Abstract
Cholestasis activates bile acid receptor farnesoid X receptor (FXR) and subsequently enhances hepatic expression of small heterodimer partner (SHP). We previously demonstrated that SHP represses the transactivation of cytochrome P450 2D6 (CYP2D6) promoter by hepatocyte nuclear factor (HNF) 4α. In this study, we investigated the effects of estrogen-induced cholestasis on CYP2D6 expression. Estrogen-induced cholestasis occurs in subjects receiving estrogen for contraception or hormone replacement, or in susceptible women during pregnancy. In CYP2D6-humanized transgenic (Tg-CYP2D6) mice, cholestasis triggered by administration of 17α-ethinylestradiol (EE2) at a high dose led to 2- to 3-fold decreases in CYP2D6 expression. This was accompanied by increased hepatic SHP expression and subsequent decreases in the recruitment of HNF4α to CYP2D6 promoter. Interestingly, estrogen-induced cholestasis also led to increased recruitment of estrogen receptor (ER) α, but not that of FXR, to Shp promoter, suggesting a predominant role of ERα in transcriptional regulation of SHP in estrogen-induced cholestasis. EE2 at a low dose (that does not cause cholestasis) also increased SHP (by ∼50%) and decreased CYP2D6 expression (by 1.5-fold) in Tg-CYP2D6 mice, the magnitude of differences being much smaller than that shown in EE2-induced cholestasis. Taken together, our data indicate that EE2-induced cholestasis increases SHP and represses CYP2D6 expression in Tg-CYP2D6 mice in part through ERα transactivation of Shp promoter.
Introduction
Cholestasis is a disease caused by impaired bile formation in liver cells (i.e., intrahepatic cholestasis) or obstruction of bile flow via bile ducts (i.e., extrahepatic cholestasis) (Trauner et al., 1999; Kullak-Ublick and Meier, 2000; Trauner and Boyer, 2003). Intrahepatic cholestasis can be caused by medications (e.g., cyclosporine A) or pregnancy, and extrahepatic cholestasis by bile duct blockade from gallstones or tumors. Regardless of the underlying causes, cholestasis results in hepatic and systemic accumulation of cytotoxic bile acids. This induces liver damage accompanied by pruritus and indigestion, ultimately leading to biliary fibrosis and cirrhosis (Trauner et al., 1998; Rodriguez-Garay, 2003). Treatment of cholestasis involves surgical removal of underlying causes, if possible, and drug therapy using ursodeoxycholic acid and immunosuppressive drugs (EASL, 2009). The management of symptoms accompanying cholestasis may also require the use of drug therapy (e.g., serotonin reuptake inhibitors) (Kremer et al., 2008).
Cytochrome P450 2D6 (CYP2D6) is a major drug-metabolizing enzyme and is responsible for eliminating approximately 20% of marketed drugs such as opioid analgesics and antidepressants. Recently, we have identified small heterodimer partner (SHP) as a novel transcriptional regulator of CYP2D6 expression (Koh et al., 2014); SHP represses hepatocyte nuclear factor (HNF) 4α transactivation of CYP2D6 promoter (Cairns et al., 1996; Koh et al., 2014). Also, in CYP2D6-humanized transgenic (Tg-CYP2D6) mice, SHP knockdown led to a significant increase in CYP2D6 expression (Koh et al., 2014). SHP is a representative target gene of the bile acid sensor, farnesoid X receptor (FXR). Upon binding to bile acids, FXR transactivates SHP promoter and upregulates SHP expression (Goodwin et al., 2000). SHP in turn represses the transcription of genes involved in bile acid synthesis such as CYP7A1 and CYP8B1 (Goodwin et al., 2000). Whether enhanced expression of SHP in cholestasis leads to altered CYP2D6 expression remains unknown.
Estrogen is the major component in oral contraceptives and hormone replacement therapy. Estrogen regulates growth and differentiation as well as multiple physiologic functions by activating its cognate receptor, estrogen receptor (ER) α and ERβ. In the liver, expression of ERβ is localized to the cholangiocytes (Alvaro et al., 2002), and ERα is the major isoform expressed in the parenchymal cells (Kuiper et al., 1997). Of note, estrogens can cause intrahepatic cholestasis in premenopausal women receiving oral contraceptives, in postmenopausal women on hormone replacement therapy (Schreiber and Simon, 1983), or in men who receive estrogen therapy for the treatment of prostate cancer (Kontturi and Sotaniemi, 1969). Estrogen-induced cholestasis can also occur during pregnancy; intrahepatic cholestasis of pregnancy is the most common liver disease in pregnant women (Reyes, 1997; Riely and Bacq, 2004). An experimental intrahepatic cholestasis model established by 17α-ethinylestradiol (EE2) administration in rodents has been commonly used to study the pathogenesis of estrogen-induced intrahepatic cholestasis (Bossard et al., 1993; Trauner et al., 1999; Rodriguez-Garay, 2003; Yamamoto et al., 2006).
In this study, we examined the effects of estrogen-induced intrahepatic cholestasis on CYP2D6-mediated drug metabolism. Our results indicate that EE2-induced cholestasis increases SHP and represses CYP2D6 expression in Tg-CYP2D6 mice. Studies of the underlying mechanisms revealed a role of ERα in CYP2D6 regulation in estrogen-induced cholestasis.
Materials and Methods
Animals.
CYP2D6-humanized transgenic (Tg-CYP2D6) mice were previously described (Corchero et al., 2001). Tg-CYP2D6 mouse harbors the wild-type CYP2D6 gene with its 2.5-kb upstream regulatory region in the mouse genome (Corchero et al., 2001). Adult male mice (8 weeks of age and weighing 20–25 g) were used for the experiments. For estrogen-induced intrahepatic cholestasis, mice received subcutaneous injections of EE2 (10 mg/kg) or vehicle (olive oil) daily for 5 days (Yamamoto et al., 2006). For the activation of ERα alone (without cholestasis), mice received intraperitoneal injections of EE2 (5 μg/kg) or vehicle daily for 5 days (Yoshikawa et al., 2012). Mice were sacrificed on the sixth day (i.e., 24 hours after the last dose), and blood and liver tissues were collected. All procedures were carried out in accordance with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the U.S. National Institutes of Health, and were approved by the Institution’s Animal Care and use Committee at the University of Illinois (Chicago, IL).
Chemicals and Reagents.
Debrisoquine, (±)-4-hydroxydebrisoquin, and paraxanthine were purchased from Biomol (Plymouth Meeting, PA). EE2 was purchased from Sigma-Aldrich (St. Louis, MO).
Western Blot.
Western blot was performed, as described previously (Koh et al., 2014), using SHP antibody from Santa Cruz Biotechnology (Dallas, TX).
Determination of CYP2D6 Activity.
Liver S9 fractions were prepared, as described previously (Koh et al., 2014). S9 fractions were incubated with debrisoquine (a CYP2D6 probe substrate; 200 μM) based on the finding that mouse endogenous CYP2Ds have minor roles in debrisoquine hydroxylation (Koh et al., 2014). The concentration of 4-hydroxydebrisoquine was determined by liquid chromatography–tandem mass spectrometry using paraxanthine as the internal standard (Koh et al., 2014).
RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction.
Total RNA was isolated from mouse liver tissues using TRIzol (Life Technologies, Carlsbad, CA) and converted to cDNA using High-Capacity cDNA Reverse Transcription Kit (Life Technologies). Using the cDNA as template, quantitative real-time polymerase chain reaction (qRT-PCR) was performed using StepOnePlus Real-Time PCR System and primers listed in Supplemental Table 1. The results are expressed as fold changes under treatment using the gene expression levels normalized to those of glyceraldehyde-3-phosphate dehydrogenase (2−ΔΔCt method).
Chromatin Immunoprecipitation Assays.
Chromatin immunoprecipitation (ChIP) assays were performed in mouse liver tissues, as described previously (Koh et al., 2014). Briefly, livers were finely minced and incubated in phosphate-buffered saline containing 1% formaldehyde at room temperature for 15 minutes, and glycine was added to stop the cross-linking reaction. Cell pellets were resuspended in hypotonic buffer (15 mM HEPES, pH 7.9, 60 mM KCl, 2 mM EDTA, 0.5% bovine serum albumin, 0.15 mM spermine, 0.5 mM spermidine, and 0.32 M sucrose) and lysed by homogenization. Nuclei were pelleted and resuspended in nuclei lysis buffer (50 mM Tris-HCl, pH 8.0, 2 mM EDTA, 1% SDS). The samples were sonicated to shear DNA to the length ranging from 100 to 500 bp by using Misonix S-4000 sonicator (Farmingdale, NY) with 80% amplitude for 12 minutes. After centrifugation, the chromatin sample was immunoprecipitated with 2 μg antibody (ERα; FXR; HNF4α; RNA polymerase II; SHP; Santa Cruz) or IgG (normal goat IgG; normal rabbit IgG; Santa Cruz) at 4°C for overnight. The immune complex was collected, the magnetic beads were extensively washed, and the bound chromatin was eluted. Genomic DNA was purified by PCR Clean-up kit (Promega, Madison, WI) and used as a template for quantitative PCR. Primer sequences are listed in Supplemental Table 2.
Alkaline Phosphatase and Alanine Aminotransferase Measurement.
Plasma alkaline phosphatase (ALP) and alanine aminotransferase (ALT) levels were measured by chemistry analyzer (Olympus AU 680; Center Valley, PA) following the manufacturer’s protocol.
Statistical Analysis.
Values were reported as mean ± S.E.M. Statistical differences were determined by using Student’s t test.
Results
EE2-Induced Cholestasis Leads to Decreased HNF4α Transactivation of CYP2D6 Promoter.
Previous studies have shown that administration of high-dose estrogen triggers intrahepatic cholestasis (Bossard et al., 1993; Trauner et al., 1999; Rodriguez-Garay, 2003; Yamamoto et al., 2006). To investigate the effects of EE2-induced cholestasis on CYP2D6 expression and activity in vivo, Tg-CYP2D6 mice were treated with EE2 or vehicle control. As expected from EE2-induced cholestasis, the plasma levels of ALP [a marker for cholestasis (Krones et al., 2014)] and ALT (a marker for liver injury) were significantly increased in EE2-treated mice (Fig. 1).
Fig. 1.
EE2 induces cholestasis in Tg-CYP2D6 mice. Tg-CYP2D6 mice were injected subcutaneously with EE2 (10 mg/kg) or vehicle (olive oil) daily for 5 days (n = 5 mice/group). ALP and ALT activities were measured in mouse plasma. **P < 0.01. conc., concentration.
To examine potential effects of EE2-induced cholestasis on hepatic CYP2D6 expression, CYP2D6 mRNA and protein levels were determined in the mice by using qRT-PCR and Western blot. mRNA expression levels of Cyp7a1 and Cyp8b1 are known to be repressed in cholestasis (Jahan and Chiang, 2005; Yamamoto et al., 2006) and were examined as controls. The results showed that EE2-induced cholestasis led to significant decreases in Cyp7a1 and Cyp8b1 expression, as expected (Fig. 2A). EE2 also decreased mRNA level of CYP2D6 2-fold (Fig. 2A). Results from S9 phenotyping (by using debrisoquine as a probe drug for CYP2D6) revealed a ∼3-fold decrease in CYP2D6 activity (Fig. 2B). The decreased CYP2D6 expression was accompanied by increased expression of SHP at mRNA and protein levels (Fig. 2, C and D). EE2 did not alter mRNA expression levels of Hnf4α (data not shown).
Fig. 2.
EE2-induced cholestasis represses CYP2D6 expression in Tg-CYP2D6 mice. Tg-CYP2D6 mice were injected subcutaneously with EE2 (10 mg/kg) or vehicle (olive oil) daily for 5 days (n = 5 mice/group). (A) Hepatic CYP2D6, Cyp7a1, Cyp8b1, and (C) Shp mRNA expression were determined by using qRT-PCR. (B) S9 fractions were prepared from the liver tissues, and CYP2D6 activity in S9 fractions was measured using debrisoquine (200 μM) as a probe drug. Data shown are metabolite production rates in pmol/min per milligram protein. (D) SHP protein expression level was determined by Western blot. The image of Western blot (right) and the quantified band intensities (left; SHP level normalized by β-actin) are shown. Values are presented as mean ± S.E.M. **P < 0.01, versus vehicle treatment.
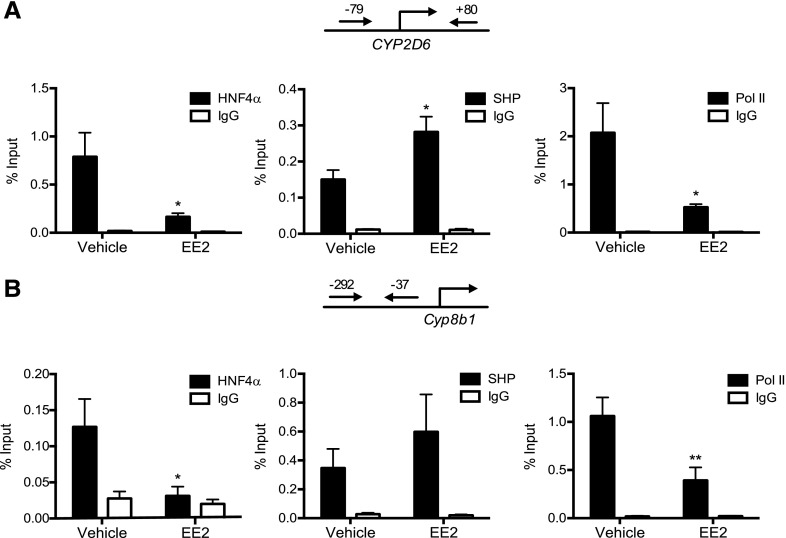
To determine whether the altered SHP expression upon EE2 administration leads to changes in HNF4α transactivation of CYP2D6 promoter, ChIP assays were performed using mouse liver tissues. Livers from vehicle- or EE2-treated mice were collected and subjected to ChIP assays using antibodies against HNF4α, SHP, or RNA polymerase II (Pol II, a marker of transcription initiation). Based on previous reports that HNF4α binds to −53/−41 of CYP2D6 (Cairns et al., 1996) and SHP suppresses HNF4α transactivation of CYP2D6 promoter (Koh et al., 2014), recruitment of the transcription factors to the proximal promoter region of CYP2D6 was examined. The results showed a significant increase in the recruitment of SHP to CYP2D6 promoter, accompanied by decreased recruitment of both HNF4α and Pol II to CYP2D6 promoter (Fig. 3A). A similar pattern of changes in SHP, HNF4α, and Pol II recruitment was observed for Cyp7a1 and Cyp8b1, the known target genes of SHP (Inoue et al., 2006) (Fig. 3B; Supplemental Fig. 1 for Cyp7a1). Such changes in the transcriptional factor recruitment were absent when a PCR primer set detecting a downstream region (+3913/+4112) of CYP2D6 gene was used as a negative control (Supplemental Fig. 2). Also, the decrease in HNF4α recruitment to the promoter region was not observed for other target genes of HNF4α (i.e., ApoC2 and Hes6 (Nikolaidou-Neokosmidou et al., 2006; Martinez-Jimenez et al., 2010) (Supplemental Fig. 3), suggesting that the repressive action of SHP on HNF4α transactivation may be target gene-specific. Together, these results suggest that EE2-induced cholestasis leads to decreased HNF4α transactivation of CYP2D6 promoter, potentially through enhanced SHP expression.
Fig. 3.
EE2-induced cholestasis represses HNF4α transactivation of CYP2D6 promoter. Tg-CYP2D6 mice were injected subcuaneously with EE2 (10 mg/kg) or vehicle (olive oil) daily for 5 days (n = 5 mice/group). Recruitment of HNF4α, SHP, and RNA Pol II to (A) CYP2D6 promoter and (B) Cyp8b1 promoter was analyzed by ChIP assay using the mouse liver tissues. Values are presented as mean ± S.E.M. *P < 0.05; **P < 0.01, versus vehicle treatment.
ERα Plays a Predominant Role in SHP Upregulation in EE2-Induced Cholestasis.
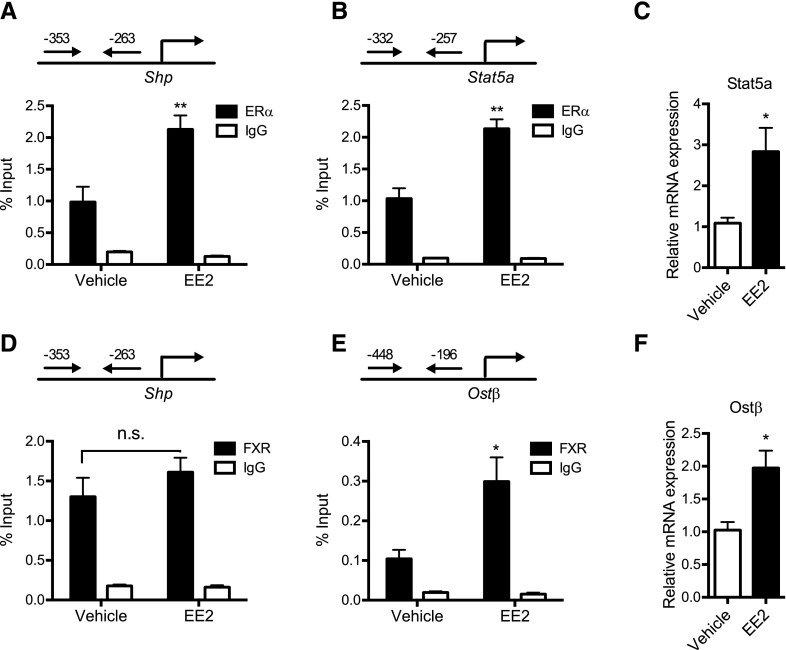
FXR transactivates SHP promoter by binding to −294/−281 of SHP in humans (Goodwin et al., 2000). Interestingly, results from a previous study indicate that ERα also transactivates SHP promoter by binding to an overlapping promoter region (Lai et al., 2003). As the SHP promoter sequence is highly conserved between humans and mice (Lai et al., 2003), we examined the comparative roles of ERα and FXR in the regulation of SHP expression in mice with EE2-induced cholestasis. To this end, recruitment of ERα and FXR to Shp promoter was compared between the livers of EE2- versus vehicle-treated mice by using ChIP assay. Previously known target genes of ERα and FXR, Stat5a and Ostβ, respectively (Zollner et al., 2006; Hewitt et al., 2010), were used as positive controls. The results showed that ERα recruitment to Shp promoter was increased significantly upon EE2 treatment (Fig. 4A). Such increase in ERα recruitment was not observed when a PCR primer set detecting a downstream region (+4018/+4209) of Shp was used as a negative control (Supplemental Fig. 4). EE2 treatment also led to a significant increase in ERα recruitment to Stat5a promoter (Fig. 4B) and increased mRNA expression of Stat5a (Fig. 4C). In contrast to the increased ERα recruitment to Shp promoter, the recruitment of FXR to the promoter region did not differ between the EE2-treated and the control groups (Fig. 4D). In contrast, FXR recruitment to Ostβ promoter and mRNA expression of Ostβ were significantly increased (Fig. 4, E and F), indicating a robust FXR activation by EE2-induced cholestasis. Together, these results suggest that when both ERα and FXR are activated in EE2-induced cholestasis, ERα may play a predominant role in the regulation of SHP expression.
Fig. 4.
ERα plays a major role in SHP upregulation in EE2-induced cholestasis. Tg-CYP2D6 mice were injected subcutaneously with EE2 (10 mg/kg) or vehicle (olive oil) daily for 5 days (n = 5 mice/group). Recruitment of ERα to (A) Shp promoter and (B) Stat5a promoter; recruitment of FXR to (D) Shp promoter and (E) Ostβ promoter was analyzed by ChIP assay using the mouse liver tissues. (C) Stat5a and (F) Ostβ mRNA expression levels in the liver tissues were determined by using qRT-PCR. Values are presented as mean ± S.E.M. *P < 0.05; **P < 0.01; n.s., not statistically significant, versus vehicle treatment.
ERα Activation Leads to CYP2D6 Repression.
To determine whether ERα activation alone (without accompanying cholestasis) can alter CYP2D6 expression via upregulating SHP, mice were administered with EE2 at a low dose (5 μg/kg) for 5 days, and CYP2D6 expression was examined. Plasma ALP levels were slightly higher in mice treated with low-dose EE2 (Fig. 5A), but within the normal range (i.e., 113 ± 49 U/l) for 8-week-old mice (Krones et al., 2014). ALT levels did not increase in EE2-treated mice (Fig. 5A). Also, the dramatic decreases in Cyp7a1 and Cyp8b1 expression or increased Ostβ expression (i.e., hallmarks of cholestasis and FXR activation) were not observed in these mice (Fig. 5B). Together, the data indicate a lack of cholestasis in mice treated with low-dose EE2.
Fig. 5.
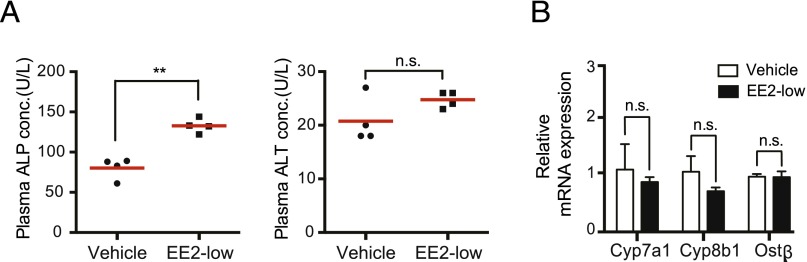
Low-dose EE2 does not induce cholestasis in Tg-CYP2D6 mice. Tg-CYP2D6 mice were injected intraperitoneally with EE2 (5 μg/kg) or vehicle control (olive oil) daily for 5 days (n = 4 mice/group). (A) ALP and ALT activities in mouse plasma were measured. (B) Hepatic mRNA levels of Cyp7a1, Cyp8b1, and Ostβ were determined by using qRT-PCR. Values are presented as mean ± S.E.M. **P < 0.01; n.s., not statistically significant. conc., concentration.
The treatment with EE2 at the low dose led to increased Shp and decreased CYP2D6 mRNA levels (Fig. 6A). The mRNA expression of Stat5a (an ERα target gene) was also increased in EE2-treated mice, as expected (Fig. 6A). In accordance with increased Shp mRNA levels, SHP protein level was increased upon EE2 treatment, but to an extent smaller than that by EE2 at the high dose (1.5-fold versus 2.5-fold for low- and high-dose EE2-treated groups, respectively) (Figs. 2D and 6B). CYP2D6 activity was decreased in the EE2-treated group only by ∼20% as compared with the vehicle control group (Fig. 6C). Consistent with the changes in SHP and CYP2D6 expression, ChIP results showed increased recruitment of SHP to CYP2D6 promoter, and decreased recruitment of HNF4α and Pol II to CYP2D6 promoter (Supplemental Fig. 5A). Such changes in transcription factor recruitment were not observed for Cyp8b1 (Supplemental Fig. 5B). ERα recruitment to Shp promoter was increased upon low-dose EE2 treatment, indicating enhanced ERα transactivation of Shp promoter (Fig. 6D). A similar pattern was observed for ERα recruitment to the promoter of Stat5a (Fig. 6D). Together, these results suggest that ERα activation leads to CYP2D6 repression through enhanced SHP expression, although the magnitude of CYP2D6 repression was smaller than that in EE2-induced cholestasis.
Fig. 6.
Low-dose EE2 represses CYP2D6 expression in Tg-CYP2D6 mice. Tg-CYP2D6 mice were injected intraperitoneally with EE2 (5 μg/kg) or vehicle control (olive oil) daily for 5 days (n = 4 mice/group). (A) Hepatic mRNA expression levels of CYP2D6, Shp, and Stat5a were measured by using qRT-PCR. (B) SHP protein expression level was determined by Western blot. The image of Western blot (right) and the quantified band intensities (left; SHP level normalized by β-actin) are shown. (C) CYP2D6 activity in hepatic S9 fractions was measured using debrisoquine (200 μM) as a probe drug. Data shown are metabolite production rates (in pmol/min per milligram protein). (D) Recruitment of ERα to Shp promoter and Stat5a promoter was analyzed by ChIP assay using the mouse liver tissues. Values are presented as mean ± S.E.M. *P < 0.05, versus vehicle treatment.
Discussion
In previous studies, we have shown that SHP is a transcriptional repressor of CYP2D6 expression (Koh et al., 2014) and that FXR activation (by using an FXR agonist GW4064 [3-[2-[2-chloro-4-[[3-(2,6-dichlorophenyl)-5-(1-methylethyl)-4-isoxazolyl]methoxy]phenyl]ethenyl]benzoic acid] leads to decreased CYP2D6 expression via enhancing SHP expression (Pan et al., 2015). In this study, we examined whether cholestasis (a condition known to enhance FXR activity) also alters CYP2D6 expression by upregulating SHP expression. To this end, we employed the estrogen-induced cholestasis model that is widely used to examine the mechanisms involved in intrahepatic cholestasis (Bossard et al., 1993; Trauner et al., 1999; Rodriguez-Garay, 2003; Yamamoto et al., 2006).
In Tg-CYP2D6 mice, EE2 at a dose of 10 mg/kg per day led to increased plasma concentrations of liver enzymes and decreased expression of Cyp7a1 and Cyp8b1, a hallmark of cholestasis. In the EE2-treated mice, CYP2D6 expression and activity were significantly repressed, suggesting that CYP2D6-mediated drug metabolism may be decreased in cholestasis. Cholestasis is caused by factors that impair bile formation or bile flow. For example, in 0.4–1% of pregnant women in North America and up to 15–20% of pregnant women in some areas of Europe, intrahepatic cholestasis occurs most likely due to high plasma concentrations of estrogen during pregnancy (Reyes et al., 1978; Reyes, 1997; Riely and Bacq, 2004; Pusl and Beuers, 2007). Based on our results, it appears possible that CYP2D6-mediated drug metabolism is lower in pregnant women with increased hepatic bile acid levels (than in pregnant women with normal bile acid levels), and this may lead to greater interindividual variability in CYP2D6 activity in pregnant women as compared with the nonpregnant subjects. Indeed, it was previously shown that CYP2D6 activity (i.e., clearance of metoprolol) exhibited greater variability during pregnancy than after delivery (Hogstedt et al., 1985). Whether altered hepatic bile acid levels are indeed responsible for the increased interindividual variability in CYP2D6 activity during pregnancy by controlling hepatic CYP2D6 expression remains to be determined.
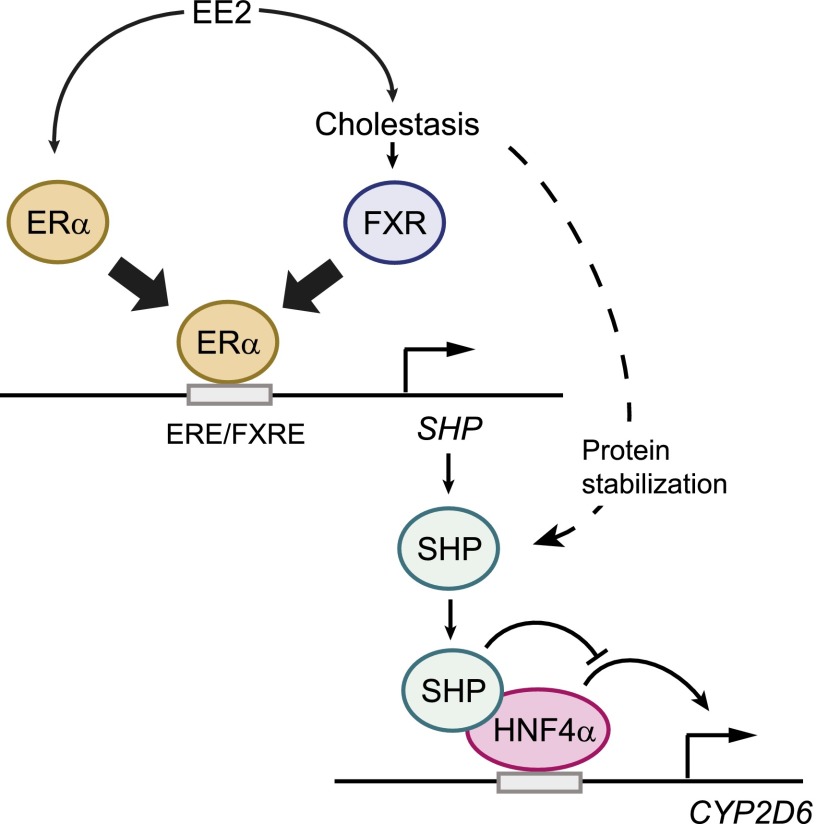
CYP2D6 repression in EE2-induced cholestasis was accompanied by increased SHP expression and subsequent changes in transcription factor recruitment to CYP2D6 promoter, that is, increased SHP and decreased HNF4α recruitment to the promoter. These results suggest increased SHP expression may be responsible for CYP2D6 repression in cholestasis. SHP promoter is transactivated by FXR and ERα (Goodwin et al., 2000; Lai et al., 2003), both of which are most likely activated in EE2-induced cholestasis. Of note, FXR and ERα bind to overlapping promoter regions (Lai et al., 2003), suggesting that the roles of ERα and FXR in SHP upregulation could be mutually exclusive in EE2-induced cholestasis. To determine which one of two transcription factors (ERα or FXR) plays a major role in SHP induction in EE2-induced cholestasis, the extent of ERα and FXR recruitment to Shp promoter was examined. Our results showed that ERα recruitment (but not that of FXR) to Shp promoter was increased in mice administered with EE2 at a high dose. The results suggest that ERα transactivation of Shp promoter is potentially responsible for CYP2D6 repression in EE2-induced cholestasis.
Based on the results indicating an important role of ERα in the regulation of SHP expression, we further examined the effects of ERα activation alone (without cholestasis and subsequent FXR activation) on CYP2D6 expression. To this end, Tg-CYP2D6 mice were administered with EE2 at a low dose that does not cause cholestasis or liver damage. At the low dose, EE2 increased SHP and decreased CYP2D6 expression; however, the extent of CYP2D6 repression in these mice was much smaller than that in mice with EE2-induced cholestasis (2.6- versus 1.2-fold decrease in CYP2D6 activity for high- and low-dose EE2 groups, respectively). The greater magnitude of CYP2D6 repression in cholestatic mice could be in part due to bile acid–induced stabilization of SHP protein; bile acids increase stability of hepatic SHP protein by inhibiting its proteasomal degradation in an extracellular signal-regulated kinase–dependent manner (Miao et al., 2009). Indeed, the extent of increases in SHP protein level was greater in mice administered with high-dose EE2 than in those with low-dose EE2. Cholestasis triggers hepatic inflammation that is known to downregulate CYP2D6 expression (Hara and Adachi, 2002), and this may have also contributed to CYP2D6 repression in EE2-induced cholestasis. Our qRT-PCR results showed that expression levels of inflammatory cytokines (i.e., TNF-α, IL-1β, and IL-6) were significantly higher in the livers of mice treated with high-dose EE2 as compared with the vehicle-treated mice, and such increase was not observed in mice treated with low-dose EE2 (data not shown). Consistent with the relatively small changes in CYP2D6 activity upon administration of EE2 at the low dose, clinical data suggest minor roles of estrogen (if any) in the regulation of CYP2D6 expression/activity in humans. For example, the use of oral contraceptive steroids had no influence on the urinary metabolic ratios of CYP2D6 substrates (sparteine or dextromethorphan) (Bock et al., 1994; Tamminga et al., 1999). Also, sex did not affect the extent of CYP2D6-mediated sparteine oxidation in 194 subjects (Bock et al., 1994) nor the CYP2D6 expression levels in 300 human liver tissues (Zanger et al., 2005). Overall, these results suggest that CYP2D6 repression in estrogen-induced cholestasis is triggered in part by a combination of 1) ERα activation and 2) biologic changes accompanying cholestasis (e.g., SHP protein stabilization and inflammation) (Fig. 7).
Fig. 7.
Working model for CYP2D6 regulation in EE2-induced cholestasis. The working model illustrates the overlapping estrogen response element (ERE) and FXR response element (FXRE) in SHP promoter. In EE2-induced cholestasis, ERα recruitment to SHP promoter increases, leading to higher SHP expression. Also, cholestasis stabilizes SHP protein, further enhancing SHP expression. SHP in turn suppresses HNF4α transactivation of CYP2D6 promoter, leading to CYP2D6 repression in EE2-induced cholestasis.
We showed that EE2-induced cholestasis increases SHP and represses CYP2D6 expression in Tg-CYP2D6 mice. Importantly, this study presents estrogen and bile acids as potential contributors to the differential regulation of CYP2D6 expression. This potentially provides a mechanistic basis to identify the sources of interindividual variability in CYP2D6-mediated drug metabolism.
Supplementary Material
Acknowledgments
The authors thank Sung-joon Cho for technical assistance with animal experiments.
Abbreviations
- ALP
alkaline phosphatase
- ALT
alanine aminotransferase
- ChIP
chromatin immunoprecipitation
- EE2
17α-ethinylestradiol
- ER
estrogen receptor
- FXR
farnesoid X receptor
- GW4064
3-[2-[2-chloro-4-[[3-(2,6-dichlorophenyl)-5-(1-methylethyl)-4-isoxazolyl]methoxy]phenyl]ethenyl]benzoic acid
- HNF
hepatocyte nuclear factor
- Pol II
polymerase II
- qRT-PCR
quantitative real-time polymerase chain reaction
- SHP
small heterodimer partner
Authorship Contributions
Participated in research design: Pan, Jeong.
Conducted experiments: Pan.
Performed data analysis: Pan, Jeong.
Wrote or contributed to the writing of the manuscript: Pan, Jeong.
Footnotes
>This work was supported by National Institutes of Health National Institute of Child Health and Human Development [Grant R01-HD065532].
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Alvaro D, Alpini G, Onori P, Franchitto A, Glaser SS, Le Sage G, Folli F, Attili AF, Gaudio E. (2002) Alfa and beta estrogen receptors and the biliary tree. Mol Cell Endocrinol 193:105–108. [DOI] [PubMed] [Google Scholar]
- Bock KW, Schrenk D, Forster A, Griese EU, Mörike K, Brockmeier D, Eichelbaum M. (1994) The influence of environmental and genetic factors on CYP2D6, CYP1A2 and UDP-glucuronosyltransferases in man using sparteine, caffeine, and paracetamol as probes. Pharmacogenetics 4:209–218. [DOI] [PubMed] [Google Scholar]
- Bossard R, Stieger B, O’Neill B, Fricker G, Meier PJ. (1993) Ethinylestradiol treatment induces multiple canalicular membrane transport alterations in rat liver. J Clin Invest 91:2714–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns W, Smith CA, McLaren AW, Wolf CR. (1996) Characterization of the human cytochrome P4502D6 promoter: a potential role for antagonistic interactions between members of the nuclear receptor family. J Biol Chem 271:25269–25276. [DOI] [PubMed] [Google Scholar]
- Corchero J, Granvil CP, Akiyama TE, Hayhurst GP, Pimprale S, Feigenbaum L, Idle JR, Gonzalez FJ. (2001) The CYP2D6 humanized mouse: effect of the human CYP2D6 transgene and HNF4alpha on the disposition of debrisoquine in the mouse. Mol Pharmacol 60:1260–1267. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver (2009) EASL Clinical Practice Guidelines: management of cholestatic liver diseases. J Hepatol 51:237–267. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Jones SA, Price RR, Watson MA, McKee DD, Moore LB, Galardi C, Wilson JG, Lewis MC, Roth ME, et al. (2000) A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol Cell 6:517–526. [DOI] [PubMed] [Google Scholar]
- Hara H, Adachi T. (2002) Contribution of hepatocyte nuclear factor-4 to down-regulation of CYP2D6 gene expression by nitric oxide. Mol Pharmacol 61:194–200. [DOI] [PubMed] [Google Scholar]
- Hewitt SC, Li Y, Li L, Korach KS. (2010) Estrogen-mediated regulation of Igf1 transcription and uterine growth involves direct binding of estrogen receptor alpha to estrogen-responsive elements. J Biol Chem 285:2676–2685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Högstedt S, Lindberg B, Peng DR, Regårdh CG, Rane A. (1985) Pregnancy-induced increase in metoprolol metabolism. Clin Pharmacol Ther 37:688–692. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Yu AM, Yim SH, Ma X, Krausz KW, Inoue J, Xiang CC, Brownstein MJ, Eggertsen G, Björkhem I, et al. (2006) Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4alpha. J Lipid Res 47:215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahan A, Chiang JY. (2005) Cytokine regulation of human sterol 12alpha-hydroxylase (CYP8B1) gene. Am J Physiol Gastrointest Liver Physiol 288:G685–G695. [DOI] [PubMed] [Google Scholar]
- Koh KH, Pan X, Shen HW, Arnold SL, Yu AM, Gonzalez FJ, Isoherranen N, Jeong H. (2014) Altered expression of small heterodimer partner governs cytochrome P450 (CYP) 2D6 induction during pregnancy in CYP2D6-humanized mice. J Biol Chem 289:3105–3113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontturi M, Sotaniemi E. (1969) Effect of oestrogen on liver function of prostatic cancer patients. BMJ 4:204–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer AE, Beuers U, Oude-Elferink RP, Pusl T. (2008) Pathogenesis and treatment of pruritus in cholestasis. Drugs 68:2163–2182. [DOI] [PubMed] [Google Scholar]
- Krones E, Erwa W, Trauner M, Fickert P. (2014) Serum alkaline phosphatase levels accurately reflect cholestasis in mice. Hepatology DOI: 10.1002/hep.27622 [published ahead of print]. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson JA. (1997) Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors alpha and beta. Endocrinology 138:863–870. [DOI] [PubMed] [Google Scholar]
- Kullak-Ublick GA, Meier PJ. (2000) Mechanisms of cholestasis. Clin Liver Dis 4:357–385. [DOI] [PubMed] [Google Scholar]
- Lai K, Harnish DC, Evans MJ. (2003) Estrogen receptor alpha regulates expression of the orphan receptor small heterodimer partner. J Biol Chem 278:36418–36429. [DOI] [PubMed] [Google Scholar]
- Martinez-Jimenez CP, Kyrmizi I, Cardot P, Gonzalez FJ, Talianidis I. (2010) Hepatocyte nuclear factor 4alpha coordinates a transcription factor network regulating hepatic fatty acid metabolism. Mol Cell Biol 30:565–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao J, Xiao Z, Kanamaluru D, Min G, Yau PM, Veenstra TD, Ellis E, Strom S, Suino-Powell K, Xu HE, et al. (2009) Bile acid signaling pathways increase stability of small heterodimer partner (SHP) by inhibiting ubiquitin-proteasomal degradation. Genes Dev 23:986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolaidou-Neokosmidou V, Zannis VI, Kardassis D. (2006) Inhibition of hepatocyte nuclear factor 4 transcriptional activity by the nuclear factor kappaB pathway. Biochem J 398:439–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan X, Lee YK, Jeong H. (2015) Farnesoid X Receptor Agonist Represses Cytochrome P450 2D6 Expression by Upregulating Small Heterodimer Partner. Drug Metab Dispos DOI:10.1124/dmd.115.064758 [published ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusl T, Beuers U. (2007) Intrahepatic cholestasis of pregnancy. Orphanet J Rare Dis 2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes H. (1997) Review: intrahepatic cholestasis: a puzzling disorder of pregnancy. J Gastroenterol Hepatol 12:211–216. [DOI] [PubMed] [Google Scholar]
- Reyes H, Gonzalez MC, Ribalta J, Aburto H, Matus C, Schramm G, Katz R, Medina E. (1978) Prevalence of intrahepatic cholestasis of pregnancy in Chile. Ann Intern Med 88:487–493. [DOI] [PubMed] [Google Scholar]
- Riely CA, Bacq Y. (2004) Intrahepatic cholestasis of pregnancy. Clin Liver Dis 8:167–176. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Garay EA. (2003) Cholestasis: human disease and experimental animal models. Ann Hepatol 2:150–158. [PubMed] [Google Scholar]
- Schreiber AJ, Simon FR. (1983) Estrogen-induced cholestasis: clues to pathogenesis and treatment. Hepatology 3:607–613. [DOI] [PubMed] [Google Scholar]
- Tamminga WJ, Wemer J, Oosterhuis B, Weiling J, Wilffert B, de Leij LF, de Zeeuw RA, Jonkman JH. (1999) CYP2D6 and CYP2C19 activity in a large population of Dutch healthy volunteers: indications for oral contraceptive-related gender differences. Eur J Clin Pharmacol 55:177–184. [DOI] [PubMed] [Google Scholar]
- Trauner M, Boyer JL. (2003) Bile salt transporters: molecular characterization, function, and regulation. Physiol Rev 83:633–671. [DOI] [PubMed] [Google Scholar]
- Trauner M, Meier PJ, Boyer JL. (1998) Molecular pathogenesis of cholestasis. N Engl J Med 339:1217–1227. [DOI] [PubMed] [Google Scholar]
- Trauner M, Meier PJ, Boyer JL. (1999) Molecular regulation of hepatocellular transport systems in cholestasis. J Hepatol 31:165–178. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Moore R, Hess HA, Guo GL, Gonzalez FJ, Korach KS, Maronpot RR, Negishi M. (2006) Estrogen receptor alpha mediates 17alpha-ethynylestradiol causing hepatotoxicity. J Biol Chem 281:16625–16631. [DOI] [PubMed] [Google Scholar]
- Yoshikawa Y, Miyashita T, Higuchi S, Tsuneyama K, Endo S, Tsukui T, Toyoda Y, Fukami T, Nakajima M, Yokoi T. (2012) Mechanisms of the hepatoprotective effects of tamoxifen against drug-induced and chemical-induced acute liver injuries. Toxicol Appl Pharmacol 264:42–50. [DOI] [PubMed] [Google Scholar]
- Zanger UM, Klein K, Richter T, Toscano C, Zukunft J. (2005) Impact of genetic polymorphism in relation to other factors on expression and function of human drug-metabolizing p450s. Toxicol Mech Methods 15:121–124. [DOI] [PubMed] [Google Scholar]
- Zollner G, Wagner M, Moustafa T, Fickert P, Silbert D, Gumhold J, Fuchsbichler A, Halilbasic E, Denk H, Marschall HU, et al. (2006) Coordinated induction of bile acid detoxification and alternative elimination in mice: role of FXR-regulated organic solute transporter-alpha/beta in the adaptive response to bile acids. Am J Physiol Gastrointest Liver Physiol 290:G923–G932. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.