Abstract
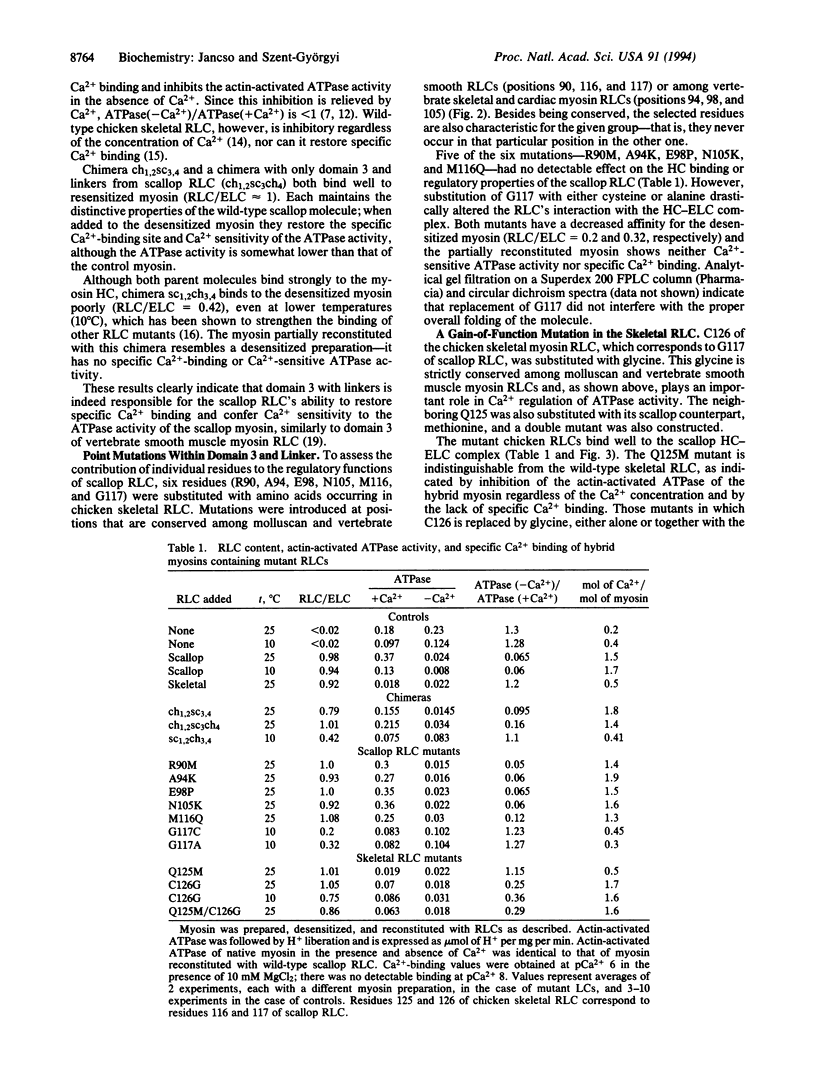
Specific Ca2+ binding and Ca2+ activation of ATPase activity in scallop myosin require a regulatory light chain (RLC) from regulated (molluscan or vertebrate smooth) myosin; hybrids containing vertebrate skeletal RLCs do not bind Ca2+ and their ATPase activity is inhibited. Chimeras between scallop and chicken skeletal RLCs restore Ca2+ sensitivity to RLC-free myosin provided that residues 81-117 are derived from scallop. Six mutants (R90M, A94K, D98P, N105K, M116Q, and G117C) were generated by replacing amino acids of the scallop RLC with the corresponding skeletal RLC residues in positions conserved in either regulated or nonregulated myosins. Ca2+ binding was abolished by a G117C and a G117A mutation; however, these mutants have a decreased affinity for the heavy chain. None of the other mutations affected RLC function. Replacement of the respective cysteine with glycine in the skeletal RLC has markedly changed the regulatory properties of the molecule. The single cysteine to glycine mutation conferred to this light chain the ability to restore Ca2+ binding and regulated ATPase activity, although Ca2+ activation of the actin-activated ATPase was lower than with scallop RLC. The presence of amino acids other than glycine at this position in vertebrate skeletal myosin RLCs may explain why these are not fully functional in the scallop system. The results are in agreement with x-ray crystallography data showing the central role of G117 in stabilizing the Ca(2+)-binding site of scallop myosin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankrett R. J., Rowe A. J., Cross R. A., Kendrick-Jones J., Bagshaw C. R. A folded (10 S) conformer of myosin from a striated muscle and its implications for regulation of ATPase activity. J Mol Biol. 1991 Jan 20;217(2):323–335. doi: 10.1016/0022-2836(91)90546-i. [DOI] [PubMed] [Google Scholar]
- Ashiba G., Szent-Györgyi A. G. Essential light chain exchange in scallop myosin. Biochemistry. 1985 Nov 5;24(23):6618–6623. doi: 10.1021/bi00344a048. [DOI] [PubMed] [Google Scholar]
- Babu Y. S., Sack J. S., Greenhough T. J., Bugg C. E., Means A. R., Cook W. J. Three-dimensional structure of calmodulin. Nature. 1985 May 2;315(6014):37–40. doi: 10.1038/315037a0. [DOI] [PubMed] [Google Scholar]
- Barouch W. W., Breese K. E., Davidoff S. A., Leszyk J., Szent-Györgyi A. G., Theibert J. L., Collins J. H. Amino acid sequences of myosin essential and regulatory light chains from two clam species: comparison with other molluscan myosin light chains. J Muscle Res Cell Motil. 1991 Aug;12(4):321–332. doi: 10.1007/BF01738587. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Brockerhoff S. E., Stevens R. C., Davis T. N. The unconventional myosin, Myo2p, is a calmodulin target at sites of cell growth in Saccharomyces cerevisiae. J Cell Biol. 1994 Feb;124(3):315–323. doi: 10.1083/jcb.124.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chantler P. D., Szent-Györgyi A. G. Regulatory light-chains and scallop myosin. Full dissociation, reversibility and co-operative effects. J Mol Biol. 1980 Apr 15;138(3):473–492. doi: 10.1016/s0022-2836(80)80013-1. [DOI] [PubMed] [Google Scholar]
- Collins J. H. Myosin light chains and troponin C: structural and evolutionary relationships revealed by amino acid sequence comparisons. J Muscle Res Cell Motil. 1991 Feb;12(1):3–25. doi: 10.1007/BF01781170. [DOI] [PubMed] [Google Scholar]
- Collins J. H., Theibert J. L., Dalla Libera L. Amino acid sequence of rabbit ventricular myosin light chain-2: identity with the slow skeletal muscle isoform. Biosci Rep. 1986 Jul;6(7):655–661. doi: 10.1007/BF01114760. [DOI] [PubMed] [Google Scholar]
- Dalla Libera L., Hoffmann E., Floroff M., Jackowski G. Isolation and nucleotide sequence of the cDNA encoding human ventricular myosin light chain 2. Nucleic Acids Res. 1989 Mar 25;17(6):2360–2360. doi: 10.1093/nar/17.6.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin E. B., Leinwand L. A., Szent-Györgyi A. G. Regulation of scallop myosin by mutant regulatory light chains. J Mol Biol. 1990 Nov 5;216(1):85–93. doi: 10.1016/S0022-2836(05)80062-2. [DOI] [PubMed] [Google Scholar]
- Goodwin E. B., Szent-Gyorgyi A. G., Leinwand L. A. Cloning and characterization of the scallop essential and regulatory myosin light chain cDNAs. J Biol Chem. 1987 Aug 15;262(23):11052–11056. [PubMed] [Google Scholar]
- Grant J. W., Taubman M. B., Church S. L., Johnson R. L., Nadal-Ginard B. Mammalian nonsarcomeric myosin regulatory light chains are encoded by two differentially regulated and linked genes. J Cell Biol. 1990 Sep;111(3):1127–1135. doi: 10.1083/jcb.111.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson S. A., Xu Y. C., Chien K. R. Nucleotide sequence of full length cDNAs encoding rat cardiac myosin light chain-2. Nucleic Acids Res. 1988 May 25;16(10):4722–4722. doi: 10.1093/nar/16.10.4722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikura M., Clore G. M., Gronenborn A. M., Zhu G., Klee C. B., Bax A. Solution structure of a calmodulin-target peptide complex by multidimensional NMR. Science. 1992 May 1;256(5057):632–638. doi: 10.1126/science.1585175. [DOI] [PubMed] [Google Scholar]
- Inoue A., Yanagisawa M., Takano-Ohmuro H., Masaki T. Two isoforms of smooth muscle myosin regulatory light chain in chicken gizzard. Eur J Biochem. 1989 Aug 15;183(3):645–651. doi: 10.1111/j.1432-1033.1989.tb21094.x. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J., Lehman W., Szent-Györgyi A. G. Regulation in molluscan muscles. J Mol Biol. 1970 Dec 14;54(2):313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- Kumar C. C., Mohan S. R., Zavodny P. J., Narula S. K., Leibowitz P. J. Characterization and differential expression of human vascular smooth muscle myosin light chain 2 isoform in nonmuscle cells. Biochemistry. 1989 May 2;28(9):4027–4035. doi: 10.1021/bi00435a059. [DOI] [PubMed] [Google Scholar]
- Kwon H., Goodwin E. B., Nyitray L., Berliner E., O'Neall-Hennessey E., Melandri F. D., Szent-Györgyi A. G. Isolation of the regulatory domain of scallop myosin: role of the essential light chain in calcium binding. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4771–4775. doi: 10.1073/pnas.87.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H., Melandri F. D., Szent-Györgyi A. G. Role of gizzard myosin light chains in calcium binding. J Muscle Res Cell Motil. 1992 Jun;13(3):315–320. doi: 10.1007/BF01766459. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maita T., Tanaka H., Konno K., Matsuda G. Amino acid sequence of the regulatory light chain of squid mantle muscle myosin. J Biochem. 1987 Nov;102(5):1151–1157. doi: 10.1093/oxfordjournals.jbchem.a122153. [DOI] [PubMed] [Google Scholar]
- Matsuda G., Maita T., Kato Y., Chen J. I., Umegane T. Amino acid sequences of the cardiac L-2A, L-2B and gizzard 17 000-Mr light chains of chicken muscle myosin. FEBS Lett. 1981 Dec 7;135(2):232–236. doi: 10.1016/0014-5793(81)80789-2. [DOI] [PubMed] [Google Scholar]
- Matsuda G., Maita T., Suzuyama Y., Setoguchi M., Umegane T. Amino acid sequence of the L-2 light chain of rabbit skeletal muscle myosin. J Biochem. 1977 Mar;81(3):809–811. doi: 10.1093/oxfordjournals.jbchem.a131520. [DOI] [PubMed] [Google Scholar]
- Meador W. E., Means A. R., Quiocho F. A. Target enzyme recognition by calmodulin: 2.4 A structure of a calmodulin-peptide complex. Science. 1992 Aug 28;257(5074):1251–1255. doi: 10.1126/science.1519061. [DOI] [PubMed] [Google Scholar]
- Messer N. G., Kendrick-Jones J. Molecular cloning and sequencing of the chicken smooth muscle myosin regulatory light chain. FEBS Lett. 1988 Jul 4;234(1):49–52. doi: 10.1016/0014-5793(88)81300-0. [DOI] [PubMed] [Google Scholar]
- Messer N., Kendrick-Jones J. Chimaeric myosin regulatory light chains: sub-domain switching experiments to analyse the function of the N-terminal EF hand. J Mol Biol. 1991 Apr 20;218(4):825–835. doi: 10.1016/0022-2836(91)90270-g. [DOI] [PubMed] [Google Scholar]
- Miyanishi T., Maita T., Morita F., Kondo S., Matsuda G. Amino acid sequences of the two kinds of regulatory light chains of adductor smooth muscle myosin from Patinopecten yessoensis. J Biochem. 1985 Feb;97(2):541–551. doi: 10.1093/oxfordjournals.jbchem.a135089. [DOI] [PubMed] [Google Scholar]
- Nudel U., Calvo J. M., Shani M., Levy Z. The nucleotide sequence of a rat myosin light chain 2 gene. Nucleic Acids Res. 1984 Sep 25;12(18):7175–7186. doi: 10.1093/nar/12.18.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrie W. T., Perry S. V. An electrophoretic study of the low-molecular-weight components of myosin. Biochem J. 1970 Aug;119(1):31–38. doi: 10.1042/bj1190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayment I., Rypniewski W. R., Schmidt-Bäse K., Smith R., Tomchick D. R., Benning M. M., Winkelmann D. A., Wesenberg G., Holden H. M. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science. 1993 Jul 2;261(5117):50–58. doi: 10.1126/science.8316857. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., Fischman D. A. Recombinant DNA approach for defining the primary structure of monoclonal antibody epitopes. The analysis of a conformation-specific antibody to myosin light chain 2. J Mol Biol. 1985 Feb 5;181(3):411–422. doi: 10.1016/0022-2836(85)90229-3. [DOI] [PubMed] [Google Scholar]
- Reinach F. C., Nagai K., Kendrick-Jones J. Site-directed mutagenesis of the regulatory light-chain Ca2+/Mg2+ binding site and its role in hybrid myosins. Nature. 1986 Jul 3;322(6074):80–83. doi: 10.1038/322080a0. [DOI] [PubMed] [Google Scholar]
- Rowe T., Kendrick-Jones J. Chimeric myosin regulatory light chains identify the subdomain responsible for regulatory function. EMBO J. 1992 Dec;11(13):4715–4722. doi: 10.1002/j.1460-2075.1992.tb05576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe T., Kendrick-Jones J. The C-terminal helix in subdomain 4 of the regulatory light chain is essential for myosin regulation. EMBO J. 1993 Dec;12(12):4877–4884. doi: 10.1002/j.1460-2075.1993.tb06177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. The "megaprimer" method of site-directed mutagenesis. Biotechniques. 1990 Apr;8(4):404–407. [PubMed] [Google Scholar]
- Scholey J. M., Taylor K. A., Kendrick-Jones J. The role of myosin light chains in regulating actin-myosin interaction. Biochimie. 1981 Apr;63(4):255–271. doi: 10.1016/s0300-9084(81)80115-0. [DOI] [PubMed] [Google Scholar]
- Sellers J. R., Chantler P. D., Szent-Györgyi A. G. Hybrid formation between scallop myofibrils and foreign regulatory light-chains. J Mol Biol. 1980 Dec 15;144(3):223–245. doi: 10.1016/0022-2836(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Simmons R. M., Szent-Györgyi A. G. Reversible loss of calcium control of tension in scallop striated muscle associated with the removal of regulatory light chains. Nature. 1978 May 4;273(5657):62–64. doi: 10.1038/273062a0. [DOI] [PubMed] [Google Scholar]
- Spudich J. A., Watt S. The regulation of rabbit skeletal muscle contraction. I. Biochemical studies of the interaction of the tropomyosin-troponin complex with actin and the proteolytic fragments of myosin. J Biol Chem. 1971 Aug 10;246(15):4866–4871. [PubMed] [Google Scholar]
- Stafford W. F., 3rd, Szentkiralyi E. M., Szent-Györgyi A. G. Regulatory properties of single-headed fragments of scallop myosin. Biochemistry. 1979 Nov 27;18(24):5273–5280. doi: 10.1021/bi00591a002. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Use of bacteriophage T7 lysozyme to improve an inducible T7 expression system. J Mol Biol. 1991 May 5;219(1):37–44. doi: 10.1016/0022-2836(91)90855-z. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi A. G., Szentkiralyi E. M., Kendrick-Jonas J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973 Feb 25;74(2):179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Maita T., Ojima T., Nishita K., Matsuda G. Amino acid sequence of the regulatory light chain of clam foot muscle myosin. J Biochem. 1988 Mar;103(3):572–580. doi: 10.1093/oxfordjournals.jbchem.a122308. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Grant J. W., Nadal-Ginard B. Cloning and characterization of mammalian myosin regulatory light chain (RLC) cDNA: the RLC gene is expressed in smooth, sarcomeric, and nonmuscle tissues. J Cell Biol. 1987 Jun;104(6):1505–1513. doi: 10.1083/jcb.104.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trybus K. M., Chatman T. A. Chimeric regulatory light chains as probes of smooth muscle myosin function. J Biol Chem. 1993 Feb 25;268(6):4412–4419. [PubMed] [Google Scholar]
- Vale R. D., Szent-Gyorgyi A. G., Sheetz M. P. Movement of scallop myosin on Nitella actin filaments: regulation by calcium. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6775–6778. doi: 10.1073/pnas.81.21.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way M., Pope B., Gooch J., Hawkins M., Weeds A. G. Identification of a region in segment 1 of gelsolin critical for actin binding. EMBO J. 1990 Dec;9(12):4103–4109. doi: 10.1002/j.1460-2075.1990.tb07632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X., Harrison D. H., Schlichting I., Sweet R. M., Kalabokis V. N., Szent-Györgyi A. G., Cohen C. Structure of the regulatory domain of scallop myosin at 2.8 A resolution. Nature. 1994 Mar 24;368(6469):306–312. doi: 10.1038/368306a0. [DOI] [PubMed] [Google Scholar]