from the consumption of contaminated food (4). In most cases, S. aureus enters the human body through the skin and mucous membrane and spreads via the bloodstream. It can infect every tissue and organ of the human body (5). Over the past decades, multidrug-resistant S. aureus strains have emerged rapidly, and particularly, methicillin-resistant S. aureus (MRSA) is a major public health problem. MRSA can cause greater morbidity, mortality, length of hospital stay, and hospitalization costs than methicillin-susceptible S. aureus (6). Regrettably, treatment options for multidrug-resistant S. aureus strains are limited (7). Therefore, vaccination remains the best way to prevent and control S. aureus infections.
In recent years, it has become well known that bacterial ghosts (BGs) offer a promising and innovative approach in nonliving vaccine technology (8). BGs are nonliving, empty cell envelopes, and the most common method used to produce BGs from Gram-negative bacteria is controlled expression of the lysis E gene. Protein E leads to the formation of transmembrane structures on the cell surface, which results in empty cell envelopes. The resulting BGs induce strong immune responses and protect against specific infections in experimental animal models (9, 10). However, the major drawback of the protein E-induced inactivation method is that it is restricted to Gram-negative bacteria only (11). Interestingly, Ra et al. (12) have demonstrated that Escherichia coli/Streptococcus iniae glyceraldehyde 3-phosphate dehydrogenase ghosts, produced with a double-cassette vector system, protected aquatic fish from streptococcal diseases. Exceptionally, S. iniae ghosts produced by E gene-mediated lysis were being suggested as a potential vaccine candidate (13). However, a number of studies have demonstrated that the lysis efficiency of genetically inactivated BGs was 99.9% (10, 14), suggesting a potential risk of their use as a vaccine. Alternatively, the new approach used to generate E. coli ghosts by using the MICs and minimum growth concentrations of sodium hydroxide (NaOH), sodium dodecyl sulfate, and calcium carbonate (CaCO3) has been described in detail (15–17). Moreover, we have recently shown that chemically induced Salmonella enterica serovar Enteritidis BGs induce immune responses and strong protective immunity to Salmonella infection in a rat model (18). This new strategy of BG induction with NaOH was faster than the protein E-mediated lysis system.
The bacterial envelope of S. aureus is composed of peptidoglycan, teichoic acid, and proteins. Several studies have suggested that S. aureus envelope components are potential vaccine candidates in animal models (19, 20). Recently, immunization with S. aureus peptidoglycan has been found to induce protective immunity to a lethal challenge in experimental animals (21). Vaccination of rats with iron-responsive surface determinant A (IsdA) or IsdH protected against nasal carriage (22). Earlier studies have also shown that protein A (PA), a cell wall component of S. aureus, is highly immunogenic (23). Stranger-Jones et al. (24) demonstrated that immunization with a combination of four surface antigens (IsdA, IsdB, serine-aspartate repeat A [SdrA], and SdrE) induced opsonophagocytic antibodies and provided 100% protection from clinical S. aureus isolates in experimental animals. It has been reported that S. aureus cell wall components are able to induce both humoral and cell-mediated immunity (25). Altogether, these whole-cell envelope components of S. aureus represent an attractive vaccine candidate.
In the present study, we generated novel S. aureus ghosts (SAGs) by using the MIC of NaOH. Additionally, we demonstrated that immunization with SAG vaccine via the oral, subcutaneous, and intravenous routes could induce both humoral and cellular immune responses in rats. Furthermore, these immune responses provided protective immunity to a challenge with virulent S. aureus.
MATERIALS AND METHODS
Bacterial strain, medium, and culture condition.
Virulent S. aureus strain KCCM12256 was kindly provided by Ki-Sung Lee, Department of Biology and Medicinal Science, Pai Chai University, Daejeon, South Korea. S. aureus was cultured in tryptic soy broth (TSB; Difco) at 37°C in a shaking incubator at 200 rpm. Growth and lysis rates were measured spectrophotometrically by determination of optical density at 600 nm (OD600).
Determination of MIC.
Determination of the MIC of NaOH for S. aureus was performed by the 2-fold broth dilution method as described previously (26, 27), with some modifications. Briefly, a virulent culture of S. aureus was grown in TSB and adjusted to 1 × 108 CFU/ml. The initial concentration of NaOH was 60 mg/ml. Two-fold dilutions of NaOH were added to samples of the virulent bacterial culture, and they were incubated at 37°C for 18 h. After incubation, the turbidity of each individual tube was assessed visually and the MIC was determined as the lowest concentration of NaOH that completely killed the bacterial growth. Further, to determine viability, the culture that showed no visible bacterial growth was verified by spreading 100 μl of the culture onto tryptic soy agar (TSA) plates and incubating them at 37°C for 24 h. The MIC was determined in three independent experiments.
Production of SAGs.
SAGs were produced by using the MIC of NaOH as described previously (18). In brief, the biomass of 72-h-old S. aureus cells was collected by centrifugation (10,000 × g for 10 min at 4°C) and washed three times with phosphate-buffered saline (PBS; 3.2 mM Na2HPO4, 0.5 mM KH2PO4, 1.3 mM KCl, 135 mM NaCl, pH 7.4). One milliliter of the MIC of NaOH was added to 2 ml of the bacterial suspension (1 × 108 CFU/ml) and incubated at 37°C for 75 min. To determine the lysis rate, samples of cells treated with the MIC of NaOH and nontreated control cells were collected at 15-min intervals (15, 30, 45, 60, and 75 min) after treatment and spread onto TSA plates. After incubation at 37°C for 24 h, viable colonies were enumerated and results were expressed in numbers of CFU/ml. The efficiency of lysis was determined by using the following formula: lysis rate = [1 − (CFU of treated cells/CFU of nontreated control cells)] × 100%. Lysis efficiency was determined in three independent experiments. At the end of the lysis process, SAGs were harvested by centrifugation (10,000 × g for 10 min at 4°C) and washed three times with PBS. The final pellet was resuspended in sterile PBS and stored at 4°C until further use.
DNA-free SAG analysis by real-time PCR.
To analyze the degradation of DNA in S. aureus cells, samples of bacterial cells treated with the MIC of NaOH and nontreated control cells were collected at 15, 30, 45, 60, and 75 min, respectively. Their genomic DNA was prepared with a bacterial genomic DNA isolation kit (iNtRON Biotechnology), according to the manufacturer's instructions. The extracted genomic DNA was analyzed by electrophoresis in 1% agarose gel stained with ethidium bromide. The gel was examined with the UV gel documentation system and photographed (Canon PowerShot A620). Quantitative real-time PCR assays were performed with the Sybr green detection system. The genomic DNA extracted after various lysis times was used as the template for real-time PCR assays. The S. aureus 16S rRNA was amplified with specific primers (forward, 5′-AGTGTCAAGTGTTAGGGGGTTTC-3′; reverse, 5′-ATTCCTTTGAGTTTCAACCTTGCGGT-3′). The total volume of each tube was 20 μl containing 1 μl of 1:100 template, 1 μl of each primer (10 pmol/μl), 10 μl of 2× Sybr green quantitative PCR master mix (Agilent Technologies), and 7 μl of sterilized distilled water. The reaction was initiated at 95°C for 15 min, which was followed by 40 cycles of 95°C for 10 s, 60°C for 40 s, and 72°C for 60 s. Real-time PCR was carried out in a Stratagene Mx3000P real-time PCR machine (Agilent Technologies). Each sample, including a no-template control, was analyzed in triplicate.
SEM.
Morphological features of SAGs were analyzed by scanning electron microscopy (SEM) as described previously (10). SAGs and nontreated control bacterial cells were fixed in 2.5% glutaraldehyde in 0.1 M phosphate buffer (pH 7.0) for 2 h at 4°C, washed three times with the same buffer, and then postfixed in 1% osmium tetroxide for 1.5 h at 4°C. After another washing, cells were dehydrated in a graded series of ethanol concentrations (10, 30, 50, 70, and 100%). After the samples were critical point dried with liquid CO2, they were mounted on SEM stubs, subsequently sputter coated with gold-palladium, and examined with a scanning electron microscope (Leo 1455VP; Korea Basic Science Institute, Daejeon, South Korea) at an accelerating voltage of 20 kV and a magnification of ×20,000.
Experimental animals.
Ten-week-old male Sprague-Dawley rats were maintained at 23 to 25°C with a 12-h light/dark cycle and had access to a standard pellet diet and water ad libitum. All animal experimental procedures were approved by the Pai Chai University institutional animal care and use committee.
Immunization and challenge experiment.
In order to assess the immunogenicity and protective efficacy of SAGs, a group of 32 rats was divided into four groups of 8, named A, B, C, and D. Group A rats (nonimmunized control group) received sterilized PBS by the subcutaneous route. Group B, C, and D rats were immunized with SAGs (1 × 106 cells/ml) in sterile PBS by the oral, subcutaneous, and intravenous routes, respectively. Rats from all four groups were immunized three times at 2-week intervals (weeks 1, 3, and 5). Two weeks after the last immunization (week 7), all rats were challenged intravenously with virulent S. aureus at 2 × 108 CFU/ml of PBS. To determine the immune response, blood samples were taken from the tail veins of individual rats at 2-week intervals during immunization and after the challenge.
Measurement of antibody response by ELISA.
Sera from immunized and nonimmunized control rats were examined for the presence of specific immunoglobulin G (IgG) by indirect enzyme linked immunosorbent assay (ELISA). Briefly, microtiter plates were coated with 100 μl of antigen (1 × 106 cells/ml) in coating buffer (pH 9.6) and incubated for 2 h at room temperature. The plates were then washed three times with PBS containing Tween 20 (PBS-T) and then blocked with 1% bovine serum albumin (BSA) in PBS-T for 2 h at room temperature. After washing, 100 μl of serially diluted serum was added and the mixture was incubated for 2 h at room temperature. Plates were washed three times with PBS-T, and then 100 μl of alkaline phosphatase-conjugated goat anti-rat IgG (1:30,000; Sigma-Aldrich) in PBS-T with 1% BSA was added and the mixture was incubated for 2 h at room temperature. Plates were then washed three times with PBS-T, and the color was developed by using 100 μl of p-nitrophenylphosphate substrate (Sigma-Aldrich, St. Louis, MO) and incubating the plates for 30 min at room temperature in the dark. The reaction was stopped by adding 100 μl of 3 M NaOH, and the absorbance at 405 nm of the plates was read with a microplate reader (Bio-Rad).
Flow cytometric analysis.
To study the cellular immune response, T-cell markers were examined by collecting blood samples from immunized and nonimmunized control rats on the 7th day (week 6) after the final immunization. Peripheral blood mononuclear cells (PBMCs) were prepared with Histopaque-1077 (Sigma-Aldrich, St. Louis, MO) according to the manufacturer's protocol. The PBMCs were washed three times with cold PBS and stained with appropriately diluted fluorescein isothiocyanate-labeled anti-CD4 or -CD8 monoclonal antibodies at 4°C for 30 min in the dark. After incubation, all samples were washed three times with PBS and resuspended in 0.5 ml of PBS. Data were collected by fluorescence-activated cell sorter (FACS) analysis with a BD FACSCanto II flow cytometer (BD Biosciences) and BD FACSDiva software (BD Biosciences).
Determination of SBA.
Serum bactericidal activity (SBA) was determined as described previously (13, 18), with some modifications. In brief, 100 μl of a bacterial suspension (1 × 106 CFU/ml) was added to 25 μl of serum and incubated at room temperature for 1 h. After incubation, the mixture was spread onto selective medium and incubated at 37°C for 48 h. For the control, serum was replaced with PBS. The percentage of SBA was determined by using the following formula: SBA = [1 − (number of viable bacteria after serum treatment/number of viable bacteria after PBS treatment)] × 100%.
Bacteriological analysis.
To evaluate the protective efficacy of SAG vaccine against a virulent challenge, all experimental rats were sacrificed at 2 weeks (week 9) postchallenge. Their livers, lungs, spleens, and kidneys were collected and aseptically homogenized in 5 ml of sterile cold PBS by using a tissue homogenizer (Sun MI Technology). Tenfold serial dilutions of these tissue homogenates were plated onto selective medium, and the numbers of CFU were determined.
Statistical analysis.
Statistical analyses were performed by one-way analysis of variance or the Kaplan-Meier log rank test with SPSS software (version 14.0). All data are expressed as the mean ± the standard error of the mean. Differences were considered statistically significant at P < 0.05.
RESULTS AND DISCUSSION
Production and characterization of SAGs.
To produce SAGs, we first determined the MIC of NaOH for S. aureus strain KCCM12256 by the 2-fold broth dilution method. The MIC of NaOH for S. aureus was 7.50 mg/ml, and this specific concentration was used to produce SAGs. It is well known that NaOH has the ability to create transmembrane lysis tunnels on the bacterial cell surface, degrade DNA, and turn bacteria into empty cell envelopes (15, 18, 28). In our recent study, we demonstrated that S. Enteritidis ghosts could be produced within 45 min by using the MIC of NaOH (18). In the present study, S. aureus cells treated with the MIC of NaOH showed effective lysis and a rapid decrease in the number of viable cells. Most significantly, the lysis time course curve presented in Fig. 1 shows 100% lysis efficiency in S. aureus cells treated with the MIC of NaOH and no viable cells were detected after 60 min of lysis. These data suggest that the MIC of NaOH would be sufficient to produce inactivated S. aureus bacteria. Generation of BGs from S. aureus has not been reported previously. However, this new strategy has been used with a number of Gram-negative bacterial strains, such as E. coli BL21 (15), E. coli JM109 (16, 17), and S. Enteritidis (18). Therefore, it is interesting that this method might successfully be used to produce BGs from both Gram-positive and Gram-negative bacteria.
FIG 1.
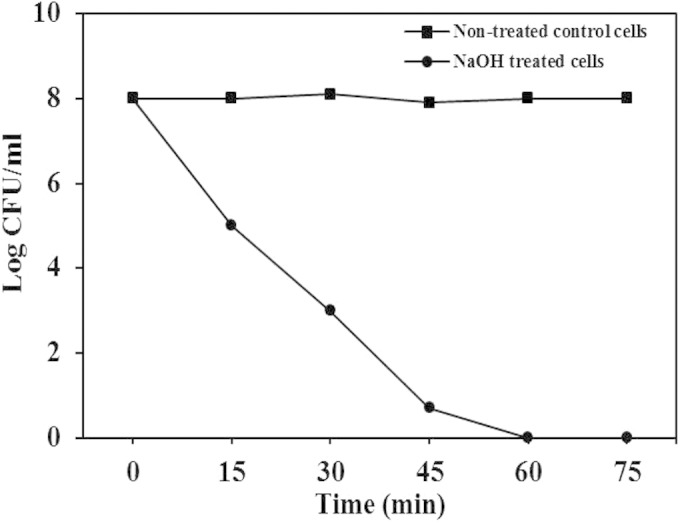
Curves of S. aureus cell lysis at different time points (15, 30, 45, 60, and 75 min). The graph shows the lysis of bacterial cells treated with the MIC of NaOH and nontreated control cells. Lysis was measured by the number of CFU at 15-min intervals after treatment.
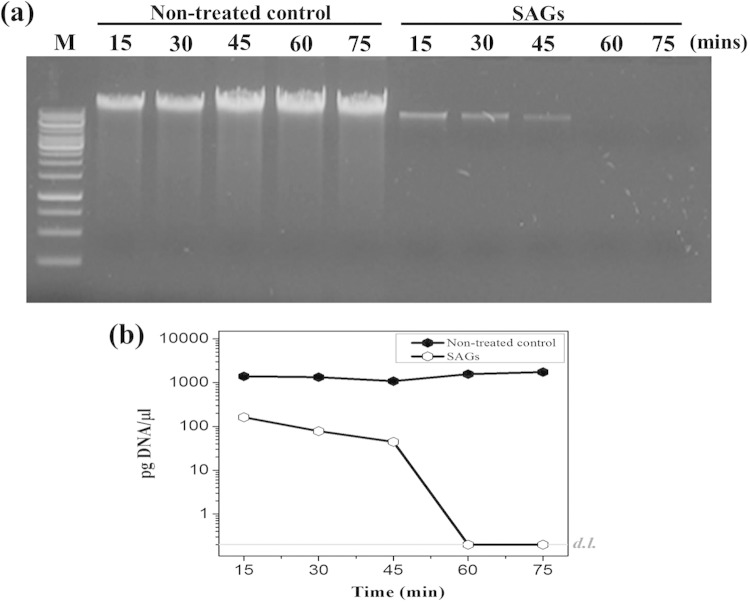
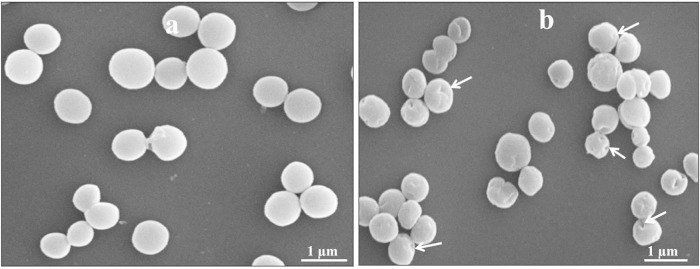
As shown in Fig. 2a, the total DNA content was well comparable to that of the nontreated control (Fig. 2a, lanes 1 to 5) and cells treated with the MIC of NaOH (Fig. 2a, lanes 6 to 10) at various lysis time points. Subsequently, we found that S. aureus cells treated with the MIC of NaOH showed gradual degradation of DNA content at successive time points during lysis, and complete absence of detectable DNA was observed after 60 min of lysis, as shown by real-time PCR analysis (Fig. 2b). This result indicates that cells treated with the MIC of NaOH degraded the genetic content of bacteria during the lysis process. Several reports have demonstrated that the synthesis of virulence and colonizing factors in S. aureus is controlled by several regulatory loci, such as an accessory gene regulator (agr), a staphylococcal accessory regulator (sarA), and an autolysis-related locus (arl) (29–31). In addition, the agr locus is mainly responsible for causing virulence and colonization in experimental animals (32, 33). In this regard, the empty cell envelope of S. aureus would not be expected to cause any problem because of the total loss of DNA content in SAGs. We also examined the formation of transmembrane lysis tunnel structures in SAGs by SEM. Electron microscopic images, as shown in Fig. 3, indicate that the MIC of NaOH induced transmembrane lysis tunnels in SAGs (Fig. 3b; arrowheads) but not in unlysed control cells (Fig. 3a). Furthermore, the MIC of NaOH did not cause any cellular morphology damage except for the lysis pore.
FIG 2.
Analysis of DNA-free SAGs. (a) Total DNA extracted from nontreated control S. aureus cells (lanes 1 to 5) and S. aureus cells treated with the MIC of NaOH (lanes 6 to 10). Lane M, 1-kb marker ladder. (b) DNA contents of nontreated control cells and cells treated with the MIC of NaOH at various time points during lysis, as quantified by real-time PCR with the Sybr green detection system. For practical reasons, the DNA concentrations that fell below the detection limit (d.l.; gray line) are illustrated as corresponding to the limit of detection and the specific concentrations are not shown.
FIG 3.
SEM images of S. aureus (a) and SAGs (b). Arrows indicate transmembrane lysis tunnels.
Humoral immune responses.
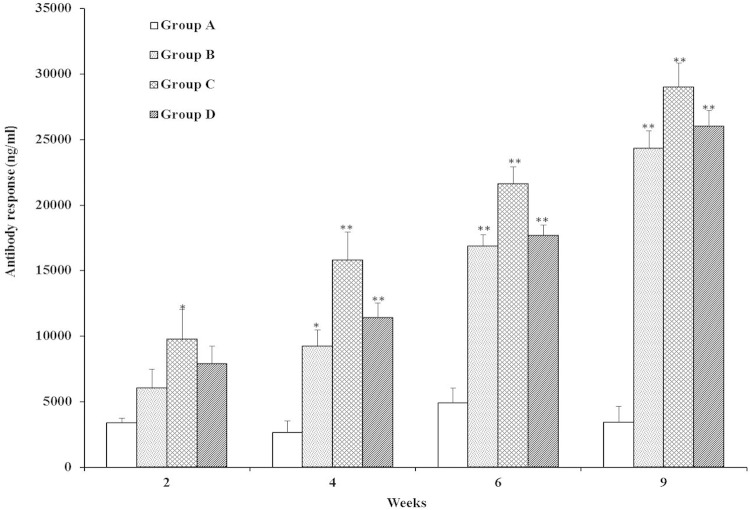
Serum IgG antibodies were induced in rats after immunization and a challenge, as shown in Fig. 4. In week 2, the serum IgG antibody responses in SAG-immunized group C rats were significantly higher than those in nonimmunized control group A rats (P < 0.05). However, no significant difference was observed between groups B and D and group C. Interestingly, the IgG antibody responses in groups B (P < 0.05), C (P < 0.01), and D (P < 0.01) were statistically significantly different from that in control group A in week 4. In week 6, the maximum IgG antibody levels were detected in immunized groups compared to group A (P < 0.01) during the immunization period. Moreover, each booster immunization with SAGs greatly increased the IgG antibody levels in the immunized groups (B, C, and D), with group C rats showing the maximum IgG antibody response. Most significantly, after a challenge with virulent S. aureus, serum IgG antibody levels increased robustly in all of the SAG-immunized groups but not in the nonimmunized group (P < 0.01). Furthermore, no significant differences in IgG antibodies were observed among the immunized groups (B, C, and D) postchallenge. This indicates that immunization with SAGs via the oral, subcutaneous, and intravenous routes was able to induce protective immunity to a virulent challenge. It has been established that BG vaccines derived from Gram-positive bacteria induced significant immune responses and protective efficacy against infections in immunized animals (12, 13). Clarke et al. (22) reported that surface proteins of S. aureus (IsdA or IsdH) stimulated greater IgG immune responses and protected rats from a virulent challenge, suggesting that the humoral immune response is essential for protection against S. aureus. It has previously been demonstrated that peptidoglycan from S. aureus is the important component of protection (34). In another study, immunization with INH-A21 (human IgG antibodies developed from the surface proteins clumping factor A [ClfA] and SdrG) induced phagocytic antibodies and protective immunity to staphylococcal infection in rats and rabbits (35). Further, the administration of various microbial surface components recognizing adhesive matrix molecules from S. aureus has been found to increase IgG specific antibody levels significantly, suggesting that the surface components can induce humoral immune responses (24).
FIG 4.
The IgG antibody response levels in rats immunized with SAGs were determined by indirect ELISA. Sera were obtained from rats in groups A (nonimmunized control), B (orally immunized), C (subcutaneously immunized), and D (intravenously immunized) at weeks 2, 4, 6, and 9. Results are expressed as means ± the standard errors of the means. The asterisks indicate significant differences between the antibody responses of the immunized and nonimmunized groups (**, P < 0.01; *, P < 0.05).
Cellular immune responses.
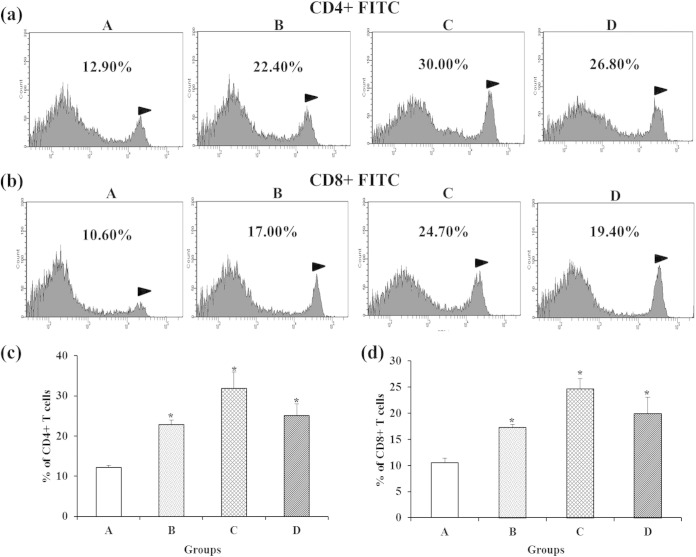
We examined changes in CD4+ and CD8+ T-cell populations in immunized groups by FACS (Fig. 5). The CD4+ and CD8+ T cells of rats in all of the immunized groups differed significantly from those of nonimmunized control group A (P < 0.05). The highest percentage of CD4+ and CD8+ T-cell populations were observed in group C rats. However, the numbers of CD4+ and CD8+ T cells did not differ significantly between groups B and D or between groups C and D. In general, CD4+ (Th1 and Th2) T-helper cells activate opsonic IgG antibodies and cell-mediated immune responses, and cytotoxic CD8+ T cells destroy the intracellular bacterial pathogens (36, 37). It has previously been suggested that CD4+ T cells are essential for the S. aureus vaccine to be effective (38). Moreover, the S. aureus envelope component of PA-immunized animals elicited higher levels of CD4+ and CD8+ T cells than that of control animals (39). Furthermore, CD4+ and CD8+ T cells were found to be involved in protection against S. aureus infections (40). These findings suggest that SAG-induced CD4+ and CD8+ T cells are capable of responding to staphylococcal antigen during a virulent challenge. Earlier attempts to develop whole-cell live or killed S. aureus vaccines had failed to induce protection against S. aureus infection (41). In most cases, administration of live-attenuated vaccines did not induce humoral immunity and protection. Furthermore, vaccination with heat-killed S. aureus was not successful in reducing bacterial loads in organs (42). Compared with other inactivated vaccines, the main advantage of BG vaccines is the ability to preserve their surface antigenic components, which themselves can provide excellent natural intrinsic adjuvant properties (43). More importantly, the empty cell envelope of SAGs contains pathogen-associated molecular patterns (PAMPs) such as peptidoglycan, lipoteichoic acid, and lipoproteins. A number of studies have suggested that these PAMPs can be recognized by Toll-like receptors (TLRs), which induce an innate immune response (44, 45). In particular, TLR2 has been widely thought to play a crucial role in the host immune response to S. aureus (46).
FIG 5.
Assessment of CD4+ and CD8+ T cells by FACS analysis. Shown are populations of CD4+ (a) and CD8+ (b) T cells and the corresponding analysis of significant differences (c and d) between immunized and nonimmunized groups 1 week (week 6) after the final immunization with SAGs. Groups: A, nonimmunized control; B, orally immunized; C, subcutaneously immunized; D, intravenously immunized. The values shown are means ± the standard errors of the means. The asterisks indicate significant differences between the T-cell populations of the immunized and nonimmunized groups (*, P < 0.05).
SBA.
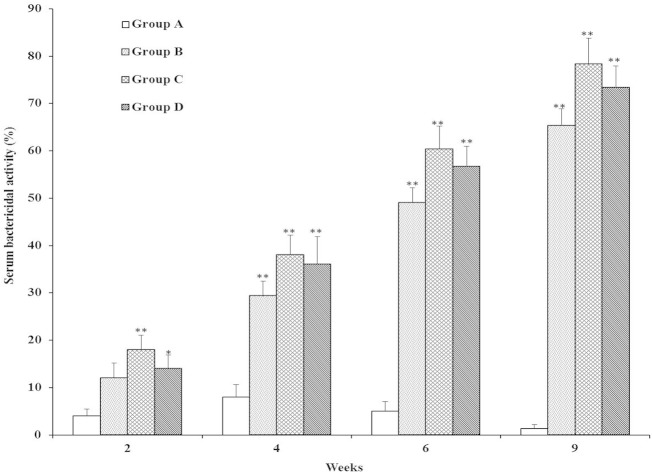
The bactericidal activities against virulent S. aureus were determined in both immunized and nonimmunized control groups (Fig. 6). In week 2, sera from groups C (P < 0.01) and D (P < 0.05) showed statistically significantly higher bactericidal activity than those from group A. Interestingly, we found that the percentage of SBA was significantly higher in all immunized groups (B, C, and D) than in control group A (P < 0.01) in weeks 4 and 6. Moreover, In weeks 4 and 6, no statistically significant differences were observed among the three immunized groups. Most importantly, bactericidal percentages were also robustly higher in SAG-immunized groups than in group A (P < 0.01) postchallenge. Bactericidal activities were not statistically significantly different between SAG-immunized groups. Overall, each booster immunization increased the SBA in all three immunized groups (B, C, and D) during the immunization period and postchallenge. These data indicated that immunization with SAGs orally, subcutaneously, or intravenously efficiently produced bacterium-killing antibodies after a challenge. Recently, it has also been reported that S. iniae ghost vaccine stimulated higher serum bactericidal antibody levels than formalin-killed vaccine and protected experimental animals from a subsequent challenge with S. iniae (13). An earlier report showed that peptidoglycan induces strong bactericidal antibodies capable of eradicating the bacterial pathogen (21).
FIG 6.
Serum bactericidal activities of rats immunized with SAGs. Groups: A, nonimmunized control; B, orally immunized; C, subcutaneously immunized; D, intravenously immunized. Data are expressed as means ± the standard errors of the means. The asterisks indicate significant differences between the serum bactericidal activities of the immunized and nonimmunized groups (**, P < 0.01; *, P < 0.05).
Protective efficacy of SAGs against S. aureus challenge.
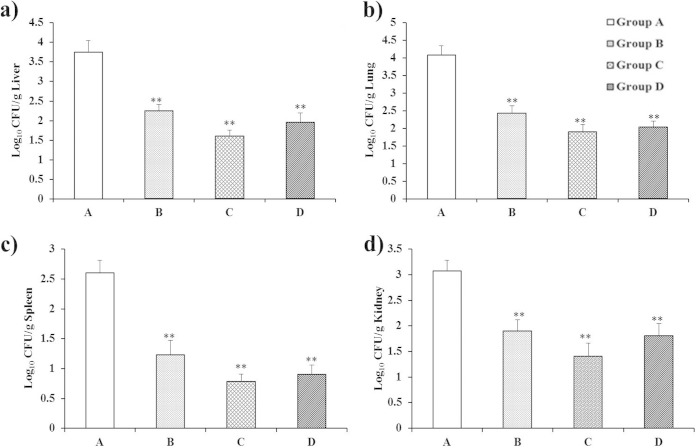
The protective efficacy of SAG vaccine against a challenge with virulent S. aureus was evaluated (Fig. 7). Rats from the immunized and nonimmunized control groups were challenged with virulent S. aureus, and their livers, lungs, spleens, and kidneys were obtained at 2 weeks postchallenge. In this study, we observed that the liver, lung, spleen, and kidney homogenates of groups B, C, and D showed bacterial loads dramatically lower than those of nonimmunized control group A (P < 0.01). Remarkably, immunization with SAGs by the oral route also efficiently stimulated protective immunity to infection. Moreover, Kaplan-Meier survival analysis (Fig. 8) showed significant differences between SAG-immunized groups B, C, and D and nonimmunized control group A (P < 0.05). These findings show that immunization with SAGs by the oral, subcutaneous, and intravenous routes elicited immune responses and protected rats against a virulent S. aureus challenge. Similar findings were reported by Capparelli et al. (21), where immunization of mice with peptidoglycan via the intramuscular, intravenous, and aerosol routes reduced colonization of the liver, lungs, spleen, and kidneys and elicited significant protection from a lethal dose of S. aureus. Further, UV-irradiated genetically attenuated vaccine decreased bacterial burdens and provided protection from a subsequent challenge with S. aureus (47). A study of S. aureus surface proteins revealed that immunization with four combined surface proteins (IsdA, IsdB, SdrA, and SdrE) provided complete protection from a staphylococcal challenge (24). In the same study, it was reported that bacterial clearance from the kidneys was more effective than that achieved by immunization with a single surface protein, indicating that the multicomponent vaccine is needed to control S. aureus infection. In addition, immunization with the multicomponent vaccine has the potential to prevent multiple organ infections, in contrast to a single-component vaccine. Specifically, recent studies suggest that a multicomponent vaccine rather than a single-component vaccine could trigger both humoral and cellular immunity and induce protective immunity to staphylococcal diseases (48, 49). Therefore, our novel strategy of using SAGs as a vaccine could be a new way to prevent S. aureus infections.
FIG 7.
Bacterial loads in liver (a), lung (b), spleen (c), and kidney (d) homogenates after a virulent S. aureus challenge. Results are expressed as means ± the standard errors of the means. The asterisks indicate significant differences between the bacterial burdens of the immunized and nonimmunized groups (**, P < 0.01).
FIG 8.
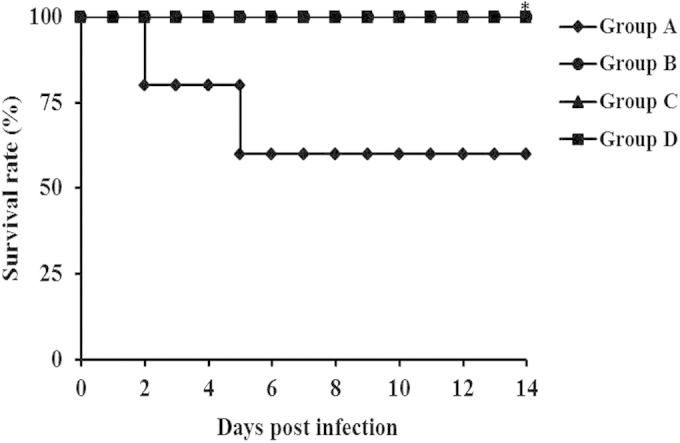
Kaplan-Meier analysis of the survival of rats immunized with SAGs (groups B, C, and D) or treated with PBS (group A) and challenged intravenously with S. aureus. Groups: A, nonimmunized control; B, orally immunized; C, subcutaneously immunized; D, intravenously immunized. The asterisks indicate significant differences between the survival rates of the immunized and nonimmunized groups (*, P < 0.05).
Conclusion.
In conclusion, nonliving SAGs have been successfully generated by using the MIC of NaOH and the approach is more rapid and cost-effective than other methods. Interestingly, the present strategy may open the door to the production of BGs from Gram-positive bacteria. Most importantly, we have shown that immunization with SAGs induced significant humoral and cellular immune responses and provided strong protection against a virulent challenge in rats. Therefore, our present findings could be useful in the future development of vaccines against S. aureus infections.
ACKNOWLEDGMENT
This work was supported by Business for Cooperative R&D between Industry, Academy, and Research Institute funded Korea Small and Medium Business Administration in 2014 (grant C0191740).
REFERENCES
- 1.Archer GL, Climo MW. 2001. Staphylococcus aureus bacteremia—consider the source. N Engl J Med 344:55–56. doi: 10.1056/NEJM200101043440110. [DOI] [PubMed] [Google Scholar]
- 2.Weems JJ., Jr 2001. The many faces of Staphylococcus aureus infection: recognizing and managing its life-threatening manifestations. Postgrad Med 110:24–36. doi: 10.3810/pgm.2001.10.1042. [DOI] [PubMed] [Google Scholar]
- 3.Mongkolrattanothai K, Boyle S, Kahana MD, Daum RS. 2003. Severe Staphylococcus aureus infections caused by clonally related community-acquired methicillin-susceptible and methicillin-resistant isolates. Clin Infect Dis 37:1050–1058. doi: 10.1086/378277. [DOI] [PubMed] [Google Scholar]
- 4.Le Loir Y, Baron F, Gautier M. 2003. Staphylococcus aureus and food poisoning. Genet Mol Res 2:63–76. [PubMed] [Google Scholar]
- 5.Lowy FD. 1998. Staphylococcus aureus infections. N Engl J Med 339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 6.Cosgrove SE, Qi Y, Kaye KS, Harbarth S, Karchmer AW, Carmeli Y. 2005. The impact of methicillin resistance in Staphylococcus aureus bacteremia on patient outcomes: mortality, length of stay, and hospital charges. Infect Control Hosp Epidemiol 26:166–174. doi: 10.1086/502522. [DOI] [PubMed] [Google Scholar]
- 7.Drew RH. 2007. Emerging options for treatment of invasive, multidrug-resistant Staphylococcus aureus infections. Pharmacotherapy 27:227–249. doi: 10.1592/phco.27.2.227. [DOI] [PubMed] [Google Scholar]
- 8.Langemann T, Koller VJ, Muhammad A, Kudela P, Mayr UB, Lubitz W. 2010. The bacterial ghost platform system: production and applications. Bioeng Bugs 1:326–336. doi: 10.4161/bbug.1.5.12540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jawale CV, Chaudhari AA, Jeon BW, Nandre RM, Lee JH. 2012. Characterization of a novel inactivated Salmonella enterica serovar Enteritidis vaccine candidate generated using a modified cI857/λ PR/gene E expression system. Infect Immun 80:1502–1509. doi: 10.1128/IAI.06264-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu W, Yang G, Zhang Y, Yuan J, An L. 2012. Generation of biotechnology-derived Flavobacterium columnare ghosts by PhiX174 gene E-mediated inactivation and the potential as vaccine candidates against infection in grass carp. J Biomed Biotechnol 2012:760730. doi: 10.1155/2012/760730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lubitz P, Mayr UB, Lubitz W. 2009. Applications of bacterial ghosts in biomedicine. Adv Exp Med Biol 655:159–170. doi: 10.1007/978-1-4419-1132-2_12. [DOI] [PubMed] [Google Scholar]
- 12.Ra CH, Kim YJ, Park SJ, Jeong CW, Nam YK, Kim KH, Kim SK. 2009. Evaluation of optimal culture conditions for recombinant ghost bacteria vaccine production with the antigen of Streptococcus iniae GAPDH. J Microbiol Biotechnol 19:982–986. doi: 10.4014/jmb.0901.007. [DOI] [PubMed] [Google Scholar]
- 13.Hossain MMM, Ehsan A, Rahman MA, Chowdhury MBR, Haq M. 2012. Responses of monosex Nile tilapia (Oreochromis niloticus) to intraperitoneal challenge by Streptococcus iniae after vaccination with ghosts of the bacterium. Bangl Vet 29:31–37. doi: 10.3329/bvet.v29i1.11889. [DOI] [Google Scholar]
- 14.Hu M, Zhang Y, Xie F, Li G, Li J, Si W, Liu S, Hu S, Zhang Z, Shen N, Wang C. 2013. Protection of piglets by a Haemophilus parasuis ghost vaccine against homologous challenge. Clin Vaccine Immunol 20:795–802. doi: 10.1128/CVI.00676-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amara AA, Salem-Bekhit MM, Alanazi FK. 2013. Sponge-like: a new protocol for preparing bacterial ghosts. Sci World J 2013:545741. doi: 10.1155/2013/545741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amro AA, Salem-Bekhit MM, Alanazi FK. 2014. Plackett-Burman randomization method for bacterial ghosts preparation from E. coli JM109. Saudi Pharm J 22:273–279. doi: 10.1016/j.jsps.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amara AA, Salem-Bekhit MM, Alanazi FK. 2013. Preparation of bacterial ghosts for E. coli JM109 using “sponge-like reduced protocol.” Asian J Biol. Sci 6:363–369. doi: 10.3923/ajbs.2013.363.369. [DOI] [Google Scholar]
- 18.Vinod N, Oh S, Kim S, Choi CW, Kim SC, Jung CH. 2014. Chemically induced Salmonella enteritidis ghosts as a novel vaccine candidate against virulent challenge in a rat model. Vaccine 32:3249–3255. doi: 10.1016/j.vaccine.2014.03.090. [DOI] [PubMed] [Google Scholar]
- 19.Verkaik NJ, van Wamel WJ, van Belkum A. 2011. Immunotherapeutic approaches against Staphylococcus aureus. Immunotherapy 3:1063–1073. doi: 10.2217/imt.11.84. [DOI] [PubMed] [Google Scholar]
- 20.Lee JC. 2003. Staphylococcus aureus vaccine, p 283–293. In Ellis RW, Brodeur BR (ed), New bacterial vaccines. Kluwer Academic/Plenum Publishers, New York, NY. [Google Scholar]
- 21.Capparelli R, Nocerino N, Medaglia C, Blaiotta G, Bonelli P, Iannelli D. 2011. The Staphylococcus aureus peptidoglycan protects mice against the pathogen and eradicates experimentally induced infection. PLoS One 6:e28377. doi: 10.1371/journal.pone.0028377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clarke SR, Brummell KJ, Horsburgh MJ, McDowell PW, Mohamad SA, Stapleton MR, Acevedo J, Read RC, Day NP, Peacock SJ, Mond JJ, Kokai-Kun JF, Foster SJ. 2006. Identification of in vivo-expressed antigens of Staphylococcus aureus and their use in vaccinations for protection against nasal carriage. J Infect Dis 193:1098–1108. doi: 10.1086/501471. [DOI] [PubMed] [Google Scholar]
- 23.Romagnani S, Giudizi MG, Biagiotti R, Almerigogna F, Maggi E, Del Prete G, Ricci M. 1981. Surface immunoglobulins are involved in the interaction of protein A with human B cells and in the triggering of B cell proliferation induced by protein A-containing Staphylococcus aureus. J Immunol 127:1307–1313. [PubMed] [Google Scholar]
- 24.Stranger-Jones YK, Bae T, Schneewind O. 2006. Vaccine assembly from surface proteins of Staphylococcus aureus. Proc Natl Acad Sci U S A 103:16942–16947. doi: 10.1073/pnas.0606863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stewart-Tull DE. 1980. The immunological activities of bacterial peptidoglycans. Annu Rev Microbiol 34:311–340. doi: 10.1146/annurev.mi.34.100180.001523. [DOI] [PubMed] [Google Scholar]
- 26.Fernandes CJ, Stevens DA, Groot Obbink DJ, Ackerman VP. 1985. A replicator method for the combined determination of minimum inhibitory concentration and minimum bactericidal concentration. J Antimicrob Chemother 15:53–60. doi: 10.1093/jac/15.1.53. [DOI] [PubMed] [Google Scholar]
- 27.Tavares LC, Vessoni Penna TC, Amaral AT. 1997. Synthesis and biological activity of nifuroxazide and analogs. Boll Chim Farm 3:244–249. [PubMed] [Google Scholar]
- 28.Rehman A-U, Stodart B, Raman H. 2007. A high throughput, non-organic method for plant genomic DNA isolation. Pak J Bot 39:831–840. [Google Scholar]
- 29.Traber K, Lee E, Benson S, Corrigan R, Cantera M, Shopsin B, Novick RP. 2008. Agr function in clinical Staphylococcus aureus isolates. Microbiology 154:2265–2274. doi: 10.1099/mic.0.2007/011874-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Novick RP. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol Microbiol 48:1429–1449. doi: 10.1046/j.1365-2958.2003.03526.x. [DOI] [PubMed] [Google Scholar]
- 31.Fournier B, Klier A, Rapoport G. 2001. The two-component system ArlS-ArlR is a regulator of virulence gene expression in Staphylococcus aureus. Mol Microbiol 41:247–261. doi: 10.1046/j.1365-2958.2001.02515.x. [DOI] [PubMed] [Google Scholar]
- 32.Abdelnour A, Arvidson S, Bremell T, Rydén C, Tarkowski A. 1993. The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model. Infect Immun 61:3879–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gillaspy AF, Hickmon SG, Skinner RA, Thomas JR, Nelson CL, Smeltzer MS. 1995. Role of the accessory gene regulator (agr) in pathogenesis of staphylococcal osteomyelitis. Infect Immun 63:3373–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y, Liu B, Yang D, Li X, Wen L, Zhu P, Fu N. 2011. Peptide mimics of peptidoglycan are vaccine candidates and protect mice from infection with Staphylococcus aureus. J Med Microbiol 60:995–1002. doi: 10.1099/jmm.0.028647-0. [DOI] [PubMed] [Google Scholar]
- 35.Vernachio JH, Bayer AS, Ames B, Bryant D, Prater BD, Syribeys PJ, Gorovits EL, Patti JM. 2006. Human immunoglobulin G recognizing fibrinogen-binding surface proteins is protective against both Staphylococcus aureus and Staphylococcus epidermidis infections in vivo. Antimicrob Agents Chemother 50:511–518. doi: 10.1128/AAC.50.2.511-518.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macatonia SE, Hsieh CS, Murphy KM, O'Garra A. 1993. Dendritic cells and macrophages are required for Th1 development of CD4 T cells from αβ TCR transgenic mice: IL-12 substitution for macrophages to stimulate IFN-γ production is IFN-γ-dependent. Int Immunol 5:1119–1128. doi: 10.1093/intimm/5.9.1119. [DOI] [PubMed] [Google Scholar]
- 37.Lee J-W, O'Brien CN, Guidry AJ, Paape MJ, Shafer-Weaver KA, Zhao X. 2005. Effect of a trivalent vaccine against Staphylococcus aureus mastitis lymphocyte subpopulations, antibody production, and neutrophil phagocytosis. Can J Vet Res 69:11–18. [PMC free article] [PubMed] [Google Scholar]
- 38.Lawrence PK, Rokbi B, Arnaud-Barbe N, Sutten EL, Norimine J, Lahmers KK, Brown WC. 2012. CD4 T cell antigens from Staphylococcus aureus Newman strain identified following immunization with heat-killed bacteria. Clin Vaccine Immunol 19:477–489. doi: 10.1128/CVI.05642-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghosh AK, Sinha P, Das T, Sa G, Ray PK. 1999. S. aureus superantigen protein A expands CD4+/CD8+/CD19+/CD34+ cells in mice: a potential immunorestorer. Biochem Biophys Res Commun 256:142–146. doi: 10.1006/bbrc.1999.0194. [DOI] [PubMed] [Google Scholar]
- 40.Gómez MI, Sordelli DO, Buzzola FR, García VE. 2002. Induction of cell-mediated immunity to Staphylococcus aureus in the mouse mammary gland by local immunization with a live attenuated mutant. Infect Immun 70:4254–4260. doi: 10.1128/IAI.70.8.4254-4260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rogers DE, Melly MA. 1965. Speculations on the immunology of staphylococcal infections. Ann N Y Acad Sci 128:274–284. [DOI] [PubMed] [Google Scholar]
- 42.Schmaler M, Jann NJ, Ferracin F, Landmann R. 2011. T and B cells are not required for clearing Staphylococcus aureus in systemic infection despite a strong TLR2-MyD88-dependent T cell activation. J Immunol 186:443–452. doi: 10.4049/jimmunol.1001407. [DOI] [PubMed] [Google Scholar]
- 43.Riedmann EM, Kyd JM, Cripps AW, Lubitz W. 2007. Bacterial ghosts as adjuvant particles. Expert Rev Vaccines 6:241–253. doi: 10.1586/14760584.6.2.241. [DOI] [PubMed] [Google Scholar]
- 44.Bubeck Wardenburg J, Williams WA, Missiakas D. 2006. Host defenses against Staphylococcus aureus infection require recognition of bacterial lipoproteins. Proc Natl Acad Sci U S A 103:13831–13836. doi: 10.1073/pnas.0603072103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Seo HS, Michalek SM, Nahm MH. 2008. Lipoteichoic acid is important in innate immune responses to Gram-positive bacteria. Infect Immun 76:206–213. doi: 10.1128/IAI.01140-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fournier B, Philpott DJ. 2005. Recognition of Staphylococcus aureus by the innate immune system. Clin Microbiol Rev 18:521–540. doi: 10.1128/CMR.18.3.521-540.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burnside K, Lembo A, Harrell MI, Klein JA, Lopez-Guisa J, Siegesmund AM, Torgerson TR, Oukka M, Molina DM, Rajagopal L. 2012. Vaccination with a UV-irradiated genetically attenuated mutant of Staphylococcus aureus provides protection against subsequent systemic infection. J Infect Dis 206:1734–1744. doi: 10.1093/infdis/jis579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spellberg B, Daum R. 2012. Development of a vaccine against Staphylococcus aureus. Semin Immunopathol 34:335–348. doi: 10.1007/s00281-011-0293-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anderson AS, Miller AA, Donald RG, Scully IL, Nanra JS, Cooper D, Jansen KU. 2012. Development of a multicomponent Staphylococcus aureus vaccine designed to counter multiple bacterial virulence factors. Hum Vaccin Immunother 8:1585–1594. doi: 10.4161/hv.21872. [DOI] [PMC free article] [PubMed] [Google Scholar]