Abstract
The role of CD19+ CD5+ and CD19+ CD5− B cell subpopulations in the antibody response to pneumococcal capsular polysaccharides (caps-PSs) is controversial. In the present study, we evaluated the role of human CD19+ CD5+ and CD19+ CD5− cell populations in the serotype-specific antibody response to caps-PS. After vaccination of 5 healthy human adults with Pneumovax (23-valent pneumococcal polysaccharide vaccine [PPV23]), IgG anti-caps-PS serotype 4 antibody-producing cells resided mainly in the CD19+ CD5− B cell subset, as assessed by enzyme-linked immunosorbent spot (ELISpot) analysis. Moreover, in a humanized SCID mouse model, CD19+ CD5− B cells were more effective than CD19+ CD5+ cells in producing IgG anti-cap-PS antibodies. Finally, an association was found between the level of IgG anti-caps-PS antibodies and the number of CD19+ CD5− B cells in 33 humans vaccinated with PPV23. Taken together, our data suggest that CD5 defines a functionally distinct population of B cells in humans in the anti-caps-PS immune response.
INTRODUCTION
Infections caused by Streptococcus pneumoniae (e.g., pneumonia, septicemia, and meningitis) are an important cause of mortality and morbidity, especially among young children, the elderly, and immunocompromised patients. Host protection against pneumococcal infections is mediated by antibodies (Abs) against pneumococcal surface proteins and capsular polysaccharides (caps-PSs) (1). Caps-PSs are a main determinant of virulence of S. pneumoniae, and antibodies to caps-PS, which are considered T lymphocyte-independent type 2 antigens, are protective against infection with S. pneumoniae. In mice, antibodies to T lymphocyte-independent antigens have been demonstrated to be generated by a distinctive B lymphocyte population, the so-called B-1 lymphocytes (2). B-1 lymphocytes represent a unique B-cell population that can be differentiated from marginal zone B cells and follicular B cells (together referred to as B-2 cells) by surface marker expression, developmental origin, self-renewing capacity, and functions (2). Furthermore, murine B-1 cells can be separated phenotypically into two functionally different subpopulations on the basis of surface expression of CD5. The CD5+ subset (referred to as B-1a cells) is responsible for the constitutive secretion of polyspecific antibodies (mainly IgM), whereas the CD5− subset (B-1b cells) produces antibodies (IgM, IgA, and IgG isotype) only after antigen-specific stimulation, including stimulation with caps-PS antigens of S. pneumoniae (3, 4).
In humans, the existence of B-1 cells has been open to question for many years. Recently, Griffin et al. (5) claimed the discovery of a small subset of human CD20+ B cells expressing CD27 and CD43 that recap the functional characteristics of murine B-1 cells. However, controversy has arisen about the nature of these cells, and we recently showed that CD27+ CD43+ B cells are in fact activated cells on their way to plasma cell differentiation (6–9).
We recently evaluated whether CD20+ CD27+ CD43+ B cells are involved in antibody production in response to pneumococcal caps-PS and found that the numbers of these cells increased in peripheral blood 1 week after vaccination with unconjugated pneumococcal polysaccharide vaccine (PPV23) (8). Interestingly, enzyme-linked immunosorbent spot (ELISpot) analysis of isolated cell populations revealed that the CD5− subset of these cells had a higher capacity than the CD5+ subset for the production caps-PS-specific antibodies (particularly IgG) (8). This finding (8) is in line with data from an older study by Barrett et al., who found that anti-type 4 pneumococcal polysaccharide antibody-secreting cells were found in the CD5− B cell subpopulation (10). Furthermore, the importance of the CD5− subset of B cells for antibody production in response to polysaccharide antigens is also suggested by peripheral blood CD5+ B cell predominance in patients with a specific antipolysaccharide antibody deficiency (11).
Taken together, the above-summarized data suggest a functional role of CD5− B cells in the antibody response to caps-PS. However, the concept that CD5 defines a functionally distinct population of B cells in the human anti-caps-PS immune response is not generally accepted. Carsetti et al. assert that human IgM memory B cells that are generated in the spleen control the immune response to pneumococcal caps-PS and claim that CD5 does not define a functionally distinct population of B cells in the human anti-caps-PS response (12, 13).
Given the controversial nature of this subject, we sought to further delineate the role of the CD19+ CD5+ and CD19+ CD5− B cell subpopulations in the human antibody response to pneumococcal caps-PS. In order to realize this, we performed studies with humans and humanized SCID mice.
MATERIALS AND METHODS
Subjects.
Thirty-three patients (median age, 4 years; age range, 2 to 41 years) who were referred (between 2008 and 2010) to our institution for increased susceptibility to respiratory infections were included in this study. Recurrent infection of the upper respiratory tract was defined as at least 5 episodes of upper respiratory tract infections complicated by otitis media or chronic draining ears (>3 weeks) in a 1-year period. Recurrent infection of the lower respiratory tract was defined as at least 3 lower respiratory tract infections in a 1-year period, with radiographic evidence of pneumonia in at least 2 episodes. These patients were immunized with a 23-valent pneumococcal polysaccharide vaccine (PPV23) (Pneumovax; Sanofi MSD) for evaluation of the humoral immune system as part of the clinical workup. Children (aged 2 to 5 years) had received a conjugated pneumococcal vaccine (Prevnar 7; Pfizer) during their first year of life.
Five healthy adults (laboratory workers and students) were immunized with PPV23 specifically for this study, which was approved by the ethics committee of the University Hospital Leuven. Seven days after immunization, blood was taken and analyzed by ELISpot analysis.
Detection of anti-caps-PS antibody-secreting cells by ELISpot assay.
A caps-PS-specific ELISpot assay was performed as previously described by Mascart-Lemone et al. (14), with slight modifications. In brief, individual wells of a nitrocellulose Millititer HA plate (Mahans 4550; Millipore) were coated overnight at 4°C in a humid chamber with 100 μl of a poly-l-lysine hydrobromide (molecular weight [MW], 30,000 to 70,000; Sigma)-coupled caps-PS-specific antigen solution. A total of 5 × 104 sorted B cells were applied per well.
Mice.
CB17 PrkdcSCID/PrkdcSCID (SCID) mice (Jackson Laboratory) were bred under sterile conditions and fed ad libitum with autoclaved food and water. SCID mice were held in a room with a 12-h/12-h light/dark cycle. SCID mice were tested for leakiness by analyzing the level of mouse IgG Abs according to a previously described enzyme-linked immunosorbent assay (ELISA) (15). Only 6- to 10-week-old SCID mice with IgG concentrations of <2 μg/ml were used in the experiments. Approval of this study was granted by the KU Leuven ethics committee for animal experiments.
Isolation of PBMCs and cell subsets.
Peripheral blood buffy coat from healthy blood donors was obtained from the Blood Transfusion Centre of the Red Cross Flanders. No information about previous exposure of human donors to any particular S. pneumoniae serotype was available, nor was the vaccination status of the blood donors known. Human peripheral blood mononuclear cells (PBMCs) were prepared by Ficoll-Hypaque density gradient centrifugation. The viability of human PBMCs (>99%) was tested by trypan blue exclusion. Enriched CD19+ B cells (obtained with CD19 human microbeads) were stained with anti-CD5 allophycocyanin (clone UCHT2) or with an isotype control (mouse IgG1) (4°C for 30 min) and sorted on a FACSAria cell sorter (BD Biosciences) under sterile conditions.
Cell transfer experiments and in vivo immunization.
SCID mice were treated intraperitoneally (i.p.) with 1 mg TMβ1 1 day before humanization to improve human cell engraftment (16). Anti-NK-treated SCID mice were immunized i.p. with 20 μl of PPV23 at the same time as they received the human cells. The CD19+/CD4+ ratio of the cells transferred corresponded to the CD19+/CD4+ ratio in the donor buffy coat. Fourteen days later, blood was drawn by heart puncture in isoflurane (Schering-Plough Animal Health)-anesthetized mice. Mice were euthanized by cervical dislocation after isoflurane inhalation.
Detection of caps-PS-specific Ig by ELISA.
Antibodies to cap-PS serotypes were measured by an ELISA, as previously described (17, 18). Sera were preabsorbed with C polysaccharide and serotype 22F to remove cross-reactive antibodies before application onto coated plates. For each sample, six 2-fold dilutions (starting from a 1:200 dilution for serum from humans and a 1:6.25 dilution for sera from humanized mice) were analyzed. For quantification of anti-caps-PS antibodies, U.S. pneumococcal reference serum lot 89-SF was used as a reference. For calculation of the concentration, a 4-parameter logistic-log curve fit model was used.
Opsonophagocytic activity.
Opsonophagocytic activity was determined for serotypes 18C, 19F, 6A, and 9V by a 4-fold multiplex opsonophagocytosis assay (19). Titers were expressed as serum dilutions with 50% killing of the given serotype. A titer below the detection limit of 8 was reported as 4 for the purpose of data analyses.
Statistical methods.
Statistical tests were performed by using Analyze-it for Microsoft Excel 3.90. A Wilcoxon-Mann-Whitney test was used to evaluate differences in antibody-secreting cells (between CD19+ CD5− and CD19+ CD5+). Differences in antibody responses and opsonophagocytic activity between CD19+ CD5− B cells and CD19+ CD5+ B cells were evaluated by use of a Kruskal-Wallis test (significance level, 5%). Correlations between antibody titers and opsonophagocytic activity and between anti-caps-PS antibody responses and the amount of CD19+ CD5− B cells were evaluated by use of the Spearman correlation (significance level, 5%).
RESULTS
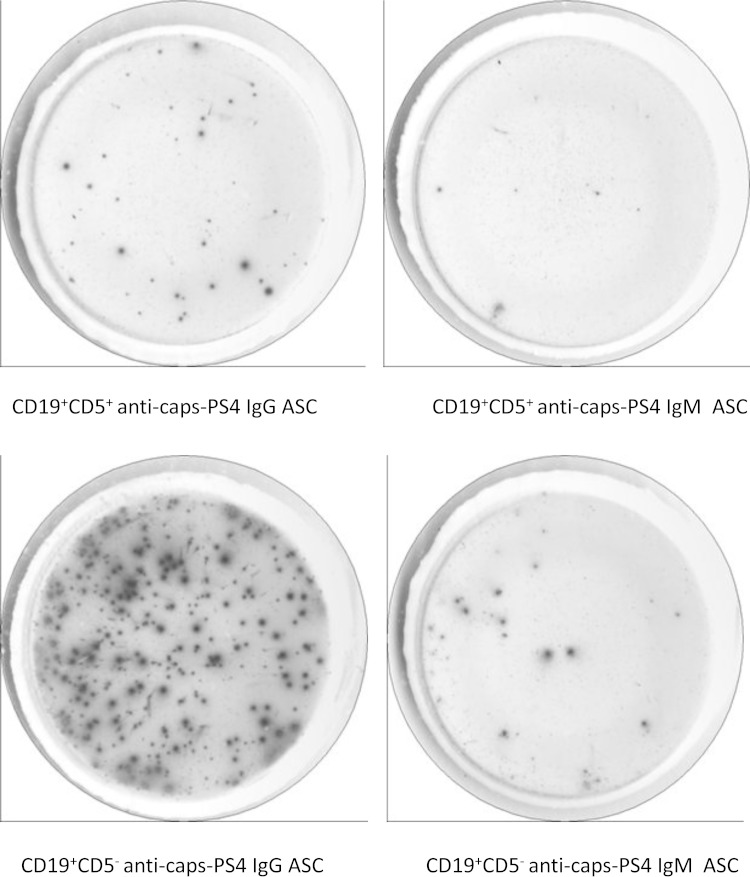
We vaccinated five healthy adults with PPV23 and evaluated the capacity of CD19+ CD5− and CD19+ CD5+ B cells to secrete serotype 4 caps-PS-specific antibodies by an ELISpot assay 7 days after vaccination. Serotype 4 was selected, as this serotype was previously used for ELISpot analysis (8, 10). Although there was a substantial variation in the number of antibody-secreting cells among the five individuals (summarized in Table 1), the number of IgG antibody-secreting cells consistently tended to be higher in the CD19+ CD5− cell subset than in the CD19+ CD5+ cell subset (P = 0.06 by Wilcoxon test). The numbers of IgM anti-caps-PS serotype 4 antibody-secreting cells were comparable between the two subsets (P = 0.8 by Wilcoxon test). Figure 1 illustrates the results from one representative individual. No anti-caps-PS antibody-secreting cells were found before vaccination (data not shown).
TABLE 1.
Capacity of CD19+ CD5− and CD19+ CD5+ cells to secrete caps-PS-specific antibodies after vaccinationa
| Healthy individual | No. of cells secreting IgG |
No. of cells secreting IgM |
||
|---|---|---|---|---|
| CD19+ CD5− | CD19+ CD5+ | CD19+ CD5− | CD19+ CD5+ | |
| 1 | 298 | 57 | 31 | 19 |
| 2 | 119 | 31 | 14 | 20 |
| 3 | 4 | 1 | 3 | 1.5 |
| 4 | 52 | 45 | 25 | 19 |
| 5 | 31 | 24 | 27 | 31 |
Five healthy individuals were vaccinated with PPV23. The capacity of CD19+ CD5− and CD19+ CD5+ cells to secrete caps-PS-specific antibodies was evaluated by ELISpot analysis 7 days after vaccination. Shown are the numbers of IgG and IgM anti-caps-PS serotype 4 antibody-secreting cells per 2.5 × 104 cells for the CD19+ CD5− and CD19+ CD5+ subsets.
FIG 1.
Caps-PS antibody secretion by CD19+ CD5− and CD19+ CD5+ B cell subsets after vaccination with PPV23. CD19+ CD5+ B cells (2.5 × 104/well) and CD19+ CD5− B cells (2.5 × 104/well) obtained from a healthy adult at 7 days post-PPV23 vaccination were analyzed for their capacity to secrete anti-caps-PS serotype 4 antibodies by an ELISpot assay. ASC, antibody-secreting cells.
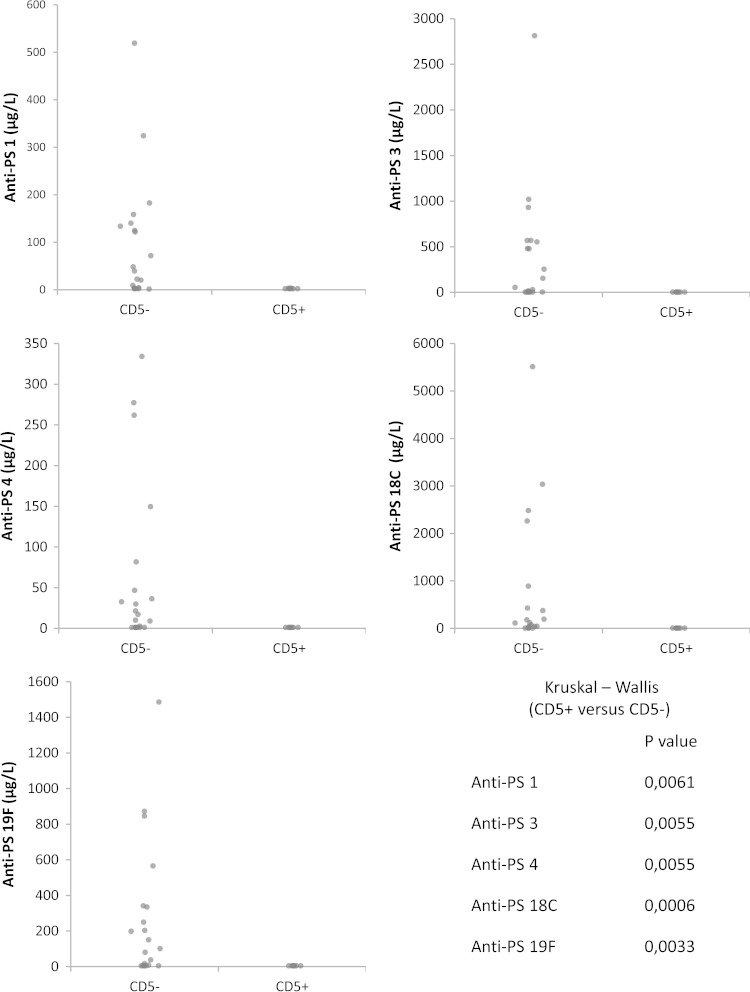
To evaluate the role of CD19+ CD5+ and CD19+ CD5− B cells further, we used a humanized SCID mouse model. TMβ1-treated SCID mice were transplanted i.p. with PBMCs (containing 20 × 106 ± 4.0 × 106 CD4+ cells) depleted of B lymphocytes in combination with either CD19+ CD5+ enriched B cells (2 × 106 to 3 × 106 cells; average purity, 79% CD19+ CD5+) or CD19+ CD5− enriched B cells (containing 2 × 106 to 3 × 106 cells; average purity, 95%). Two weeks after immunization with a reduced dose of PPV23 (20 μl; two-fifths of the dose used in humans), antibodies to serotypes 1, 3, 4, 18C, and 19F were measured by an ELISA. These serotypes were selected because they are immunogenic (20) and have been prevalent in Belgium over the last 15 years (21, 22). SCID mice reconstituted with CD19+ CD5− B cells produced significantly higher levels of IgG antibodies than did SCID mice reconstituted with CD19+ CD5+ B cells (Fig. 2). The IgM antibody response was low and comparable between CD5+ and CD5− cells (data not shown). Immunized SCID mice that had not been transplanted generated no antibodies (data not shown). Thus, CD19+ CD5− B cells were more effective than CD19+ CD5− cells for IgG antibody production.
FIG 2.
Effect of B-cell subsets on the IgG antibody response to soluble caps-PS. SCID mice were transplanted with PBMCs depleted of CD19+ B cells in combination with either CD5+ B lymphocytes or CD5− B lymphocytes and immunized with soluble caps-PS (PPV23). The immune response to caps-PS (serotypes 1, 3, 4, 18C, and 19F) was measured after 14 days. Data from five independent experiments (n = 7 for CD5+; n = 21 for CD5−) were pooled. For each experiment, the CD5+ and CD5− B lymphocytes came from a single individual (five independent experiments with cells obtained from five different individuals).
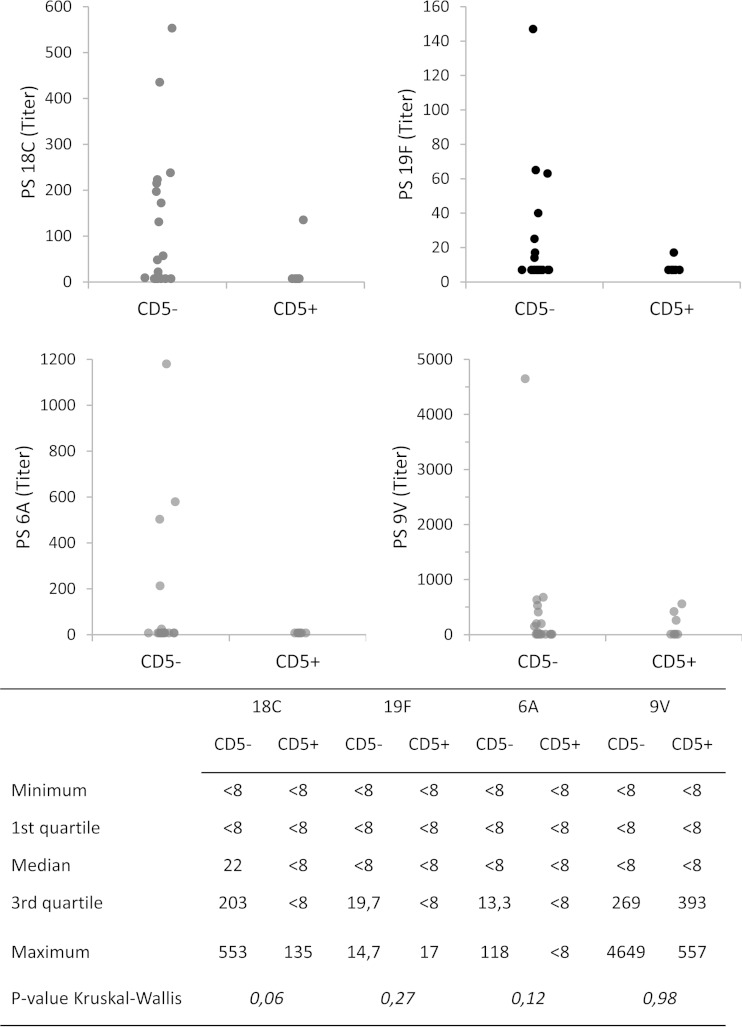
In order to evaluate the functional activity of the antibodies produced, we studied opsonophagocytosis in SCID mice reconstituted with either CD19+ CD5− B cells or CD19+ CD5+ B cells. While our focus was on serotypes 18C and 19F, for which we had ELISA values, the assay was multiplexed with serotypes 6A and 9V. Analysis of opsonophagocytosis of additional serotypes was hampered by the limited serum volumes available from the humanized SCID mice. The level of opsonophagocytosis was low in SCID mice reconstituted with CD19+ CD5+ B cells. In SCID mice reconstituted with CD19+ CD5− B cells, the level of opsonophagocytosis tended to be higher than those in SCID mice reconstituted with CD19+ CD5+ B cells for caps-PS serotype 18C (P = 0.06), caps-PS serotype 6A (P = 0.12), and caps-PS serotype 19F (P = 0.28) but not for caps-PS serotype 9V (P = 0.98 by Kruskal-Wallis test) (Fig. 3). For caps-PS serotype 18C, there was a statistically significant correlation between opsonophagocytosis and IgG titers (Pearson correlation coefficient [rs], 0.689; P = <0.0001). For caps-PS serotype 19F, the Pearson correlation coefficient was 0.321 (P = 0.09).
FIG 3.
Effect of B cell subsets on opsonophagocytic activity. SCID mice were transplanted with PBMCs depleted of CD19+ B cells in combination with either CD5+ B lymphocytes or CD5− B lymphocytes and immunized with soluble caps-PS (PPV23). The opsonophagocytic activity in response to caps-PS serotypes 18C, 19F, A, and 9V was measured after 14 days. Data from five independent experiments (n = 7 for CD5+; n = 21 for CD5−) were pooled. For each experiment, the CD5+ and CD5− B lymphocytes came from a single individual (five independent experiments with cells obtained from five different individuals). The y axis represents titers expressed as serum dilutions with 50% killing.
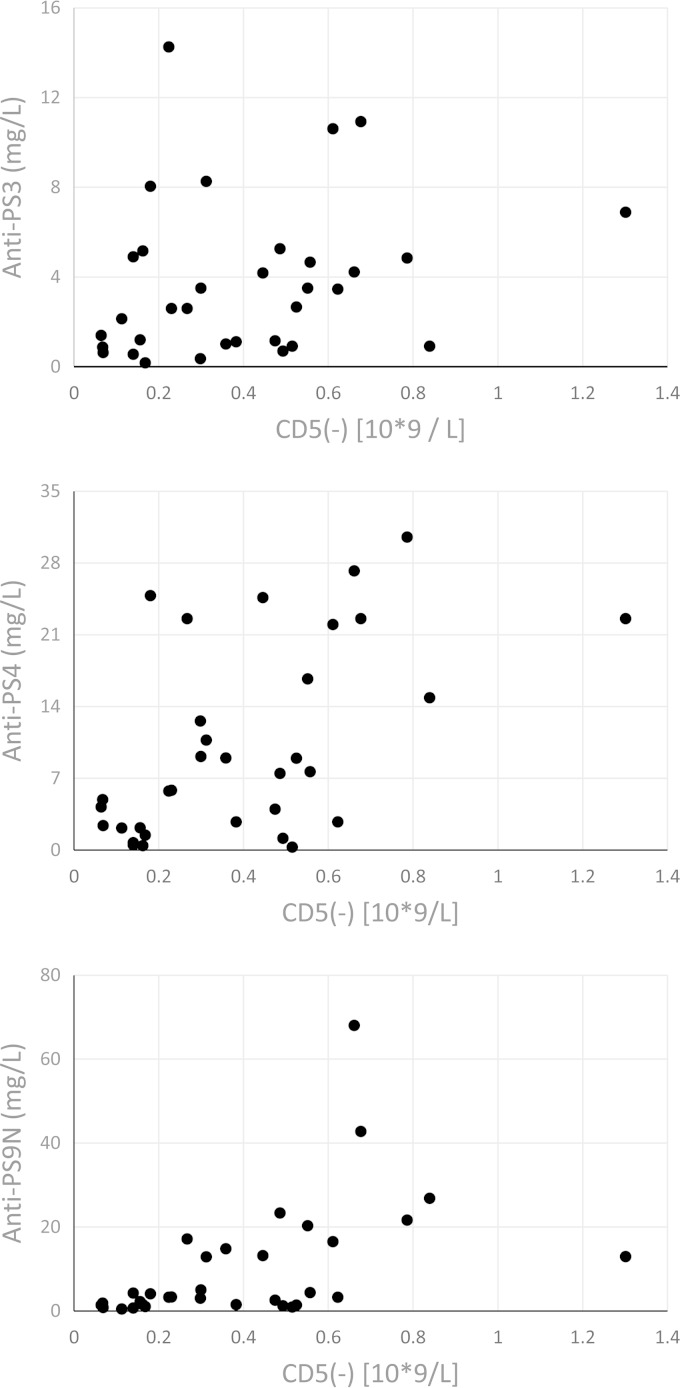
Based on the above-described data, we hypothesized that individuals with high numbers of CD19+ CD5− cells generate more IgG antibodies to soluble caps-PS than do individuals with low numbers of CD19+ CD5− B cells. In order to test this hypothesis, we prospectively quantified CD19+ CD5− cells in 33 individuals vaccinated with PPV23. These children and young adults were being investigated for frequent infections, and their characteristics (age and sex) are described in Table 2. The numbers of CD19+ CD5− B cells and the IgG response to pneumococcal polysaccharides were measured 3 weeks after vaccination with PPV23. For these studies, data from the laboratory clinical immunological investigation were used. This included data on antibodies to serotype 3 (strong immunogen), serotype 4 (intermediate immunogen), and serotype 9N (low immunogen) (18). The results are shown in Fig. 4. There was a correlation (Spearman) between the numbers of CD19+ CD5− cells and the postvaccination IgG antibody levels for serotype 3 (rs, 0.336; P = 0.056), serotype 4 (rs, 0.535; P = 0.0013), and serotype 9N (rs, 0.626; P = <0.0001).
TABLE 2.
Age and sex distribution of patients vaccinated with PPV23
FIG 4.
Antibody response to PPV23 as a function of CD5− B cell subset. Thirty-three individuals suffering from increased susceptibility to respiratory infections were vaccinated with PPV23. The IgG antibody response to caps-PS serotype 3 (top), caps-PS serotype 4 (middle), and caps-PS serotype 9N (bottom) was measured 3 weeks after vaccination. Shown is the antibody level (y axis) as a function of the number of CD19+ CD5− B cells.
Data on prevaccination antibody levels were available for 26 patients for serotype 3 and serotype 9N and for 27 patients for serotype 4. The vaccination-induced fold increase of the specific antibody level (postvaccination antibody level divided by the prevaccination antibody level) was calculated and correlated with the number of CD19+ CD5− cells. There was a significant correlation (Spearman) between the number of CD19+ CD5− cells and the fold increase of IgG antibody levels for serotype 3 (rs, 0.462; P = 0.0175), serotype 4 (rs, 0.446; P = 0.00198), and serotype 9N (rs, 0.567; P = 0.0025).
There was also a significant correlation between the total number of B lymphocytes and the number of CD5− B cells (rs, 0.735; P = <0.0001).
Analysis of patients <5 years old (n = 22) (who received a conjugated pneumococcal vaccine during their first year of life) also revealed a significant correlation between the numbers of CD19+ CD5− cells and the postvaccination IgG antibody levels for serotype 4 (rs, 0.523; P = 0.015) and serotype 9N (rs, 0.643; P = 0.0017). For serotype 3, the correlation (rs) was 0.345 (P = 0.125). For subjects >5 years old, no significant correlation was found (P > 0.5), probably because of the limited number of subjects included (n = 11).
DISCUSSION
The role of the CD19+ CD5+ and CD19+ CD5− B cell subpopulations in the antibody response to caps-PS is controversial. In the present study, we evaluated the role of the CD19+ CD5+ and CD19+ CD5− B cell subpopulations in the antibody response to caps-PS using three approaches.
First, we found that after vaccination with PPV23, the IgG anti-caps-PS serotype 4 antibody-producing cells in healthy adults resided mainly in the CD19+ CD5− B-cell subset. This confirms a previously reported observation that anti-type 4 pneumococcal polysaccharide antibody-secreting cells were found in the CD5− B cell subpopulation (10) and is consistent with our recent observation that the CD5− fraction of CD20+ CD27+ CD43+ B cells generated anti-caps-PS serotype 4 antibodies (8).
Second, in a humanized mouse model, IgG anti-caps-PS antibodies (for serotypes 1, 3, 4, 18C, and 19F) derived mainly from CD19+ CD5− cells. Since the main mechanism by which anti-caps-PS antibodies confer protection is through activation of the complement system and opsonization for phagocytosis (23), we also measured opsonophagocytic activity of the antibodies produced by the humanized mice. Levels of opsonophagocytic activity for caps-PS serotypes 18C, 6A, and 19F, but not serotype 9V, tended to be higher in SCID mice reconstituted with CD19+ CD5− B cells than in SCID mice reconstituted with CD19+ CD5+ B cells, although this difference was not statistically significant and serotype dependent. The dynamic range of the opsonophagocytosis assay is lower than the dynamic range of ELISAs. This could explain why the ELISA better discriminated between the antibody responses of CD5− and CD5+ cells in a humanized SCID model.
Finally, we found an association between the number of CD19+ CD5− B cells and the level of IgG anti-caps-PS antibodies or the fold increase of the specific antibody level in individuals immunized with PPV23. This association was found for the three serotypes tested (serotypes 3, 4, and 9N), independent of whether the serotype was included in the conjugated vaccine or not. The young children (aged 2 to 5 years) included in our study had received a conjugated pneumococcal vaccine (Prevnar 7) during their first year of life. This might affect the subsequent antibody response to PPV23 for serotype 4, which is contained within the conjugated vaccine, but not for serotypes 3 and 9N (not contained within the conjugated 7-valent vaccine). Analysis of the subgroup of children younger than 5 years of age revealed a statistically significant correlation between the number of CD19+ CD5− B cells and the antibody level for serotype 4 and serotype 9N. It should be noted that these studies were performed on patients with a history of frequent infection.
We used different approaches to study the role of CD5− B cells in the anti-caps-PS antibody response. Although the serotypes tested were not fully consistent across the different approaches, the overall results largely indicate that for several serotypes, the antibody response was dependent on CD19+ CD5− human B cells.
Although it has been suggested that CD5 does not define a functionally distinct population of B cells in the human anti-caps-PS response (12, 13), our data argue in favor of the idea that CD19+ CD5− characterizes a functionally separate population of B cells in the human serotype-specific IgG anti-caps-PS immune response. Our data therefore confirm previous suggestions that there may be an association between CD19+ CD5+ B cell predominance and specific antipolysaccharide deficiency (10) and that human IgG caps-PS-secreting cells bear the CD19+ CD5− phenotype (8). Serotype-dependent differences, however, may exist.
Under conditions of pneumococcal disease susceptibility, diminished numbers of CD5− B cells could be a possible basis for immunodeficiency. For example, it has been reported that the number of CD19+ CD5− B cells was lower in patients with common variable immunodeficiency than in controls (24). Moreover, HIV-infected patients, who have a high risk of pneumococcal disease (25), have a striking increase in the percentage of CD5+ B lymphocytes (54.7% ± 19% of circulating B cells) compared with HIV-negative drug users (35.5% + 14%) and with healthy controls (17% ± 5%) (26).
The CD5+ B cell population is prominent in early life, and the percentage of CD5+ B cells diminishes with age (27). Human B cells gradually lose CD5 and acquire CD27 during maturation from immature transitional cells via mature naive cells to memory cells (28). CD5+ B cells have fewer somatic hypermutations than do CD5− B cells and predominantly produce spontaneous low-affinity IgM Abs (29, 30). CD5 expression probably denotes immature/naive B cells. The relative absence of CD5− B cells in young infants could be a reason why young children do not respond to pneumococcal polysaccharides until they are older. Taken together, one could hypothesize that a predominance of CD5+ B cells and a lack of CD5− B cells are associated with susceptibility to pneumococcal disease.
In conclusion, the results described in this report implicate a role for the CD19+ CD5− cell population in the human IgG antibody response to pneumococcal caps-PS.
ACKNOWLEDGMENTS
This work was supported by grants from KU Leuven (GOA/13/013). X.B. was a senior clinical investigator of the Fund for Scientific Research-Flanders. I.M. is a postdoctoral researcher of the Klinisch Onderzoeksfonds Universitaire Ziekenhuizen Leuven. B.V. and K.C. were supported through a grant from the Flemish Institute for Innovation through Science and Technology.
There were no financial or commercial conflicts of interest.
REFERENCES
- 1.Bruyn GA, Zegers BJ, van Furth R. 1992. Mechanisms of host defense against infection with Streptococcus pneumoniae. Clin Infect Dis 14:251–262. doi: 10.1093/clinids/14.1.251. [DOI] [PubMed] [Google Scholar]
- 2.Baumgarth N. 2011. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 11:34–46. doi: 10.1038/nri2901. [DOI] [PubMed] [Google Scholar]
- 3.Haas KM, Poe JC, Steeber DA, Tedder TF. 2005. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity 23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Haas KM. 2011. Programmed cell death 1 suppresses B-1b cell expansion and long-lived IgG production in response to T cell-independent type 2 antigens. J Immunol 187:5183–5195. doi: 10.4049/jimmunol.1101990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffin DO, Holodick NE, Rothstein TL. 2011. Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+ CD27+ CD43+ CD70. J Exp Med 208:67–80. doi: 10.1084/jem.20101499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Andres M, Grosserichter-Wagener C, Teodosio C, van Dongen JJ, Orfao A, van Zelm MC. 2011. The nature of circulating CD27+CD43+ B cells. J Exp Med 208:2565–2566. doi: 10.1084/jem.20112203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Descatoire M, Weill JC, Reynaud CA, Weller S. 2011. A human equivalent of mouse B-1 cells? J Exp Med 208:2563–2564. doi: 10.1084/jem.20112232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verbinnen B, Covens K, Moens L, Meyts I, Bossuyt X. 2012. Human CD20+CD27+CD43+CD5- B cells generate antibodies against capsular polysaccharides of Streptococcus pneumoniae. J Allergy Clin Immunol 130:272–275. doi: 10.1016/j.jaci.2012.04.040. [DOI] [PubMed] [Google Scholar]
- 9.Covens K, Verbinnen B, Geukens N, Meyts I, Schuit F, Van Lommel L, Jacquemin M, Bossuyt X. 2013. Characterization of proposed human B-1 cells reveals pre-plasmablast phenotype. Blood 121:5176–5183. doi: 10.1182/blood-2012-12-471953. [DOI] [PubMed] [Google Scholar]
- 10.Barrett DJ, Sleasman JW, Schatz DA, Steinitz M. 1992. Human anti-pneumococcal polysaccharide antibodies are secreted by the CD5- B cell lineage. Cell Immunol 143:66–79. doi: 10.1016/0008-8749(92)90006-B. [DOI] [PubMed] [Google Scholar]
- 11.Antall PM, Meyerson H, Kaplan D, Venglarcik J, Hostoffer RW. 1999. Selective antipolysaccharide antibody deficiency associated with peripheral blood CD5+ B-cell predominance. J Allergy Clin Immunol 103:637–641. doi: 10.1016/S0091-6749(99)70236-8. [DOI] [PubMed] [Google Scholar]
- 12.Kruetzmann S, Rosado MM, Weber H, Germing U, Tournilhac O, Peter HH, Berner R, Peters A, Boehm T, Plebani A, Quinti I, Carsetti R. 2003. Human immunoglobulin M memory B cells controlling Streptococcus pneumoniae infections are generated in the spleen. J Exp Med 197:939–945. doi: 10.1084/jem.20022020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carsetti R, Rosado MM, Wardemann H. 2004. Peripheral development of B cells in mouse and man. Immunol Rev 197:179–191. doi: 10.1111/j.0105-2896.2004.0109.x. [DOI] [PubMed] [Google Scholar]
- 14.Mascart-Lemone F, Gérard M, Libin M, Crusiaux A, Franchioly P, Lambrechts A, Goldman M, Clumeck N. 1995. Differential effect of human immunodeficiency virus infection on the IgA and IgG antibody responses to pneumococcal vaccine. J Infect Dis 172:1253–1260. doi: 10.1093/infdis/172.5.1253. [DOI] [PubMed] [Google Scholar]
- 15.Steinsvik TE, Gaarder PI, Aaberge IS, Lovik M. 1995. Engraftment and humoral immunity in SCID and RAG2-deficient mice transplanted with human peripheral blood lymphocytes. Scand J Immunol 42:607–616. doi: 10.1111/j.1365-3083.1995.tb03703.x. [DOI] [PubMed] [Google Scholar]
- 16.Tournoy KG, Depraetere S, Meuleman P, Leroux-Roels G, Pauwels RA. 1998. Murine IL-2 receptor beta chain blockade improves human leukocyte engraftment in SCID mice. Eur J Immunol 28:3221–3230. doi:. [DOI] [PubMed] [Google Scholar]
- 17.Moens L, Wuyts M, Meyts I, De Boeck K, Bossuyt X. 2008. Human memory B lymphocyte subsets fulfill distinct roles in the anti-polysaccharide and anti-protein immune response. J Immunol 181:5306–5312. doi: 10.4049/jimmunol.181.8.5306. [DOI] [PubMed] [Google Scholar]
- 18.Jeurissen A, Moens L, Raes M, Wuyts G, Willebrords L, Sauer K, Proesmans M, Ceuppens JL, De Boeck K, Bossuyt X. 2007. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens. Clin Chem 53:505–510. doi: 10.1373/clinchem.2006.080051. [DOI] [PubMed] [Google Scholar]
- 19.Burton RL, Nahm MH. 2006. Development and validation of a fourfold multiplexed opsonization assay (MOPA4) for pneumococcal antibodies. Clin Vaccine Immunol 13:1004–1009. doi: 10.1128/CVI.00112-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borgers H, Moens L, Picard C, Jeurissen A, Raes M, Sauer K, Proesmans M, De Boeck K, Casanova JL, Meyts I, Bossuyt X. 2010. Laboratory diagnosis of specific antibody deficiency to pneumococcal capsular polysaccharide antigens by multiplexed bead assay. Clin Immunol 134:198–205. doi: 10.1016/j.clim.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 21.Verhaegen J, Van De Ven J, Verbiest N, Van Eldere J, Verbist L. 2000. Evolution of Streptococcus pneumoniae serotypes and antibiotic resistance in Belgium—update (1994-98). Clin Microbiol Infect 6:308–315. doi: 10.1046/j.1469-0691.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 22.Verhaegen J, Flamaing J, De Backer W, Delaere B, Van Herck K, Surmont F, Van Laethem Y, Van Damme P, Peetermans W. 2014. Epidemiology and outcome of invasive pneumococcal disease among adults in Belgium, 2009-2011. Euro Surveill 19(31):pii=20869. http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20869. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Steiner S, Frasch CE, Carlone G, Fleck RA, Goldblatt D, Nahm MH. 2006. Use of opsonophagocytosis for serological evaluation of pneumococcal vaccines. Clin Vaccine Immunol 13:165–169. doi: 10.1128/CVI.13.2.165-169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patuzzo G, Mazzi F, Vella A, Ortolani R, Barbieri A, Tinazzi E, Marchi G, Codella O, Beri R, Puccetti A, Lunardi C. 2013. Immunophenotypic analysis of B lymphocytes in patients with common variable immunodeficiency: identification of CD23 as a useful marker in the definition of the disease. ISNR Immunol 2013:512527. doi: 10.1155/2013/512527. [DOI] [Google Scholar]
- 25.Nunes MC, von Gottberg A, de Gouveia L, Cohen C, Kuwanda L, Karstaedt AS, Klugman KP, Madhi SA. 2011. Persistent high burden of invasive pneumococcal disease in South African HIV-infected adults in the era of an antiretroviral treatment program. PLoS One 6:e27929. doi: 10.1371/journal.pone.0027929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sampalo A, López-Gómez M, Jiménez-Alonso J, Ortiz F, Samaniego F, Garrido F. 1993. CD5+ B lymphocytes in HIV infection: relationship to immunological progression of disease. Clin Immunol Immunopathol 66:260–268. [DOI] [PubMed] [Google Scholar]
- 27.Ibegbu CC, Nahmias AJ, Spira TJ, Stoll BJ, Jones B, Symbas N, Nesheim S, Mendez H, Keyserling H, Lee FK. 1992. CD5+ B cells in normal newborns and infants, and in those with HIV and intrauterine infections. Ann N Y Acad Sci 651:572–575. doi: 10.1111/j.1749-6632.1992.tb24665.x. [DOI] [PubMed] [Google Scholar]
- 28.Cuss AK, Avery DT, Cannons JL, Yu LJ, Nichols KE, Shaw PJ, Tangye SG. 2006. Expansion of functionally immature transitional B cells is associated with human-immunodeficient states characterized by impaired humoral immunity. J Immunol 176:1506–1516. doi: 10.4049/jimmunol.176.3.1506. [DOI] [PubMed] [Google Scholar]
- 29.Mackenzie LE, Youinou PY, Hicks R, Yuksel B, Mageed RA, Lydyard PM. 1991. Auto- and polyreactivity of IgM from CD5+ and CD5- cord blood B cells. Scand J Immunol 33:329–335. doi: 10.1111/j.1365-3083.1991.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 30.Brezinschek HP, Foster SJ, Brezinschek RI, Dörner T, Domiati-Saad R, Lipsky PE. 1997. Analysis of the human VH gene repertoire. Differential effects of selection and somatic hypermutation on human peripheral CD5(+)/IgM+ and CD5(-)/IgM+ B cells. J Clin Invest 99:2488–2501. [DOI] [PMC free article] [PubMed] [Google Scholar]