Abstract
The orphan nuclear receptor RORγ is a key regulator for T helper 17 (TH17) cell differentiation, which regulates metabolic and circadian rhythm genes in peripheral tissues. Previously, it was shown that the small molecule inverse agonist of RORγ SR1555 [1-(4-((4′-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-[1,1′-biphenyl]-4-yl)methyl)piperazin-1-yl) ethanone] suppressed TH17 differentiation and stimulated induced T regulatory (iTreg) cells. Here, we show that treatment of cultured pre-adipocyctes with SR1555 represses the expression of RORγ while leading to increased expression of FGF21 and adipoQ. Chronic administration of SR1555 to obese diabetic mice resulted in a modest reduction in food intake accompanied with significant reduction in fat mass, resulting in reduced body weight and improved insulin sensitivity. Analysis ex vivo of treated mice demonstrates that SR1555 induced expression of the thermogenic gene program in fat depots. Further studies in cultured cells showed that SR1555 inhibited activation of hormone-sensitive lipase and increased fatty acid oxidation. Combined, these results suggest that pharmacological repression of RORγ may represent a strategy for treatment of obesity by increasing thermogenesis and fatty acid oxidation, while inhibition of hormone-sensitive lipase activity results in a reduction of serum free fatty acids, leading to improved peripheral insulin sensitivity.
Introduction
The percentage of the global population categorized as overweight or obese has increased dramatically over the last few decades. This trend is predicted to continue as developed and developing nations increasingly adopt more sedentary lifestyles and gain easier access to high calorie diets. The metabolic syndrome is associated with obesity, and patients with this syndrome are at a significant increased risk of suffering from cardiovascular disease and stroke (Moller and Kaufman, 2005). There are two major underlying drivers for the development of metabolic syndrome: excess adiposity (obesity) and type 2 diabetes mellitus (Grundy et al., 2005). Type 2 diabetes mellitus is a chronic metabolic disorder that results in part by the inability of the body to respond adequately to circulating insulin, a state of insulin resistance. In the obese state, free fatty acids (FFAs) are elevated in plasma and in all insulin responsive organs including skeletal muscle, liver, and endothelial cells. As such, elevated FFAs are linked to the development of the metabolic syndrome. Obesity is also closely associated with a low-grade state of inflammation characterized by elevated pro-inflammatory cytokines in blood and tissues (Tataranni and Ortega, 2005).
Treatments for metabolic syndrome included modification of diet and increased exercise (Grundy et al., 2004). However, pharmacologic intervention is typically required because weight loss and exercise often are not sufficient due to poor compliance and confounding genetic factors (Bouchard, 1988; Moller et al., 1996). Members of the nuclear receptor (NR) superfamily are ligand-controlled transcription factors that regulate a wide range of metabolic, endocrine, and immunologic functions, and this protein superfamily has proven to be a rich source of targets for the development of therapeutics for a wide range of human diseases including inflammation and diabetes. A subset of NRs is classified as orphan receptors due to lack of a characterized or agreed upon endogenous ligand (Kliewer et al., 1999). The retinoic acid receptor–related orphan receptor (ROR) NR1F subfamily was identified based on sequence similarities to the retinoic acid and retinoid X receptors (Becker-André et al., 1993; Giguère et al., 1994). The T cell–specific γ isoform, RORγt, has been the focus of considerable attention due to its role in the development of T helper 17 (TH17) cells and in the pathology of autoimmune disease. While RORγt is highly expressed in immune cells and thymus, RORγ variants are expressed in the liver, skeletal muscle, adipose tissue, and kidney (Jetten, 2009), and its expression can be induced in macrophages during acute inflammatory responses (Barish et al., 2005; Gu et al., 2008; Chang et al., 2014). Furthermore, genetic deletion of RORγ results in profound effects on adipose depots with increased adipocyte numbers, yet reduced hypertrophy accompanied by improved insulin sensitivity. These RORγ-deficient mice were shown to be resistant to diet-induced insulin resistance (Meissburger et al., 2011).
Recently, synthetic modulators of the ROR subfamily have been described (Kojetin and Burris, 2014; Marciano et al., 2014), including a series of reports on the development of potent and selective inverse agonists of RORγ. These studies demonstrate their utility in reducing the severity of inflammation in mouse models of multiple sclerosis (experimental autoimmune encephalomyelitis) (Huh et al., 2011; Solt et al., 2011), and rheumatoid arthritis (collagen-induced arthritis) (Chang et al., 2014). One such compound, SR1555 [1-(4-((4′-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-[1,1′-biphenyl]-4-yl)methyl)piperazin-1-yl) ethanone], was previously shown to inhibit TH17 cell development while increasing the frequency of T regulatory (Treg) cells (Solt et al., 2012). Thus, SR1555 was evaluated in the collagen-induced arthritis mouse model (Chang et al., 2014). In addition to observing a reduction in joint inflammation, effects on body weight and adiposity were noted. Based on this observation SR1555 was evaluated in the diet-induced obesity (DIO) murine model of obesity and type 2 diabetes mellitus, and the results of these studies are presented here.
Materials and Methods
Chemicals.
SR1555 (Fig. 1A) was synthesized as previously described (Solt et al., 2012).
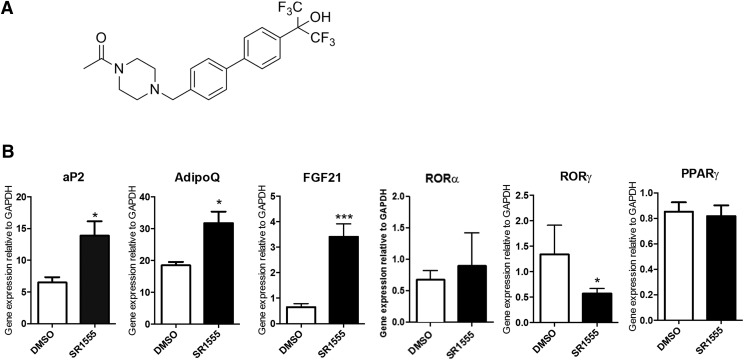
Fig. 1.
SR1555 impacts adipogenic markers. (A) Chemical structure of SR1555 and (B) gene expression analysis in differentiated adipocytes. 3T3-L1 cells were grown to confluence, and then incubated with dexamethasone, insulin, and isobutylmethylxanthine for 2 days to induce differentiation. Cells were then treated with 20 μM SR1555 and insulin for 5 days, after which the expression of thermogenic genes and several nuclear receptors were analyzed by quantitative polymerase chain reaction. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression was used for normalization. Values are the mean ± S.E.M. of three samples per group. *P < 0.05, ***P < 0.001 versus dimethylsulfoxide (DMSO), as determined using Student’s unpaired t test.
LanthaScreen Time-Resolved Fluorescence Resonance Energy Transfer Competitive Binding Assay for PPARγ.
The PPARγ competitive binding assay (Invitrogen, Carlsbad, CA) was performed according to the manufacturer’s protocol. A mixture of 0.5 nM GST-PPARγ LBD, 5 nM Tb-GST-antibody, 5 nM fluormone Pan-PPAR Green, and serial dilutions of the compound beginning at 10 µM downward was added in 384-well low-volume plates (Greiner Bio-One, Monroe, NC) to a total volume of 18 µl (2% dimethylsulfoxide in all wells). Dimethylsulfoxide at 2% concentration was used as a no-ligand control. All dilutions were made in time-resolved fluorescence resonance energy transfer (TR-FRET) PPAR assay buffer. Experiments were performed in triplicate and incubated for 2 hours in the dark before analysis in a PerkinElmer ViewLux Ultra HTS Microplate Reader (PerkinElmer, Waltham, MA). The TR-FRET signal was measured by excitation at 340 nm with emission at 520 nm for fluorescein and 490 nm for terbium. Data were plotted as the TR-FRET ratio 520/490 nm using the GraphPad Prism software (GraphPad Software, La Jolla, CA).
Animals.
Seventeen-week-old male C57BL/6 DIO mice were purchased from Jackson Laboratories (Bar Harbor, ME). All procedures were approved and conducted in accordance with the Scripps Florida Institutional Animal Care and Use Committee. Mice were placed on a high-fat diet (60% kcal derived from fat) for 7 weeks until they reached an average weight of ∼40 g. Prior to and during compound administration mice were maintained on a high-fat diet. Animals were sham dosed with vehicle for 3 days prior to compound administration. SR1555 was administered intraperitoneally twice a day at 10 or 20 mg/kg or by oral gavage at 20 mg/kg once a day. Body weight and food intake were monitored daily. Pre- and post experiment whole-body composition analysis was performed on vehicle only–treated and SR1555-treated mice using NMR (Minispec mq 7.5 NMR analyzer; Bruker Optics, Billerica, MA). Blood samples were collected for plasma cholesterol, triglyceride, FFA, and insulin measurements. For insulin tolerance tests, mice were injected (intraperitoneally) with 10 mg/kg SR1555 for 18 days, and fasted 6 hours before intraperitoneal injection of insulin (0.75 U/kg, Sigma-Aldrich, St. Louis, MO). Blood glucose levels were measured at 0, 15, 30, 60, 90, and 120 minutes using a glucometer (LifeScan, Milpitas, CA). Mice were allowed to recover from the stress associated with the insulin tolerance test for 7 days prior to performing the glucose tolerance test. Here, mice were fasted overnight before injection of 2 g/kg d-glucose intraperitoneally, and blood glucose levels were measured at 0, 15, 30, 60, 90, and 120 minutes.
In Vitro Adipocyte Differentiation.
3T3-L1 and C3H10T1/2 cells were maintained in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (FBS) (Gibco BRL/Life Technologies, Grand Island, NY). Twenty-four hours postconfluence, the 3T3-L1 cells were differentiated with isobutylmethylxanthine (0.5 mM), dexamethasone (1 μM), insulin (10 μg/ml), and 10% FBS for 48 hours. After induction of adipogenesis, the cells were maintained with insulin (10 μg/ml) and 20 μM of SR1555 for 3 or 4 days. The stromal vascular fraction (SVF) within adipose tissue was isolated from the epididymal adipose tissue of C57BL/6 mice to avoid contamination from nonadipose tissues as previously described (Koh et al., 2007). To exclude blood contamination in the adipose tissue, systemic perfusion with heparinized phosphate-buffered saline (PBS) was performed before harvesting or washing isolated adipose tissues with PBS. Then, the adipose tissues were incubated in Hanks’ balanced salt solution (Sigma-Aldrich) containing 0.2% collagenase type 2 for 60 minutes at 37°C with constant shaking. After inactivation of collagenase activity with 10% FBS containing Dulbecco’s modified eagle medium, the cell suspension was filtered through a 40 μm nylon mesh (BD Biosciences, San Jose, CA) followed by centrifugation at 420g for 5 minutes. Floating adipocytes and supernatant were removed from the SVF pellet. The SVF pellet was washed and resuspended in sterilized PBS.
Quantitative Real-Time Polymerase Chain Reaction.
Total RNA was extracted from 3T3-L1 cells or tissues using TRIzol reagent (Invitrogen). The RNA was reverse transcribed using the ABI reverse transcription kit (Applied Biosystems/ThermoFisher Scientific, Waltham, MA). Quantitative polymerase chain reactions were performed with SYBR green fluorescent dye using an ABI9300 polymerase chain reaction machine. Relative mRNA expression was determined by the ΔΔ-Ct method normalized to glyceraldehyde-3-phosphate dehydrogenase levels. The sequences of primers used in this study are found in Supplemental Fig. 1.
MitoTracker Fluorescence-Activated Cell Sorting Quantification.
C2C12 cells were washed with PBS, trypsinized, and incubated at 37°C for 20 minutes with 100 nM MitoTracker Green FM and Red GM dyes (Molecular Probes, Eugene, OR). The MitoTracker Green probe preferentially accumulates in mitochondria, allowing estimation of mitochondrial content. The MitoTracker Red probe is a red-fluorescent dye that stains mitochondria in living cells and its accumulation is dependent on the membrane potential (Molecular Probes). Samples were washed three times in PBS and subjected to flow cytometric analysis on a BD LSRII instrument (Becton Dickinson, San Jose, CA). The results were analyzed using FlowJo software (Tree Star, Ashland, OR).
Oxygen Consumption.
Immortalized SVF cells were seeded at 1 × 104 cells per well in a differentiation medium containing Dulbecco’s modified Eagle’s medium with 10% FBS on Seahorse XF96 well culture plates (Seahorse Bioscience, North Billerica, MA). Cellular oxygen consumption rate was measured using the Seahorse XF96 at 4 to 5 days differentiation with 20 μM SR1555. The concentration of fatty acid (oleic and linoleic acids), oligomycin, carbonilcyanide p-triflouromethoxyphenylhydrozone, and rotenone were 1 mg/ml, 10 mg/ml, 6 mM, and 3 mM, respectively.
Resting Whole-Body Metabolic Parameters.
The daily average whole-body volume of oxygen (VO2) (ml/kg) per hour, and cage activity (movement counts) were measured in DIO mice using a comprehensive laboratory animal monitoring system (CLAMS) (Oxymax series; Columbus Instruments, Columbus, OH). Each sealed chamber (CLAMS unit) is equipped with an O2 electrochemical sensor, a CO2 infrared sensor, and infrared beam activity sensors. The units allow the VO2 consumed (ml/kg) per hour to be measured. Infrared beam interruptions in both horizontal (X) and vertical (Z) directions were measured to quantify the motor activity of mice in both directions. Total horizontal beam breaks were summed to provide the total horizontal activity count. Two or more consecutive horizontal beam breaks were recorded as the ambulatory activity count. All vertical beam breaks were summed to provide the total vertical activity count. The CLAMS studies began after animals were acclimated to the metabolic chambers and VO2 data were collected at 16 minute intervals over a 5 day period under a consistent environmental temperature (22°C). During these studies, mice were allowed free access to food and water.
Western Blot Analysis.
Primary antibodies against phosphorylated hormone-sensitive lipase (HSL) (S660), total HSL, and β-actin were purchased from Cell Signaling Technology (Danvers, MA). A rabbit fast western blotting kit (Pierce/Thermo Scientific, Rockford, IL) was used for detection of primary antibody.
Plasma Concentration of FGF21 and Insulin.
Blood samples were collected in heparin tubes on ice and centrifuged at 4°C. Plasma FGF21 and insulin levels were determined by a specific mouse enzyme-linked immunosorbent assay (Millipore, Billerica, MA) following the manufacturer’s instructions.
Plasma Lipid Profile.
Blood samples were collected in heparin tubes on ice and centrifuged at 4°C. Cholesterol, triglyceride, and FFA were quantitated using a Cobas c311 clinical chemistry analyzer (Roche Diagnostics, Basel, Switzerland).
Pharmacokinetics.
The oral pharmacokinetic profile of SR1555 was determined in male C57Bl6 mice. SR1555 was formulated at a concentration of 1 mg/ml in 15% Cremophore EL (Sigma-Aldrich) in water and dosed by oral gavage to three mice at a final dose of 20 mg/kg. Blood was collected in lithium-heparin–coated capillary tubes using a microsampling technique at 15, 30, 60, 120, 240, 360, 480, and 1440 minutes and plasma was generated using standard centrifugation methods. Plasma concentrations were determined via liquid chromatography–tandem mass spectrometry on an ABSciex 5500 (Sciex, Framingham, MA). SR1555 was detected using the mass transition 461 → 333, and concentrations were determined by comparison with a 9-point standard curve between 2 and 2000 ng/ml prepared in mouse plasma. Pharmacokinetic analysis was done with WinNonLin (Pharsight/Cetara, Princeton, NJ) using a noncompartmental model. All procedures described are covered under existing protocols and have been approved by the Scripps Florida Institutional Animal Care and Use Committee to be conducted in the Scripps Vivarium, which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Statistical Analysis.
The data are reported as the mean ± S.E.M. Statistical significance was evaluated using the Student’s unpaired t test. P values less than 0.05 were considered significant.
Results
SR1555 Alters Adipogenesis.
Previously, SR1555 (Fig. 1A) was shown to be a RORγ-specific inverse agonist that inhibited TH17 cell development and increased the Treg cell population (Solt et al., 2012). Because genetic deletion of RORγ results in profound effects on adipose tissue we investigated the effects of SR1555 on adipogenesis using differentiated 3T3L1 cells. Treatment of these cells with SR1555 resulted in decreased expression of RORγ (Fig. 1B). Consistent with observations from RORγ-depleted mice, SR1555 treatment led to increased expression of FABP4 (aP2) and adiponectin (adipoQ); however, it had no effect on the expression of PPARγ, a key adipogenic regulator. Interestingly, the expression of FGF21 was increased in SR1555-treated 3T3L1 adipocytes. FABP4 is a PPARG (NR1C3) target gene. Therefore, we tested the ability of SR1555 to bind to this NR. Consistent with results from broad selectivity profiling, and as shown in Supplemental Fig. 2A, in a TR-FRET competitive binding assay SR1555 was not able to displace labeled ligand at all concentrations tested. Furthermore, SR1555 was not able to transactivate the expression of luciferase in a reporter gene assay at all concentrations tested (Supplemental Fig. 2B).
RORγ-deficient mice are resistant to obesity and insulin resistance (Meissburger et al., 2011). Based on these observations, we sought to determine the effects of pharmacological repression of RORγ in the DIO mouse model of obesity and diabetes. Mice were fed a high-fat diet until reaching obesity (average weight of ∼40 g). DIO mice were then treated with vehicle (15% Cremophor, i.p.) for 3 days prior to initiating administration of SR1555. As shown in Supplemental Fig. 3, bolus administration of SR1555 to mice at 5 and 10 mg/kg i.p., and 20 mg/kg by oral gavage afforded plasma concentrations of the compound 4 hours postadministration in excess of 4, 8, and 16 μM, respectively. The calculated pharmacokinetic parameters are presented in Supplemental Fig. 4. With the intent of only partially repressing RORγ and based on the plasma exposure from the pharmacokinetic studies shown in Supplemental Fig. 3, SR1555 was administered intraperitoneally to obese mice at 5 or 10 mg/kg twice a day. Body weight and food intake were recorded daily. Following 20 days of compound administration, DIO mice receiving vehicle only maintained body weight but animals treated with SR1555 at 5 mg/kg had lost 15% of their body weight (Fig. 2A). SR1555 demonstrated a dose-dependent impact on body weight because mice administered compound at 10 mg/kg lost an additional 8% body weight (23% loss). Compound treatment did cause a modest reduction in food intake by DIO mice compared with control animals (Fig. 2B). However, no change in food intake was observed in lean mice, suggesting SR1555 is not driving weight loss by systemic toxicity. It is possible that SR1555 treatment results in an improvement in leptin sensitivity.
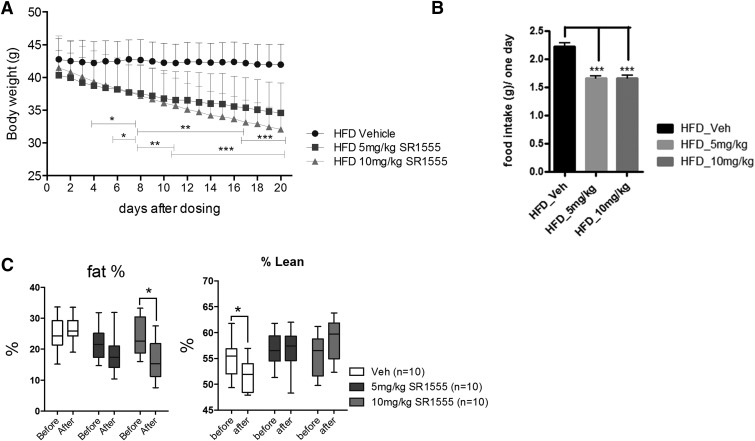
Fig. 2.
SR1555 administration mice are resistant to obesity. (A) Body weight of high-fat diet (HFD) controls and SR1555-treated obese mice, (B) food intake, and (C) whole-body NMR. Twenty-one week old DIO mice were administered SR1555 intraperitoneally at 5 or 10 mg/kg for 20 days. Body weight and food intake were monitored daily. Percentage fat was measured just prior to the start of compound administration and again after 17 days of compound administration. Values are the mean ± S.E.M. of 10 samples per group. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle or before start dosing, by Student’s unpaired t test. Veh, vehicle.
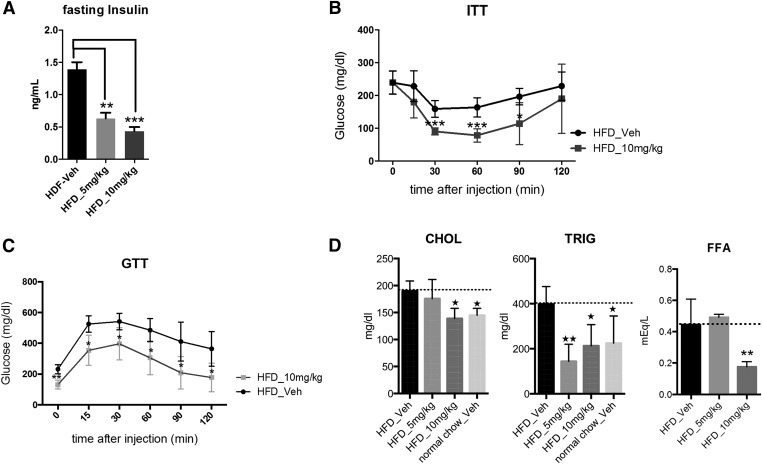
Whole-body composition of DIO mice was analyzed using NMR prior to the start of vehicle or compound administration and again at the conclusion of the study. As shown in Fig. 2C, lean mass was unaffected by SR1555 treatment; however, there was a statistically significant loss of fat mass in the 10 mg/kg SR1555-treated DIO mice. On day 18 of treatment, mice were fasted for 8 hours and then subjected to an insulin tolerance test. As shown in Fig. 3A fasting insulin was significantly reduced in both the 5 and 10 mg/kg arms of the study. Figure 3B illustrates that glucose disposal was improved with a quick decline in plasma glucose 15 minutes post insulin administration in the 10 mg/kg arm of the study. Statistically significant differences in plasma glucose were observed at 30, 60, and 90 minutes post insulin administration. Following the insulin tolerance test, compound administration was terminated and mice were allowed to recover from the stress of fasting. At 7 days post compound administration a glucose tolerance test was performed to determine if SR1555 treatment results in improved disposal of plasma glucose in response to a bolus intake of a glucose solution. As shown in Fig. 3C, DIO mice treated with SR1555 retained improved insulin sensitivity 7 days post compound treatment. Analysis of plasma lipids at this time point showed that SR1555 treatment reduced cholesterol and triglyceride levels below vehicle only–treated mice and to a level similar to that in control lean mice maintained on a normal chow diet (Fig. 3D). The FFA levels were also reduced in SR1555-treated animals (Fig. 3D). Although SR1555 increased expression of FGF21 in adipocytes, compound treatment in DIO mice did not result in an increase in plasma FGF21 (data not shown) secreted from the liver.
Fig. 3.
Metabolic parameters in DIO mice were improved by SR1555 administration. (A) Cholesterol (CHOL), triglyceride (TRIG), and FFAs were measured in SR1555-treated DIO mice after 20 days dosing. (B) Fasting insulin and insulin (0.75 U/kg) tolerance test (ITT, n = 10) were measured on DIO mice and (C) glucose tolerance test (GTT) performed 7 days following last dose of SR1555. Values are the mean ± S.E.M. of 10 samples per group. *P < 0.05, **P < 0.01, ***P < 0.001 versus vehicle, by Student’s unpaired t test. Veh, vehicle.
We next investigated whether whole-body energy expenditure and ambulatory activity were impacted by SR1555 treatment. DIO mice were acclimated to single animal housing for 7 days prior to the start of this study. SR1555 was then administered by oral gavage at 20 mg/kg for 7 days before placing the animals in CLAMS chambers. Once inside the CLAMS chambers, mice were administered SR1555 orally each day at 20 mg/kg and metabolic parameters and activity were evaluated over a 7 day period.
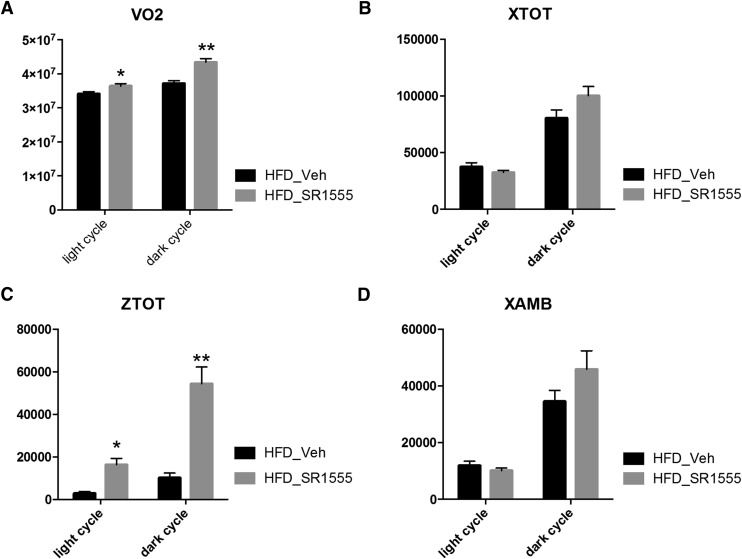
As shown in Fig. 4A, oxygen consumption (VO2) in compound-treated DIO mice was increased during both the light and dark cycles compared with the vehicle only–treated group. Furthermore, body temperature was significantly increased in SR1555-treated mice (Supplemental Fig. 5, A and B). No difference in the total horizontal activity count and ambulatory activity count was observed when comparing SR1555-treated mice to vehicle only–treated mice; however, the total vertical activity count was increased in compound-treated DIO mice (Fig. 4, B–D). It is possible that this increase in vertical activity may reflect increased exploration of the environment (attention), cognitive enhancement, or perhaps anxiety. Regardless, the data obtained using CLAMS suggests that SR1555 stimulated activity and increased energy expenditure in DIO mice.
Fig. 4.
In vivo measurement of energy expenditure and motor activity. (A) Total oxygen consumption (VO2), (B) total horizontal motor activity (XTOT), (C) total vertical motor activity (ZTOT), and (D) ambulatory activity count (XAMB). Mice were placed into the CLAMS for 5 days after one week (SR1555-treated obese mice). Values from all animals were pooled and averaged with an N = 5 per group. Data were analyzed based on light and dark cycles. The light cycle occurred from 7:00 AM to 7:00 PM and the dark cycle occurred from 7:00 PM to 7:00 AM in the metabolic cages. Values are the mean ± S.E.M. of 10 samples per group. *P < 0.05, **P < 0.01 versus vehicle (Veh), by Student’s unpaired t test.
Induction of Thermogenesis by SR1555.
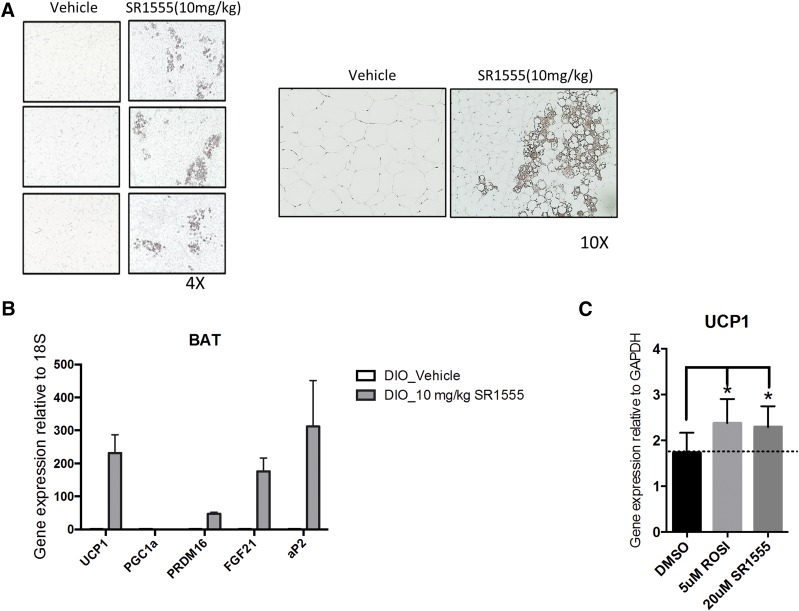
UCP1 (uncoupling protein 1) is critical for heat generation, and the protein is selectively expressed in brown and beige adipose cells. Classic brown adipose tissue and inducible white adipocytes (beige cells) are capable of increasing energy expenditure through the uncoupling of oxidative metabolism from ATP (Wu et al., 2012, 2013). Also, previous physiologic studies have shown that diet can induce thermogenesis to maintain body weight (Rothwell and Stock, 1997). Therefore, we asked whether SR1555 induces thermogenic genes in adipose depots in DIO mice. Inguinal white adipose tissue and brown adipose tissue were collected from DIO mice. Induction of UCP1 protein in inguinal white adipose tissue and overall adipose size were smaller in SR1555-treated inguinal white adipose tissue (Fig. 5A). Additionally, gene expression of UCP1, PRDM16, and FGF21 was increased in brown adipose tissue from SR1555-treated mice compared with tissue from vehicle only–treated mice. The expression of the 18S gene was used as a control to determine the relative expression of thermogenic genes (Fig. 5B).
Fig. 5.
Improvement of thermogenic genes by SR1555 and UCP1 induction. (A) Immunohistochemical examination of the presence of UCP1. Inguinal adipose tissues from vehicle-treated DIO mice or 10 mg/kg SR1555-treated DIO mice were stained brown for UCP1 immunoreactivity. (B) mRNA expression of thermogenic genes in brown fat tissue (BAT) of mice fed with either vehicle-treated DIO or 10 mg/kg SR1555-treated DIO. (C) UCP1 mRNA expression in adipogenic C3H10T1/2 cell line. C3H10T1/2 cells were induced adipogenesis with dexamethasone, insulin, and isobutylmethylxanthine for 2 days, and then maintained with insulin and each compound for 5 days. DMSO, dimethylsulfoxide; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; ROSI, Rosiglitazone.
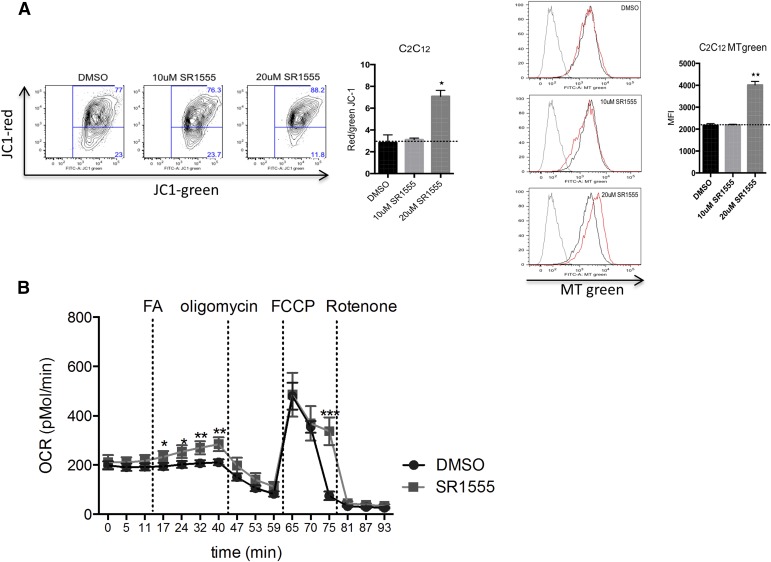
FABP4 (aP2) gene expression was also increased by SR1555 treatment, and this phenotype was similar to that observed with genetic depletion of RORγ (Meissburger et al., 2011). SR1555 treatment induced UCP1 gene expression in C3H10T1-2 cells (Fig. 5C), where the effect of SR1555 treatment was similar to that of thiazolidinedione treatment, which was the positive control for the assay (Ohno et al., 2012). Furthermore, SR1555 increased the mitochondrial membrane potential and mass in C2C12 cells (Fig. 6A). Taken together, we conclude that SR1555 induces the thermogenic gene program and alters mitochondrial potential in adipose and muscle cell lines.
Fig. 6.
Fatty acid oxidation and modulation of mitochondrial respiration. (A) Functional mitochondria content in C2C12 cells was analyzed by flow cytometry using JC-1 and red/green JC-1 (the high-low mitochondrial membrane potential) mean fluorescent index (MFI) ratio (right, n = 3 per condition). Mitochondria content was measured by flow cytometry analysis using Mito Tracker (MT) Green staining (n = 3 per condition). (B) SVF cells were grown to confluence; incubated with dexamethasone, insulin, isobutylmethylxanthine, triiodothyronine (T3), and Rosiglitazone for 2 days to induce adipocyte differentiation; and then cultured with 20 μM SR1555, insulin, and T3. Oxygen consumption rates (OCRs) were measured by a Seahorse XF-96 flux analyzer (n = 10, three different conditions). Values are the mean ± S.E.M. of 10 samples per group. *P < 0.05, **P < 0.01, and ***P < 0.001 versus dimethylsulfoxide (DMSO), by Student’s unpaired t test. FA; fatty acid (oleic and linoleic acids); FCCP, carbonilcyanide p-triflouromethoxyphenylhydrozone.
Fatty Acid Oxidation and Lipolysis.
The reduction of FFAs observed in obese mice treated with SR1555 could be the result of increased FFA storage, increased degradation of FFAs by β-oxidation, a decrease in lipolysis of triglycerides, or a combination of these events. The effect of SR1555 on fatty acid oxidation was monitored in immortalized SVF cells. Differentiation was achieved in vitro by sequential application of adipogenic factors to preadipocytes over a period of several days. Following differentiation, adipocytes were characterized using the XF Analyzer from Seahorse Bioscience to measure four essential parameters of mitochondrial function: basal respiration, ATP turnover, proton leak, and maximal respiration. Fatty acid oxidation in mitochondria is mainly controlled by cpt1 (carnitine palmitoyl transferase I), which facilitates transport of long chain fatty acids into mitochondria. cpt1 was unchanged in SR1555-treated SVF cells. However, oxygen consumption was increased at four independent time points following addition of a fatty acid mixture (oleic and linoleic acids), whereas no increase was observed in dimethylsulfoxide only–treated SVF cells (Fig. 6B). Next, we evaluated the activity of HSL in differentiated 3T3L1 cells following SR1555 treatment (Supplemental Fig. 6). Elevated plasma levels of FFAs are thought to play a major role in the pathogenesis of insulin resistance and type 2 diabetes by inhibiting glucose uptake and utilization by muscle and causing increased glucose output by the liver (Claus et al., 2005; Girousse et al., 2013). Here, we observed that HSL gene expression and phosphorylated HSL (S660) were reduced in fully differentiated 3T3L1 cells. Therefore, we suggest that decreasing phosphorylated HSL might drive the reduction of FFA release from white adipocytes.
Discussion
The NR superfamily of ligand regulated transcription factors has proven to be a rich source of targets for the development of therapeutics for a wide range of human diseases. Within the NR1F subfamily of NRs, RORγt has garnered much attention due to its role in TH17 cells. However, its contribution to obesity and insulin resistance has been recently demonstrated (Meissburger et al., 2011; Takeda et al., 2014). The discovery that the potent LXR agonist T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide] also functioned as an inverse agonist for RORα and RORγ (Kumar et al., 2010) led to an explosion in medicinal chemistry efforts focused on the NR1F subfamily of receptors. This effort is exemplified by publications of the RORα/γ agonist SR1078 [N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-4-(trifluoromethyl)benzamide] (Wang et al., 2010), the selective RORα inverse agonist SR3335 [N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-2-thiophenesulfonamide] (Kumar et al., 2011), the dual RORα/γ inverse agonist SR1001 [N-[4-methyl-5-[[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]amino]sulfonyl]-2-thiazolylacetamide] (Solt et al., 2011), and the selective RORγ inverse agonists SR2211 [1,1,1,3,3,3-hexafluoro-2-(4′-((4-(pyridin-4-ylmethyl)piperazin-1-yl)methyl)-[1,1′-biphenyl]-4-yl)propan-2-ol] (Kumar et al., 2012) and SR1555 (Solt et al., 2012). Here, we demonstrate that the selective RORγ inverse agonist SR1555 recapitulates well the RORγ depletion phenotype particularly in terms of adiposity and metabolic parameters. Specifically, SR1555 treatment of obese and diabetic mice modulated adipocyte function, leading to a reduction in body weight exclusively as a result of reduced adiposity leading to improved insulin sensitivity and glucose disposal.
It is possible that SR1555-mediated improvement in metabolic phenotype involves the modulation of Treg development. Previously, it was shown that in cultured cells SR1555 repressed TH17 cell differentiation with concomitant expansion of inducible Tregs (Solt et al., 2012), and induction of Tregs has been shown to be associated with metabolic disorders (Michalek et al., 2011; Bhat et al., 2014). Additionally, obese individuals with insulin resistance display a sharp reduction of Tregs (DeFuria et al., 2013), and depletion of Treg cells in mice using an anti-CD25 monoclonal antibody exacerbates insulin resistance (Eller et al., 2011). Previously, we had shown that SR1555 was capable of suppressing inflammatory cytokine production such as IL-6 and TNF-α in lipopolysaccharide-stimulated RAW264.7 cells (Chang et al., 2014). Therefore, it is possible that the anti-inflammatory actions of SR1555 are associated with its ability to improve adiposity and metabolic parameters.
Treatment of 3T3L1 cells with SR1555 resulted in inhibition of the enzyme HSL (EC 3.1.1.79). HSL is highly expressed in adipose tissue and is a major contributor to the formation of FFAs in fat. Release of these FFAs provides a source of energy for most tissues. HSL activity is the rate-limiting step in catecholamine-induced lipolysis, and the level of HSL expression is directly related to the lipolytic capacity of mature fat cells (Large et al., 1998). These initial findings suggested that increased HSL activity may contribute to the increased plasma FFAs observed in obesity. Interestingly, partial inhibition of lipolysis in adipose tissue in HSL haplo-insufficient mice (HSL+/−) resulted in improved insulin sensitivity (Girousse et al., 2013). Analysis of the promoter region of HSL (LIPE) suggests it contains four putative RORE sequences (Supplemental Fig. 7, A and B). Thus, it is plausible that HSL expression and activity are regulated by RORγ. Previous chromatin immunoprecipitation sequencing data demonstrated that RORγ binds to the promoter of family members of HSL. It remains to be determined if SR1555-mediated repression of HSL activity is direct or indirect.
Furthermore, SR1555 treatment increased gene expression and protein levels of UCP1, altered mitochondrial potential, and increased the level of fatty acid oxidation in both brown adipocytes and beige cells, suggesting that RORγ regulates the oxidative phosphorylation process in mitochondria. In summary, the selective RORγ inverse agonist SR1555 may provide a useful starting point for the development of peripheral acting therapeutics for the treatment of diabetes and obesity.
Supplementary Material
Acknowledgments
The authors thank Melissa Kazantzis for assistance with metabolic studies and Mohammad Fallahi-Sichani for bioinformatic support.
Abbreviations
- CLAMS
comprehensive laboratory animal monitoring system
- DIO
diet-induced obesity
- FBS
fetal bovine serum
- FFA
free fatty acid
- HSL
hormone-sensitive lipase
- NR
nuclear receptor
- PBS
phosphate-buffered saline
- ROR
retinoic acid receptor–related orphan receptor
- SR1001
N-[4-methyl-5-[[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]amino]sulfonyl]-2-thiazolylacetamide
- SR1078
N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-4-(trifluoromethyl)benzamide
- SR1555
1-(4-((4′-(1,1,1,3,3,3-hexafluoro-2-hydroxypropan-2-yl)-[1,1′-biphenyl]-4-yl)methyl)piperazin-1-yl) ethanone
- SR2211
1,1,1,3,3,3-hexafluoro-2-(4′-((4-(pyridin-4-ylmethyl)piperazin-1-yl)methyl)-[1,1′-biphenyl]-4-yl)propan-2-ol
- SR3335
N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-2-thiophenesulfonamide
- SVF
stromal vascular fraction
- T0901317
N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide
- TH17
T helper 17
- Treg
T regulatory
- TR-FRET
time-resolved fluorescence resonance energy transfer
- VO2
volume of oxygen
Authorship Contributions
Participated in research design: Chang, Kamenecka, White, Griffin, Cameron.
Conducted experiments: Chang, Garcia-Ordonez, Kuruvilla, T. Khan, S. Khan, Lin, Unger.
Contributed new reagents or analytic tools: He, Kamenecka.
Performed data analysis: Chang, Unger, White, Griffin, Cameron.
Wrote or contributed to the writing of the manuscript: Chang, Corzo, Griffin, Cameron.
Footnotes
>This work was supported in part by the by the Intramural Research Program of the National Institutes of Health National Institute of Mental Health [Grant U54-MH074404].
None of the authors have conflicts of interest with the work presented in this paper. P.R.G. was a cofounder of Ember Therapeutics, which has recently undergone a merger and P.R.G. is no longer associated with the new company. T.J.U. and D.W.W. were (but are no longer) employees of Ember Therapeutics.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Barish GD, Downes M, Alaynick WA, Yu RT, Ocampo CB, Bookout AL, Mangelsdorf DJ, Evans RM. (2005) A Nuclear Receptor Atlas: macrophage activation. Mol Endocrinol 19:2466–2477. [DOI] [PubMed] [Google Scholar]
- Becker-André M, André E, DeLamarter JF. (1993) Identification of nuclear receptor mRNAs by RT-PCR amplification of conserved zinc-finger motif sequences. Biochem Biophys Res Commun 194:1371–1379. [DOI] [PubMed] [Google Scholar]
- Bhat PK, Navin HK, Idris M, Christopher P, Rai N. (2014) Modified distal shoe appliance for premature loss of multiple deciduous molars: A case report. J Clin Diagn Res 8:ZD43–ZD45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard C. (1988) Genetic factors in the regulation of adipose tissue distribution. Acta Med Scand Suppl 723:135–141. [DOI] [PubMed] [Google Scholar]
- Chang MR, Lyda B, Kamenecka TM, Griffin PR. (2014) Pharmacologic repression of retinoic acid receptor-related orphan nuclear receptor γ is therapeutic in the collagen-induced arthritis experimental model. Arthritis Rheum (Munch) 66:579–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus TH, Lowe DB, Liang Y, Salhanick AI, Lubeski CK, Yang L, Lemoine L, Zhu J, Clairmont KB. (2005) Specific inhibition of hormone-sensitive lipase improves lipid profile while reducing plasma glucose. J Pharmacol Exp Ther 315:1396–1402. [DOI] [PubMed] [Google Scholar]
- DeFuria J, Belkina AC, Jagannathan-Bogdan M, Snyder-Cappione J, Carr JD, Nersesova YR, Markham D, Strissel KJ, Watkins AA, Zhu M, et al. (2013) B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci USA 110:5133–5138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eller K, Kirsch A, Wolf AM, Sopper S, Tagwerker A, Stanzl U, Wolf D, Patsch W, Rosenkranz AR, Eller P. (2011) Potential role of regulatory T cells in reversing obesity-linked insulin resistance and diabetic nephropathy. Diabetes 60:2954–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V, Tini M, Flock G, Ong E, Evans RM, Otulakowski G. (1994) Isoform-specific amino-terminal domains dictate DNA-binding properties of RORα, a novel family of orphan hormone nuclear receptors. Genes Dev 8:538–553. [DOI] [PubMed] [Google Scholar]
- Girousse A, Tavernier G, Valle C, Moro C, Mejhert N, Dinel AL, Houssier M, Roussel B, Besse-Patin A, Combes M, et al. (2013) Partial inhibition of adipose tissue lipolysis improves glucose metabolism and insulin sensitivity without alteration of fat mass. PLoS Biol 11:e1001485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, et al. American Heart Association. National Heart, Lung, and Blood Institute (2005) Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112:2735–2752. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Hansen B, Smith SC, Jr, Cleeman JI, Kahn RA, American Heart Association. National Heart, Lung, and Blood Institute. American Diabetes Association (2004) Clinical management of metabolic syndrome: report of the American Heart Association/National Heart, Lung, and Blood Institute/American Diabetes Association conference on scientific issues related to management. Circulation 109:551–556. [DOI] [PubMed] [Google Scholar]
- Gu Y, Yang J, Ouyang X, Liu W, Li H, Yang J, Bromberg J, Chen SH, Mayer L, Unkeless JC, et al. (2008) Interleukin 10 suppresses Th17 cytokines secreted by macrophages and T cells. Eur J Immunol 38:1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh JR, Leung MW, Huang P, Ryan DA, Krout MR, Malapaka RR, Chow J, Manel N, Ciofani M, Kim SV, et al. (2011) Digoxin and its derivatives suppress TH17 cell differentiation by antagonizing RORγt activity. Nature 472:486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetten AM. (2009) Retinoid-related orphan receptors (RORs): critical roles in development, immunity, circadian rhythm, and cellular metabolism. Nucl Recept Signal 7:e003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer SA, Lehmann JM, Willson TM. (1999) Orphan nuclear receptors: shifting endocrinology into reverse. Science 284:757–760. [DOI] [PubMed] [Google Scholar]
- Koh YJ, Kang S, Lee HJ, Choi TS, Lee HS, Cho CH, Koh GY. (2007) Bone marrow-derived circulating progenitor cells fail to transdifferentiate into adipocytes in adult adipose tissues in mice. J Clin Invest 117:3684–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojetin DJ, Burris TP. (2014) REV-ERB and ROR nuclear receptors as drug targets. Nat Rev Drug Discov 13:197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Kojetin DJ, Solt LA, Kumar KG, Nuhant P, Duckett DR, Cameron MD, Butler AA, Roush WR, Griffin PR, et al. (2011) Identification of SR3335 (ML-176): a synthetic RORα selective inverse agonist. ACS Chem Biol 6:218–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Lyda B, Chang MR, Lauer JL, Solt LA, Burris TP, Kamenecka TM, Griffin PR. (2012) Identification of SR2211: a potent synthetic RORγ-selective modulator. ACS Chem Biol 7:672–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar N, Solt LA, Conkright JJ, Wang Y, Istrate MA, Busby SA, Garcia-Ordonez RD, Burris TP, Griffin PR. (2010) The benzenesulfoamide T0901317 [N-(2,2,2-trifluoroethyl)-N-[4-[2,2,2-trifluoro-1-hydroxy-1-(trifluoromethyl)ethyl]phenyl]-benzenesulfonamide] is a novel retinoic acid receptor-related orphan receptor-α/γ inverse agonist. Mol Pharmacol 77:228–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large V, Arner P, Reynisdottir S, Grober J, Van Harmelen V, Holm C, Langin D. (1998) Hormone-sensitive lipase expression and activity in relation to lipolysis in human fat cells. J Lipid Res 39:1688–1695. [PubMed] [Google Scholar]
- Marciano DP, Chang MR, Corzo CA, Goswami D, Lam VQ, Pascal BD, Griffin PR. (2014) The therapeutic potential of nuclear receptor modulators for treatment of metabolic disorders: PPARγ, RORs, and Rev-erbs. Cell Metab 19:193–208. [DOI] [PubMed] [Google Scholar]
- Meissburger B, Ukropec J, Roeder E, Beaton N, Geiger M, Teupser D, Civan B, Langhans W, Nawroth PP, Gasperikova D, et al. (2011) Adipogenesis and insulin sensitivity in obesity are regulated by retinoid-related orphan receptor gamma. EMBO Mol Med 3:637–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek RD, Gerriets VA, Jacobs SR, Macintyre AN, MacIver NJ, Mason EF, Sullivan SA, Nichols AG, Rathmell JC. (2011) Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J Immunol 186:3299–3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller DE, Bjørbaek C, Vidal-Puig A. (1996) Candidate genes for insulin resistance. Diabetes Care 19:396–400. [DOI] [PubMed] [Google Scholar]
- Moller DE, Kaufman KD. (2005) Metabolic syndrome: a clinical and molecular perspective. Annu Rev Med 56:45–62. [DOI] [PubMed] [Google Scholar]
- Ohno H, Shinoda K, Spiegelman BM, Kajimura S. (2012) PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab 15:395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell NJ, Stock MJ. (1997) A role for brown adipose tissue in diet-induced thermogenesis. Obes Res 5:650–656. [DOI] [PubMed] [Google Scholar]
- Solt LA, Kumar N, He Y, Kamenecka TM, Griffin PR, Burris TP. (2012) Identification of a selective RORγ ligand that suppresses TH17 cells and stimulates T regulatory cells. ACS Chem Biol 7:1515–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solt LA, Kumar N, Nuhant P, Wang Y, Lauer JL, Liu J, Istrate MA, Kamenecka TM, Roush WR, Vidović D, et al. (2011) Suppression of TH17 differentiation and autoimmunity by a synthetic ROR ligand. Nature 472:491–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda Y, Kang HS, Freudenberg J, DeGraff LM, Jothi R, Jetten AM. (2014) Retinoic acid-related orphan receptor γ (RORγ): a novel participant in the diurnal regulation of hepatic gluconeogenesis and insulin sensitivity. PLoS Genet 10:e1004331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Ortega E. (2005) A burning question: does an adipokine-induced activation of the immune system mediate the effect of overnutrition on type 2 diabetes? Diabetes 54:917–927. [DOI] [PubMed] [Google Scholar]
- Wang Y, Kumar N, Nuhant P, Cameron MD, Istrate MA, Roush WR, Griffin PR, Burris TP. (2010) Identification of SR1078, a synthetic agonist for the orphan nuclear receptors RORα and RORγ. ACS Chem Biol 5:1029–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, Khandekar M, Virtanen KA, Nuutila P, Schaart G, et al. (2012) Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150:366–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Cohen P, Spiegelman BM. (2013) Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev 27:234–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.