Abstract
UDP-sugars, which are indispensable for protein glycosylation reactions in cellular secretory pathways, also act as important extracellular signaling molecules. We discuss here the broadly expressed P2Y14 receptor, a G-protein–coupled receptor targeted by UDP sugars, and the increasingly diverse set of physiologic responses discovered recently functioning downstream of this receptor in many epithelia as well as in immune, inflammatory, and other cells.
Introduction
UDP-sugars are key components of glycosylation reactions in the lumen of the endoplasmic reticulum (ER) and Golgi apparatus, and they also are important extracellular signaling molecules acting as potent agonists of the broadly expressed P2Y14 receptor (P2Y14R) (Harden et al., 2010). The P2Y14R is activated by UDP-glucose, UDP-galactose, UDP-N-acetylglucosamine, and other UDP-sugars. UDP also acts as an agonist on this receptor, but ATP, UTP, or other naturally occurring diphosphate or triphosphate nucleotides have no P2Y14R activity (Chambers et al., 2000; Carter et al., 2009; Harden et al., 2010). The P2Y14R is a subtype in the eight-member family of nucleotide-activated P2Y receptors, which are all G-protein–coupled receptors (GPCRs) (Ralevic and Burnstock, 1998; Abbracchio et al., 2006; von Kugelgen and Harden, 2011). These receptors can be subclassified based on sequence and cell signaling response similarities into two subgroups, the P2Y12 receptor–like and the P2Y1 receptor–like subgroups. The P2Y14R is a member of the P2Y12 receptor–like subgroup, which also includes the ADP-activated P2Y12 and P2Y13 receptors. An orphan GPCR, GPR87, with high homology to the P2Y14R, also exists in the P2Y12-like subfamily of receptors. These GPCRs share >40% primary sequence identity, and all three couple to the Gi family of heterotrimeric G proteins. They exhibit much lower sequence identity (<25%) with the P2Y1 receptor–like subgroup, which includes the ADP-activated P2Y1 receptor, the UTP/ATP-activated P2Y2 receptor, the UTP-activated P2Y4 receptor, the UDP-activated P2Y6 receptor and the ATP-activated P2Y11 receptor. All members of the P2Y1 receptor–like subgroup couple to heterotrimeric proteins of the Gq family and activate phospholipase C.
Mechanisms of Release and Metabolism of Nucleotide Sugars
The initial characterization of the P2Y14R as a UDP-sugar–activated receptor of widespread tissue distribution (Chambers et al., 2000; Lee et al., 2003; Moore et al., 2003; Skelton et al., 2003) suggested that nucleotide sugars are released from cells in a regulated manner to accomplish autocrine/paracrine signaling functions. Furthermore, functional studies overexpressing recombinant P2Y14R in null cell lines revealed the presence of endogenous agonists in extracellular solutions. Using a chimeric Gα-subunit (Gαq/i) that couples Gi-linked GPCRs to phospholipase C-β, robust P2Y14R-dependent inositol phosphate production was shown to occur in COS-7 cells in the absence of added agonist. That is, expression of the P2Y14R resulted in a large increase in basal [3H]inositol phosphate levels that were further elevated by the addition of UDP-glucose to the medium (Lazarowski et al., 2003). The elevated levels of [3H]inositol phosphates observed in the absence of exogenous agonists were markedly reduced by addition to the medium of Crotalus adamentus phosphodiesterase, which hydrolyzes nucleotides and nucleotide sugars; apyrase, which hydrolyzes nucleotides but not nucleotide sugars, had no effect (Lazarowski et al., 2003). Using highly sensitive radioenzymatic/high-performance liquid chromatography–based methodology, UDP-glucose, UDP-N-acetylglucosamine, and UDP-galactose were identified and quantified in extracellular solutions (Lazarowski et al., 2003, 2010; Sesma et al., 2009).
Actual concentrations of UDP-sugars in the physiologic extracellular milieu are not known owing to difficulties in sampling the thin cell-surface liquid without causing cell damage and uncontrolled nucleotide release. When assessed together in diluted culture medium (300 µl/cm2 culture surface), steady-state concentration values of UDP-glucose and UDP-N-acetylglucosamine on various airway epithelial cell types were similar (both in the 5–10 nM range) (Sesma et al., 2009), and, as discussed later herein, they increased considerably in response to environmental challenges, likely attaining micromolar levels at the surface of stressed cells.
UDP-sugars are synthesized in the cytosol and translocated into the lumen of the ER and Golgi apparatus where they serve as donor substrates of glycosylation reactions (Abeijon and Hirschberg, 1992; Berninsone and Hirschberg, 1998). Vesicular UDP-sugars cannot be transported back to the cytosol and thus are likely delivered as cocargo with exported glycoconjugates (Fig. 1). Several lines of evidence support this hypothesis. Gene deletion of Yea4, a major UDP-N-acetylglucosamine transporter in Saccharomyces cerevisiae, resulted in impaired release of this nucleotide sugar and was associated with reduced production of chitin, an acetylglucosamine-rich component of the yeast wall. Complementation of yea4-deficient cells with yea4 restored UDP-N-acetylglucosamine release and chitin formation (Sesma et al., 2009). Similarly, overexpression of the human UDP-N-acetylglucosamine transporter HFRC1/SLC35D2 in bronchial epithelial cells resulted in enhanced release of UDP-N-acetylglucosamine and enhanced cell-surface expression of acetylglucosamine-containing glycans (Sesma et al., 2009). Studies with mucin-producing goblet epithelial cells indicated that UDP-glucose release was associated with mucin secretion. For example, 1) goblet-like Calu-3 cells exhibited Ca2+-regulated exocytosis of mucin granules, which was accompanied by enhanced release of UDP-glucose (Kreda et al., 2007); and 2) both mucin secretion and UDP-glucose levels increased concurrently in human bronchial epithelial cell cultures that were induced to develop goblet (mucous) cell metaplasia (Okada et al., 2011). Lastly, 1321N1 human astrocytoma cells exhibit both constitutive and protease-activated receptor 1–promoted release of UDP-glucose, which were Ca2+-dependent and markedly reduced by brefeldin A1, a fungal metabolite that disrupts retrograde movements along the secretory pathway (Kreda et al., 2008).
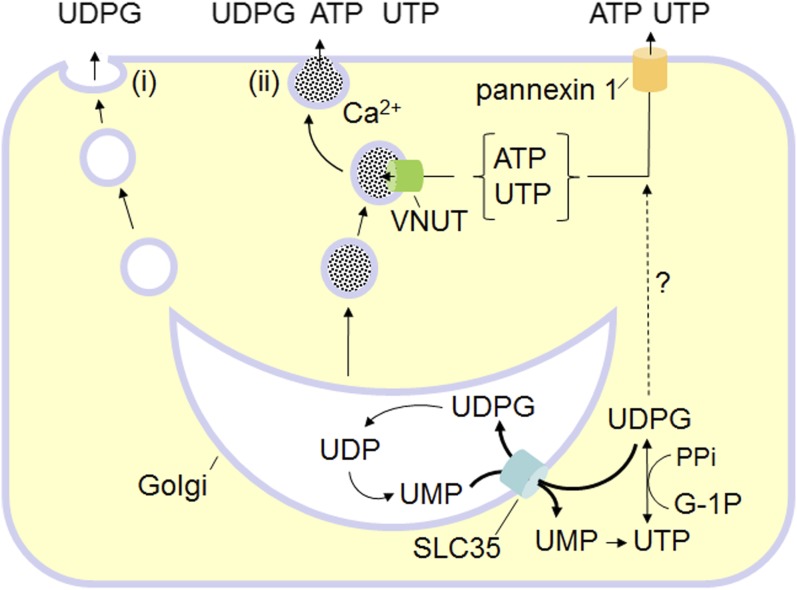
Fig. 1.
Pathways for UDP-sugar release. UDP-glucose (UDPG) and other UDP-sugars are synthesized in the cytosol and enter the secretory pathway via ER/Golgi-resident SLC35 transporters, using UMP as antiporter substrates. ER/Golgi UDP-sugars are used for glycosylation, yielding UDP as a reaction product, and UDP is metabolized to UMP. (i) Residual UDP-sugars in the ER/Golgi lumen are released as cargo via the constitutive secretory pathway. (ii) Unlike ATP and UTP, UDP-sugars are not predicted to be substrates of the vesicular nucleotide transporter (VNUT), but Golgi-derived vesicles are a source of UDP-sugars in specialized granules susceptible to Ca2+-regulated exocytosis. Not shown: Golgi UDP is released with UDP-sugars. Whether pannexin 1 mediates the release of cytosolic UDP-sugars is not known. PPi, pyrophosphate; G-1P, glucose-1P.
Collectively, these observations suggest that the secretory pathway is a major contributor to the cellular release of UDP-sugars (Fig. 1). However, nonvesicular mechanisms that contribute to the cellular release of ATP in many cell types also could be involved in the release of UDP-sugars. For example, caspase 3-mediated proteolytic irreversible activation of plasma membrane pannexin 1 channels resulted in robust release of cytosolic ATP, as well as ADP, AMP, and UTP, from apoptotic lymphocytes (Elliott et al., 2009; Chekeni et al., 2010; Boyd-Tressler et al., 2014). These data suggest that the pannexin 1 pore exhibits no specificity toward nucleotide species. Thus, it is conceivable that, at least in apoptotic cells, cytosolic UDP-glucose is released together with adenine and uracil nucleotides via pannexin 1.
It is worth noting that, in most cases where regulated nucleotide release was assessed, rates of UDP-glucose release were markedly lower than ATP release rates, whereas steady-state levels of UDP-glucose were often higher than ATP levels. Differences in the rates of extracellular metabolism account for this apparent discrepancy. UDP-sugars are not metabolized by ecto-ATPase (ecto-nucleoside triphospho diphosphohydrolase; NTPDase) activities, which are abundantly expressed on the surface of most cell types (Zimmermann et al., 2012). Instead, ecto-nucleotide pyrophosphatases (ENPPs) break down UDP-sugars, according to the following reaction:
ENPPs also metabolize nucleoside triphosphates (to nucleoside monophosphates + pyrophosphate), as well as nicotine adenine dinucleotide, ADP-ribose, and dinucleoside polyphosphates. ENPP expression has been reported in several tissues (Zimmermann et al., 2012), but only a few reports have examined its possible action in extracellular metabolism of UDP-sugars. UDP-[3H]glucose added as a radiotracer to confluent cultures of 1321N1 human astrocytoma cells was metabolized at rates that were much lower than ATP hydrolysis rates (Lazarowski et al., 2003). UDP-[3H]glucose remained unchanged after a 1-hour incubation with purified human neutrophils (Sesma et al., 2012) or primary cultures of human airway epithelial cells (Lazarowski, unpublished), whereas [3H]ATP was completely hydrolyzed.
The high stability of UDP-glucose on airway epithelia and potentially other tissues exhibiting regulated release of UDP-sugars provides a mechanism by which these molecules could accumulate in extracellular environments at pathophysiologically relevant levels. For example, UDP-glucose and UDP-N-acetylglucosamine concentrations in diluted bronchioalveolar lavages from patients with chronic lung obstruction and inflammation reached the 200–400 nM range. As discussed further in subsequent sections of this article, these observations suggest a link between local UDP-glucose release and inflammation.
Targeting of the P2Y14R by Native Agonists and High-Affinity Molecular Probes
The work of Chambers and colleagues (Chambers et al., 2000) led to the realization that the orphan receptor GPR105, now P2Y14R, is activated by nucleotide sugars with a relative potency order of UDP-glucose > UDP-galactose > UDP-glucuronic acid > UDP-N-acetylglucosamine, and all these molecules exhibited potencies in the 0.1–1.0 µM range of concentrations. One potential caveat from this and additional early studies was that they were accomplished using overexpressed G-protein α-subunits, either with Gα16, which mediates coupling of essentially all GPCR to phospholipase C-β isozymes, or with the engineered protein, Gαq/i, which selectively couples Gi-linked GPCR to activation of phospholipase C-β (Chambers et al., 2000; Freeman et al., 2001; Lazarowski et al., 2003; Fricks et al., 2008). Nonetheless, results from work studying the agonist selectivity of the P2Y14R in cell systems in which it coupled through native Gi heterotrimers to promote inhibition of adenylyl cyclase confirmed the nucleotide sugar selectivity established by Chambers and coworkers; that is, UDP-glucose is the most potent agonist, but the activities of other nucleotide sugars are within an order of magnitude (Fricks et al., 2009).
The studies of Carter and coworkers (Carter et al., 2009) examining P2Y14R-dependent activities measured downstream of natively expressed Gi also revealed that UDP is a very potent agonist of the P2Y14R. Indeed, this nucleotide was 5-fold more potent than UDP-glucose in studies with several different cell lines. Although direct comparisons have not been published, it is unlikely that the concentration of extracellular UDP approaches that of UDP-sugars under most physiologic and pathologic conditions. UDP generated in the ER/Golgi as a product of glycosylation reactions is predicted to be either degraded to UMP by Golgi resident UDPase or released with UDP-sugars via vesicle exocytosis; UDP also is generated extracellularly from the hydrolysis of released UTP; however, cell surface NTPDases rapidly metabolize UDP but not UDP-sugars. Thus, we discuss the P2Y14R as a nucleotide sugar-activated GPCR with the realization that UDP may also play an important, albeit not yet clearly established, role in its physiologic regulation.
Although nucleotide sugars are potent P2Y14R agonists that are present at receptor-activating concentrations in extracellular medium, the existence of other physiologic agonists of this receptor cannot be entirely ruled out. The realization that at least one orphan GPR87 with high (∼47%) homology to the P2Y14R exists in the P2Y12-like subclass is not inconsistent with this possibility.
Both synthetic agonists and high-affinity competitive antagonists have been developed that selectively target the P2Y14R (Table 1). For example, 2-thio-UDP-glucose (MRS2690) exhibits 30-fold greater potency for the P2Y14R than does UDP-glucose (Ko et al., 2007, 2009). Several analogs of UDP also have been developed that exhibit high potency at the P2Y14R (Das et al., 2010). These include α,β-difluoromethylene-UDP (MRS2802) and α,β-methylene-2-thio-UDP (MRS2905). The importance of these molecules lies in their selectivity for activation of the P2Y14R at concentrations that are inactive at the UDP-activated P2Y6 receptor, as well as at other P2Y receptors (Carter et al., 2009; Das et al., 2010).
TABLE 1.
Agonists and antagonists of the P2Y14R
| Molecule | Potency | |
|---|---|---|
| Natural agonistsa | UDP-glucose | EC50 = 132 nM |
| UDP | EC50 = 33 nM | |
| Selective synthetic agonistsb,c | 2-thio-UDP-glucose (MRS2690) | EC50 = 49 nM |
| α,β-methylene-2-thio-UDP (MRS2905) | EC50 = 0.92 nM | |
| Selective competitive antagonistd | PPTN | KB = 400 pM |
EC50 values are from experiments measuring inhibition of cAMP accumulation in P2Y14R-expressing CHO cells (Carter et al., 2009).
Values are from experiments measuring inositol phosphate accumulation in Cos-7 cells expressing the human P2Y14R and Gαq/i (Ko et al., 2007).
Values are from experiments measuring inhibition of cAMP accumulation in P2Y14R-expressing HEK293 cells (Das et al., 2010).
KB for PPTN was obtained in Schild analyses measuring antagonism of UDP-glucose–promoted inhibition of cyclic AMP accumulation in P2Y14R-expressing CHO cells (Barrett et al., 2013).
Black and coworkers applied high-throughput screens to identify dihydropyridopyrimidine (Guay et al., 2011) and naphthoic acid (Gauthier et al., 2011) compounds as potential P2Y14R antagonists. Analogs of each type of molecule subsequently were synthesized. Whereas dihydropyridopyrimidine derivatives appeared to be noncompetitive antagonists of the P2Y14R, the naphthoic acid derivatives inhibited [3H]UDP binding, suggesting antagonism of the orthosteric ligand binding site of the receptor (Gauthier et al., 2011; Robichaud et al., 2011). Problems with high-affinity binding to serum proteins observed with several of these naphthoic acid analogs were partially circumvented in the analog PPTN [4-((piperidin-4-yl)-phenyl)-7-(4-(trifluoromethyl)-phenyl)-2-naphthoic acid] that retained activity as a P2Y14R antagonist. A prodrug based on PPTN also was developed to increase bioavailability. Barrett et al. (2013) illustrated that PPTN is indeed a high-affinity (KB = ∼400 pM) competitive antagonist of the P2Y14R that exhibits no activity at the other seven P2Y receptors at concentrations as high as 10 µM. PPTN was applied subsequently to selectively block P2Y14R-dependent responses. Thus, UDP-glucose promoted chemotaxis of human neutrophils (Barrett et al., 2013), P2Y14R agonist-promoted release of β-hexosaminidase from human LAD2 mast cells (Gao et al., 2013), and promoted contractile effects on porcine vascular smooth muscle (Alsaqati et al., 2014); all were antagonized by low concentrations of PPTN. Several other potential P2Y14R inhibitors have been reported by Hamel and coworkers (Hamel et al., 2011).
Jacobson and colleagues examined a series of chain-elongated alkynyl or amino derivatives of PPTN with the goal of generating a fluorescent probe for the P2Y14R (Kiselev et al., 2014). Many of these retained activity at the P2Y14R, and an Alexa Fluor 488 (AF488)–derivatized version (MRS4174) of one of these exhibited remarkably high (Ki = 80 pM) binding activity. This molecule selectively labeled the P2Y14R in live cells as quantified by flow cytometry.
P2Y14R-Mediated Cell Signaling Responses
The idea that the P2Y14R is Gi-coupled was initially suggested by the observation that UDP-glucose stimulated [35S]GTPγS binding in membranes from P2Y14R-expressing HEK293 cells, which was inhibited by pretreatment of cells with pertussis toxin (Chambers et al., 2000). As discussed already, this notion was further supported by studies using chimeric Gαq/i proteins that translate Gi signaling into phospholipase C-β activation (Freeman et al., 2001; Lazarowski et al., 2003; Fricks et al., 2008). Fricks and coworkers (Fricks et al., 2009) showed that the human P2Y14R indeed couples through natively occurring Gi (Fig. 2) and that its activation results in robust inhibition of the classic effector of Gi, adenylyl cyclase.
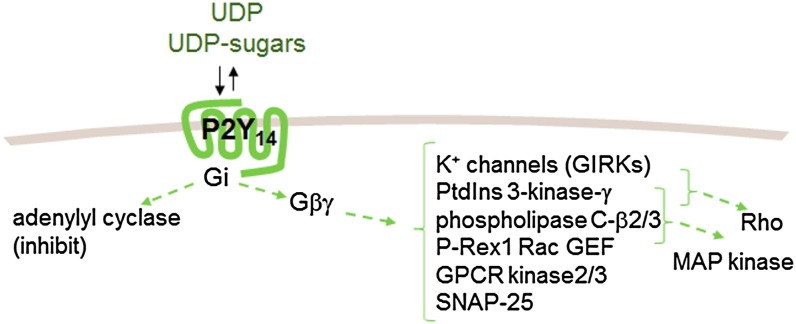
Fig. 2.
Signaling pathways associated with P2Y14R activation. The P2Y14R couples to Gi, thus promoting Gαi- and Gβγ-mediated inhibition of adenylyl cyclases 1, 3, 5, 6, 8, and 9. Additional direct and indirect effectors of Gβγ include K+ channels (GIRK), phosphatidylinositol 3-kinase-γ, GPCR kinase 2 and 3, and phospholipase C β2 and β3, P-rex1 Rac GEF, and SNAP-25. Rho, MAPKs, and potentially other effectors not listed represent examples of signaling proteins that are also activated downstream of Gβγ.
Gi heterotrimers are expressed at high levels in plasma membranes compared with other Gα-subunits and largely, but not entirely, signal through the release of Gβγ-subunits from the heterotrimer (Smrcka, 2008). Thus, Gβγ-activated effectors can be expected to be activated downstream of activated P2Y14R, although data directly illustrating responses from most of these are not available. Activation of mitogen-activated protein kinase (MAPK) signaling pathways predictably occurs downstream of Gi-coupled GPCRs (Crespo et al., 1994), and therefore it is not surprising that P2Y14R-dependent phosphorylation of MAPKs also is well established. UDP-glucose promoted extracellular signal–regulated kinase (ERK) 1/2 phosphorylation in P2Y14R-expressing human embryonic kidney (HEK293) cells with a potency similar to that observed in the same cells for inhibition of adenylyl cyclase and by a mechanism that was blocked by pertussis toxin pretreatment (Fricks et al., 2009). Differentiation of undifferentiated HL-60 promyelocytic leukemia cells results in expression of P2Y14R mRNA (Fricks et al., 2009), and whereas the effects of UDP-glucose were not observed in undifferentiated cells, ERK1/2 phosphorylation occurred in response to UDP-glucose in differentiated HL-60 cells. Again, pertussis toxin pretreatment blocked UDP-glucose–promoted ERK activation. Activation of ERK also was reported for UDP-glucose–treated human neutrophils (Scrivens and Dickenson, 2006).
Sesma and coworkers showed that UDP-glucose promotes activation of Rho and chemotaxis of human neutrophils, and the latter was blocked by inhibitors of Rho kinase. Activation of Rho and chemotaxis also occurred in differentiated but not wild-type HL-60 cells. These responses were absent after pretreatment of differentiated HL-60 cells with pertussis toxin and were also inhibited by pharmacologic inhibitors of phosphatidylinositol (PtdIns)-3-kinase or Rho kinase. PtdIns-3-kinase is a well established effector of Gβγ (Stephens et al., 1994), and this work suggests that neutrophils respond to P2Y14R activation in a pathway that involves Gβγ-dependent activation of PtdIns-3-kinase, plasma membrane recruitment of an exchange factor for Rho, production of GTP-Rho, actin cytoskeleton rearrangement, and enhanced neutrophil shape change and locomotion. Additional effectors of Gβγ (Fig. 2) that may also function downstream of activated P2Y14R include K+ channels, GPCR kinase 2 and 3, phospholipase C-β2 and -β3, SNAP-25, and P-Rex 1 Rac GEF (Smrcka, 2008).
Physiologic and Pathophysiologic Consequences of P2Y14 Receptor Activation
Whereas several rigorous studies indicate a role of P2Y14R in UDP-sugar–promoted physiologic responses, unambiguous evidence of the involvement of the P2Y14R in cellular functions often has been difficult to assimilate. For example, although commercial sources of UDP-glucose induced inflammatory effects in N9 microglia, this action was observed only with bacterially derived, but not synthetic, UDP-glucose, and other nucleotide sugars had no effect (Brautigam et al., 2008). As pointed out by the authors, these results likely reflected an effect that is due to a contaminant of UDP-glucose solutions and were independent of P2Y14R activation (Brautigam et al., 2008). Scrivens and Dickenson reported that UDP-glucose promoted inhibition of cAMP formation in forskolin-stimulated human neuroblastoma SH-SY5Y cells, but they noted that reverse transcription polymerase chain reaction analysis showed no P2Y14R mRNA expression in these cells (Scrivens and Dickenson, 2005b). In another report, intraperitoneal administration of high concentrations of UDP-glucose to wild-type mice resulted in an increase in gastric emptying, but this effect was retained in P2Y14R knockout mice (Bassil et al., 2009). Thus, caution should be taken in interpreting studies of UDP-sugar–evoked responses that are not validated by data with receptor gene deletion or knockdown or by selective pharmacologic antagonism. Conversely, weak or no responses to exogenous UDP-glucose in tissues known to express P2Y14R mRNA could be due to receptor desensitization or elevated baselines caused by cellular UDP-sugar release, low receptor protein abundance, robust ENPP activity, or a combination of these factors. Caution also should be taken when analyzing results from P2Y14R-targeted mice, as epigenetic changes may contribute to phenotypes not necessarily associated with P2Y14R lack of function. Further, antisera purportedly generated against P2Y14R have been used to assess receptor protein expression in various tissues, but strict validation of these antibodies has not been provided. Bearing in mind these concerns, evidence supporting physiologic or pathophysiologic roles for the P2Y14R is discussed below.
Immune or Inflammatory Cells
P2Y14R transcripts are robustly expressed in leukocytes and other immune/inflammatory cells and are upregulated in the rat brain after immunologic challenge with lipopolysaccharide (Moore et al., 2003), suggesting that the P2Y14R plays an important role in immune or inflammatory responses.
Monocytes and Macrophages.
Macrophages play important roles in both innate and adaptive immunity and are critical effector cells in proinflammatory processes. The release of proinflammatory cytokines from macrophages in obesity is associated with insulin receptor phosphorylation and insulin resistance (Olefsky and Glass, 2010), and a recent study suggests contribution of the P2Y14R to this process (Xu et al., 2012). Specifically, 1) wild-type mice developed severe glucose intolerance when fed a high-fat diet (HFD), whereas P2Y14R knockout mice were protected from HFD-induced glucose intolerance; 2) P2Y14R-deficient mice exhibited improved systemic insulin sensitivity after a HFD and enhanced insulin action in adipose tissue, liver, and skeletal muscles; 3) after a HFD, P2Y14R knockout mice exhibited a reduced number of macrophages in the liver compared with wild-type animals; 4) P2Y14R-deficient monocytes showed reduced migration to the liver of HFD-fed wild-type mice; 5) plasma levels of UDP-glucose were elevated 4-fold in HFD-fed animals compared with controls; 6) UDP-glucose–promoted migration of bone marrow–derived macrophages in vitro was reduced with P2Y14R knockout-derived bone marrow–derived macrophages; and 7) transplantation of P2Y14R-deficient bone marrow cells reestablished insulin sensitivity in HFD-fed wild-type mice. Taken together, these results suggest that UDP-glucose released from liver cells in obese states, possibly via hepatocellular apoptosis (Wang et al., 2008), acts as a chemoattractant to recruit monocytes/macrophages, leading to liver inflammation and insulin resistance.
Neutrophils.
Polymorphonuclear neutrophils (PMNs) constitute the first line of defense to eradicate bacterial infection. Circulating PMNs are among the peripheral human cell types that most abundantly expresses P2Y14R mRNA (Moore et al., 2003), but initial attempts to identify P2Y14R-promoted responses in neutrophils were inconclusive. UDP-glucose promoted a modest inhibition of forskolin-stimulated cAMP formation in neutrophils, but other UDP-sugars had no effect (Scrivens and Dickenson, 2006). UDP-glucose also evoked a modest increase in ERK phosphorylation in these cells, but this response was observed only at high micromolar concentrations and was not mimicked by other UDP-sugars; neutrophil elastase secretion was not enhanced by UDP-glucose (Scrivens and Dickenson, 2006). Lastly, neutrophils exhibited no migration response toward UDP-glucose when assessed via the classic transwell filter staining method (Arase et al., 2009).
Subsequent studies have provided more compelling evidence for functional expression of P2Y14R in neutrophils. As discussed already, expression of native P2Y14R is induced during differentiation of neutrophil-like HL-60 human myeloid leukemia cells, and UDP-glucose and other UDP-sugars promoted activation of MAPK and Rho signaling in neutrophil-differentiated (but not in undifferentiated) HL60 cells in a pertussis toxin-sensitive manner (Fricks et al., 2009; Sesma et al., 2012). UDP-glucose–promoted migration of differentiated HL60 cells was observed using a modified Boyden chamber under conditions that quantified chemotaxis of fluorescently labeled cells with high sensitivity. UDP-glucose also promoted Rho kinase-dependent chemotaxis of freshly isolated human neutrophils (Sesma et al., 2012), and this response was abolished when the P2Y14R antagonist PPTN was added to the PMN suspension (Barrett et al., 2013).
Observation of P2Y14R-dependent PMN migration and potentially additional neutrophil functions could be relevant to chronic inflammatory lung diseases associated with elevated levels of extracellular UDP-sugars in the lung (Sesma et al., 2009). As discussed further below, an additional mechanism by which UDP-sugars may evoke PMN recruitment in the lung involves activation of P2Y14R on epithelial cells and release of IL-8 (Muller et al., 2005).
Mast Cells.
Mast cells are bone marrow–derived immune effector cells that play important roles in allergic diseases such as bronchial asthma, rhinitis, anaphylaxis, and urticaria. IgE (Fc)-stimulated mast cells release histamine, proteases, and other hydrolytic activities from secretory granules and generate eicosanoid mediators that promote the production and secretion of proinflammatory cytokines and chemokines. Whether the P2Y14R is expressed in primary mast cells is not known, but studies with mast cell–like cell lines suggest that the P2Y14R is a mediator of degranulation in these cells. P2Y14R mRNA was abundantly present in RBL-2H3 rat mast cells, and UDP-glucose, as well as the potent P2Y14R agonist 2-thio-UDP-glucose, enhanced the release of β-hexosaminidase, an indicator of granule secretion (Gao et al., 2013). Hexosaminidase secretion was reduced by knocking down the P2Y14R via small interfering RNA (siRNA). Similar results were reported using the human bone marrow–derived mast cell line LAD2, and incubation of these cells with PPTN (10 µM) resulted in reduced UDP-sugar–potentiated C3a-promoted hexaminidase secretion (Gao et al., 2013).
Dendritic Cells.
P2Y14R mRNA was detected at high levels in immature human monocyte-derived dendritic cells that mature and migrate to sites of inflammation (Skelton et al., 2003). Exposure of immature monocyte-derived dendritic cells to UDP-glucose resulted in transient mobilization of Ca2+ and increased expression of CD86, suggesting that the P2Y14R regulates dendritic cell maturation (Skelton et al., 2003).
Lymphocytes.
T-lymphocytes express P2Y14R transcripts (Moore et al., 2003), and UDP-glucose was reported to cause a modest decrease in cAMP levels in these cells as well as to inhibit T-cell proliferation in response to anti-CD3 antibody or interleukin 2 (Scrivens and Dickenson, 2005a).
Hematopoietic Stem Cells
The P2Y14R was reported to be selectively expressed in subpopulations of primitive bone marrow hematopoietic stem cells (HSCs), suggesting a role for this receptor in bone marrow cell localization and compartmentalization (Lee et al., 2003). Subsequent studies illustrated that subcutaneous administration of UDP-glucose to mice resulted in enhanced mobilization of HSCs into the peripheral blood and spleen (Kook et al., 2013a). In a separate study (Kook et al., 2013b), the authors reported that subcutaneous administration of UDP-glucose to in utero-irradiated pregnant mice attenuated the effect of irradiation on the growth and mortality rates of the offspring. Whether these effects of UDP-glucose reflected a P2Y14R-dependent mechanism is not known. Strikingly, prenatal irradiation of P2Y14R knockout mice resulted in enhanced growth and survival rates compared with wild-type mice (Kook et al., 2013b).
In a more recent study, this group showed that HSCs from P2Y14R knockout mice exhibit increased senescence, a state of permanent irreversible growth arrest in response to aging, radiation, chemotherapy, and other environmental stresses (Cho et al., 2014). Enhanced senescence was associated with increased reactive oxygen species, elevated p16INK4a expression, and reduced hyperphosphorylation of the retinoblastoma protein pRb, an inducer of cell senescence. Treatment of wild-type cells with pertussis toxin recapitulated the phenotype observed in P2Y14R-deficient mice. Furthermore, primitive HSCs lacking P2Y14R exhibited impaired ability to restore hematopoiesis in irradiated mice. These observations suggest that the P2Y14R on stem or progenitor hematopoietic cells promotes regenerative responses after injury (Cho et al., 2014; Garrison and Rossi, 2014).
Osteoclast Differentiation
Osteoclasts are multinucleated cells that develop from the fusion of bone marrow–derived monocyte/macrophage progenitors; receptor activator of nuclear factor-κB ligand (RANKL) is a key regulator of osteoclast differentiation and function (Teitelbaum, 2000). Using tartrate-resistant acid phosphatase as an osteoclast gene expression marker, Lee and coworkers showed that the P2Y14R is selectively transcribed during RANKL-induced osteoclastogenesis (Lee et al., 2013). P2Y14R siRNA markedly decreased P2Y14R protein expression and caused a >50% reduction of RANKL-induced osteoclast formation, suggesting that P2Y14R expression is required for the osteoclast differentiation program. This hypothesis was further supported by the observation that RANKL-induced osteoclast formation is markedly potentiated by coincubating bone marrow–derived monocyte/macrophage precursors with UDP-glucose, UDP-galactose, UDP-N-acetylglucosamine, or UDP-glucuronic acid (Lee et al., 2013). In line with this work, the above-mentioned study of Kook and colleagues indicated that UDP-glucose–stimulated hematopoietic stem cell mobilization was associated with enhanced bone marrow RANKL expression and enhanced osteoclastogenesis (Kook et al., 2013a).
Glia and Astrocytes
Initial reports indicated broad expression of P2Y14R mRNA in various areas of the brain (Chambers et al., 2000), and strong immunohistochemical staining of brain astrocytes and glia with an affinity-purified P2Y14R antiserum was subsequently reported (Moore et al., 2003). Cultured rat primary astrocytes and murine microglia N9 cells express P2Y14R mRNA, and addition of 100 µM UDP-glucose to rat astrocytes resulted in transient elevation of intracellular calcium (Fumagalli et al., 2003; Bianco et al., 2005). More recently, Kinoshita et al. (2013) reported that UDP-glucose–promoted Ca2+ signaling was reduced by ∼50% in astrocytes transfected with P2Y14R siRNA, although the extent of P2Y14R suppression was not reported. In the absence of exogenous agonists, P2Y14R siRNA-transfected cells displayed increased release of tumor necrosis factor-α (TNFα) and expression of matrix metalloprotease-9 (MMP-9), suggesting that astrocyte remodeling events are suppressed by constitutively active P2Y14R (Kinoshita et al., 2013).
Innate Mucosal Immunity
Female Reproductive Tract Epithelia.
P2Y14R mRNA is highly expressed in the surface epithelium of the female reproductive tract, and immunohistochemical studies also were consistent with prominent expression of this receptor in epithelium but not stromal tissues (Arase et al., 2009). Similarly, UDP-glucose caused a concentration-dependent increase in interleukin-8 (IL-8) secretion in primary cultures of human glandular cells but had no effect on stromal cells (Arase et al., 2009). Ishikawa endometrial epithelial cells also express P2Y14R, and UDP-glucose caused an increase in IL-8 production and secretion in these cells, which was reduced by pretreatment of cells with pertussis toxin and by siRNA-mediated knockdown of the P2Y14R. Conditioned medium from UDP-glucose–stimulated Ishikawa cells exhibited neutrophil chemoattractant activity, which was blocked by a neutralizing anti–IL-8 antibody (Arase et al., 2009).
Incubation of mouse uterine tissues with 10 nM UDP-glucose resulted in enhanced expression of two functional homologs of IL-8, MIP-2 and KC (Arase et al., 2009). Surprisingly, the effect of UDP-glucose was not observed at higher (1000 nM) concentrations, and UDP-N-acetylglucosamine had no effect. Direct injection of UDP-glucose (but not saline) into one horn of the mouse uterus in vivo induced MIP-2 and KC protein. Importantly, induction of these two chemokines was restricted to the endometrial luminal and glandular cells as was observed with IL-8 in the human endometrium. Finally, administration of UDP-glucose to mouse uterus resulted in a ∼5-fold increase in the number of neutrophils present in the endometrium after 48 hours, which was reduced by sonoporation of P2Y14R siRNA into the mouse uterus (Arase et al., 2009). Lipopolysaccharide also induced leukocytosis in mouse uterus, and this effect was attenuated by P2Y14R siRNA.
Arase and coworkers also noted that lipopolysaccharide induced upregulation of P2Y14R in Ishikawa cells as well as in mouse uterus, which is consistent with the observation of P2Y14R upregulation in the human endometrium during inflammation. For example, messages for the P2Y14R as well as for IL-1β, IL-8, and TNF-α all were upregulated in endometria from patients with pelvic inflammatory disease (Arase et al., 2009). Reports of P2Y14R mRNA upregulation in the mouse uterus after estradiol treatment of 7 days (Crabtree et al., 2006, 2008) also suggest that P2Y14R expression may be regulated by circulating hormone levels.
In sum, these studies strongly suggest that the P2Y14R promotes innate mucosal immunity in the female reproductive tract by inducing IL-8.
Lung Epithelial Cells.
P2Y14R mRNA is expressed in primary cultures of human alveolar epithelial type 2 cells, as well as in an human adenocarcinoma alveolar epithelial cell line (A549 cells) and immortalized human bronchial epithelial BEAS-2B cells (Muller et al., 2005). UDP-glucose dose-dependently evoked Ca2+ mobilization and IL-8 secretion in A549 and BEAS-2 cells, and pertussis toxin abolished these effects. Alveolar type II cells are located at the boundary between the alveolar airspace and the interstitium, and addition of UDP-glucose to primary alveolar type 2 cells resulted in enhanced IL-8 secretion, suggesting that the P2Y14R on type 2 cells senses UDP-sugars accumulating in the alveolar space, potentially triggering neutrophil recruitment via IL-8 secretion (Muller et al., 2005).
Gastrointestinal Functions
Message for the P2Y14R is widely expressed in the rodent gastrointestinal tract (Chambers et al., 2000), with the highest levels observed in the forestomach (Bassil et al., 2009). UDP-glucose and UDP-galactose promoted concentration-dependent increases in baseline muscle tension in isolated forestomach longitudinal muscle preparations (Bassil et al., 2009). The effects of UDP-glucose were lost in tissue isolated from the p2y14 knockout mouse (generated by interrupting the open reading frame of the P2Y14R with an IRES-LacZ-polyA reporter expression cassette), and strong β-galactosidase staining was observed in the muscularis externa of the forestomach of these mice (Bassil et al., 2009). P2Y14R-deficient mice exhibited no apparent alteration in gastric clearance of a nutrient meal (Bassil et al., 2009). Interestingly, using a similarly generated P2Y14R-deficient mouse model, Meister and colleagues reported that robust β-galactosidase activity was observed in subsets of smooth muscle cells from the ileum to the colon, and excretion of orally administrated dextran blue in feces was delayed in P2Y14R knockout mice compared with wild-type mice (Meister et al., 2014). These observations suggest that P2Y14R function is associated with meal passage throughout the entire gastrointestinal tract.
Endocrine Pancreas
Meister and coworkers also found high levels of P2Y14R mRNA in the pancreas of wild-type mice, with robust β-galactosidase expression in the islets as well as the exocrine pancreas of P2Y14R-targeted mice; the cell type in which the receptor is expressed was not identified (Meister et al., 2014). Insulin immunostaining of pancreatic tissue was similar in both genotypes, but glucose-promoted insulin secretion in perfused islets was markedly reduced in P2Y14R-deficient mice. P2Y14R knockout mice kept under a Western-type diet (rich in fat and cholesterol) for 12 weeks exhibited reduced serum insulin levels in response to glucose administration compared with wild-type animals. Perfusion of wild-type islets with UDP-glucose promoted no insulin secretion, suggesting that the P2Y14R regulates functions that are necessary but not sufficient to promote insulin granule exocytosis. Transcriptome analysis of pancreatic islets indicated that P2Y14R deletion resulted in downregulation of more than 300 genes, including the glucose transporter Slc2a2 and several components of the Ca2+-triggered and GPCR-modulated insulin release pathways (Meister et al., 2014). In addition, more than 400 transcripts were upregulated in these preparations. Notably, no differences in insulin sensitivity were observed between Western diet–fed wild-type and P2Y14R-deficient mice (Meister et al., 2014), an observation that is in apparent conflict with those described by Xu and colleagues with HFD-fed mice (Xu et al., 2012). The discrepancy may reflect different contributions of liver inflammation to insulin sensitivity between the two mouse models.
Vascular and Airway Tone
Vascular Tone.
P2Y14R transcripts are localized in middle meningeal and rat aortic smooth muscle cells (Govindan et al., 2010; Haanes and Edvinsson, 2014b). Meister and collaborators found strong β-galactosidase staining in smooth muscle cells of venous blood vessels as well as in a few arteries, such as lung arteries and central nervous system circulus arteriosus Willisii. P2Y14R-targeted mice exhibited a mild increase in blood pressure and heart rates (Meister et al., 2014).
Haanes and Edvinsson (2014a) reported that UDP-glucose promoted contraction of mouse coronary arteries, and this effect was reduced by pertussis toxin. High micromolar concentrations of UDP-glucose were used to induce contraction of freshly excised arteries, but UDP-glucose potency increased considerably in coronary arteries that were maintained in culture medium for 24 hours; all these responses were reproduced with arteries isolated from P2Y2 receptor knockout mice, ruling out the possibility that traces of ATP or UTP in UDP-glucose solutions accounted for the observed results (Haanes and Edvinsson, 2014a). The enhanced potency of UDP-glucose effects on precultured coronary arteries correlated with an enhanced immunohistochemistry staining and increased intensity of a 70-kDa band on Western blots assessed with a commercial antibody against P2Y14R. Results similar to those with coronary arteries were observed with basilar arteries, except that in the latter, treatment with pertussis toxin resulted in UDP-glucose–evoked relaxation rather than contraction (Haanes and Edvinsson, 2014a). The availability of P2Y14R antagonists and P2Y14R knockout mouse models should now provide tools to more conclusively define the involvement of the P2Y14R in UDP-glucose–promoted contraction of coronary and basilar arteries.
Alsaqati and coworkers reported that UDP-glucose (1–1000 µM) and the synthetic P2Y14R agonist MRS2690 (0.1–30 µM) elicited vasoconstriction of porcine pancreatic arteries, and the P2Y14R antagonist PPTN (10 µM) inhibited these responses (Alsaqati et al., 2014). In contrast, UTP-promoted contraction was not affected by PPTN. UDP-sugar–promoted contraction was attenuated by removal of the endothelium as well as by antagonists of endothelin ETA and thromboxane/prostaglandin receptors and by inhibitors of intracellular signaling (e.g., Ca2+, Rho kinase). UDP-glucose (1 mM) and MRS2690 (10 µM) promoted a decrease of cAMP formation in pancreatic arterial rings costimulated with forskolin and the prostaglandin analog U46619 [(5Z)-7-[(1R,4S,5S,6R)-6-[(1E,3S)-3-hydroxy-1-octenyl]-2-oxabicyclo[2.2.1]hept-5-yl]-5-heptenoic acid]. By promoting endothelium-dependent artery contraction in the pancreas, the P2Y14R may influence pancreatic functions by regulating blood flow into the pancreas.
Airway Tone.
In addition to vascular and gastrointestinal smooth muscles, Meister and coworkers observed P2Y14R reporter expression (β-galactosidase) in smooth muscle cells of bronchioles. In line with this P2Y14R expression pattern, reduced lung resistance and increased dynamic compliance in response to methacholine were observed in P2Y14R knockout mice (Meister et al., 2014). These observations may be relevant to altered airway responsiveness associated with obstructive lung diseases.
Conclusions
Fifteen years have elapsed since the initial characterization of the product of the orphan gene GPR105 (KIAA0001) as P2Y14R, a Gi-coupled receptor potently activated by UDP-sugars (Chambers et al., 2000). The abundant message expression of the human P2Y14R in adipose tissue, stomach, intestine, some brain regions, skeletal muscles, spleen, lung, and heart suggests that released UDP-sugars fulfill important extracellular signaling roles in these tissues. Realization that mRNA for the GPR105 rat ortholog in brain and spleen is regulated by immunologic challenge and that the P2Y14R mRNA is also prominently expressed in peripheral inflammatory cells further suggests a role for this receptor in immunity and inflammation (Chambers et al., 2000; Moore et al., 2003).
Using P2Y14R gene deletion or downregulation approaches, as well as newly developed highly selective P2Y14R agonists and antagonists, considerable progress was achieved during recent years to further support and expand those early observations. The work of Arase et al. (2009) successfully targeting P2Y14-R expression by introducing siRNA in mouse uterus in vivo revealed a mechanism relevant to mucosal inflammation (i.e., UDP-glucose–promoted P2Y14R-mediated IL-8 release and neutrophil recruitment in the uterus). Studies by Sesma and coworkers (2012) and Barrett and coworkers (2013) support an additional scenario for UDP-sugar–promoted neutrophil inflammation (i.e., direct activation of the neutrophil P2Y14R).
ATP released from stressed cells acts as a “find-me” signal to recruit macrophages at the site of injury (Elliott et al., 2009; Chekeni et al., 2010), and the work by Xu and colleagues (2012) suggests a similar role for UDP-glucose. That is, HFD-induced macrophage inflammation in the liver was attenuated in P2Y14R-targeted mice. Moreover, blood levels of UDP-glucose were elevated in HFD-fed mice, and UDP-glucose promoted chemotaxis of wild-type mice but not P2Y14-deficient monocytes.
In addition to promoting recruitment of circulating neutrophils and monocytes, UDP-glucose induced the mobilization and enhanced relocation to extramedullary organs of a subset of bone marrow stem cells (Kook et al., 2013a). P2Y14R-mediated hematopoietic stem cell mobilization provides a potential tool to improve the efficiency and outcome of hematopoietic peripheral stem cell transplantation. Furthermore, primitive hematopoietic stem cells lacking P2Y14R exhibited impaired ability to restore hematopoiesis in irradiated mice, suggesting that the P2Y14R on progenitor stem cells promotes regenerative responses after injury (Cho et al., 2014).
P2Y14R-targeted mice have been useful to identify functions of this receptor that are associated with gastrointestinal and digestive functions: 1) UDP-glucose–promoted baseline muscle tension in isolated forestomach longitudinal muscle preparations was abolished in P2Y14 knockout mice (Bassil et al., 2009); 2) P2Y14R knockout mice exhibited delayed excretion of orally administrated dextran blue in feces (Meister et al., 2014), suggesting that the P2Y14R regulates meal passage through the gastrointestinal tract; and 3) P2Y14R knockout mice exhibited impaired glucose tolerance and deficient insulin secretion in response to glucose (Meister et al., 2014).
Realization that the P2Y14R is abundantly expressed in subsets of smooth muscle cells of the vasculature and airways and that P2Y14R-deficient mice exhibited increased blood pressure and heart rates as well as reduced lung resistance and increased dynamic compliance in response to methacholine (Meister et al., 2014) suggest important additional areas of potential pharmacologic intervention.
Indeed, potent and selective P2Y14R antagonists (e.g., PPTN) are now available (Kiselev et al., 2014), and one of them, PPTN, significantly reduced UDP-glucose–promoted neutrophil chemotaxis, mast cell degranulation, and pancreatic artery contraction. This finding suggests novel strategies for the treatment of inflammation and, potentially, other disorders associated with the increasing number of P2Y14R-regulated functions recently identified (Table 2).
TABLE 2.
Proposed physiologic effects of P2Y14R activation
| P2Y14R-Associated Responses | References |
|---|---|
| Regulation of dendritic cell maturation | Skelton et al., 2003 |
| Inhibition of T-lymphocyte proliferation | Scrivens and Dickenson, 2005a |
| Recruitment of monocytes and macrophages in inflammation | Xu et al., 2012 |
| Stimulation of neutrophil chemotaxis | Sesma et al., 2012; Barrett et al., 2013 |
| Enhancement of degranulation of mast cells | Gao et al., 2013 |
| Promotion of hematopoietic regenerative responses | Cho et al., 2014 |
| Regulation of osteoclast differentiation | Lee et al., 2013; Kook et al., 2013a |
| Regulation of astrocyte remodeling | Kinoshita et al., 2013 |
| Stimulation of innate mucosal immunity in the female reproductive tract | Arase et al., 2009 |
| Promotion of lung inflammation | Muller et al., 2005 |
| Regulation of gastrointestinal muscle contraction and meal passage | Bassil et al., 2009; Meister et al., 2014 |
| Regulation of insulin secretion | Meister et al., 2014 |
| Regulation of smooth muscle contraction in some vascular beds | Meister et al., 2014; Haanes and Edvinsson, 2014a; Alsaqati et al., 2014 |
Abbreviations
- ENPP
ectonucleotide pyrophosphatase
- ER
endoplasmic reticulum
- ERK
extracellular signal–regulated kinase
- HFD
high-fat diet
- HSC
hematopoietic stem cell
- GPCR
G-protein–coupled receptor
- MAPK
mitogen-activated protein kinase
- MRS2690
2-thio-UDP-glucose
- PMN
polymorphonuclear neutrophil
- P2Y14R
P2Y14 receptor
- PPTN
4-((piperidin-4-yl)-phenyl)-7-(4-(trifluoromethyl)-phenyl)-2-naphthoic acid
- RANKL
receptor activator of nuclear factor-κB ligand
- siRNA
small interfering RNA
- U46619
(5Z)-7-[(1R,4S,5S,6R)-6-[(1E,3S)-3-hydroxy-1-octenyl]-2-oxabicyclo[2.2.1]hept-5-yl]-5-heptenoic acid
Authorship Contributions
Wrote or contributed to the writing of the manuscript: Lazarowski, Harden.
Footnotes
>This work was supported by the National Institutes of Health (NIH) National Institute of General Medical Sciences [Grant R01-GM38213] and the NIH National Heart, Lung, and Blood Institute [Grant P01-HL110873].
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, et al. (2006) International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev 58:281–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeijon C, Hirschberg CB. (1992) Topography of glycosylation reactions in the endoplasmic reticulum. Trends Biochem Sci 17:32–36. [DOI] [PubMed] [Google Scholar]
- Alsaqati M, Latif ML, Chan SL, Ralevic V. (2014) Novel vasocontractile role of the P2Y14 receptor: characterization of its signalling in porcine isolated pancreatic arteries. Br J Pharmacol 171:701–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arase T, Uchida H, Kajitani T, Ono M, Tamaki K, Oda H, Nishikawa S, Kagami M, Nagashima T, Masuda H, et al. (2009) The UDP-glucose receptor P2RY14 triggers innate mucosal immunity in the female reproductive tract by inducing IL-8. J Immunol 182:7074–7084. [DOI] [PubMed] [Google Scholar]
- Barrett MO, Sesma JI, Ball CB, Jayasekara PS, Jacobson KA, Lazarowski ER, Harden TK. (2013) A selective high-affinity antagonist of the P2Y14 receptor inhibits UDP-glucose-stimulated chemotaxis of human neutrophils. Mol Pharmacol 84:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil AK, Bourdu S, Townson KA, Wheeldon A, Jarvie EM, Zebda N, Abuin A, Grau E, Livi GP, Punter L, et al. (2009) UDP-glucose modulates gastric function through P2Y14 receptor-dependent and -independent mechanisms. Am J Physiol Gastrointest Liver Physiol 296:G923–G930. [DOI] [PubMed] [Google Scholar]
- Berninsone P, Hirschberg CB. (1998) Nucleotide sugars, nucleotide sulfate, and ATP transporters of the endoplasmic reticulum and Golgi apparatus. Ann N Y Acad Sci 842:91–99. [DOI] [PubMed] [Google Scholar]
- Bianco F, Fumagalli M, Pravettoni E, D’Ambrosi N, Volonte C, Matteoli M, Abbracchio MP, Verderio C. (2005) Pathophysiological roles of extracellular nucleotides in glial cells: differential expression of purinergic receptors in resting and activated microglia. Brain Res Brain Res Rev 48:144–156. [DOI] [PubMed] [Google Scholar]
- Boyd-Tressler A, Penuela S, Laird DW, Dubyak GR. (2014) Chemotherapeutic drugs induce ATP release via caspase-gated pannexin-1 channels and a caspase/pannexin-1-independent mechanism. J Biol Chem 289:27246–27263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brautigam VM, Dubyak GR, Crain JM, Watters JJ. (2008) The inflammatory effects of UDP-glucose in N9 microglia are not mediated by P2Y14 receptor activation. Purinergic Signal 4:73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RL, Fricks IP, Barrett MO, Burianek LE, Zhou Y, Ko H, Das A, Jacobson KA, Lazarowski ER, Harden TK. (2009) Quantification of Gi-mediated inhibition of adenylyl cyclase activity reveals that UDP is a potent agonist of the human P2Y14 receptor. Mol Pharmacol 76:1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers JK, Macdonald LE, Sarau HM, Ames RS, Freeman K, Foley JJ, Zhu Y, McLaughlin MM, Murdock P, McMillan L, et al. (2000) A G protein-coupled receptor for UDP-glucose. J Biol Chem 275:10767–10771. [DOI] [PubMed] [Google Scholar]
- Chekeni FB, Elliott MR, Sandilos JK, Walk SF, Kinchen JM, Lazarowski ER, Armstrong AJ, Penuela S, Laird DW, Salvesen GS, et al. (2010) Pannexin 1 channels mediate ‘find-me’ signal release and membrane permeability during apoptosis. Nature 467:863–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Yusuf R, Kook S, Attar E, Lee D, Park B, Cheng T, Scadden DT, Lee BC. (2014) Purinergic P2Y14 receptor modulates stress-induced hematopoietic stem/progenitor cell senescence. J Clin Invest 124:3159–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree JS, Peano BJ, Zhang X, Komm BS, Winneker RC, Harris HA. (2008) Activity of three selective estrogen receptor modulators on hormone-dependent responses in the mouse uterus and mammary gland. Mol Cell Endocrinol 287:40–46. [DOI] [PubMed] [Google Scholar]
- Crabtree JS, Zhang X, Peano BJ, Zhang Z, Winneker RC, Harris HA. (2006) Development of a mouse model of mammary gland versus uterus tissue selectivity using estrogen- and progesterone-regulated gene markers. J Steroid Biochem Mol Biol 101:11–21. [DOI] [PubMed] [Google Scholar]
- Crespo P, Xu N, Simonds WF, Gutkind JS. (1994) Ras-dependent activation of MAP kinase pathway mediated by G-protein beta gamma subunits. Nature 369:418–420. [DOI] [PubMed] [Google Scholar]
- Das A, Ko H, Burianek LE, Barrett MO, Harden TK, Jacobson KA. (2010) Human P2Y14 receptor agonists: truncation of the hexose moiety of uridine-5′-diphosphoglucose and its replacement with alkyl and aryl groups. J Med Chem 53:471–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott MR, Chekeni FB, Trampont PC, Lazarowski ER, Kadl A, Walk SF, Park D, Woodson RI, Ostankovich M, Sharma P, et al. (2009) Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 461:282–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman K, Tsui P, Moore D, Emson PC, Vawter L, Naheed S, Lane P, Bawagan H, Herrity N, Murphy K, et al. (2001) Cloning, pharmacology, and tissue distribution of G-protein-coupled receptor GPR105 (KIAA0001) rodent orthologs. Genomics 78:124–128. [DOI] [PubMed] [Google Scholar]
- Fricks IP, Carter RL, Lazarowski ER, Harden TK. (2009) Gi-dependent cell signaling responses of the human P2Y14 receptor in model cell systems. J Pharmacol Exp Ther 330:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fricks IP, Maddileti S, Carter RL, Lazarowski ER, Nicholas RA, Jacobson KA, Harden TK. (2008) UDP is a competitive antagonist at the human P2Y14 receptor. J Pharmacol Exp Ther 325:588–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fumagalli M, Brambilla R, D’Ambrosi N, Volonté C, Matteoli M, Verderio C, Abbracchio MP. (2003) Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia 43:218–03. [DOI] [PubMed] [Google Scholar]
- Gao ZG, Wei Q, Jayasekara MP, Jacobson KA. (2013) The role of P2Y14 and other P2Y receptors in degranulation of human LAD2 mast cells. Purinergic Signal 9:31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrison BS, Rossi DJ. (2014) Loss of P2Y₁₄ results in an arresting response to hematological stress. J Clin Invest 124:2846–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauthier JY, Belley M, Deschênes D, Fournier JF, Gagné S, Gareau Y, Hamel M, Hénault M, Hyjazie H, Kargman S, et al. (2011) The identification of 4,7-disubstituted naphthoic acid derivatives as UDP-competitive antagonists of P2Y14. Bioorg Med Chem Lett 21:2836–2839. [DOI] [PubMed] [Google Scholar]
- Govindan S, Taylor EJ, Taylor CW. (2010) Ca2+ signalling by P2Y receptors in cultured rat aortic smooth muscle cells. Br J Pharmacol 160:1953–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guay D, Beaulieu C, Belley M, Crane SN, DeLuca J, Gareau Y, Hamel M, Henault M, Hyjazie H, Kargman S, et al. (2011) Synthesis and SAR of pyrimidine-based, non-nucleotide P2Y14 receptor antagonists. Bioorg Med Chem Lett 21:2832–2835. [DOI] [PubMed] [Google Scholar]
- Haanes KA, Edvinsson L. (2014a) Characterization of the contractile P2Y14 receptor in mouse coronary and cerebral arteries. FEBS Lett 588:2936–2943. [DOI] [PubMed] [Google Scholar]
- Haanes KA, Edvinsson L. (2014b) Expression and characterization of purinergic receptors in rat middle meningeal artery-potential role in migraine. PLoS ONE 9:e108782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamel M, Henault M, Hyjazie H, Morin N, Bayly C, Skorey K, Therien AG, Mancini J, Brideau C, Kargman S. (2011) Discovery of novel P2Y14 agonist and antagonist using conventional and nonconventional methods. J Biomol Screen 16:1098–1105. [DOI] [PubMed] [Google Scholar]
- Harden TK, Sesma JI, Fricks IP, Lazarowski ER. (2010) Signalling and pharmacological properties of the P2Y14 receptor. Acta Physiol (Oxf) 199:149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita M, Nasu-Tada K, Fujishita K, Sato K, Koizumi S. (2013) Secretion of matrix metalloproteinase-9 from astrocytes by inhibition of tonic P2Y14-receptor-mediated signal(s). Cell Mol Neurobiol 33:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselev E, Barrett MO, Katritch V, Paoletta S, Weitzer CD, Brown KA, Hammes E, Yin AL, Zhao Q, Stevens RC, et al. (2014) Exploring a 2-naphthoic acid template for the structure-based design of P2Y14 receptor antagonist molecular probes. ACS Chem Biol 9:2833–2842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Das A, Carter RL, Fricks IP, Zhou Y, Ivanov AA, Melman A, Joshi BV, Kovác P, Hajduch J, et al. (2009) Molecular recognition in the P2Y14 receptor: probing the structurally permissive terminal sugar moiety of uridine-5′-diphosphoglucose. Bioorg Med Chem 17:5298–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H, Fricks I, Ivanov AA, Harden TK, Jacobson KA. (2007) Structure-activity relationship of uridine 5′-diphosphoglucose analogues as agonists of the human P2Y14 receptor. J Med Chem 50:2030–2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook S, Cho J, Lee SB, Lee BC. (2013a) The nucleotide sugar UDP-glucose mobilizes long-term repopulating primitive hematopoietic cells. J Clin Invest 123:3420–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook SH, Cho JS, Morrison A, Wiener E, Lee SB, Scadden D, Lee BC. (2013b) The purinergic P2Y14 receptor axis is a molecular determinant for organism survival under in utero radiation toxicity. Cell Death Dis 4:e703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, Davis CW, Boucher RC, Lazarowski ER. (2007) Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol 584:245–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Seminario-Vidal L, van Heusden C, Lazarowski ER. (2008) Thrombin-promoted release of UDP-glucose from human astrocytoma cells. Br J Pharmacol 153:1528–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER. (2010) Quantification of extracellular UDP-galactose. Anal Biochem 396:23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarowski ER, Shea DA, Boucher RC, Harden TK. (2003) Release of cellular UDP-glucose as a potential extracellular signaling molecule. Mol Pharmacol 63:1190–1197. [DOI] [PubMed] [Google Scholar]
- Lee BC, Cheng T, Adams GB, Attar EC, Miura N, Lee SB, Saito Y, Olszak I, Dombkowski D, Olson DP, et al. (2003) P2Y-like receptor, GPR105 (P2Y14), identifies and mediates chemotaxis of bone-marrow hematopoietic stem cells. Genes Dev 17:1592–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SA, Park JH, Lee SY. (2013) Selective induction of P2Y14 receptor by RANKL promotes osteoclast formation. Mol Cells 36:273–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister J, Le Duc D, Ricken A, Burkhardt R, Thiery J, Pfannkuche H, Polte T, Grosse J, Schöneberg T, Schulz A. (2014) The G protein-coupled receptor P2Y14 influences insulin release and smooth muscle function in mice. J Biol Chem 289:23353–23366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DJ, Murdock PR, Watson JM, Faull RL, Waldvogel HJ, Szekeres PG, Wilson S, Freeman KB, Emson PC. (2003) GPR105, a novel Gi/o-coupled UDP-glucose receptor expressed on brain glia and peripheral immune cells, is regulated by immunologic challenge: possible role in neuroimmune function. Brain Res Mol Brain Res 118:10–23. [DOI] [PubMed] [Google Scholar]
- Müller T, Bayer H, Myrtek D, Ferrari D, Sorichter S, Ziegenhagen MW, Zissel G, Virchow JC, Jr, Luttmann W, Norgauer J, et al. (2005) The P2Y14 receptor of airway epithelial cells: coupling to intracellular Ca2+ and IL-8 secretion. Am J Respir Cell Mol Biol 33:601–609. [DOI] [PubMed] [Google Scholar]
- Okada SF, Zhang L, Kreda SM, Abdullah LH, Davis CW, Pickles RJ, Lazarowski ER, Boucher RC. (2011) Coupled nucleotide and mucin hypersecretion from goblet-cell metaplastic human airway epithelium. Am J Respir Cell Mol Biol 45:253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olefsky JM, Glass CK. (2010) Macrophages, inflammation, and insulin resistance. Annu Rev Physiol 72:219–246. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. (1998) Receptors for purines and pyrimidines. Pharmacol Rev 50:413–492. [PubMed] [Google Scholar]
- Robichaud J, Fournier JF, Gagné S, Gauthier JY, Hamel M, Han Y, Hénault M, Kargman S, Levesque JF, Mamane Y, et al. (2011) Applying the pro-drug approach to afford highly bioavailable antagonists of P2Y14. Bioorg Med Chem Lett 21:4366–4368. [DOI] [PubMed] [Google Scholar]
- Scrivens M, Dickenson JM. (2005a) Functional expression of the P2Y14 receptor in murine T-lymphocytes. Br J Pharmacol 146:435–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrivens M, Dickenson JM. (2005b) Pharmacological effects mediated by UDP-glucose that are independent of P2Y14 receptor expression. Pharmacol Res 51:533–538. [DOI] [PubMed] [Google Scholar]
- Scrivens M, Dickenson JM. (2006) Functional expression of the P2Y14 receptor in human neutrophils. Eur J Pharmacol 543:166–173. [DOI] [PubMed] [Google Scholar]
- Sesma JI, Esther CR, Jr, Kreda SM, Jones L, O'Neal W, Nishihara S, Nicholas RA, Lazarowski ER. (2009) Endoplasmic reticulum/golgi nucleotide sugar transporters contribute to the cellular release of UDP-sugar signaling molecules. J Biol Chem 284:12572–12583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesma JI, Kreda SM, Steinckwich-Besancon N, Dang H, García-Mata R, Harden TK, Lazarowski ER. (2012) The UDP-sugar-sensing P2Y14 receptor promotes Rho-mediated signaling and chemotaxis in human neutrophils. Am J Physiol Cell Physiol 303:C490–C498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelton L, Cooper M, Murphy M, Platt A. (2003) Human immature monocyte-derived dendritic cells express the G protein-coupled receptor GPR105 (KIAA0001, P2Y14) and increase intracellular calcium in response to its agonist, uridine diphosphoglucose. J Immunol 171:1941–1949. [DOI] [PubMed] [Google Scholar]
- Smrcka AV. (2008) G protein βγ subunits: central mediators of G protein-coupled receptor signaling. Cell Mol Life Sci 65:2191–2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens L, Smrcka A, Cooke FT, Jackson TR, Sternweis PC, Hawkins PT. (1994) A novel phosphoinositide 3 kinase activity in myeloid-derived cells is activated by G protein beta gamma subunits. Cell 77:83–93. [DOI] [PubMed] [Google Scholar]
- Teitelbaum SL. (2000) Bone resorption by osteoclasts. Science 289:1504–1508. [DOI] [PubMed] [Google Scholar]
- von Kügelgen I, Harden TK. (2011) Molecular pharmacology, physiology, and structure of the P2Y receptors. Adv Pharmacol 61:373–415. [DOI] [PubMed] [Google Scholar]
- Wang Y, Ausman LM, Russell RM, Greenberg AS, Wang XD. (2008) Increased apoptosis in high-fat diet-induced nonalcoholic steatohepatitis in rats is associated with c-Jun NH2-terminal kinase activation and elevated proapoptotic Bax. J Nutr 138:1866–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Morinaga H, Oh D, Li P, Chen A, Talukdar S, Mamane Y, Mancini JA, Nawrocki AR, Lazarowski E, et al. (2012) GPR105 ablation prevents inflammation and improves insulin sensitivity in mice with diet-induced obesity. J Immunol 189:1992–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann H, Zebisch M, Sträter N. (2012) Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal 8:437–502. [DOI] [PMC free article] [PubMed] [Google Scholar]