Abstract
Emergence of resistance to pentavalent antimonials has become a severe obstacle in the treatment of visceral leishmaniasis (VL) in the Indian subcontinent. Mitogen-activated protein kinases (MAPKs) are well-known mediators of signal transduction of eukaryotes, regulating important processes, like proliferation, differentiation, stress response, and apoptosis. In Leishmania, MAPK1 has been shown to be consistently downregulated in antimony-resistant field isolates, suggesting that it has a role in antimony resistance. The present work investigates the molecular mechanism of MAPK1 in antimony resistance in Leishmania donovani. The L. donovani MAPK1 (LdMAPK1) single-allele replacement mutants exhibited increased resistance to Sb(III) (5.57-fold) compared to wild-type promastigotes, while overexpressing parasites became much more susceptible to antimony. The LdMAPK1-mediated drug sensitivity was directly related to antimony-induced apoptotic death of the parasite, as was evidenced by a 4- to 5-fold decrease in cell death parameters in deletion mutants and a 2- to 3-fold increase in MAPK1-overexpressing cells. LdMAPK1-underexpressing parasites also exhibited increased P-glycoprotein (P-gp)-mediated efflux pump activity, while a significant decrease in pump activity was observed in overexpressing cells. This change in efflux pump activity was directly related to expression levels of P-gp in all cell lines. However, episomal complementation of the gene restored normal growth, drug sensitivity, P-gp expression, and efflux pump activity. The data indicate that LdMAPK1 negatively regulates the expression of P-glycoprotein-type efflux pumps in the parasite. The decrease in efflux pump activity with an increase in LdMAPK1 expression may result in increased antimony accumulation in the parasite, making it more vulnerable to the drug.
INTRODUCTION
Leishmania, a protozoan parasite, causes leishmaniasis, a group of diseases with clinical manifestations that range from self-healing cutaneous and mucocutaneous skin ulcers to a fatal visceral form (visceral leishmaniasis [VL]). The disease imposes a significant burden of mortality and morbidity, affecting 12 million people in more than 88 countries in tropical and subtropical zones of the world (1). Since antileishmanial vaccines are still under development, control of the disease is dependent mostly on chemotherapy (2). However, the efficacy of Sb(V), the first-line treatment, is now threatened by the emergence of drug-resistant Leishmania parasites, as described in several regions of endemicity (3–5). During the last decade, several novel formulations of conventional antileishmanials, as well as new drugs, including the oral agent miltefosine, became available or were under investigation. However, their widespread use in poor countries is hindered by their high cost and also by concerns about toxicity and the emergence of resistance (6, 7). The present-day requirement in the treatment of leishmaniasis is to battle escalating lack of responsiveness to antimony, and hence, an urgent need exists to define the mechanisms of resistance in the field.
Several studies have been conducted on resistant field isolates, mostly from the Indian subcontinent, to understand the molecular mechanism of clinical antimony resistance. The suggested mechanisms include decreased uptake, increased efflux/sequestration, and modulation of the unique parasite thiol metabolism (8, 9). These studies also inferred that natural antimonial resistance is multifactorial and hence requires wider exploration. Microarray technology and proteomic screening have been employed to elucidate a global picture of the mechanisms leading to resistance in the field (10–12). One such study, carried out by our group through transcriptome analysis, identified a gene encoding mitogen-activated protein kinase 1 (MAPK1) of the kinetoplast protozoan Leishmania donovani (LdMAPK1) that was consistently downregulated in antimony-resistant field isolates (13). Interestingly, overexpression of the gene in wild-type sensitive or resistant parasites resulted in increased sensitivity of the transfectants to potassium antimony tartrate, suggesting that MAPK1 has a role in antimony resistance.
MAP kinases (MAPKs) are serine/threonine-specific protein kinases that are highly conserved in all eukaryotes. They are the last kinases in signal transduction cascades relaying signals augmented by environmental stress or stimuli that can ultimately lead to changes in gene expression profiles. The MAPKs regulate critical cellular activities, like cell growth, differentiation, cell shape, mortality, cellular response to stress, and apoptosis (14). The roles of MAPKs in chemoresistance in cancer cells have been studied extensively. It is well established that Sb(III) and the related metal As(III) induce apoptosis in lymphoid tumor cells, human myeloid leukemia HL60 cells, other mammalian cells, and Leishmania parasites (15–18). Traditionally, upregulation of MAPKs (JNK and p38) is associated with antimony- and arsenite-stimulated apoptosis (15, 19–21). Interestingly, downregulation of a MAP kinase, ERK2, has also been reported in multidrug-resistant (MDR) hepatocellular carcinoma cells, suggesting that downregulation of ERK2 has a role in the toxicity pathway and resistance (22). Interestingly, MDR in cancer cells may be reversed by modulation of ERK activation, which induces apoptosis (23). In other studies, either ERK activation in human breast cancer cells or enhancement of JNK in human gastric and pancreatic carcinoma cells resulted in downregulation of P-glycoprotein (P-gp) (24, 25). Recently, MAPK2 of Leishmania major (LmjMAPK2) has been shown to modulate the influx pump aquaglycoporin (AQP1), which is responsible for drug uptake. Phosphorylation of LmjAQP1 at Thr-197 is required for its pump activity, and LmjMAPK2 activity is responsible for it. Inhibition of AQP1 phosphorylation results in reduced influx of Sb(III), slower volume recovery of cells, and, hence, increased drug resistance (26).
In the present study, for the first time, we have established the role of LdMAPK1 in antimony-mediated apoptosis by modulating the functionality of P-gp-type efflux pumps.
MATERIALS AND METHODS
Parasites and cell culture.
L. donovani promastigotes (WHO designation, MHOM/IN/80/Dd8), originally obtained as a gift from the late Prof. P. C. C. Garnham and routinely maintained at the Central Drug Research Institute, Lucknow, India, in golden hamsters, were used in the present study. Promastigotes were grown in medium 199 (M199) (Sigma) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (Gibco) (27). An LdMAPK1 construct cloned into the PKS-neo shuttle vector was transfected in laboratory strain promastigotes (wild type [Dd8+/+]) as described previously (13). Transfectants with LdMAPK1 construct (Dd8++/++) and vector control (Dd8Vc) were selected and maintained under G418 (40 μg/ml) drug pressure in M199 supplemented with 10% FBS.
Development of LdMAPK1 gene replacement mutants.
The upstream (5′-FLK [5FLK]) and downstream (3′-FLK [3FLK]) flanking-region sequences (approximately 1 kb in length) of LdMAPK1 were amplified using L. donovani genomic DNA as the template and primers (P1, P2, P3, and P4 [Table 1]) designed on the basis of the L. donovani genomic sequence available at the NCBI site. The amplified products were sequenced. Internal primers with the desired restriction sites were designed from the sequenced region (Table 1). The 5′-FLK and 3′-FLK were further amplified using primers P5 and P6 (for 5FLK) and P7 and P8 (for 3FLK), and the amplified products were cloned in the pCR II-TOPO vector (Invitrogen). The clones that had 5FLK and 3FLK in right orientation (namely, the TOPO-5FLK and TOPO-3FLK constructs) were selected. Hygromycin phosphotransferase (HYG) and neomycin phosphotranferase (NEO) genes were amplified from the pCDNA3 and pXG-GFP vectors (gifts from S. M. Beverley). These resistance marker genes were amplified using primers P9 and P10, and P11 and P12, respectively, and cloned in the pCR II-TOPO vector. The 3′-FLK fragment was directionally subcloned in the TOPO-5FLK construct using XmaI and EcoRV sites, resulting in the TOPO-5/3FLK construct. HYG and NEO gene cassettes were inserted directionally in the TOPO-5/3 FLK construct using SalI and XmaI sites, resulting in the final gene deletion constructs, TOPO-5FLK/HYG/3FLK and TOPO-5FLK/NEO/3FLK. Both the 5FLK/HYG/3FLK and 5FLK/NEO/3FLK fragments were taken out of the pCR-TOPO vector by digestion with SpeI and EcoRV and purified. First, 5FLK/HYG/3FLK was transfected in wild-type Dd8+/+ cells and selected in the presence of 10 μg/ml hygromycin in M199 supplemented with 20% FBS to obtain the Dd8+/− MAPK1 single-allele replacement mutant. The single-allele replacement mutant was further transfected with a second allele deletion fragment, 5FLK/NEO/3FLK, and selected and maintained under 10 μg/ml hygromycin and 20 μg/ml neomycin drug pressure in M199 supplemented with 20% (vol/vol) FBS.
TABLE 1.
Sequences of primers used in development of knockout constructs and verification of LdMAPK1 allele replacement
| Primer no. | Primer name | Sequencea | Restriction site(s) |
|---|---|---|---|
| P1 | 5FLK-F | 5′-CACTGCGTTTGCTTGAATTGTC-3′ | None |
| P2 | 5FLK-R | 5′-GTCGATGCCATAGGAGGTCAT-3′ | None |
| P3 | 3FLK-F | 5′-CACATAACAGACGTGTATTAGCG-3′ | None |
| P4 | 3FLK-R | 5′-CACGCCAGCATCGATACACAT-3′ | None |
| P5 | 5FLKS-F | 5′-GCCCACTAGTCCACGAGCAATAATGC-3′ | SpeI |
| P6 | 5FLKX-R | 5′-ATTACCCGGGAGGAGGTCATGATGG-3′ | XmaI |
| P7 | 3FLKX-F | 5′-TACCCGGGATCTATGTCGACCGACAGACTGAAGTT-3′ | XmaI and SalI |
| P8 | 3FLKE-R | 5′-GCACGATATCCACGCCAGCATCGATACACATG-3′ | EcoRV |
| P9 | HYGX-F | 5′-ATCGCCCGGGATGAAAAAGCCTGAA-3′ | XmaI |
| P10 | HYGS-R | 5′-TAGCGTCGACCTATTCCTTTGCCCT-3′ | SalI |
| P11 | NEOX-F | 5′-ATTACCCGGGATGGGATCGGCCATT-3′ | XmaI |
| P12 | NEOS-R | 5′-GAGCGTCGACTCAGAAGAACTCGTC-3′ | SalI |
| P13 | MAPK1-F | 5′-ATGGCCTCCTATGGCATCGAC-3′ | None |
| P14 | MAPK1-R | 5′-AACGTCTGTAATGTGATG-3′ | None |
Restriction sites are in boldface.
Verification of transfection and homologous recombination of the MAPK1 single allele/double allele with the hygromycin phosphotransferase or neomycin phosphotransferase gene at the correct position (between 5′-FLK and 3′-FLK) was done by PCR using genomic DNA of Dd8+/+, Dd8+/−, and Dd8−/− parasites as the templates. HYG was amplified with primers P9 and P10, NEO with P11 and P12 (Table 1), and MAPK1 with P13 and P14. The PCR product was checked on a 1% agarose gel and stained with ethidium bromide.
For episomal complementation, the LdMAPK1 gene cloned in the pKS-neo shuttle vector was transfected in Dd8+/− parasites. The gene add-back mutants (Dd8+/−/+) were selected and maintained under 20-μg/ml G418 drug pressure in M199 supplemented with 10% (vol/vol) FBS.
SDS-PAGE and Western blotting.
The expression levels of LdMAPK1 in different mutants were compared through Western blotting. Proteins from equivalent numbers of cells (4 × 106) were analyzed by SDS-PAGE, transferred onto nitrocellulose membranes, and processed for Western blot analysis with anti-LdMAPK1 antibodies raised in rabbit (28, 29).
Macrophage infection.
The infectivity of the parasites and the growth of intracellular amastigotes were evaluated as described previously (27). Briefly, 0.5 × 105 macrophages were layered in 16-well chamber slides (Nunc) and allowed to adhere for 24 h in RPMI medium supplemented with 10% FBS at 37°C in a 5% CO2-95% air mixture. The adhered macrophages were infected with stationary-phase promastigotes at a 1:10 ratio for 24 h. The noninternalized parasites were washed from the wells, and infected macrophages were incubated for 48 h in RPMI medium supplemented with 10% FBS at 37°C in a 5% CO2-95% air mixture. The macrophages were fixed with 90% methanol after 24 and 72 h of infection, stained with Giemsa stain, and observed under a microscope. The number of infected macrophages per 100 cell nuclei (percent infectivity) was determined at 24 h of infection. The numbers of amastigotes per 100 macrophages at 24 h and 72 h of infection were determined for intracellular amastigote proliferation.
Drug sensitivity assay.
Mid-log-phase promastigotes of the wild type and different mutants were seeded in 96-well culture plates to a final concentration of 0.5 × 106 cells/ml in M199 alone or in the presence of increasing concentrations of trivalent antimony [Sb(III)] in triplicate. The cells were allowed to grow in the presence or absence of Sb(III) for 72 h at 24 ± 1°C. The number of viable cells per well was determined microscopically (30), and the 50% inhibitory concentration (IC50) was calculated by probate analysis.
Cell cycle analysis.
Cells (1 × 106), untreated and treated with trivalent antimony (350 μM), were washed with 1× phosphate-buffered saline (PBS) (1,000 × g for 10 min at 4°C) and fixed in 90% chilled methanol at −20°C overnight. The fixed cells were washed with 1× PBS and incubated with RNase for 1 h at 37°C. The pellet was stained with propidium iodide (PI) (40 μg/ml) for 15 min in the dark. Data were acquired on a FACSCalibur (BD Biosciences), and the fluorescence intensity was measured on FL2-A. Analyses were done using the ModFit program (BD Biosciences).
In situ detection of DNA fragmentation by TUNEL assay.
In situ detection of DNA fragments following treatment of promastigotes was measured by terminal deoxynucleotidyltransferase (TdT)-mediated dUTP nick end labeling (TUNEL) using a Cell Death Detection kit (Roche) according to the manufacturer's instructions. Cells were washed and fixed in 2% paraformaldehyde and incubated on ice for 1 h, washed with PBS, and resuspended in permeabilization solution for 2 min on ice. Thereafter, the cells were washed twice with PBS and incubated with 50 μl reaction mixture containing TdT and dUTP-fluorescein isothiocyanate (FITC) for 1 h at 37°C. The cells were washed twice with PBS and resuspended in 500 μl PBS and acquired on the FACSCalibur. Analyses were done on the FL1 channel using Cellquest Pro software.
Analysis of externalization of phosphatidylserine.
Double staining for annexin V-FITC and PI was performed as described previously (31). The data were acquired on a FACSCalibur, and the fluorescence intensity was measured on the FL1-H channel for annexin V and FL2-H for propidium iodide. Analyses were performed on 10,000 gated events using Cellquest Pro software.
Measurement of ROS.
To measure the change in the level of reactive oxygen species (ROS), the cell-permeable, nonfluorescent probe 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) (Molecular Probes) was used. H2DCFDA is a nonpolar compound that, on entering the cell, is converted into nonfluorescent, nonpermeable dichlorodihyrofluorescein by cellular esterases. With the availability of oxidant species, it oxidizes to fluorescent 2,7-dichlorofluorescein. ROS measurement was done according to the manufacturer's instructions.
Analysis of mitochondrial transmembrane potential.
To determine the mitochondrial transmembrane electrochemical gradient (Δψm), JC-1 (5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethyl benzimidazolyl carbocyanine iodide) was used. JC-1 is a lipophilic, potential-sensitive cation that can be used to measure mitochondrial membrane potential. At higher potential, it is incorporated into the mitochondrial membrane, where it forms red-fluorescent “J aggregates.” Upon loss of mitochondrial potential, as in case of apoptosis, it is released into the cytosol and converted into a green-fluorescent monomer. Decrease in the ratio of red to green fluorescence of JC-1 is an indicator of a loss of mitochondrial transmembrane potential.
Accordingly, to evaluate the effect of Sb(III) on the mitochondrial membrane potential of the wild type and mutants, the cells were pelleted and washed with PBS. The cells were stained with 5 μM JC-1 in 500 μl PBS for 15 min at room temperature. The data were acquired on a FACSCalibur and analyzed with the Cellquest Pro software on the basis of a quadrant dot plot to determine monomers and J aggregates.
Activation of caspase-like proteases.
A PhiPhiLux (PPL) G1D2 substrate (Calbiochem) was used to study caspase-3 activation in live parasites. The substrate is a peptide with the caspase-3-specific sequence GDEVDGI and is conjugated to two fluorophores, G1 and D2, which fluoresce upon cleavage of the peptide by activated caspase-3. The cells were treated with trivalent antimony (350 μM) and amphotericin B (0.2 μM). Untreated and treated cells (1 × 106) were incubated for 30 min at 37°C in the presence of 25 μl PPL substrate according to the manufacturer's protocol. The data were acquired on a FACSCalibur and analyzed on the FL1 channel for fluorescence using CellQuest software.
Accumulation and retention of the dye Rhodamine123.
Accumulation of Rhodamine123 (Rho123) was studied by incubating the promastigotes (wild-type and mutant strains) with 0.2 μg/ml Rho123 in M199 at 24 ± 1°C for 1 h in the presence or absence of efflux pump inhibitors, 100 μM verapamil and 20 μM trifluoperazine (TFP), as described previously (8). Efflux of the dye was studied after washing the Rho123-loaded parasites twice with chilled PBS, pH 7.4, and resuspending them in plain M199 in the presence or absence of inhibitor(s). At the 0-h and 1-h time points, the cells were analyzed on a FACSCalibur to evaluate dye retention or efflux.
P-glycoprotein expression.
Mid-log-phase cells (1 × 106; wild-type and mutant strains) in 50 μl PBS were immune stained with 20 μl anti-P-gp antibody conjugated to phycoerythrin or its isotype anti-mouse antibody (BD Biosciences) at room temperature for 1 h in the dark. Thereafter, the cells were washed and suspended in PBS. Flow cytometric analysis was performed on a FACSCalibur FL2 channel. An isotype control for each sample was used to measure the background fluorescence.
Immunofluorescence microscopy.
Log-phase promastigotes (1 × 106) were harvested, washed thrice with ice-cold PBS, and suspended in 200 μl PBS. The cells were allowed to adhere to a poly-l-lysine-coated coverslip for 15 min at 25°C. The adhered cells were fixed with 4% (wt/vol) paraformaldehyde for 30 min at room temperature and washed thrice with 0.5% (wt/vol) glycine-containing PBS. The cells were permeabilized with 0.5% (vol/vol) Triton X-100 for 5 min and blocked with 0.5% (wt/vol) bovine serum albumin (BSA) in PBS-glycine buffer. Then, the cells were incubated with anti-P-gp antibody (1:200) conjugated to phycoerythrin or its isotype anti-mouse antibody (BD Biosciences) at 4°C overnight. The coverslips were washed five times with blocking buffer and mounted on glass slides with Prolong Gold antifade reagent (Molecular Probes) to stain double-stranded DNA in the kinetoplast and nucleus. The images were acquired using a Leica TCS-SP8 confocal microscope. The images, at 532- and 488-nm excitation, were acquired separately and merged for presentation using Image J software.
Flow cytometric analysis.
All flow cytometric analyses were done on a BD FACSCalibur equipped with an argon laser tuned to 488 nm. All the samples were analyzed at <300 cells/s, and a typical analysis was done on 10,000 cells. Fluorescence was measured on a photomultiplier tube (FL1 with a 530/30-nm band pass filter or FL2 with a 585/40-nm band pass filter as indicated for each experiment).
Statistical analysis.
Data are expressed as means ± standard deviations (SD) unless otherwise indicated. Comparisons were made using two-way analysis of variance (ANOVA), and differences were considered significant at a 0.001 (***) or 0.01 (**) level of confidence; a P value of >0.05 was considered nonsignificant (ns).
Nucleotide sequence accession numbers.
The complete sequences of the cloned 5′ and 3′ flanking regions of LdMAPK1 are available in GenBank under accession no. KP126514 and KP126515, respectively.
RESULTS
MAPK1 is essential for growth and survival of L. donovani promastigotes.
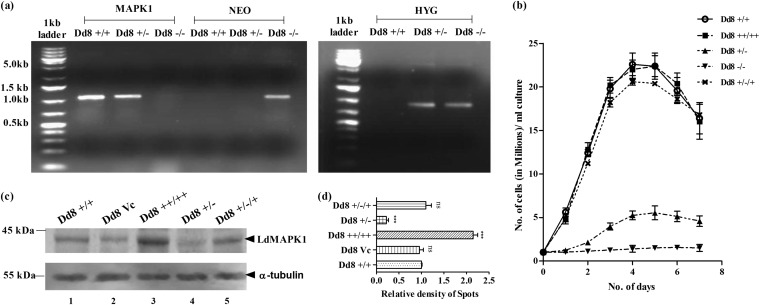
For functional characterization of MAPK1 of L. donovani, the gene was replaced in the parasite genome with a resistance marker gene(s). Since the gene is present in a single copy in the haploid genome (13), two replacements were required to knock off the gene completely from the diploid genome of the parasite. The two alleles were replaced by resistance gene markers conferring resistance to hygromycin or neomycin. Gene replacement constructs were prepared by cloning approximately 1-kb upstream (5′-FLK) and downstream (3′-FLK) flanking regions of the MAPK1 open reading frame (ORF) with a resistance marker gene (HYG or NEO). Transfection of HYG-containing constructs in wild-type parasites and selection under 10-μg/ml hygromycin pressure resulted in parasites with single-allele replacement of the MAPK1 gene (Dd8+/−). The replacement of the MAPK1 allele with HYG was confirmed by PCR of the respective genomic DNA using primers for MAPK1 and HYG (Fig. 1a). The parasites with a single allele replaced were further transfected with a deletion construct containing a NEO marker gene and selected under 10-μg/ml hygromycin and 20-μg/ml neomycin pressure. The multiplication rate of parasites with two alleles replaced was greatly reduced, and these parasites failed to survive after 30 days under the culture conditions. Even after single-allele replacement, multiplication of mutant parasites was also greatly retarded compared to that of wild-type Dd8+/+ cells (Fig. 1b). On episomal complementation of the LdMAPK1 gene in Dd8+/− mutants, the Dd8+/−/+ cells regained normal growth (Fig. 1b). However, overexpression of MAPK1 did not modulate the growth pattern of the parasite (13). Since Dd8−/− parasites could not be maintained even after several attempts at transfection, further experiments were carried out with a single-allele replacement mutant, Dd8+/−.
FIG 1.
Modulation of LdMAPK1 expression in Leishmania parasites. (a) Agarose gel stained with ethidium bromide showing PCR products of MAPK1, NEO, and HYG genes with Dd8+/+ (wild-type parasites), Dd8+/− (parasites with MAPK1 single-allele replacement), and Dd8−/− (parasites with MAPK1 double-allele replacement). (From left) Lanes 1 and 8, 1 kb plus DNA ladders; lanes 2 to 4, MAPK1 PCR products from Dd8+/+, Dd8+/−, and Dd8−/− parasites; lanes 5 to 7, NEO PCR products from Dd8+/+, Dd8+/−, and Dd8−/− parasites; lanes 9 to 11, HYG PCR products from Dd8+/+, Dd8+/−, and Dd8−/− parasites. (b) Effect of modulation of LdMAPK1 expression on promastigote growth. (c) Changes in expression of LdMAPK1 in wild-type (Dd8+/+) parasites, vector control (Dd8Vc) parasites, overexpressing transfectants (Dd8++/++), single-allele replacement mutants (Dd8+/−), and add-back mutants (Dd8+/−/+) by Western blot analysis using anti-LdMAPK1 antibodies. α-Tubulin was used as a control. (d) Quantitative estimation of expression of LdMAPK1 in cells by measuring the relative density of each band. The data are representative of the results of 3 independent experiments. The error bars represent SD. ***, P < 0.001; ns, not significant.
Figure 1c and d depicts the expression of LdMAPK1 in all cell types: wild-type (Dd8+/+) cells, expressing endogenous levels of MAPK1; LdMAPK1 transfectant (Dd8++/++) cells, overexpressing the MAPK1 gene; mutant (Dd8+/−) cells with single-allele replacement, down-expressing the MAPK1 gene; and the gene add-back mutant (Dd8+/−/+), regaining the expression of LdMAPK1. As expected, MAPK1-overexpressing transfectants (Dd8++/++) exhibited 2.15-fold-higher expression than wild-type (Dd8+/+) cells (Fig. 1c and d). Similarly, MAPK1 expression was decreased 4.54-fold in single-replacement (Dd8+/−) mutants. Dd8+/−/+ mutants displayed expression of LdMAPK1 comparable to that of the wild-type (Dd8+/+) parasite, thus confirming the regaining of LdMAPK1 expression. Dd8Vc cells, taken as the vector control, showed no significant change in LdMAPK1 expression compared to Dd8+/+ wild-type cells.
Change in expression of LdMAPK1 modulates infectivity and amastigote multiplication.
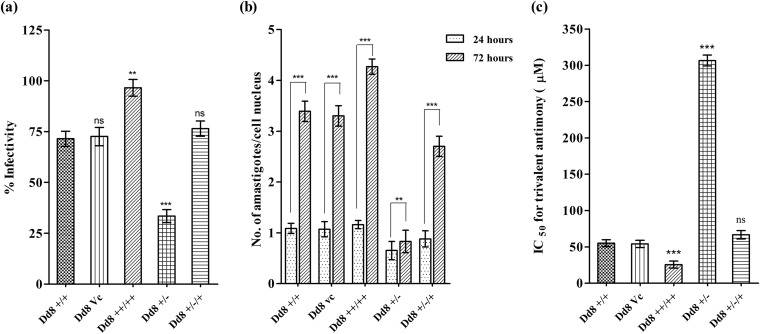
Approximately 20% increase in the infectivity of promastigotes to macrophages was observed with increase in expression of LdMAPK1 (Fig. 2a). While single-allele replacement mutants exhibited significantly decreased infectivity (33.5% ± 2.5%) compared to wild-type Dd8+/+ (71.5% ± 3.2%) cells. On complementing the LdMAPK1 gene in Dd8+/−/+, the parasite infectivity became comparable (76.5% ± 6.5%) to that of wild-type Dd8+/+ parasites.
FIG 2.
Effect of modulation of expression of LdMAPK1 on percent infectivity (number of infected macrophages × 100 cell nuclei) (a), number of intracellular amastigotes per cell nucleus after 24 and 72 h of infection (b), and antimony [Sb(III)] susceptibility of the parasite (c). The data represent means ± SD of the results of 3 independent experiments. **, P < 0.01; ***, P < 0.001; ns, not significant.
Further, loss of the LdMAPK1 allele(s) not only reduced promastigote growth (Fig. 1b), but also significantly affected intracellular amastigote multiplication. Amastigote proliferation of Dd8+/− mutants was reduced to 1.33-fold compared to 3.12-fold proliferation of wild-type Dd8+/+ amastigotes (Fig. 2b). On episomal complementation of the LdMAPK1 gene, amastigote multiplication was recovered to 2.86-fold. This regaining of amastigote proliferation confirmed that the observed decrease in the growth rate of single-replacement mutant amastigotes was due to a decrease in LdMAPK1 expression. However, only a marginal increase in amastigote multiplication of Dd8++/++ parasites was observed compared to wild-type amastigotes.
Effect of differential expression of LdMAPK1 on parasite susceptibility to trivalent antimony.
The overexpressing promastigotes exhibited a 1.92-fold lower IC50 for Sb(III) than wild-type cells (Fig. 2c). The IC50 of deletion mutants was increased by 5.57-fold (306.6 μM) compared with wild-type cells (55.07 μM). Moreover, Dd8+/−/+ add-back mutants regained drug susceptibility (IC50, 66.6 μM), suggesting that development of antimony resistance in Dd8+/− mutants was due to underexpression of LdMAPK1.
For evaluation of apoptotic parameters (DNA fragmentation, phosphatidylserine exposure, mitochondrial transmembrane depolarization, and ROS generation), the cells were exposed to Sb(III) for 6 h at 350 μM concentration. Viability of the parasite was assessed by propidium iodide labeling of the cells; cells unlabeled by propidium iodide were considered viable. All cell types were more than 90% viable after this treatment (see Table S1 in the supplemental material).
Modulation of LdMAPK1 expression affected neither the cell cycle nor apoptosis in the cell.
Cell cycle analysis of Dd8+/+, Dd8Vc, Dd8++/++, and Dd8+/− cells did not exhibit any significant change among all the cell types (Fig. 3a).
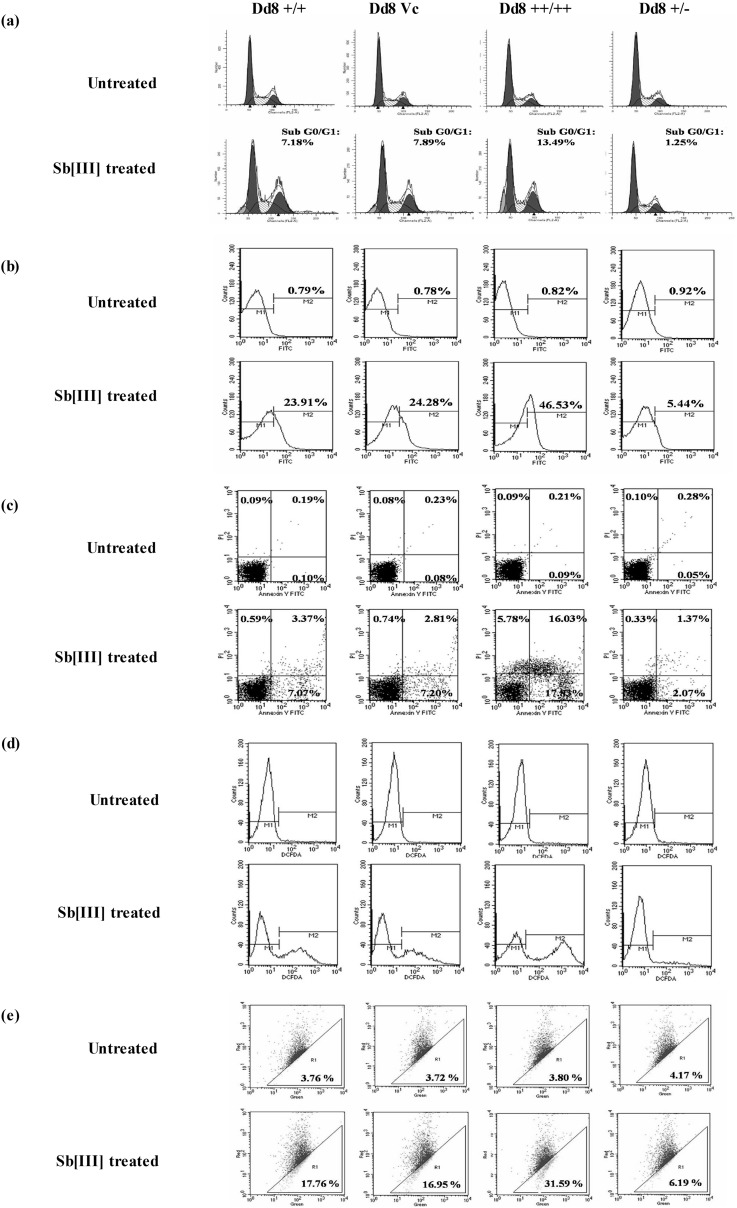
FIG 3.
Effect of modulation of LdMAPK1 expression on antimony-induced apoptosis. (a) Cell cycle analysis by flow cytometry. The dark-gray peaks represent the G0/G1 and G2/M phases, and the shaded region represents S phase, while the light-gray peaks represent the sub-G0/G1 (apoptotic) phase. (b) Histogram depicting DNA fragmentation by TUNEL assay. M1 is marked according to unstained cells, while M2 represents fluorescence of dUTP-FITC bound to nicked DNA ends. (c) Dot plot of annexin V and PI staining. The lower left quadrant represents unstained (healthy) cells, the upper left represents PI-positive (necrotic) cells, the lower right represents annexin V-positive (early apoptotic) cells, and the upper right represents annexin- and PI-positive (late apoptotic) cells. (d) ROS generation by H2CDCF-DA staining. (e) Dot plot representing loss of mitochondrial transmembrane potential upon antimony treatment. The regions were marked on the basis of untreated cells. The dots outside region R1 represent red-fluorescent J aggregates (for healthy cells), and the dots within region R1 represent green-fluorescent monomers (for cells with depolarizing mitochondria). The data are representative of the results of three independent experiments.
Further, the overexpression or underexpression of the LdMAPK1 gene did not affect apoptosis in the parasite, as no significant change was observed in key apoptotic parameters: DNA fragmentation, plasma membrane permeabilization, mitochondrial depolarization, and ROS generation (Fig. 3b to e).
Change in LdMAPK1 expression changes antimony-stimulated DNA fragmentation.
DNA fragmentation is the hallmark of metazoan apoptosis and has been reported in free-swimming parasites, as well as intracellular amastigotes, upon exposure to apoptotic stimuli. During apoptosis, activated nucleases degrade the chromatin structure of DNA into small DNA pieces of about 200 bp and its multiples in length. DNA fragmentation was studied by cell cycle analysis through PI labeling and TUNEL assay. A significant hypodiploid population was observed on PI staining of Sb(III)-treated wild-type Dd8+/+ cells. Interestingly, this antimony-induced hypodiploid population was increased in MAPK1-overexpressing parasites from 7.2% to 13.5% but was reduced to 1.25% in deletion mutants (Fig. 3a).
In accordance with this, parasites overexpressing LdMAPK1 (Dd8++/++) exhibited 46.9% ± 5.09% TUNEL-positive cells compared to 24.4% ± 3.9% and 23.9% ± 2.3% TUNEL-positive cells in wild-type Dd8+/+ and vector control Dd8Vc (Fig. 3b) cells. Thus, overexpression of LdMAPK1 results in a 1.9-fold increase in TUNEL-positive cells compared to wild-type parasites. On the other hand, LdMAPK1 deletion mutants (Dd8+/−) exhibited only a 5.44% ± 1.1% TUNEL-positive population, indicating that MAPK1 may have a role in antimony-induced apoptotic death of the parasite.
LdMAPK1 expression modulates phosphatidylserine externalization in L. donovani promastigotes on Sb(III) treatment.
The phospholipid composition of the plasma membrane is not identical on both sides. The outer leaflet is predominantly composed of choline phospholipids, while the inner leaflet is populated with aminophospholipids. However, in cells undergoing apoptosis, this asymmetry is lost, and phosphatidylserine is exposed on the outer surface. This is identified as an early step in apoptosis, which is usually followed by permeabilization of the membrane. However, in the case of necrosis, there is direct permeabilization of the membrane, which results in the cell bursting. On externalization, phosphatidylserine becomes bound to annexin V conjugated to FITC. Apoptotic cell death can be differentiated from necrotic cell death by counterstaining with PI, a nonpermeable stain with high affinity for nucleic acids. PI selectively enters necrotic cells. Therefore, costaining with annexin V and PI can differentiate between cells undergoing early apoptosis (annexin V-positive and PI-negative) and late-apoptotic (annexin V-positive and PI-positive), necrotic (PI-positive and annexin V-negative), and live (PI- and annexin V-negative) cells. On treatment with Sb(III), the wild type (Dd8+/+) and LdMAPK1 tranfectants (Dd8++/++) exhibited both annexin V-positive cells and a few PI-positive cells. However, in transfectants, the percentages of annexin V-positive and PI-negative cells were 2.3 ± 0.4 times greater than in wild-type parasites. The deletion mutants (Dd8+/−) showed 3.4- ± 0.7-fold decreases in annexin V-positive cells (Fig. 3c). No significant change was observed in a transfectant control.
Antimony-induced ROS generation changes with expression levels of LdMAPK1.
Fig. 3d depicts ROS generation in various MAPK1-expressing cell types on exposure to trivalent antimony. Reactive oxygen species are primary messengers of apoptosis. Inside the cell, H2CDCFDA is converted into nonpermeable probe, which, on reacting with reactive oxygen species, is converted into fluorescent probe. High fluorescence represents ROS accumulation in the cell. On antimony treatment, the mean fluorescence intensity in wild-type (Dd8+/+) cells was twofold less (128.77 ± 12.89 relative fluorescence units [RFU]) than in overexpressing Dd8++/++ parasites (274.76 ± 23.4 RFU). Thus, 2.1-times-higher ROS generation in Dd8++/++ parasites was observed. As expected, Dd8+/− parasites generated a minimal level of ROS (55.25 ± 11.23 RFU).
A change in LdMAPK1 expression levels changes the depolarization of the mitochondrial transmembrane potential of the parasite on antimony treatment.
Maintenance of the mitochondrial transmembrane potential is essential for survival of Leishmania parasites, as the parasite possesses single mitochondria. Loss of mitochondrial transmembrane potential is analyzed using JC-1 lipophilic dye. In normal cells, JC-1 forms red-fluorescent aggregates, which on depolarization diffuse in the cytosol as green-fluorescent monomers.
In dot plot analysis, JC-1 fluorescence is observed in both red (FL2) and green (FL1) channels. The green-fluorescent population was gated as R1 and represents the apoptotic population. In untreated culture, the parasites were predominantly outside the R1 gate. Only 3.8% ± 0.6% of cells fluoresced green. On treatment with 350 μM Sb(III) for 6 h, the R1 population increased to 17.5% ± 1.9% for the wild type and 17.67% ± 1.7% for the transfection control compared to 32.43% ± 2.69% in LdMAPK1 transfectants (Fig. 3e). The red/green ratio dropped from 25.59 to 4.63 in Dd8+/+, while it dropped from 25.31 to 2.35 in LdMAPK1 transfectants (Dd8++/++). On the other hand, in MAPK1single-deletion mutants, the ratio dropped only from 24.58 to 17.15 (Fig. 3e), suggesting a role of LdMAPK1 in antimony-induced apoptosis.
Caspase-like activation.
In metazoans, apoptosis involves the activation of a class of cysteine proteases known as caspases. These caspases have not yet been reported in Leishmania parasites. However, caspase-like activity has been observed in stationary-phase promastigotes and amphotericin-treated axenic amastigotes (32), while antimony-induced apoptosis has been reported to be a caspase-independent process. On treatment with Sb(III), even up to 48 h, no caspase-like activity was observed in all cell types (see Fig. S1a in the supplemental material), while amphotericin B treatment for 6 h led to approximately 45% PPL activity-positive cells in both wild-type and transfectant parasites. This suggests that antimony induced apoptosis is a caspase-independent pathway and that LdMAPK1 is not associated with caspase activation.
LdMAPK1 regulates antimony-induced apoptosis.
The above-mentioned results showed that a decrease in LdMAPK1 expression results in a decrease in antimony-induced apoptosis. To confirm this, the parasites with LdMAPK1 single-allele replacement were complemented with the LdMAPK1 gene through episomal expression (Dd8+/−/+). When Dd8+/−/+ parasites were treated with 350 μM Sb(III), the percentage of TUNEL-positive cells was 21.8% ± 1.2%, comparable to that of wild-type Dd8+/+ parasites (25.6% ± 1.8%) (see Fig. S1b in the supplemental material). Thus, restoration of LdMAPK1 expression in gene add-back mutants results in their increased antimony-induced apoptosis. The data further support the role of LdMAPK1 in regulating the antimony sensitivity of the parasite.
Rhodamine123 accumulation and retention.
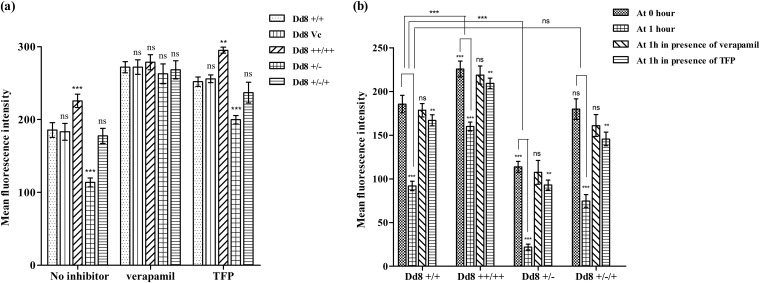
Rhodamine123 is a fluorescent cationic dye that accumulates in mitochondria and can be examined by flow cytometric analysis. It is used as an established substrate of P-gp-type efflux pumps. The fluorescence after 1 h of dye loading represents the accumulation of Rho123 in the parasites. In the absence of any efflux pump inhibitor, MAPK1 transfectants exhibited significantly greater accumulation of Rho123 (1.3-fold) than wild-type cells. On the other hand, single-allele replacement mutants exhibited significantly decreased (1.6-fold; P < 0.001) accumulation of dye (Fig. 4a). On adding back the LdMAPK1 gene in Dd8+/−/+ parasites, the dye accumulation again became comparable to that of wild-type Dd8+/+ parasites. In the presence of verapamil (a strong inhibitor of P-gp-type efflux pumps), the accumulation levels of Rho123 were almost comparable in all the cell types. However, in the presence of TFP (a partial inhibitor of P-gp-type pumps), the difference in accumulation of dye in all cell types was reduced, but not to comparable extents (Fig. 4a).
FIG 4.
Graphical representation of a rhodamine assay. (a) Mean fluorescence intensities of accumulated Rhodamine123 after 1 h of loading in the absence or presence of verapamil or trifluoperazine. (b) Mean fluorescence intensities representing rhodamine123 retention at 0 and 1 h of efflux in the absence or presence of verapamil or trifluoperazine. The data represent means ± SD of the results of 3 independent experiments. ***, P < 0.001; **, P < 0.05; ns, not significant.
For efflux studies, noninternalized dye was washed off, cells were suspended in fresh medium, and the accumulated dye was allowed to efflux for 1 h. Dd8+/+ cells retained 49.5% ± 1.38% of the dye, and the rest (50.4% ± 2.9%) was effluxed. On the other hand, in Dd8++/++ cells, only 29.2% ± 0.9% of the accumulated dye was effluxed, while in replacement mutants (Dd8+/−), 80.6% ± 2.09% of the accumulated dye was effluxed (Fig. 4b). As expected, the Rho123 efflux in Dd8+/−/+ add-back mutants (53.7% ± 1.2%) became comparable to that in Dd8+/+ wild-type cells. In the presence of verapamil, the efflux of dye was almost 90% blocked in all cell types. In the presence of TFP, the efflux was reduced to 13.08% ± 0.58% for Dd8+/+ cells. In LdMAPK1 transfectants, the efflux decreased to 9.21% ± 0.87%, and in LdMAPK1 deletion mutants, efflux was changed to 18.6% ± 1.05%, thus reducing the difference but still significantly retained (P < 0.05) (Fig. 4b). These data suggest that the change in expression of LdMAPK1 modulates the functionality of P-gp-type efflux pumps in Leishmania parasites.
P-glycoprotein expression and localization.
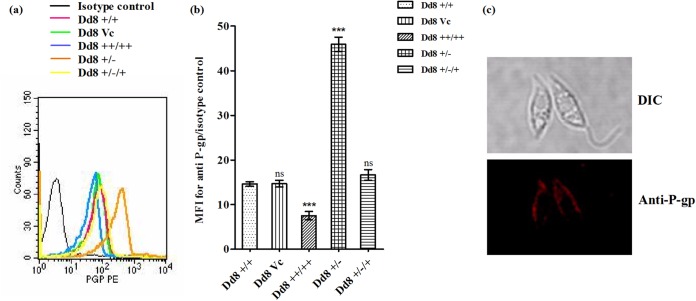
Wild-type parasites exhibited higher P-glycoprotein expression than MAPK1-overexpressing transfectants (Fig. 5a). The expression in Dd8++/++ cells was reduced to half of that in wild-type cells (Fig. 5b). Moreover, on downexpression of LdMAPK1 in Dd8+/− cells, the P-gp expression was increased by 2.7-fold, showing negative regulation of the expression and activity of P-gp-type pumps by LdMAPK1. On reviving the LdMAPK1 expression in Dd8+/−/+ cells, the P-gp expression levels became comparable (1.1-fold; P > 0.05) to that in Dd8+/+ cells, confirming that the change in P-gp expression was due to the change in LdMAPK1 expression.
FIG 5.
P-glycoprotein expression and localization. (a) Histogram depicting cells stained with anti-P-glycoprotein antibody conjugated to phycoerythrin or its isotype antibody. (b) Ratio of mean fluorescence intensity of P-gp versus the isotype control. The data represent means ± SD of the results of 3 independent experiments. (c) Subcellular localization of P-gp on the plasma membrane of the cell by immunofluorescence microscopy, using anti-P-gp antibody conjugated to phycoerythrin. ***, P < 0.001; ns, not significant. DIC, differential interference contrast.
In addition, immunofluorescence microscopy confirmed that the analyzed pump proteins are localized on the plasma membrane of the parasite, not on the membrane of the vacuole present inside the cell (Fig. 5c).
DISCUSSION
Antimony resistance is one of the major obstacles in successful management of visceral leishmaniasis in the Indian subcontinent (3) and is associated with decreased influx and increased efflux or sequestration of the drug (8, 9). However, the regulation of expression of these pathway-related genes is almost unknown in Leishmania. Recently, MAPK2 of L. major has been shown to modulate the influx pump, AQP1, responsible for antimony uptake and osmoregulation. LmjMAPK2 phosphorylates LmjAQP1 at Thr-197, which is required for its pump activity. Inhibition of AQP1 phosphorylation results in reduced influx of Sb(III), slower volume recovery of cells, and, hence, increased drug resistance (26). In another study from our laboratory, MAPK1 of L. donovani was demonstrated to be downregulated in antimony-resistant clinical isolates, and its overexpression led to an increase in parasite susceptibility to antimony by 2-fold (13). The present study was carried out to understand the molecular mechanism of MAPK1-mediated antimony resistance in L. donovani in order to improve the chemotherapeutic efficacy of this first-line treatment. The expression of LdMAPK1 was modulated in L. donovani promastigotes by either overexpressing the gene by episomal expression or decreasing expression by replacing a single allele (Fig. 1) with a hygromycin gene.
Mammalian MAP kinases are categorized into ERKs, JNKs, and p38 MAPKs. Interestingly, Leishmania mexicana MAPK1 (LmxMAPK1) exhibits significant homology to ERK2 in three-dimensional (3D) structure (33). Previously, ERKs were thought to be antiapoptotic in function (34–36), but some recent reports have suggested their proapoptotic nature (37, 38). Stress-activated JNKs and p38 MAPKs have also been shown to play anti- and proapoptotic roles (39–42). In Leishmania, a change in expression of LdMAPK1 did not correlate with the change in apoptotic death of parasites in the absence of antimony treatment. No significant changes were observed in cell cycle and apoptotic signals (DNA fragmentation, externalization of phosphatidylserine, loss of mitochondrial transmembrane potential, and ROS generation) in overexpressing transfectants or single-replacement mutants compared to wild-type cells (Fig. 3). Further, the residual MAPK1 expressed in single-allele replacement mutants may be sufficient to regulate the process; hence, the cell cycle was not disturbed. Therefore, it is hard to suggest the role of leishmanial MAPK1 in regulation of the cell cycle and apoptosis of the parasite.
Antimony has been reported to induce apoptosis in Leishmania parasites (18, 32, 43, 44). Other antileishmanial drugs are also known to cause apoptosis in a similar fashion (32, 45, 46). On exposure to trivalent antimony, significant changes in apoptotic parameters were observed in LdMAPK1-overexpressing or -underexpressing (Dd8++/++ and Dd8+/−) cell types. All apoptotic parameters were 2- to 3-fold elevated in overexpressing transfectants (Dd8++/++) compared to wild-type (Dd8+/+) cells (Fig. 3) and 4- to 5-fold reduced in single-deletion (Dd8+/−) mutants. Interestingly, on add back of the LdMAPK1 gene by episomal complementation in Dd8+/−/+ cells, the decreased apoptosis in Dd8+/− cells reverted to levels exhibited by wild-type Dd8+/+ cells. Interestingly, treatment with either 0.2 μM amphotericin B or 10 μM miltefosine did not cause any significant change in apoptotic parameters among all three cell types (data not shown). The data clearly suggest that changes in expression of LdMAPK1 are directly correlated with the antimony-induced apoptosis of the Leishmania promastigotes without affecting amphotericin B- or miltefosine-mediated apoptosis (Fig. 3). Hence, an increase/decrease in expression of LdMAPK1 renders the cells more sensitive/resistant only to antimony without changing their sensitivity to other standard antileishmanial drugs. Traditionally, in cancerous cells, upregulation of MAPKs (JNK and p38) is associated with antimony- and arsenite-stimulated apoptosis (15, 19–21). In metazoans, most of the apoptosis involves activation of a class of cysteine proteases known as caspases. No caspase activation was observed on exposure to antimony (see Fig. S1a in the supplemental material) in any cell type, i.e., wild-type (Dd8+/+), Dd8++/++, and Dd8+/− cells, which is in agreement with a previous report (32). Even in LdMAPK1-overexpressing cells, increase in apoptosis on antimony treatment is not accompanied by caspase activation.
To understand whether LdMAPK1 has any role in efflux pump-mediated antimony resistance, the functionality of P-gp-type efflux pumps was compared in all the cell types of the parasite using Rhodamine123, a substrate for P-gp-type efflux pumps. Interestingly, we observed that the change in expression of LdMAPK1 is inversely related to the functionality of efflux pumps in parasites (Fig. 4). In the absence of any efflux pump inhibitor (verapamil or TFP), increased accumulation of Rho123 in overexpressing mutants (Dd8++/++) and decreased accumulation in single-deletion mutants compared to wild-type cells (Dd8+/+) was observed (Fig. 4a). Add-on mutants exhibited accumulation of Rho123 comparable to that in wild-type cells. Verapamil is a known inhibitor of P-gp-type efflux pumps; it binds to the P-gp channels and blocks the binding of cytotoxic drugs and their efflux (47, 48) outside the cell. When we used this P-gp pump inhibitor at the time of influx, the difference in the accumulated dye concentrations in all four cell types was nullified (Fig. 4a), suggesting that the observed change in dye accumulation was due to changed efflux pump activity rather than influx pump activity.
Two types of efflux pumps have been identified in Leishmania: MRPA (also called PGPA; ABCC subfamily) (49) and P-gp-type efflux pumps (ABCB subfamily) (50). MRPA pumps are reported to be present on the membranes of vesicles/vacuoles present inside the cells near the flagellar pocket. These pumps sequester the antimonials in conjugation with thiols inside the vacuole and then outside the cell through exocytosis (51). Thus, MRPA is an intracellular pump involved in sequestration of the metal-thiol complex and protects the parasite from metal toxicity (51), while P-gp-type efflux pumps are present on the plasma membranes of the cells and are responsible for MDR (52). A 170-kDa transmembrane protein has been reported in Leishmania and was found to be overexpressed in antimony-resistant (53) and arsenite-resistant laboratory mutants, and their activity, like mammalian pumps, is regulated by a calcium channel blocker, verapamil (54). These P-gp-type pumps have been shown to be responsible for antimony resistance (55). Recently, altered functionality of the P-gp-type plasma membrane efflux transporter(s) has also been shown to be responsible for antimony resistance in L. donovani field isolates (8). TFP is a partial inhibitor of both P-gp- and MRPA-like pumps. Since TFP was unable to mirror the effect of verapamil, this further suggests that MAPK1 modulates the functionality of only P-gp-type, and not MRPA-type, pumps.
These findings were further confirmed by dye retention assays. All four cell types were able to efflux Rho123. However, the efflux activity was significantly decreased in overexpressing cells but increased in a single-deletion mutant compared to wild-type cells (Fig. 4b). Concentration of retained dye after 1 h of efflux was greatest in overexpressing transfectants and least in the single-allele replacement mutant. On episomal complementation of the LdMAPK1 gene in Dd8+/−/+ cells, the pump activity became comparable to that of the wild-type parasite. Addition of verapamil at the time of efflux inhibited the pumps completely, and the cells maintained the accumulated dye concentration after 1 h of efflux. The pumps were not blocked completely by TFP, and some efflux was observed in all cell types.
The altered functionality of efflux pumps with changes in expression of LdMAPK1 can be due to either modulation of the pump activity by LdMAPK1 or LdMAPK1 regulation of the expression of P-gp. Interestingly, modulation of LdMAPK1 expression results in altered P-gp expression (Fig. 5). The marked decrease in P-gp expression in LdMAPK1-overexpressing transfectants and increased levels in single-allele replacement mutants clearly suggests that MAPK1 negatively regulates P-gp expression. Reversal of P-gp expression in add-back mutants further confirmed this observation. Further, the differentially expressed protein was found to be localized on the plasma membrane, further confirming it to be P-gp type in nature (Fig. 5c). These observations are in agreement with earlier reports on multidrug-resistant hepatocellular carcinoma cells (22). In these cells, the drug resistance is mainly attributed to overexpression of P-gp, accompanied by downregulation of ERK1 and ERK2. Further, P-glycoprotein-overexpressing MDR gastric and pancreatic carcinoma cell lines, EPG85-257RDB and EPP85-181RDB, respectively, had lower JNK levels and activities than their parental counterparts. It has also been reported that downregulation of the MDR-1 gene at the mRNA level is itself controlled by the catalytic activity of JNK through its substrate, c-Jun, and thus downregulating P-glycoprotein expression and increasing susceptibility to drugs (25). Mammalian MAP kinases work by phosphorylating the transcription factors at their active sites. Since Leishmania lacks the transcription factors, the pathway by which LdMAPK1 regulates the expression of other genes, including the P-gp gene, remains to be elucidated.
Altogether, the present study revealed for the first time a novel role of MAPK1 of L. donovani in negative regulation of the expression of P-glycoprotein-type efflux pumps. The decreased activity of the P-gp pump with increased expression of MAPK1 may be responsible for increased accumulation of antimonials inside the parasite. This results in increased antimony-induced apoptosis in the parasite and, hence, in increased sensitivity of the parasite to the drug.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by the Department of Science and Technology, India (SB/SO/BB-010/2014), and CSIR-Network Project HOPE (BSC0114). The Council of Scientific and Industrial Research is gratefully acknowledged for financial support to Mansi Garg.
We thank A. L. Vishwakarma for technical assistance in flow cytometry and Rima Ray Saraar for confocal microscopy.
Footnotes
This article is communication number 8960 of the Central Drug Research Institute, Lucknow, India.
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.04816-14.
REFERENCES
- 1.Alvar J, Yactayo S, Bern C. 2006. Leishmaniasis and poverty. Trends Parasitol 22:552–557. doi: 10.1016/j.pt.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Kedzierski L, Sakthianandeswaren A, Curtis JM, Andrews PC, Junk PC, Kedzierska K. 2009. Leishmaniasis: current treatment and prospects for new drugs and vaccines. Curr Med Chem 16:599–614. doi: 10.2174/092986709787458489. [DOI] [PubMed] [Google Scholar]
- 3.Sundar S. 2001. Drug resistance in Indian visceral leishmaniasis. Trop Med Int Health 6:849–854. doi: 10.1046/j.1365-3156.2001.00778.x. [DOI] [PubMed] [Google Scholar]
- 4.Abdo MG, Elamin WM, Khalil EA, Mukhtar MM. 2003. Antimony-resistant Leishmania donovani in eastern Sudan: incidence and in vitro correlation. East Mediterr Health J 9:837–843. [PubMed] [Google Scholar]
- 5.Hadighi R, Mohebali M, Boucher P, Hajjaran H, Khamesipour A, Ouellette M. 2006. Unresponsiveness to glucantime treatment in Iranian cutaneous leishmaniasis due to drug-resistant Leishmania tropica parasites. PLoS Med 3:e162. doi: 10.1371/journal.pmed.0030162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashutosh, Sundar S, Goyal N. 2007. Molecular mechanisms of antimony resistance in Leishmania. J Med Microbiol 56:143–153. doi: 10.1099/jmm.0.46841-0. [DOI] [PubMed] [Google Scholar]
- 7.Maltezou HC. 2010. Drug resistance in visceral leishmaniasis. J Biomed Biotechnol 2010:617521. doi: 10.1155/2010/617521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rai S, Bhaskar, Goel SK, Nath K, Dwivedi UN, Sundar S, Goyal N. 2013. Role of efflux pumps and intracellular thiols in natural antimony resistant isolates of Leishmania donovani. PLoS One 8:e74862. doi: 10.1371/journal.pone.0074862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haldar AK, Sen P, Roy S. 2011. Use of antimony in the treatment of leishmaniasis: current status and future directions. Mol Biol Int 2011:571242. doi: 10.4061/2011/571242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leprohon P, Legare D, Raymond F, Madore E, Hardiman G, Corbeil J, Ouellette M. 2009. Gene expression modulation is associated with gene amplification, supernumerary chromosomes and chromosome loss in antimony-resistant Leishmania infantum. Nucleic Acids Res 37:1387–1399. doi: 10.1093/nar/gkn1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singh N, Almeida R, Kothari H, Kumar P, Mandal G, Chatterjee M, Venkatachalam S, Govind MK, Mandal SK, Sundar S. 2007. Differential gene expression analysis in antimony unresponsive Indian kala azar (visceral leishmaniasis) clinical isolates by DNA microarray. Parasitology 134:777–787. doi: 10.1017/S0031182007002284. [DOI] [PubMed] [Google Scholar]
- 12.Vergnes B, Gourbal B, Girard I, Sundar S, Drummelsmith J, Ouellette M. 2007. A proteomics screen implicates HSP83 and a small kinetoplastid calpain-related protein in drug resistance in Leishmania donovani clinical field isolates by modulating drug-induced programmed cell death. Mol Cell Proteomics 6:88–101. doi: 10.1074/mcp.M600319-MCP200. [DOI] [PubMed] [Google Scholar]
- 13.Ashutosh, Garg M, Sundar S, Duncan R, Nakhasi HL, Goyal N. 2012. Downregulation of mitogen-activated protein kinase 1 of Leishmania donovani field isolates is associated with antimony resistance. Antimicrob Agents Chemother 56:518–525. doi: 10.1128/AAC.00736-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Z, Gibson TB, Robinson F, Silvestro L, Pearson G, Xu B, Wright A, Vanderbilt C, Cobb MH. 2001. MAP kinases. Chem Rev 101:2449–2476. doi: 10.1021/cr000241p. [DOI] [PubMed] [Google Scholar]
- 15.Davison K, Mann KK, Waxman S, Miller WH Jr. 2004. JNK activation is a mediator of arsenic trioxide-induced apoptosis in acute promyelocytic leukemia cells. Blood 103:3496–3502. doi: 10.1182/blood-2003-05-1412. [DOI] [PubMed] [Google Scholar]
- 16.Huang H, Shu SC, Shih JH, Kuo CJ, Chiu ID. 1998. Antimony trichloride induces DNA damage and apoptosis in mammalian cells. Toxicology 129:113–123. doi: 10.1016/S0300-483X(98)00073-0. [DOI] [PubMed] [Google Scholar]
- 17.Lecureur V, Le Thiec A, Le Meur A, Amiot L, Drenou B, Bernard M, Lamy T, Fauchet R, Fardel O. 2002. Potassium antimonyl tartrate induces caspase and reactive oxygen species-dependent apoptosis in lymphoid tumoral cells. Br J Haematol 119:608–615. doi: 10.1046/j.1365-2141.2002.03863.x. [DOI] [PubMed] [Google Scholar]
- 18.Sudhandiran G, Shaha C. 2003. Antimonial-induced increase in intracellular Ca2+ through non-selective cation channels in the host and the parasite is responsible for apoptosis of intracellular Leishmania donovani amastigotes. J Biol Chem 278:25120–25132. doi: 10.1074/jbc.M301975200. [DOI] [PubMed] [Google Scholar]
- 19.Huang C, Ma WY, Li J, Dong Z. 1999. Arsenic induces apoptosis through a c-Jun NH2-terminal kinase-dependent, p53-independent pathway. Cancer Res 59:3053–3058. [PubMed] [Google Scholar]
- 20.Kajiguchi T, Yamamoto K, Hossain K, Akhand AA, Nakashima I, Naoe T, Saito H, Emi N. 2003. Sustained activation of c-jun-terminal kinase (JNK) is closely related to arsenic trioxide-induced apoptosis in an acute myeloid leukemia (M2)-derived cell line, NKM-1. Leukemia 17:2189–2195. doi: 10.1038/sj.leu.2403120. [DOI] [PubMed] [Google Scholar]
- 21.Mann KK, Davison K, Colombo M, Colosimo AL, Diaz Z, Padovani AM, Guo Q, Scrivens PJ, Gao W, Mader S, Miller WH Jr. 2006. Antimony trioxide-induced apoptosis is dependent on SEK1/JNK signaling. Toxicol Lett 160:158–170. doi: 10.1016/j.toxlet.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 22.Yan F, Wang XM, Pan C, Ma QM. 2009. Down-regulation of extracellular signal-regulated kinase 1/2 activity in P-glycoprotein mediated multidrug resistant hepatocellular carcinoma cells. World J Gastroenterol 15:1443–1451. doi: 10.3748/wjg.15.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Y, Li S, Han Y, Liu J, Zhang J, Li F, Wang Y, Liu X, Yao L. 2008. Calebin-A induces apoptosis and modulates MAPK family activity in drug resistant human gastric cancer cells. Eur J Pharmacol 591:252–258. doi: 10.1016/j.ejphar.2008.06.065. [DOI] [PubMed] [Google Scholar]
- 24.Katayama K, Yoshioka S, Tsukahara S, Mitsuhashi J, Sugimoto Y. 2007. Inhibition of mitogen-activated protein kinase pathway results in the down-regulation of P-glycoprotein. Mol Cancer Ther 6:2092–2102. doi: 10.1158/1535-7163.MCT-07-0148. [DOI] [PubMed] [Google Scholar]
- 25.Zhou J, Liu M, Aneja R, Chandra R, Lage H, Joshi HC. 2006. Reversal of P-glycoprotein-mediated multidrug resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer Res 66:445–452. doi: 10.1158/0008-5472.CAN-05-1779. [DOI] [PubMed] [Google Scholar]
- 26.Mandal G, Sharma M, Kruse M, Sander-Juelch C, Munro LA, Wang Y, Vilg JV, Tamás MJ, Bhattacharjee H, Wiese M, Mukhopadhyay R. 2012. Modulation of Leishmania major aquaglyceroporin activity by a mitogen-activated protein kinase. Mol Microbiol 85:1204–1218. doi: 10.1111/j.1365-2958.2012.08169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ashutosh, Gupta S, Ramesh, Sundar S, Goyal N. 2005. Use of Leishmania donovani field isolates expressing the luciferase reporter gene in in vitro drug screening. Antimicrob Agents Chemother 49:3776–3783. doi: 10.1128/AAC.49.9.3776-3783.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laemmli UK. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Towbin H, Staehelin T, Falgout B. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some application. Proc Natl Acad Sci U S A 76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mittal MK, Rai S, Ashutosh, Ravinder, Gupta S, Sundar S, Goyal N. 2007. Characterization of natural antimony resistance in Leishmania donovani isolates. Am J Trop Med Hyg 76:681–688. [PubMed] [Google Scholar]
- 31.Dutta A, Bandhopadhyay S, Mandal C, Chatterjee M. 2007. Aloe vera leaf exudates induce a caspase-independent cell death in Leishmania donovani promastigotes. J Med Microbiol 56:629–636. doi: 10.1099/jmm.0.47039-0. [DOI] [PubMed] [Google Scholar]
- 32.Lee N, Bertholet S, Debrabant A, Muller J, Duncan R, Nakhasi HL. 2002. Programmed cell death in the unicellular protozoan parasite Leishmania. Cell Death Differ 9:53–64. doi: 10.1038/sj.cdd.4400952. [DOI] [PubMed] [Google Scholar]
- 33.Awale M, Kumar V, Saravanan P, Mohan CG. 2010. Homology modeling and atomic level binding study of Leishmania MAPK with inhibitors. J Mol Model 16:475–488. doi: 10.1007/s00894-009-0565-3. [DOI] [PubMed] [Google Scholar]
- 34.Wang X, Martindale JL, Liu Y, Holbrook NJ. 1998. The cellular response to oxidative stress: influences of mitogen-activated protein kinase signalling pathways on cell survival. Biochem J 333:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SJ, Ju JW, Oh CD, Yoon YM, Song WK, Kim JH, Yoo YJ, Bang OS, Kang SS, Chun JS. 2002. ERK-1/2 and p38 kinase oppositely regulate nitric oxide-induced apoptosis of chondrocytes in association with p53, caspase-3, and differentiation status. J Biol Chem 277:1332–1339. doi: 10.1074/jbc.M107231200. [DOI] [PubMed] [Google Scholar]
- 36.Anderson CN, Tolkovsky AM. 1999. A role for MAPK/ERK in sympathetic neuron survival: protection against a p53-dependent, JNK-independent induction of apoptosis by cytosine arabinoside. J Neurosci 19:664–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang D, Wu D, Hirao A, Lahti JM, Liu L, Mazza B, Kidd VJ, Mak TW, Ingram AJ. 2002. ERK activation mediates cell cycle arrest and apoptosis after DNA damage independently of p53. J Biol Chem 277:12710–12717. doi: 10.1074/jbc.M111598200. [DOI] [PubMed] [Google Scholar]
- 38.Hayakawa J, Ohmichi M, Kurachi H, Ikegami H, Kimura A, Matsuoka T, Jikihara H, Mercola D, Murata Y. 1999. Inhibition of extracellular signal-regulated protein kinase or c-Jun N-terminal protein kinase cascade, differentially activated by cisplatin, sensitizes human ovarian cancer cell line. J Biol Chem 274:31648–31654. doi: 10.1074/jbc.274.44.31648. [DOI] [PubMed] [Google Scholar]
- 39.Leppa S, Bohmann D. 1999. Diverse functions of JNK signalling and c-Jun in stress response and apoptosis. Oncogene 18:6158–6162. doi: 10.1038/sj.onc.1203173. [DOI] [PubMed] [Google Scholar]
- 40.Yu C, Minemoto Y, Zhang J, Liu J, Tang F, Bui TN, Xiang J, Lin A. 2004. JNK suppresses apoptosis via phosphorylation of the proapoptotic Bcl-2 family protein BAD. Mol Cell 13:329–340. doi: 10.1016/S1097-2765(04)00028-0. [DOI] [PubMed] [Google Scholar]
- 41.Birkenkamp KU, Dokter WH, Esselink MT, Jonk LJ, Kruijer W, Vellenga E. 1999. A dual function for p38 MAP kinase in hematopoietic cells: involvement in apoptosis and cell activation. Leukemia 13:1037–1045. [DOI] [PubMed] [Google Scholar]
- 42.Zhuang S, Demirs JT, Kochevar IE. 2000. p38 mitogen-activated protein kinase mediates Bid cleavage, mitochondrial dysfunction, and caspase-3 activation during apoptosis induced by singlet oxygen, but not by hydrogen peroxide. J Biol Chem 275:25939–25948. doi: 10.1074/jbc.M001185200. [DOI] [PubMed] [Google Scholar]
- 43.Sereno D, Holzmuller P, Mangot I, Cuny G, Ouaissi A, Lemesre JL. 2001. Antimonial-mediated DNA fragmentation in Leishmania infantum amastigotes. Antimicrob Agents Chemother 45:2064–2069. doi: 10.1128/AAC.45.7.2064-2069.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sarkar A, Mandal G, Singh N, Sundar S, Chatterjee M. 2009. Flow cytometric determination of intracellular non-protein thiols in Leishmania promastigotes using 5-chloromethyl fluorescein diacetate. Exp Parasitol 122:299–305. doi: 10.1016/j.exppara.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Verma NK, Dey CS. 2004. Possible mechanism of miltefosine mediated death of Leishmania donovani. Antimicrob Agents Chemother 48:3010–3015. doi: 10.1128/AAC.48.8.3010-3015.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moreira W, Leprohon P, Ouellette M. 2011. Tolerance to drug-induced cell death favors the acquisition of multidrug resistance in Leishmania. Cell Death Dis 2:e201. doi: 10.1038/cddis.2011.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cornwell MM, Pastan I, Gottesman MM. 1987. Certain calcium channel blockers bind specifically to multidrug resistant human KB carcinoma membrane vesicles and inhibit drug binding to P-glycoprotein. J Biol Chem 262:2166–2170. [PubMed] [Google Scholar]
- 48.Neal RA, vanBueren J, McCoy NG, Iwobi M. 1989. Reversal of drug resistance in Trypanosoma cruzi and Leishmania donovani by verapamil. Trans R Soc Trop Med Hyg 83:197–198. doi: 10.1016/0035-9203(89)90642-1. [DOI] [PubMed] [Google Scholar]
- 49.Leprohon P, Légaré D, Ouellette M. 2009. Intracellular localization of the ABCC proteins of Leishmania and their role in resistance to antimonials. Antimicrob Agents Chemother 53:2646–2649. doi: 10.1128/AAC.01474-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Henderson DM, Sifri CD, Rodgers M, Wirth DF, Hendrickson N, Ullman B. 1992. Multidrug resistance in Leishmania donovani is conferred by amplification of a gene homologous to the mammalian mdr1 gene. Mol Cell Biol 12:2855–2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Légaré D, Richard D, Mukhopadhyay R, Stierhof YD, Rosen BP, Haimeur A, Papadopoulou B, Ouellette M. 2001. The Leishmania ATP-binding cassette protein PGPA is an intracellular metal-thiol transporter ATPase. J Biol Chem 276:26301–26307. doi: 10.1074/jbc.M102351200. [DOI] [PubMed] [Google Scholar]
- 52.Hamada H, Tsuruo T. 1988. Characterization of the ATPase activity of the Mr 170,000 to 180,000 membrane glycoprotein (P-glycoprotein) associated with multidrug resistance in K562/ADM cells. Cancer Res 48:4926–4932. [PubMed] [Google Scholar]
- 53.Grogl M, Martin RK, Oduola AM, Milhous WK, Kyle DE. 1991. Characteristics of multidrug resistance in Plasmodium and Leishmania: detection of P-glycoprotein-like components. Am J Trop Med Hyg 45:98–111. [DOI] [PubMed] [Google Scholar]
- 54.Kaur J, Dey CS. 2000. Putative P-glycoprotein expression in arsenite-resistant Leishmania donovani down-regulated by verapamil. Biochem Biophys Res Commun 271:615–619. doi: 10.1006/bbrc.2000.2680. [DOI] [PubMed] [Google Scholar]
- 55.Messaritakis I, Christodoulou V, Mazeris A, Koutala E, Vlahou A, Papadogiorgaki S, Antoniou M. 2013. Drug resistance in natural isolates of Leishmania donovani s.l. promastigotes is dependent of Pgp170 expression. PLoS One 8:e65467. doi: 10.1371/journal.pone.0065467. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.