Abstract
Dantrolene is the first line therapy of malignant hyperthermia. Animal studies suggest that dantrolene also protects against heart failure and arrhythmias caused by spontaneous Ca2+ release. Although dantrolene inhibits Ca2+ release from the sarcoplasmic reticulum of skeletal and cardiac muscle preparations, its mechanism of action has remained controversial, because dantrolene does not inhibit single ryanodine receptor (RyR) Ca2+ release channels in lipid bilayers. Here we test the hypothesis that calmodulin (CaM), a physiologic RyR binding partner that is lost during incorporation into lipid bilayers, is required for dantrolene inhibition of RyR channels. In single channel recordings (100 nM cytoplasmic [Ca2+] + 2 mM ATP), dantrolene caused inhibition of RyR1 (rabbit skeletal muscle) and RyR2 (sheep) with a maximal inhibition of Po (Emax) to 52 ± 4% of control only after adding physiologic [CaM] = 100 nM. Dantrolene inhibited RyR2 with an IC50 of 0.16 ± 0.03 µM. Mutant N98S-CaM facilitated dantrolene inhibition with an IC50 = 5.9 ± 0.3 nM. In mouse cardiomyocytes, dantrolene had no effect on cardiac Ca2+ release in the absence of CaM, but reduced Ca2+ wave frequency (IC50 = 0.42 ± 0.18 µM, Emax = 47 ± 4%) and amplitude (IC50 = 0.19 ± 0.04 µM, Emax = 66 ± 4%) in the presence of 100 nM CaM. We conclude that CaM is essential for dantrolene inhibition of RyR1 and RyR2. Its absence explains why dantrolene inhibition of single RyR channels has not been previously observed.
Introduction
Dantrolene is a well known inhibitor of Ca2+ release in skeletal muscle (Hainaut and Desmedt, 1974) that has been used clinically as the treatment of malignant hyperthermia (MH). MH is a potentially fatal inherited disorder of skeletal muscle in which mutations in the proteins involved in excitation-contraction coupling (e.g., RyR1 and DHPR) (McCarthy et al., 1990; Monnier et al., 1997; Jung et al., 2012) cause uncontrolled sarcoplasmic reticulum (SR) calcium release and muscle contracture in the presence of volatile anesthetics. Notably, mutations in the cardiac ryanodine receptor (RyR) isoform (RyR2) that correspond to the MH mutations in RyR1 cause catecholaminergic polymorphic ventricular tachycardia (Yano, 2005). Recent in vitro and animal studies suggest that dantrolene has antiarrhythmic effects in catecholaminergic polymorphic ventricular tachycardia and possibly also in heart failure (Jung et al., 2012; Kobayashi et al., 2009, 2010; Maxwell et al., 2012).
Dantrolene acts on skeletal and cardiac muscle by inhibiting Ca2+ release from the SR (Hainaut and Desmedt, 1974; Kobayashi et al., 2005; Uchinoumi et al., 2010). Assays of Ca2+ release in intact myocytes and cell homogenates containing SR vesicles (Fruen et al., 1997) suggest that dantrolene inhibits the SR Ca2+ release channel with a half-inhibiting concentration (IC50) of 0.3 µM (Kobayashi et al., 2009). Even though a dantrolene binding site has been identified in the DP1 regions in RyR1 and RyR2 (Parness and Palnitkar, 1995; Paul-Pletzer et al., 2002, 2005; Kobayashi et al., 2009), there has been only one direct observation of RyR inhibition by dantrolene in bilayer-based single channel recordings (Nelson et al., 1996). Studies since then find no effect of dantrolene in single channel recordings (Szentesi et al., 2001; Cherednichenko et al., 2008; Diaz-Sylvester et al., 2008; Wagner et al., 2014). Hence, it is not clear if dantrolene acts directly on the RyR or some other protein involved in excitation-contraction coupling such as the DHPR (Salata et al., 1983; Chou et al., 2014).
Calmodulin (CaM) is known to regulate the activity of RyR1 and RyR2 (Tripathy et al., 1995; Balshaw et al., 2001). CaM inhibits RyR2 directly by binding to residues 3583–3603 of each RyR2 subunit (Huang et al., 2013) with high affinity (Kd 20–100 nM) (Guo et al., 2011). Similarly, CaM may either increase RyR1 activity at resting cytoplasmic [Ca2+] or decrease activity at higher [Ca2+] (Tripathy et al., 1995). Fruen and colleagues (Fruen et al., 1997; Zhao et al., 2001) found that dantrolene reduces the effect of RyR1 activators (but interestingly, not in RyR2) including CaM, suggesting that CaM might augment dantrolene inhibition of RyR1. During the process of RyR2 isolation from the heart and their incorporation into artificial lipid bilayers, the RyR macromolecular complex stays mostly intact (Marks et al., 2002), except for CaM, which is reported to dissociate from the RyR complex with a time constant of less than 1 minute (Guo et al., 2011). Hence, bilayer-based channel studies would generally have been made devoid of this important regulatory molecule in the RyR complex, whereas CaM is abundant in intact cell and cell homogenates. Therefore, we hypothesize that CaM is the missing protein and that its absence in bilayer experiments provides an explanation as to why dantrolene inhibition has not been observed in single channel RyR recording experiments. We test this hypothesis by examining the effects of dantrolene, in the absence and presence of CaM, on the gating of RyR1 and RyR2 Ca2+ release channels incorporated into artificial lipid bilayers and on the frequency and amplitude of Ca2+ waves in permeabilized cardiomyocytes.
Materials and Methods
Chemicals.
SR vesicles containing RyR1 were isolated from rabbit skeletal muscle, and RyR2 were isolated from sheep hearts (Laver et al., 1995) and incorporated in artificial bilayer membranes composed of a lipid mixture of phosphatidylethanolamine and phosphatidylcholine (8:2 wt/wt, Avanti Polar Lipids, Alabaster, AL) in n-decane (50 mg/ml, ICN Biomedicals, Irvine, CA). Experimental solutions contained (in millimolar) 150 Cs+ (130 CsCH3O3S + 20 CsCl). All solutions were pH buffered using N-tris[hydroxymethyl] methyl-2-aminoethanesulfonic acid (ICN Biomedicals) and titrated to pH 7.4 using CsOH (ICN Biomedicals). Cytoplasmic solutions were buffered to a redox potential of −232 mV with reduced glutathione disulfide (0.2 mM) and glutathione (GSH; 4 mM), and luminal solutions were buffered to a redox potential of −180 mV with reduced glutathione disulfide (3 mM) and GSH (2 mM). A Ca2+ electrode (Radiometer, Brea, CA) was used in our experiments to determine the purity of Ca2+ buffers and Ca2+ stock solutions as well as free [Ca2+] > 100 nM. The cesium salts were obtained from Sigma-Aldrich (St. Louis, MO). CaCl2 was obtained from BDH Chemicals (VWR, Radnor, PA). Calmodulin was obtained from two sources, Sigma-Aldrich (prepared from bovine testes) and Enzo Life Sciences (Farmingdale, NY; prepared from pig brain). Dantrolene (powder) was obtained from Sigma. Dantrolene was prepared as stock solutions in dimethylsulfoxide, and calmodulin was prepared in milliQ (EMD Millipore, Billerica, MA. During experiments, the concentrations of calmodulin, dantrolene, and Ca2+ in the cytoplasmic solution were altered by a local perfusion system (O'Neill et al., 2003), which allowed exposure of a single channel to multiple bathing conditions applied in any chosen sequence with an exchange time of ∼3 seconds.
Data Acquisition and Analysis.
Experiments were carried out at room temperature (23 ± 2°C). Electric potentials are expressed using standard physiologic convention (i.e., cytoplasm relative to SR lumen at virtual ground). Control of the bilayer potential and recording of unitary currents was done using an Axopatch 200B amplifier (Axon Instruments/Molecular Devices, Sunnyvale, CA). The current signal was digitized at 5 kHz and low pass-filtered at 1 kHz. Single channel dwell-time histograms of open and closed time, open probability, and mean open time and mean closed time, were measured using a threshold discriminator at 50% of channel amplitude (Channel3 software; N. W. Laver, nic@niclaver.com). Individual readings were derived from 45-120 seconds of RyR2 recording. Hill equations were fitted to the dose-response data by the method of least squares. Average data are given as mean ± S.E.M. The statistical significance of differences was tested using Student’s t test.
Ca2+ Wave Experiments in Ventricular Myocytes.
Single ventricular myocytes from 12- to 16-week-old C57Bl/6 mice were isolated using an enzymatic digestion method as previously described (Knollmann et al., 2006). Myocytes were first exposed to a Ca2+-free relaxing solution and then permeabilized with saponin (40 μg/ml) for 60 seconds and placed in internal solution composed (in millimolar) of 120 K-aspartate, 15 KCl, 5 KH2PO4, 0.75 MgCl2, 4% dextran (40,000), 10 HEPES, 5 Mg2ATP, 10 glutathione (reduced), 0.025 Fluo-4, and 10 phosphocreatine (di-Na). These solutions also contained 10 U/ml creatine phosphokinase (Hwang et al., 2014) and had free [Ca2+] = 120 nM. To allow complete removal of CaM binding to RyR2 in permeabilized myocytes (Yang et al., 2014), all Ca2+ wave recordings were done after 30-minute incubation with either dantrolene alone or dantrolene + CaM. Free [CaM] was kept at the physiologic concentration of 100 nM. Ca2+ waves in myocytes were imaged with a confocal microscope (LSM 510; Zeiss, Thornwood, NY) in line scan mode. Ca2+ wave analysis was performed as described (Hwang et al., 2014). Given the variability between different experimental days, the Ca2+ wave frequency and amplitude data were normalized to the mean of vehicle group obtained on the same day.
Results
Essential Role of CaM on RyR1 and RyR2 Inhibition by Dantrolene.
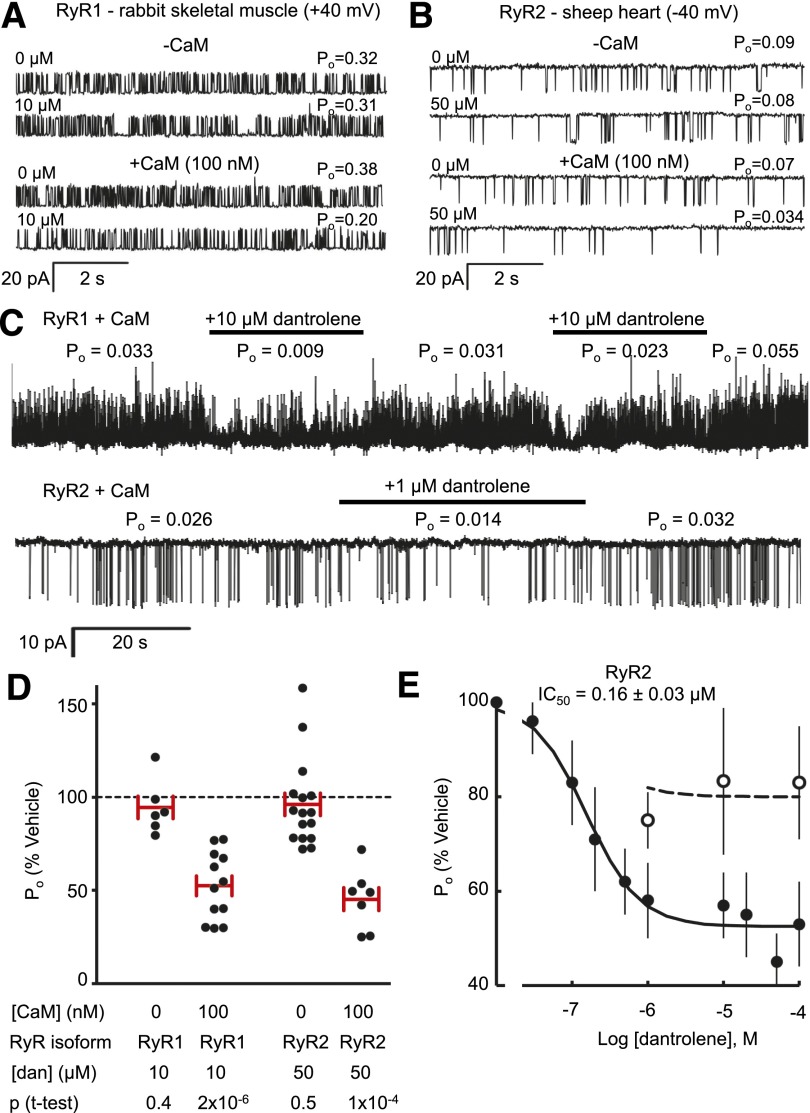
To investigate if CaM binding to RyR1 and RyR2 is a prerequisite for their inhibition by dantrolene, RyR activity was measured in the presence of 100 nM cytoplasmic Ca2+ (+2 mM ATP) for periods of 1 minute (vehicle) and then during 1-minute exposure to added dantrolene and then again after dantrolene washout. This sequence was repeated in the absence and presence of exogenous 100 nM CaM as shown in Fig. 1A for rabbit RyR1 and Fig. 1B for sheep RyR2. In the absence of CaM, dantrolene had no observable effect on the channel open probability (Po) of either RyR1 or RyR2. However, when CaM was present in the experimental solutions, dantrolene reduced the Po of both RyR isoforms. This effect of dantrolene on RyR1 and RyR2 was reversible on washout and inhibition could be seen in multiple applications (Fig. 1C). The data summary from application-washout experiments in Fig. 1D shows that dantrolene at both 10 and 50 µM significantly reduced the open probability of RyR1 to 50% and RyR2 to 45% of control (i.e., vehicle alone), respectively. When CaM was subsequently washed out by perfusion with CaM-free solutions for 1 minute, dantrolene inhibition was abolished (RyR2 open probability 95 ± 9% of vehicle, P = 0.24). Therefore, dantrolene inhibition of RyR1 and RyR2 requires the presence of CaM. The concentration-dependence of dantrolene inhibition of RyR2 is shown in Fig. 1E. In the presence of 100 nM CaM (●), inhibition exhibited a sigmoidal dependence on log-concentration with an IC50 of 0.16 ± 0.03 µM, a Hill coefficient of ∼1 and with a saturating RyR2 open probability (Emax) of 52 ± 4% compared with the absence of dantrolene. Reducing the CaM concentration to 10 nM approximately halved the magnitude of dantrolene inhibition (Fig. 1E, ○, Emax = 80 ± 5%).
Fig. 1.
Dantrolene inhibits RyR1 and RyR2 only in the presence of CaM. (A) Representative, 10-second segments of activity of RyR1 from rabbit skeletal muscle illustrating the inhibitory effect of 10 μM dantrolene in the absence (−CaM) and presence (+CaM) of 100 nM CaM. (B) Corresponding activity of RyR2 showing inhibition by 50 μM dantrolene. Open probabilities (Po) for 60-second periods of activity are given at the end of each. Experiments were done at +40 mV, and upward current jumps represent the channel openings. (C) 140-second recordings of RyR1 and RyR2, showing channel activity during dantrolene application (bars) and washout. Values of open probability (Po) are given for each segment of recording. (D) Relative inhibition of RyR1 by 10 μM dantrolene and RyR2 by 50 μM dantrolene. Each sample is RyR Po in the presence of dantrolene relative to the mean Po bracketing periods in the absence of dantrolene. Mean values are indicated by the horizontal bars and S.E.M. by the vertical bars. P values indicate significant difference of the mean from 100%. (E) Concentration-dependence of dantrolene inhibition of RyR2 in the presence of 10 nM (○; mean ± S.E.M., n = 3 to 4) and 100 nM CaM (●; mean ± S.E.M., n = 7 to 20). The luminal [Ca2+] is 0.1 mM and cytoplasmic [Ca2+] is 100 nM. The solid curve shows the Hill fit to the data using the equation: where IC50 = 0.16 ± 0.03 μM, H = 1.3 ± 0.3, and Emax = 52 ± 4%. The dashed curve uses the same parameter values, except Emax = 80 ± 5%.
Effect of Dantrolene on RyR Dwell Times.
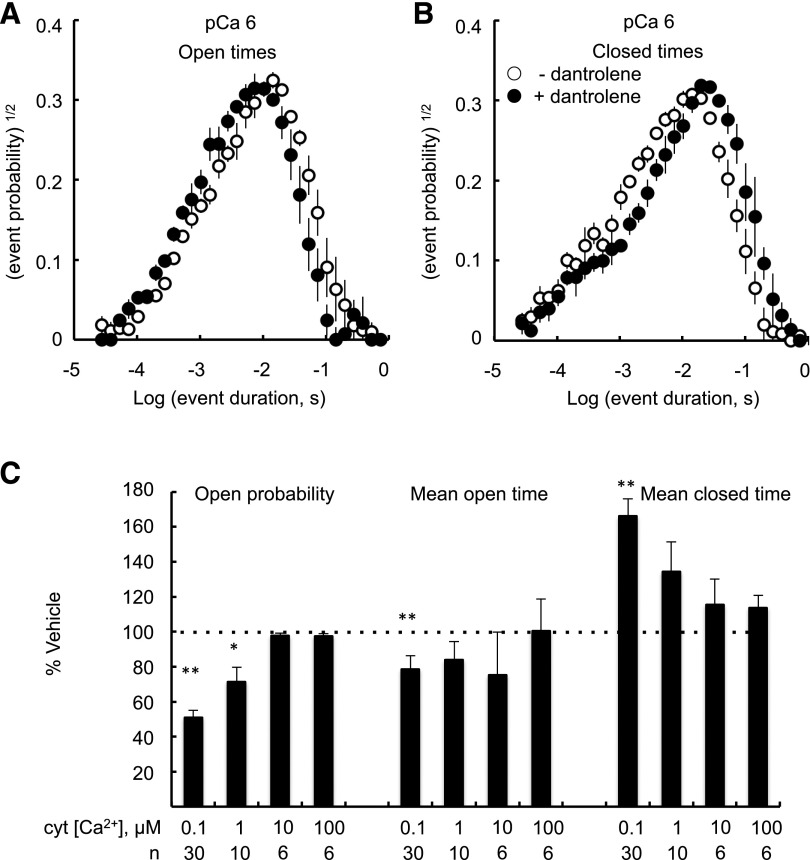
To gain more insight into the mechanism of dantrolene inhibition, we compiled dwell-time histograms of channel open and closed events of sheep RyR2 at four cytoplasmic [Ca2+], ranging from 0.1 µM (end diastolic) to 100 µM (systolic) (Fig. 2, A and B; Supplemental Fig. 1). Histograms are displayed using the log-bin method of Sigworth and Sine (1987), where individual exponential components appear as peaks centered on their time constant value. In the absence of dantrolene, open and closed dwell times in 1 µM cytoplasmic Ca2+ exhibited peaked distributions that were fitted by two exponential components (see Supplemental Table 1). Addition of dantrolene (10 µM) shifted the peak of the open distributions to shorter times and closed distribution to longer times. Dantrolene had a similar effect in 0.1 µM cytoplasmic Ca2+ but had no effect at 100 µM cytoplasmic Ca2+ (Supplemental Fig. 1). It was not possible to resolve significant differences in the parameters of the exponential fits except for the slow time-constant of the closed times at 0.1 µM cytoplasmic Ca2+ (T2; Supplemental Table 1). However, it was possible to resolve relative changes in the RyR2 mean open and closed durations (Fig. 2C). In 0.1 µM cytoplasmic Ca2+, dantrolene reduced RyR2 Po via a decrease in mean channel open duration and an increase in mean closed duration. At 1 µM cytoplasmic Ca2+, the effect of dantrolene was diminished and there was no significant inhibition occurring at higher [Ca2+]. The effect of dantrolene was to shift the Ca2+-activation response of RyR2 to higher [Ca2+].
Fig. 2.
Effect of dantrolene on open and closed dwell times of RyR2. Open (A) and closed (B) dwell-time histograms compiled using the log-bin method of Sigworth and Sine (1987) as described in the text. Histograms are averages of three experiments obtained in 1 μM cytoplasmic [Ca2+] in the absence (○) or presence (●) of 10 µM dantrolene. (C) Statistical analysis of open and closed times of dwell-time histograms showing relative changes in mean dwell times induced by 10 µM dantrolene over a range of cytoplasmic (cyt) [Ca2+]. Also shown is the relative inhibition of channel Po. Asterisks indicate significantly different than 100% (*P < 0.05; **P < 0.01).
Effect of Dantrolene on Ca2+ Waves in Mouse Cardiomyocytes.
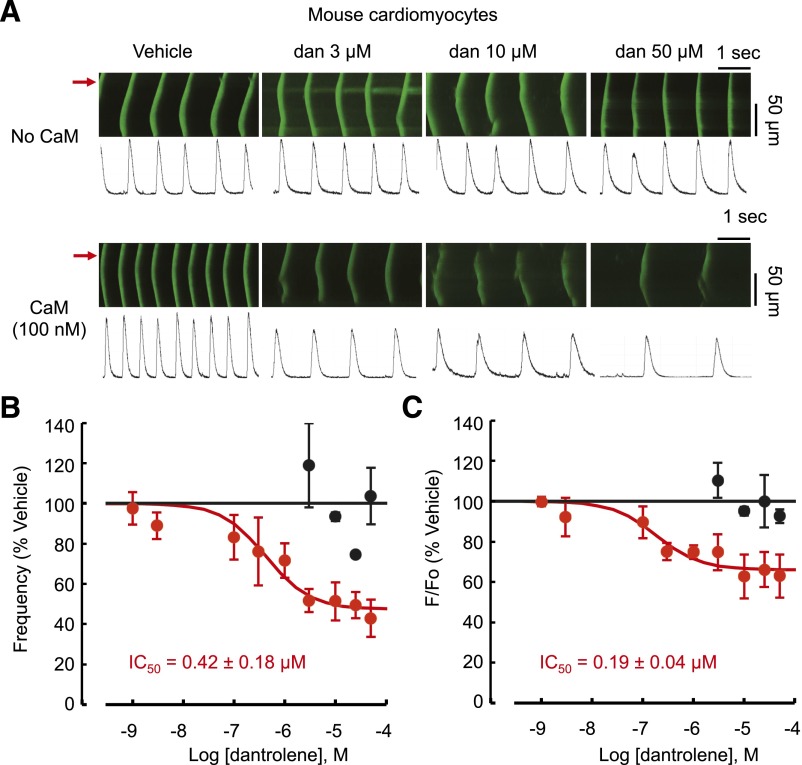
The amplitude and frequency of spontaneous Ca2+ waves, two parameters that have been implicated as independent predictors of arrhythmogenicity (Galimberti and Knollmann, 2011), were measured in mouse ventricular myocytes. Examples of the effect of 30-minute exposure to dantrolene (3, 10, 50 µM) on Ca2+ waves recorded in the presence or absence of CaM are presented in Fig. 3A. Dantrolene reduced Ca2+ wave amplitude and frequency in the presence of CaM but had no effect in the absence of CaM (Fig. 3A). This finding is consistent with the single channel experiments with the concentration dependence of these effects (Fig. 3, B and C) exhibiting remarkably similar IC50 and Emax values to that measured in the single channel experiments (Fig. 1).
Fig. 3.
Dantrolene reduces spontaneous Ca2+ wave frequency and amplitude only in the presence of CaM. Presence of CaM is required for dantrolene action on arrhythmogenic Ca2+ waves in cardiomyocytes. (A) Representative confocal microscope line scans from permeabilized mouse ventricular myocytes after 30-minute incubation with either dantrolene (dan) alone or dantrolene + CaM (100 nM). Red arrows indicate the location of the line scans plotted below each confocal image. (B and C) Concentration response curves for Ca2+ wave frequency (B) and amplitude relative to vehicle (C) in the absence (black ●) or presence (red ●) of CaM 100 nM. The solid curves show the Hill fit to the data using the equation in the caption to Fig. 1. (B) IC50 = 0.42 ± 0.18 μM and Emax = 47 ± 4% and (C) IC50 = 0.19 ± 0.04 μM and Emax = 66 ± 2%.
Dantrolene Inhibition Can Be Mediated by CaM Mutants.
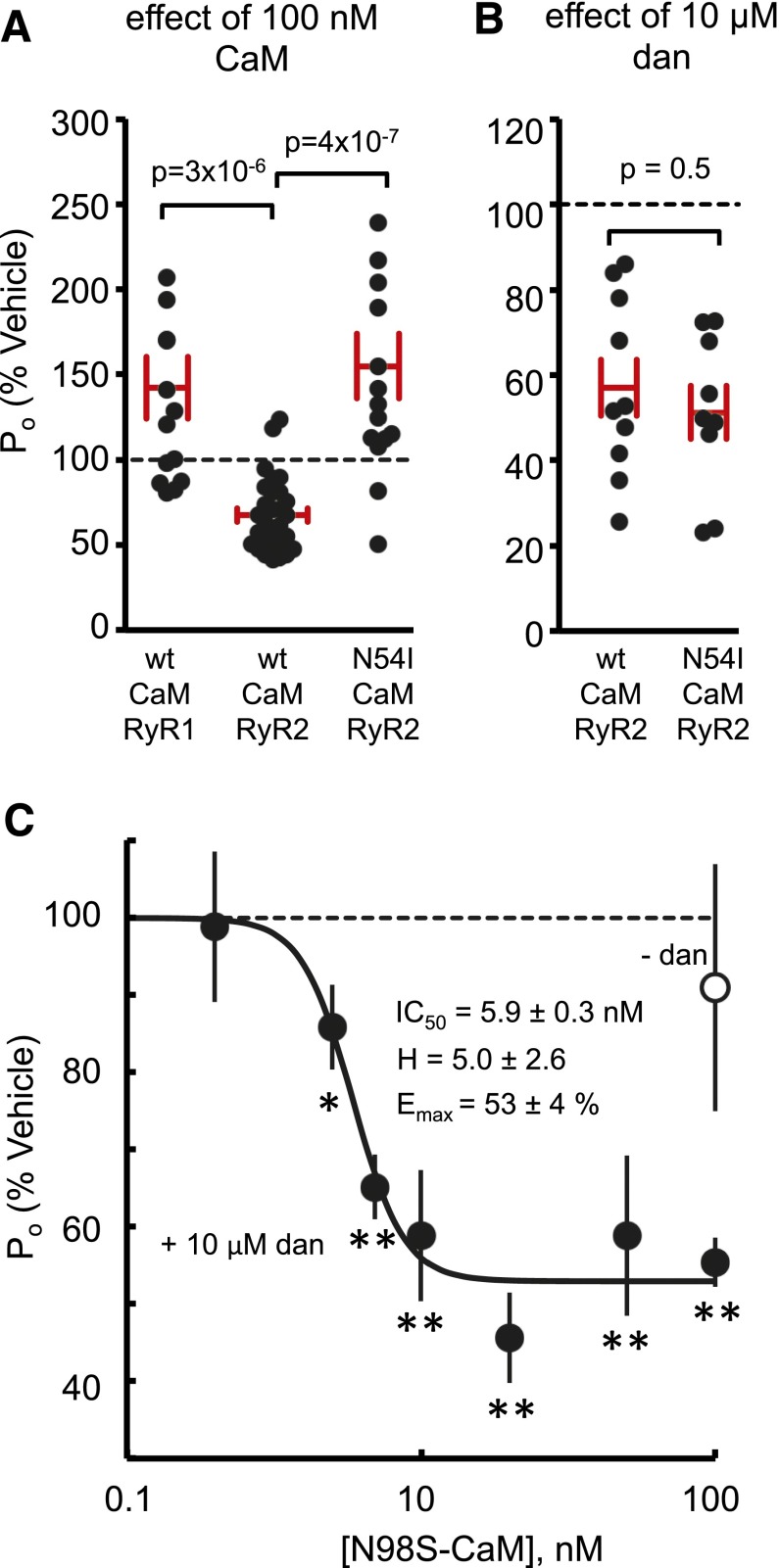
Because both dantrolene and CaM are RyR2 inhibitors, we investigated the possibility that dantrolene acts by amplifying CaM inhibitory action on RyR2. To test this possibility we measured dantrolene inhibition of sheep RyR2 in the presence of 100 nM N54I-CaM. This mutation, as shown in Fig. 4A and in our previous work, increases RyR2 Po, the opposite effect to wild-type (wt)–CaM (Hwang et al., 2014). If dantrolene merely amplifies the action of CaM, then one would expect dantrolene to be an activator in the presence of N54I-CaM. This was not the case. Dantrolene had the same inhibitory action in the presence of wt- and N54I-CaM (Fig. 4B). We also show in Fig. 4A that addition of wt-CaM to RyR1 caused channel activation in accord with previous findings (Tripathy et al., 1995).
Fig. 4.
Dantrolene inhibition of RyR in the presence of wt-CaM and mutant CaM. (A) Relative effect of wt-CaM (100 nM) on the open probability of RyR1 and RyR2 and N54I-CaM on RyR2. (B) Relative effect of 10 μM dantrolene (dan) on RyR2 Po in the presence of wt- and mutant-CaM. Mean values are indicated by the horizontal bars and S.E.M. by the vertical bars. P values indicate significant differences between wt- and mutant-CaM. (C) Facilitation of dantrolene (10 μM) inhibition by N98S-CaM (●). In the absence of dantrolene (○), N98S-CaM has no inhibiting action on RyR2. Asterisks indicate significantly different than 100% (*P < 0.05; **P < 0.01). The solid curve shows the fit of the Hill equation (see legend to Fig. 1) to the data.
We also investigated dantrolene inhibition in the presence of N98S-CaM that is a CaM mutant that has no inhibitory effect on RyR2 in the absence of dantrolene (Fig. 4C, ○). The advantage of this CaM mutant is that we can examine the effect of varying its concentration on facilitating dantrolene inhibition without the confounding effect of CaM inhibition. In the absence of CaM, dantrolene (10 µM) had no effect on RyR Po. Figure 4C shows that addition of only 6 nM N98S-CaM was sufficient to facilitate significant dantrolene inhibition of RyR2. The N98S-CaM facilitation of dantrolene inhibition had a sigmoidal dependence on log-concentration with an IC50 of 5.9 ± 0.3 nM, a Hill coefficient of 5 ± 2.6, and an Emax of 53 ± 4%.
Discussion
Our study presents the first demonstration of dantrolene inhibition of mammalian RyR1 and RyR2 from recordings of single RyR and permeabilized cardiomyocytes. The finding that a physiologic concentration of CaM is required for dantrolene inhibition of these RyRs provides an answer to the long-standing question of why dantrolene, an inhibitor of SR Ca2+ release, had no effect on the activity of mammalian RyR1 and RyR2 in previous single channel studies (Szentesi et al., 2001; Diaz-Sylvester et al., 2008; Wagner et al., 2014). Because CaM readily dissociates from the RyR complex (Guo et al., 2011), CaM would have been absent during those experiments. IC50 for CaM facilitation of dantrolene inhibition appears to be ∼10 nM for wt-CaM (Fig. 1E) and 5.9 nM for N98S-CaM (Fig. 4C). These values are ∼2-fold lower than the binding affinities for these CaMs on RyR2 (Guo et al., 2011; Hwang et al., 2014).
[3H]ryanodine binding assays have demonstrated a reduction of CaM activation of purified pig RyR1 by dantrolene (Fruen et al., 1997). However, that finding was contradicted by a single channel study (Cherednichenko et al., 2008) that, using similar experimental conditions (100 nM cytoplasmic Ca2+ and 35°C), reported no inhibition by dantrolene (20 µM) of purified rabbit RyR1 channels in bilayers in the presence of exogenous FKBP12 and CaM. Together with the findings reported here, these results suggest that the inhibitory effect of dantrolene on RyR not only requires CaM but also other RyR-associated proteins that are present in native preparations but presumably absent in some purified RyR preparations.
The maximum RyR2 inhibition (Emax = 52%) and IC50 (0.16 ± 0.03 µM; Fig. 1D) are in close agreement with dantrolene inhibition of Ca2+ wave frequency and amplitude in saponin permeablized cardiomyocytes (Fig. 3) and inhibition of Ca2+ release in SR vesicles from failing dog heart [IC50 = 0.3 ± 0.07 µM (Kobayashi et al., 2009)] and activity of purified RyR1 in [3H]ryanodine binding assays [0.15 ± 0.02 µM (Fruen et al., 1997)]. The dantrolene IC50 reported here coincides with the binding affinity of dantrolene to skeletal muscle SR vesicles [0.277 ± 0.025 µM (Parness and Palnitkar, 1995)] and its IC50 (0.3 ± 0.11 µM) for inhibiting the unzipping of the central and N-terminal domains of RyR2 (Kobayashi et al., 2009). The potency of dantrolene in our study is also consistent with the inhibitory action of 1 µM dantrolene on Ca2+ spark frequency in isoproterenol-stimulated cardiomyocytes from R2474S knock-in mice (Kobayashi et al., 2010). However, the therapeutic actions of dantrolene in skeletal and cardiac muscle occur at much higher concentrations than required for inhibition of Ca2+ release from the SR. For example, 20 µM or more dantrolene was required to prevent exercise-induced cardiac arrhythmias in R2474S knock-in mice (Kobayashi et al., 2010), increase survival after ventricular fibrillation (Zamiri et al., 2014), and prevent anesthetic induced–MH in skeletal muscle (Podranski et al., 2005). This has lead others to consider alternative therapeutic mechanisms for dantrolene such as modulating store-operated Ca2+ entry (Cherednichenko et al., 2008) or by acting as an antioxidant (Buyukokuroglu et al., 2001) or regulating antioxidant enzymes (Buyukokuroglu et al., 2002; Ucuncu et al., 2005).
Our finding that dantrolene inhibition is seen only at cytoplasmic [Ca2+] ≤ 1 µM (Fig. 2C) is consistent with previous findings that dantrolene (1 µM) inhibits the frequency Ca2+ sparks (and hence SR leak) yet does not inhibit the amplitude of Ca2+ transients (Maxwell et al., 2012; Zamiri et al., 2014). Thus dantrolene is a diastolic inhibitor of Ca2+ release in failing heart, which has the beneficial actions of increasing diastolic Ca2+ loading of the SR (Maxwell et al., 2012) and reducing diastolic SR Ca2+ leak after ventricular fibrillation (Zamiri et al., 2014). Because dantrolene is not an effective RyR inhibitor at high cytoplasmic [Ca2+], it is not surprising that other dantrolene mechanisms may be more important for suppressing Ca2+ release during skeletal muscle twitches (Flewellen et al., 1983) or suppressing MH episodes.
Single channel recordings of dantrolene inhibition provide a unique opportunity to probe the mechanism of dantrolene inhibition. RyR2 dwell-time distributions (Fig. 2, A and B) indicate that dantrolene decreases the duration of channel openings and increases the duration of closures, characteristics typical of an allosteric inhibitor rather than a channel blocker like the local anesthetics that cause distinct blocking events in single channel recordings that introduce new exponential components in closed time distributions (Tinker and Williams, 1993; Xu et al., 1993; Tsushima et al., 2002). Like CaM, dantrolene inhibits RyR2 by destabilizing their open state and stabilizing their closed state. By using a CaM mutation that causes CaM to activate RyR2, we show that dantrolene does not merely increase the efficacy of CaM, but is an inhibitor in its own right (Fig. 4). The RyR has a homotetrametic structure that includes four dantrolene binding sites and at least four CaM binding sites. The dantrolene dose-response (Fig. 1D) exhibited a Hill coefficient of ∼1, consistent with values obtained from [3H]ryanodine binding assays (Fruen et al., 1997). Such a value indicates that the binding of only one dantrolene molecule is sufficient to cause inhibition of RyR2 activity. Interestingly, the dose-response of N98S-CaM facilitation of dantrolene inhibition (Fig. 4C) had a much higher Hill coefficient, consistent with a requirement for multiple CaM molecules on RyR2. The mechanism by which CaM facilitates dantrolene inhibition remains unclear. It is unlikely that dantrolene acts by binding to a site on CaM, because that would not explain the different Hill coefficients for the dantrolene and N98S-CaM dose responses. Also, given the redox buffering of our experimental solutions (4 mM GSH in bilayer experiments and 10 mM GSH in myocyte experiments) it is unlikely that the reducing properties of dantrolene underlie its inhibition. However, one possibility is that CaM puts the RyR into a conformation that gives dantrolene access to its binding site on the RyR. Two studies have demonstrated that dantrolene has restricted access to its binding site that is regulated by RyR conformation and on the presence of RyR ligands such as Ca2+ and ATP (Paul-Pletzer et al., 2001, 2005). An alternative possibility is that CaM is a part of the signaling pathway that transduces dantrolene binding into RyR inhibition. Several studies present evidence that dantrolene modulates interdomain interactions in RyR1 (Kobayashi et al., 2005) and RyR2 (Kobayashi et al., 2009; Uchinoumi et al., 2010; Suetomi et al., 2011; Maxwell et al., 2012) between the N-terminal (1–619 aa), central (2000–2500 aa), and C-terminal domains (3900–end). Our data are consistent with both these possibilities.
In conclusion, we show that CaM binding to the RyR is required to produce dantrolene inhibition in both RyR1 and RyR2. It is likely that other, as yet undefined, factors play a similar role in facilitating dantrolene inhibition.
Supplementary Material
Acknowledgments
The authors thank Paul Johnson for assisting with the experiments.
Abbreviations
- CaM
calmodulin
- GSH
glutathione
- MH
malignant hyperthermia
- RyR
ryanodine receptor
- SR
sarcoplasmic reticulum
- wt
wild-type
Author Contributions
Participated in research design: Oo, Imtiaz, Knollmann, Laver.
Conducted experiments: Oo, Gomez-Hurtado, Walweel.
Contributed to new reagents or analytic tools: Knollmann, Laver.
Performed data analysis: Oo, Gomez-Hurtado, Knollmann, Laver.
Wrote or contributed to the writing of the manuscript: Oo, Gomez-Hurtado, van Helden, Knollmann, Laver.
Footnotes
This work was funded by a New South Wales Health Infrastructure grant through the Hunter Medical Research Institute to D.R.L.; the National Health and Medical Research Council Project [Grant APP 1005974] to D.R.L. and B.C.K.; National Institutes of Health National Heart, Lung, and Blood Institute [Grant R01-HL88635] to B.C.K.; and an American Heart Association Innovative Research Grant [13IRG13680003] to B.C.K.
>The authors declare that there is no conflict of interest in this report.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Balshaw DM, Xu L, Yamaguchi N, Pasek DA, Meissner G. (2001) Calmodulin binding and inhibition of cardiac muscle calcium release channel (ryanodine receptor). J Biol Chem 276:20144–20153. [DOI] [PubMed] [Google Scholar]
- Buyukokuroglu ME, Gulcin I, Oktay M, Kufrevioglu OI. (2001) In vitro antioxidant properties of dantrolene sodium. Pharmacol Res 44:491–494. [DOI] [PubMed] [Google Scholar]
- Buyukokuroglu ME, Taysi S, Polat F, Gocer F. (2002) Mechanism of the beneficial effects of dantrolene sodium on ethanol-induced acute gastric mucosal injury in rats. Pharmacol Res 45:421–425. [DOI] [PubMed] [Google Scholar]
- Cherednichenko G, Ward CW, Feng W, Cabrales E, Michaelson L, Samso M, López JR, Allen PD, Pessah IN. (2008) Enhanced excitation-coupled calcium entry in myotubes expressing malignant hyperthermia mutation R163C is attenuated by dantrolene. Mol Pharmacol 73:1203–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou CC, Wen MS, Lee HL, Chang PC, Wo HT, Yeh SJ, Wu D. (2014) Dantrolene suppresses ventricular ectopy and arrhythmogenicity with acute myocardial infarction in a langendorff-perfused pacing-induced heart failure rabbit model. J Cardiovasc Electrophysiol 25:431–439. [DOI] [PubMed] [Google Scholar]
- Diaz-Sylvester PL, Porta M, Copello JA. (2008) Halothane modulation of skeletal muscle ryanodine receptors: dependence on Ca2+, Mg2+, and ATP. Am J Physiol Cell Physiol 294:C1103–C1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flewellen EH, Nelson TE, Jones WP, Arens JF, Wagner DL. (1983) Dantrolene dose response in awake man: implications for management of malignant hyperthermia. Anesthesiology 59:275–280. [DOI] [PubMed] [Google Scholar]
- Fruen BR, Mickelson JR, Louis CF. (1997) Dantrolene inhibition of sarcoplasmic reticulum Ca2+ release by direct and specific action at skeletal muscle ryanodine receptors. J Biol Chem 272:26965–26971. [DOI] [PubMed] [Google Scholar]
- Galimberti ES, Knollmann BC. (2011) Efficacy and potency of class I antiarrhythmic drugs for suppression of Ca2+ waves in permeabilized myocytes lacking calsequestrin. J Mol Cell Cardiol 51:760–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo T, Fruen BR, Nitu FR, Nguyen TD, Yang Y, Cornea RL, Bers DM. (2011) FRET detection of calmodulin binding to the cardiac RyR2 calcium release channel. Biophys J 101:2170–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainaut K, Desmedt JE. (1974) Effect of dantrolene sodium on calcium movements in single muscle fibres. Nature 252:728–730. [DOI] [PubMed] [Google Scholar]
- Huang X, Liu Y, Wang R, Zhong X, Liu Y, Koop A, Chen SR, Wagenknecht T, Liu Z. (2013) Two potential calmodulin-binding sequences in the ryanodine receptor contribute to a mobile, intra-subunit calmodulin-binding domain. J Cell Sci 126:4527–4535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HS, Nitu FR, Yang Y, Walweel K, Pereira L, Johnson CN, Faggioni M, Chazin WJ, Laver D, George AL, Jr, et al. (2014) Divergent regulation of ryanodine receptor 2 calcium release channels by arrhythmogenic human calmodulin missense mutants. Circ Res 114:1114–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CB, Moretti A, Mederos y Schnitzler M, Iop L, Storch U, Bellin M, Dorn T, Ruppenthal S, Pfeiffer S, Goedel A, et al. (2012) Dantrolene rescues arrhythmogenic RYR2 defect in a patient-specific stem cell model of catecholaminergic polymorphic ventricular tachycardia. EMBO Mol Med 4:180–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knollmann BC, Chopra N, Hlaing T, Akin B, Yang T, Ettensohn K, Knollmann BE, Horton KD, Weissman NJ, Holinstat I, et al. (2006) Casq2 deletion causes sarcoplasmic reticulum volume increase, premature Ca2+ release, and catecholaminergic polymorphic ventricular tachycardia. J Clin Invest 116:2510–2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Bannister ML, Gangopadhyay JP, Hamada T, Parness J, Ikemoto N. (2005) Dantrolene stabilizes domain interactions within the ryanodine receptor. J Biol Chem 280:6580–6587. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Yano M, Suetomi T, Ono M, Tateishi H, Mochizuki M, Xu X, Uchinoumi H, Okuda S, Yamamoto T, et al. (2009) Dantrolene, a therapeutic agent for malignant hyperthermia, markedly improves the function of failing cardiomyocytes by stabilizing interdomain interactions within the ryanodine receptor. J Am Coll Cardiol 53:1993–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Yano M, Uchinoumi H, Suetomi T, Susa T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, et al. (2010) Dantrolene, a therapeutic agent for malignant hyperthermia, inhibits catecholaminergic polymorphic ventricular tachycardia in a RyR2(R2474S/+) knock-in mouse model. Circ J 74:2579–2584. [DOI] [PubMed] [Google Scholar]
- Laver DR, Roden LD, Ahern GP, Eager KR, Junankar PR, Dulhunty AF. (1995) Cytoplasmic Ca2+ inhibits the ryanodine receptor from cardiac muscle. J Membr Biol 147:7–22. [DOI] [PubMed] [Google Scholar]
- Marks AR, Marx SO, Reiken S. (2002) Regulation of ryanodine receptors via macromolecular complexes: a novel role for leucine/isoleucine zippers. Trends Cardiovasc Med 12:166–170. [DOI] [PubMed] [Google Scholar]
- Maxwell JT, Domeier TL, Blatter LA. (2012) Dantrolene prevents arrhythmogenic Ca2+ release in heart failure. Am J Physiol Heart Circ Physiol 302:H953–H963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy TV, Healy JM, Heffron JJ, Lehane M, Deufel T, Lehmann-Horn F, Farrall M, Johnson K. (1990) Localization of the malignant hyperthermia susceptibility locus to human chromosome 19q12-13.2. Nature 343:562–564. [DOI] [PubMed] [Google Scholar]
- Monnier N, Procaccio V, Stieglitz P, Lunardi J. (1997) Malignant-hyperthermia susceptibility is associated with a mutation of the alpha 1-subunit of the human dihydropyridine-sensitive L-type voltage-dependent calcium-channel receptor in skeletal muscle. Am J Hum Genet 60:1316–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TE, Lin M, Zapata-Sudo G, Sudo RT. (1996) Dantrolene sodium can increase or attenuate activity of skeletal muscle ryanodine receptor calcium release channel. Clinical implications. Anesthesiology 84:1368–1379. [DOI] [PubMed] [Google Scholar]
- O’Neill ER, Sakowska MM, Laver DR. (2003) Regulation of the calcium release channel from skeletal muscle by suramin and the disulfonated stilbene derivatives DIDS, DBDS, and DNDS. Biophys J 84:1674–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parness J, Palnitkar SS. (1995) Identification of dantrolene binding sites in porcine skeletal muscle sarcoplasmic reticulum. J Biol Chem 270:18465–18472. [DOI] [PubMed] [Google Scholar]
- Paul-Pletzer K, Palnitkar SS, Jimenez LS, Morimoto H, Parness J. (2001) The skeletal muscle ryanodine receptor identified as a molecular target of [3H]azidodantrolene by photoaffinity labeling. Biochemistry 40:531–542. [DOI] [PubMed] [Google Scholar]
- Paul-Pletzer K, Yamamoto T, Bhat MB, Ma J, Ikemoto N, Jimenez LS, Morimoto H, Williams PG, Parness J. (2002) Identification of a dantrolene-binding sequence on the skeletal muscle ryanodine receptor. J Biol Chem 277:34918–34923. [DOI] [PubMed] [Google Scholar]
- Paul-Pletzer K, Yamamoto T, Ikemoto N, Jimenez LS, Morimoto H, Williams PG, Ma J, Parness J. (2005) Probing a putative dantrolene-binding site on the cardiac ryanodine receptor. Biochem J 387:905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podranski T, Bouillon T, Schumacher PM, Taguchi A, Sessler DI, Kurz A. (2005) Compartmental pharmacokinetics of dantrolene in adults: do malignant hyperthermia association dosing guidelines work? Anesth Analg 101:1695–1699. [DOI] [PubMed] [Google Scholar]
- Salata JJ, Wasserstrom JA, Jalife J. (1983) Dantrolene sodium: effects on isolated cardiac tissues. J Mol Cell Cardiol 15:233–243. [DOI] [PubMed] [Google Scholar]
- Sigworth FJ, Sine SM. (1987) Data transformations for improved display and fitting of single-channel dwell time histograms. Biophys J 52:1047–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suetomi T, Yano M, Uchinoumi H, Fukuda M, Hino A, Ono M, Xu X, Tateishi H, Okuda S, Doi M, et al. (2011) Mutation-linked defective interdomain interactions within ryanodine receptor cause aberrant Ca²⁺release leading to catecholaminergic polymorphic ventricular tachycardia. Circulation 124:682–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi P, Collet C, Sárközi S, Szegedi C, Jona I, Jacquemond V, Kovács L, Csernoch L. (2001) Effects of dantrolene on steps of excitation-contraction coupling in mammalian skeletal muscle fibers. J Gen Physiol 118:355–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A, Williams AJ. (1993) Charged local anesthetics block ionic conduction in the sheep cardiac sarcoplasmic reticulum calcium release channel. Biophys J 65:852–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy A, Xu L, Mann G, Meissner G. (1995) Calmodulin activation and inhibition of skeletal muscle Ca2+ release channel (ryanodine receptor). Biophys J 69:106–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima RG, Kelly JE, Wasserstrom JA. (2002) Subconductance activity induced by quinidine and quinidinium in purified cardiac sarcoplasmic reticulum calcium release channels. J Pharmacol Exp Ther 301:729–737. [DOI] [PubMed] [Google Scholar]
- Uchinoumi H, Yano M, Suetomi T, Ono M, Xu X, Tateishi H, Oda T, Okuda S, Doi M, Kobayashi S, et al. (2010) Catecholaminergic polymorphic ventricular tachycardia is caused by mutation-linked defective conformational regulation of the ryanodine receptor. Circ Res 106:1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ucuncu H, Taysi S, Aktan B, Buyukokuroglu ME, Elmastas M. (2005) Effect of dantrolene on lipid peroxidation, lutathione and glutathione-dependent enzyme activities in experimental otitis media with effusion in guinea pigs. Hum Exp Toxicol 24:567–571. [DOI] [PubMed] [Google Scholar]
- Wagner LE, 2nd, Groom LA, Dirksen RT, Yule DI. (2014) Characterization of ryanodine receptor type 1 single channel activity using “on-nucleus” patch clamp. Cell Calcium 56:96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Jones R, Meissner G. (1993) Effects of local anesthetics on single channel behavior of skeletal muscle calcium release channel. J Gen Physiol 101:207–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Guo T, Oda T, Chakraborty A, Chen L, Uchinoumi H, Knowlton AA, Fruen BR, Cornea RL, Meissner G, et al. (2014) Cardiac myocyte Z-line calmodulin is mainly RyR2-bound, and reduction is arrhythmogenic and occurs in heart failure. Circ Res 114:295–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M. (2005) [Abnormal ryanodine receptor function in heart failure]. Nihon Yakurigaku Zasshi 126:372–376. [DOI] [PubMed] [Google Scholar]
- Zamiri N, Massé S, Ramadeen A, Kusha M, Hu X, Azam MA, Liu J, Lai PF, Vigmond EJ, Boyle PM, et al. (2014) Dantrolene improves survival after ventricular fibrillation by mitigating impaired calcium handling in animal models. Circulation 129:875–885. [DOI] [PubMed] [Google Scholar]
- Zhao F, Li P, Chen SR, Louis CF, Fruen BR. (2001) Dantrolene inhibition of ryanodine receptor Ca2+ release channels. Molecular mechanism and isoform selectivity. J Biol Chem 276:13810–13816. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.