Abstract
Azole resistance in Aspergillus fumigatus is an emerging public health concern. Recently, a novel fungicide-driven mutation in the cyp51A gene and its promoter, TR46/Y121F/T289A, leading to high-level resistance to voriconazole has been identified in The Netherlands, Belgium, Germany, Denmark, Tanzania, and India in both clinical and environmental samples. Here we report the first description of A. fumigatus carrying this mutation in France, in a cystic fibrosis patient, underlining the need for extensive monitoring of Aspergillus resistance.
TEXT
Azole-resistant Aspergillus fumigatus isolates have been increasingly reported in Europe since the later years of the first decade of the 2000s. This emerging public health concern occurs through two distinct routes of acquisition: in vivo selection of resistance as a consequence of long-term azole treatment and de novo acquisition of a resistant isolate directly from the environment, linked to the widespread use of azole fungicides in agriculture. Besides the TR34/L98H mutation in the cyp51A gene first described in The Netherlands, a novel fungicide-driven mutation, TR46/Y121F/T289A, has been recently identified. Until now, the TR46/Y121F/T289A mutation has been reported in both environmental and clinical samples in four countries across Europe (1–7), in Asia (8), and, more recently, in Africa (9), suggesting a large geographical spread. Here we provide the first description of A. fumigatus carrying a TR46/Y121F/T289A mutation in a cystic fibrosis patient in France.
A 23-year-old male cystic fibrosis patient with follow-up at the Pneumology Department at Rouen University Hospital, France, was seen in consultation in March 2014. This patient had high levels of total IgE and Aspergillus-specific IgE with positive Aspergillus-specific IgG antibodies, suggesting a diagnosis of allergic bronchopulmonary aspergillosis. He had a history of A. fumigatus airway colonization and exposure to mold-active azoles (itraconazole and voriconazole) since 2002. At the time of the consultation, he was treated with voriconazole. Mycological cultures of the sputum collected during the consultation grew A. fumigatus (strain 1). Species identification was obtained by both macroscopic and microscopic characteristics on Sabouraud's agar medium together with sequencing of the beta-tubulin gene (10). In accordance with a local research protocol aiming at the surveillance of azole resistance, this isolate was tested for antifungal susceptibility by the Etest method (bioMérieux, Marcy l'Etoile, France). Unexpectedly, this strain exhibited high-level resistance to voriconazole (MIC = >32 μg/ml) in comparison with itraconazole (MIC = 8 μg/ml) and posaconazole (MIC = 1 μg/ml). Antifungal susceptibility was confirmed by the EUCAST broth microdilution reference method (Table 1) (11, 12). Nucleotide sequencing of the cyp51A gene and its promoter, using previously described primers (13, 14) and in-house-designed primers (CYP51AF-F1 [5′-ATTTCCCTCATCACTGCAA], CYP51AF-R1 [5′-CATCATGTGCGCAATCTCTT], CYP51AF-F2 [5′-AGAAGCGAGATGCTGCTCAT], and CYP51AF-R2 [5′-CCTTTGAAGTCCTCGATGGT]), showed the TR46/Y121F/T289A mutation. Antifungal therapy was therefore switched to posaconazole in April 2014 and then to caspofungin (50 mg per day) in July 2014 because of pulmonary exacerbation.
TABLE 1.
Overview of the characteristics of all Aspergillus fumigatus strains isolated from sputum samples of the patient
| Strain no. | Reference in the dendrogram | Mo/yr of isolation | MIC (mg/liter) (EUCAST)a |
cyp51A mutations | |
|---|---|---|---|---|---|
| ITC | VRC | ||||
| 1 | 14-105-2468 | March 2014 | 8 | >8 | TR46/Y121F/T289A |
| 2 | 14-148-2457 | November 2013 | 0.5 | 0.5 | Wild type |
| 3 | 14-148-2460 | February 2013 | >8 | >8 | TR46/Y121F/T289A |
| 4 | 141428-459 | January 2013 | 0.5 | 1 | Wild type |
| 5 | 14-148-2458 | January 2013 | 0.25 | 0.25 | Wild type |
| 6 | 14-148-2456 | December 2010 | Not determined | Not determined | F46Y, G89G, M172V, N248T, D255E, L358L, E427K, C454C |
| 7 | 14-148-2455 | September 2010 | Not determined | Not determined | F46Y, G89G, M172V, N248T, D255E, L358L, E427K, C454C |
| 8 | 14-148-2454 | July 2010 | Not determined | Not determined | F46Y, G89G, M172V, N248T, D255E, L358L, E427K, C454C |
| 9 | None | July 2009 | Not determined | Not determined | F46Y, G89G, M172V, N248T, D255E, L358L, E427K, C454C |
| 10 | 14-148-2450 | July 2009 | 0.25 | 1 | Wild type |
| 11 | 14-148-2448 | March 2009 | 0.25 | 1 | Wild type |
| 12 | 14-148-2447 | December 2007 | 0.25 | 1 | Wild type |
| 13 | 14-148-2445 | May 2007 | 0.5 | 2 | Wild type |
| 14 | None | February 2007 | 0.5 | 1 | F46Y, G89G, M172V, N248T, D255E, L358L, E427K, C454C |
ITC, itraconazole; VRC, voriconazole.
Given these findings, we retrospectively analyzed all A. fumigatus strains that had been isolated from this patient since 2007 (n = 13) for itraconazole and voriconazole susceptibility, cyp51A sequencing, and microsatellite genotyping. As shown in Table 1, our patient had already been colonized by a TR46/Y121F/T289A isolate 1 year before, in February 2013 (strain 3). All remaining isolates collected before February 2013 were azole susceptible, either being wild type for the cyp51A gene or carrying mutations previously found in both azole-resistant and azole-susceptible isolates (15). As only a single colony was subjected to in vitro susceptibility testing, other azole-resistant isolates could have been missed. Microsatellite genotyping was performed using a panel of nine short tandem repeats as described previously (16). As illustrated in Table 1, both TR46/Y121F/T289A isolates from our patient had the same genotype as a strain previously isolated in Germany (7) (Table 1). To gain further insights into the route of acquisition of this azole-resistant isolate in our patient, we conducted an environmental study by performing soil samplings next to the patient's home as described previously (17), as well as surface samplings (contact agar plates) in his office. Neither A. fumigatus carrying TR46/Y121F/T289A nor A. fumigatus carrying TR34/L98H was identified.
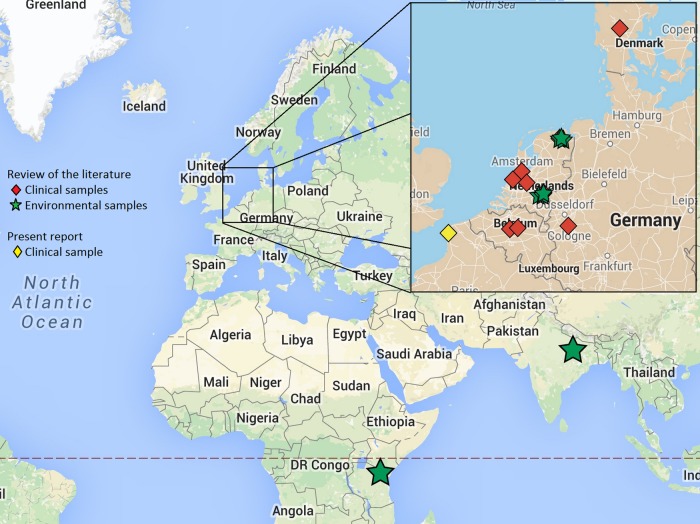
Aspergillus fumigatus isolates carrying the TR46/Y121F/T289A mutation were first described in December 2009 in The Netherlands (2). Since then, such isolates have been evidenced in three other European countries, namely, Belgium (1, 5), Germany (3, 7), and Denmark (4), and recently in India (8) and Tanzania (9) (Table 2 and Fig. 1). Taken together, these findings suggest, as discussed previously for TR34/L98H isolates, a large geographical spread of this resistance mechanism. Several lines of evidence indicate that, as in the case of TR34/L98H, TR46/Y121F/T289A has emerged through a fungicide-driven route (18), such isolates being found in both azole-naive patients (1, 2, 6, 7) and azole-exposed patients (2, 3, 5, 7) as well as in samples from the environment (2, 8, 9). Here we report the first description of A. fumigatus carrying the TR46/Y121F/T289A mutation isolated from a French patient.
TABLE 2.
Literature review of all studies reporting TR46/Y121F/T289A A. fumigatus isolatesa
| Reference | Date of isolation | Type of sample | Underlying condition(s) | Infection | Antifungal susceptibility MIC (mg/liter)b |
Outcome | Country | ||
|---|---|---|---|---|---|---|---|---|---|
| VRC | ITC | POS | |||||||
| 1 | July 2012 | BAL fluid | HSCT | Probable IA | >16 | 4 | 1 | Death | Belgium |
| 2 | December 2009 | Sputum | HSCT | Probable IA | >16 | 4 | 0.25 | Persistent infection | The Netherlands |
| January 2010 | Ear | Chronic otitis externals/sinusitis | IA | >16 | >16 | 2 | Persistent infection | The Netherlands | |
| January 2010 | Abdominal abscess | SOT | Proven IA | >16 | 2 | 0.5 | Death | The Netherlands | |
| February 2010 | Sputum | Cystic fibrosis | No IA | >16 | 4 | 0.5 | Survival | The Netherlands | |
| February 2010 | Sputum | Lung carcinoma | No IA | >16 | >16 | 2 | Survival | The Netherlands | |
| March 2010 | Sputum | HSCT | Probable IA | >16 | 1 | 0.25 | Death | The Netherlands | |
| March 2010 | Sputum | Cystic fibrosis, SOT | Proven IA | >16 | >16 | 0.5 | Survival | The Netherlands | |
| May 2010 | Biopsy specimen | Chronic otitis, surgery | Proven IA | >16 | 4 | 1 | Survival | The Netherlands | |
| May 2010 | Sputum | Lung fibrosis | None | >16 | >16 | 1 | Survival | The Netherlands | |
| June 2010 | Sputum | Traumatism | None | >16 | 1 | 0.25 | Death | The Netherlands | |
| July 2010 | Brain biopsy specimen | Beta thalassemia, diabetes mellitus | Proven IA | >16 | 4 | 1 | Death | The Netherlands | |
| September 2010 | Sputum | Cystic fibrosis | ABPA | >16 | 2 | 0.5 | Survival | The Netherlands | |
| Oct 2010 | Sputum | COPD, SOT | None | >16 | >16 | 2 | Survival | The Netherlands | |
| November 2010 | Sputum | COPD | No IA | >16 | >16 | 2 | Survival | The Netherlands | |
| January 2011 | Sputum | HSCT | Probable IA | >16 | >16 | 1 | Death | The Netherlands | |
| December 2009 to January 2011 | Air sampling | ND | ND | ND | The Netherlands | ||||
| 8 | 2012–2013 | Soil sampling | >16 | 1 to 2 | 0.25 to 0.5 | India | |||
| 3 | September 2012 | Sputum | Cystic fibrosis | Colonization | >8* | >8* | 2* | Survival | Germany |
| 4 | January 2014 | Sputum | Bruton's agammaglobulinemia, SOT | Probable IA | >4* | 0.25 to 0.5* | 0.125 to 0.25* | Death | Denmark |
| 9 | Not reported | Soil sampling | 16 to >16 | 1 to 2 | 0.25 to 0.5 | Tanzania | |||
| 5 | November 2013 | BAL fluid | HSCT | Probable IA | >8 | >16 | 1 | Death | Belgium |
| 7 | September 2012 | BAL fluid | HSCT | Probable IA | 16* | >16* | 0.5* | Death | Germany |
| July 2012 | BAL fluid | HSCT | Proven IA | 1* | >16* | 0.5* | Death | Germany | |
| Present report | February 2013 | Sputum | Cystic fibrosis | Colonization | >8* | >8* | ND | Survival | France |
| March 2014 | Sputum | Cystic fibrosis | Colonization | >8* | 8* | ND | Survival | France | |
POS, posaconazole; BAL, bronchoalveolar lavage; HSCT, hematopoietic stem cell transplantation; IA, invasive aspergillosis; SOT, solid-organ transplantation; ABPA, allergic bronchopulmonary aspergillosis; COPD, chronic obstructive pulmonary disease; ND, not determined.
Data represent results determined using CLSI breakpoints, except those indicated with an asterisk, which represent results determined using EUCAST breakpoints.
FIG 1.
Geographical spread of the TR46/Y121F/T289A resistance mechanism (for each strain, the exact location and origin [clinical or environmental] are indicated). Map data: Google GeoBasis-DE/BKG and Google, INEGI.
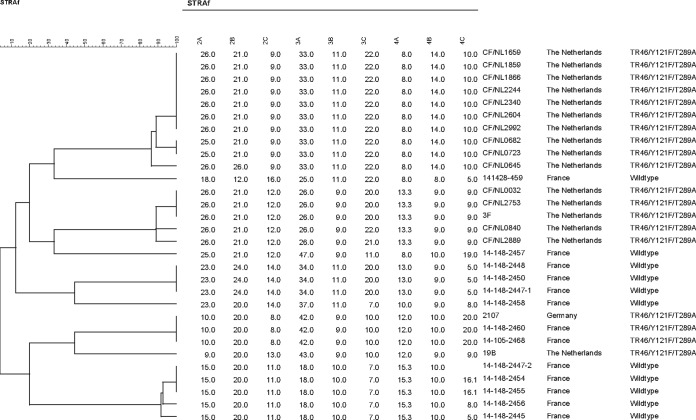
Interestingly, our patient organized trips to The Netherlands as a tour operator. For these working purposes, he traveled to Amsterdam in November 2012, 3 months before the first isolation of the TR46/Y121F/T289A strain from his sputum (February 2013). Moreover, he regularly received advertising postal packages from Dutch flower producers which were opened in his office. Three hypotheses can explain the route of acquisition of this TR46/Y121F/T289A strain in our patient. (i) The first hypothesis is that he inhaled spores carrying TR46/Y121F/T289A during his trip to The Netherlands (2). (ii) The second hypothesis is that colonization occurred after he inhaled spores carrying TR46/Y121F/T289A from his environment in France. Our environmental study conducted next to the patient's home, less than 100 km from Belgium (where TR46/Y121F/T289A strains have been recently identified [1]), failed to detect TR46/Y121F/T289A environmental isolates. Nevertheless, environmental isolates carrying this mutation have been recently identified by our team in the same region in France, supporting this hypothesis (unpublished data). (iii) The last hypothesis is that colonization occurred after he inhaled A. fumigatus spores carrying TR46/Y121F/T289A that had escaped while he was opening the packages received from The Netherlands. Though the French strains are genetically indistinguishable from the German isolates and genetically different from the Dutch isolates (Fig. 2), the route of acquisition in our patient is unclear, as the spores probably followed an airborne migration pattern as hypothesized previously for TR34/L98H (19, 20).
FIG 2.
STRAf dendrogram highlighting the genetic relatedness between the Aspergillus fumigatus isolate collected from our patient and previously reported TR46/Y121F/T289A isolates.
The present report provides evidence that A. fumigatus voriconazole-resistant isolates carrying the TR46/Y121F/T289A mutation can be now isolated from clinical samples in France. As observed with TR34/L98H, a geographical spread of this resistance mechanism is ongoing across Europe and possibly worldwide. These findings, together with the high-level voriconazole resistance of the TR46/Y121F/T289A strains both in vitro and in vivo (1, 2, 4–6), underline the need for intensive investigations to determine the prevalence of the mutation in both clinical and environmental samples. In line with this, as recommended by a European Centre for Disease Prevention and Control (ECDC) technical report (18), antifungal susceptibility testing of triazoles should be performed on all clinical A. fumigatus isolates before starting antifungal therapy.
ACKNOWLEDGMENTS
We are grateful to all the technicians of the Parasitology and Mycology Laboratories at Nantes University Hospital and Rouen University Hospital for technical assistance.
P.L.P. received grants from Astellas and Pfizer and speaker's fees from Merck and Gilead. F.M. received speaker's fees from Gilead and MSD and travel grants from Gilead, MSD, Pfizer, and Astellas. J.F.M. has received grants from Astellas, Basilea, and Merck, has been a consultant to Astellas, Basilea, and Merck, and has received speaker's fees from Merck and Gilead.
REFERENCES
- 1.Vermeulen E, Maertens J, Schoemans H, Lagrou K. 2012. Azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation emerging in Belgium, July 2012. Euro Surveill 17(48):pii=20326 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20326. [PubMed] [Google Scholar]
- 2.van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Rijnders BJ, Kuijper EJ, van Tiel FH, Varga J, Karawajczyk A, Zoll J, Melchers WJ, Verweij PE. 2013. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin Infect Dis 57:513–520. doi: 10.1093/cid/cit320. [DOI] [PubMed] [Google Scholar]
- 3.Fischer J, van Koningsbruggen-Rietschel S, Rietschel E, Vehreschild MJ, Wisplinghoff H, Kronke M, Hamprecht A. 2014. Prevalence and molecular characterization of azole resistance in Aspergillus spp. isolates from German cystic fibrosis patients. J Antimicrob Chemother 69:1533–1536. doi: 10.1093/jac/dku009. [DOI] [PubMed] [Google Scholar]
- 4.Astvad KM, Jensen RH, Hassan TM, Mathiasen EG, Thomsen GM, Pedersen UG, Christensen M, Hilberg O, Arendrup MC. 2014. First detection of TR46/Y121F/T289A and TR34/L98H alterations in Aspergillus fumigatus isolates from azole-naive patients in Denmark despite negative findings in the environment. Antimicrob Agents Chemother 58:5096–5101. doi: 10.1128/AAC.02855-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Montesinos I, Dodemont M, Lagrou K, Jacobs F, Etienne I, Denis O. 2014. New case of azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation in Belgium. J Antimicrob Chemother 69:3439–3440. doi: 10.1093/jac/dku289. [DOI] [PubMed] [Google Scholar]
- 6.Kuipers S, Bruggemann RJ, de Sevaux RG, Heesakkers JP, Melchers WJ, Mouton JW, Verweij PE. 2011. Failure of posaconazole therapy in a renal transplant patient with invasive aspergillosis due to Aspergillus fumigatus with attenuated susceptibility to posaconazole. Antimicrob Agents Chemother 55:3564–3566. doi: 10.1128/AAC.01544-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinmann J, Hamprecht A, Vehreschild MJ, Cornely OA, Buchheidt D, Spiess B, Koldehoff M, Buer J, Meis JF, Rath PM. 27 January 2015, posting date Emergence of azole-resistant invasive aspergillosis in HSCT recipients in Germany. J Antimicrob Chemother doi: 10.1093/jac/dku566. [DOI] [PubMed] [Google Scholar]
- 8.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. 2014. Azole-resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J Antimicrob Chemother 69:555–557. doi: 10.1093/jac/dkt397. [DOI] [PubMed] [Google Scholar]
- 9.Chowdhary A, Sharma C, van den Boom M, Yntema JB, Hagen F, Verweij PE, Meis JF. 2014. Multi-azole-resistant Aspergillus fumigatus in the environment in Tanzania. J Antimicrob Chemother 69:2979–2983. doi: 10.1093/jac/dku259. [DOI] [PubMed] [Google Scholar]
- 10.Balajee SA, Gribskov JL, Hanley E, Nickle D, Marr KA. 2005. Aspergillus lentulus sp. nov., a new sibling species of A. fumigatus. Eukaryot Cell 4:625–632. doi: 10.1128/EC.4.3.625-632.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodriguez-Tudela JL, Alcazar-Fuoli L, Mellado E, Alastruey-Izquierdo A, Monzon A, Cuenca-Estrella M. 2008. Epidemiological cutoffs and cross-resistance to azole drugs in Aspergillus fumigatus. Antimicrob Agents Chemother 52:2468–2472. doi: 10.1128/AAC.00156-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.European Committee on Antimicrobial Susceptibility Testing. 2014. Antifungal agents—breakpoint tables for interpretation of MICs, version 7.1. http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/AFST/Antifungal_breakpoints_v_7.0.pdf.
- 13.Morio F, Aubin GG, Danner-Boucher I, Haloun A, Sacchetto E, Garcia-Hermoso D, Bretagne S, Miegeville M, Le Pape P. 2012. High prevalence of triazole resistance in Aspergillus fumigatus, especially mediated by TR/L98H, in a French cohort of patients with cystic fibrosis. J Antimicrob Chemother 67:1870–1873. doi: 10.1093/jac/dks160. [DOI] [PubMed] [Google Scholar]
- 14.Alanio A, Sitterle E, Liance M, Farrugia C, Foulet F, Botterel F, Hicheri Y, Cordonnier C, Costa JM, Bretagne S. 2011. Low prevalence of resistance to azoles in Aspergillus fumigatus in a French cohort of patients treated for haematological malignancies. J Antimicrob Chemother 66:371–374. doi: 10.1093/jac/dkq450. [DOI] [PubMed] [Google Scholar]
- 15.Snelders E, Karawajczyk A, Schaftenaar G, Verweij PE, Melchers WJ. 2010. Azole resistance profile of amino acid changes in Aspergillus fumigatus CYP51A based on protein homology modeling. Antimicrob Agents Chemother 54:2425–2430. doi: 10.1128/AAC.01599-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Valk HA, Meis JF, Curfs IM, Muehlethaler K, Mouton JW, Klaassen CH. 2005. Use of a novel panel of nine short tandem repeats for exact and high-resolution fingerprinting of Aspergillus fumigatus isolates. J Clin Microbiol 43:4112–4120. doi: 10.1128/JCM.43.8.4112-4120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Snelders E, Huis In 't Veld RA, Rijs AJ, Kema GH, Melchers WJ, Verweij PE. 2009. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl Environ Microbiol 75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.European Centre for Disease Prevention and Control. 2013. Technical report. Risk assessment on the impact of environmental usage of triazoles on the development and spread of resistance to medical triazoles in Aspergillus species. European Centre for Disease Prevention and Control, Solna, Sweden. doi: 10.2900/76274. [DOI] [Google Scholar]
- 19.Vermeulen E, Lagrou K, Verweij PE. 2013. Azole resistance in Aspergillus fumigatus: a growing public health concern. Curr Opin Infect Dis 26:493–500. doi: 10.1097/QCO.0000000000000005. [DOI] [PubMed] [Google Scholar]
- 20.Chowdhary A, Kathuria S, Xu J, Meis JF. 2013. Emergence of azole-resistant Aspergillus fumigatus strains due to agricultural azole use creates an increasing threat to human health. PLoS Pathog 9:e1003633. doi: 10.1371/journal.ppat.1003633. [DOI] [PMC free article] [PubMed] [Google Scholar]