Abstract
The therapeutic efficacies of smallpox vaccine ACAM2000 and antiviral tecovirimat given alone or in combination starting on day 3 postinfection were compared in a cynomolgus macaque model of lethal monkeypox virus infection. Postexposure administration of ACAM2000 alone did not provide any protection against severe monkeypox disease or mortality. In contrast, postexposure treatment with tecovirimat alone or in combination with ACAM2000 provided full protection. Additionally, tecovirimat treatment delayed until day 4, 5, or 6 postinfection was 83% (days 4 and 5) or 50% (day 6) effective.
TEXT
Historically, the window for fully effective postexposure therapeutic vaccination in humans appears to close within the few days following smallpox exposure. Limited epidemiological data from the smallpox eradication era suggest that vaccination 1 to 3 days following exposure to smallpox might be effective at reducing the rate of occurrence and/or severity of smallpox (1, 2). Afterwards, vaccine efficacy appears to progressively decline throughout the incubation phase of the disease (∼12 days), and vaccination appears to be wholly ineffective following onset of clinical symptoms. The orthopoxvirus-specific antiviral tecovirimat (ST-246) is currently in late-stage development as a smallpox therapeutic under the U.S Food and Drug Administration's “Animal Rule” (21 CFR 314.600 [drugs]) since efficacy studies on human subjects are not ethical or feasible (3). Tecovirimat targets the product of the vaccinia virus F13L gene (which is highly conserved among other orthopoxviruses) and prevents virus egress from infected cells by reducing the intracellular production and release of enveloped virus (4, 5). In numerous animal models (6–10), tecovirimat has been shown to be highly effective at reducing morbidity and preventing mortality even when treatment is initiated after onset of clinical signs of orthopoxvirus-induced disease. To date, treatment with tecovirimat has not been directly compared to smallpox vaccination to evaluate the relative efficacy of each, nor has there been any investigation into the potential for tecovirimat to negate the efficacy of vaccination (or vice versa) upon combined administration postexposure (as current policy dictates that vaccine-contraindicated individuals would still be vaccinated in the case of a smallpox emergency [11]). Of note, tecovirimat treatment as an adjunct to prophylactic smallpox vaccination (Dryvax or ACAM2000) was found not to compromise vaccine-induced short- or long-term protective immunity in both mice (12, 13) and monkeys (unpublished data).
Antiviral tecovirimat treatment (either alone or as an adjunct to vaccination), but not vaccination alone, is fully protective when administered 3 days after monkeypox virus (MPXV) infection.
A study (Fig. 1) was designed to model a potential postexposure treatment scenario in which individuals have been exposed to variola virus (the causative agent of smallpox), are past the ∼12-day incubation period, and are exhibiting fever as a clinical sign of the prodromal disease phase but have not yet developed a visible rash. To do this, 32 cynomolgus macaques (16 females and 16 males) were randomized into four treatment groups (n = 8 macaques/group) based on weight and sex and intravenously (i.v.) infected with a lethal dose of MPXV Zaire-79 (∼5 × 107 PFU) on study day 0. At 3 days postinfection (p.i.), the animals were either mock vaccinated or vaccinated with a standard human dose of ACAM2000 (2.5 μl/2.5 × 105 to 12.5 × 105 PFU) by percutaneous scarification and orally treated concurrently with either a placebo or tecovirimat (10 mg/kg of body weight) in a fed state. Tecovirimat or placebo treatment was continued once daily for a total of 14 consecutive days. Animals that survived the initial MPXV challenge were then rechallenged with the same lethal dose of MPXV 2 months later (study day 63) to evaluate the acquisition of protective immunity against reinfection. During both the challenge and the rechallenge periods, temperature and weight measurements, body lesion counts, and detailed clinical observations were conducted, including monitoring of depression/weakness, recumbency, dehydration, dyspnea, cough, inappetence, nasal/ocular discharge, and edema. With the exception of recumbency and inappetence, which were scored as a “yes” or “no” answer, severities for the remaining clinical signs of disease were graded using a numerical scoring system with a range from 1 (being mild) to 3 (being severe). At regular intervals, blood was collected for the assessment of viral load (by MPXV hemagglutinin gene-specific quantitative PCR [qPCR] assay) and induction of humoral and cellular immune responses, which were evaluated by MPXV plaque reduction neutralization titer (PRNT50; the serum titer that results in a 50% reduction in plaque counts) and vaccinia virus-stimulated gamma interferon (IFN-γ) enzyme-linked immunosorbent spot (ELISPOT) assays, respectively.
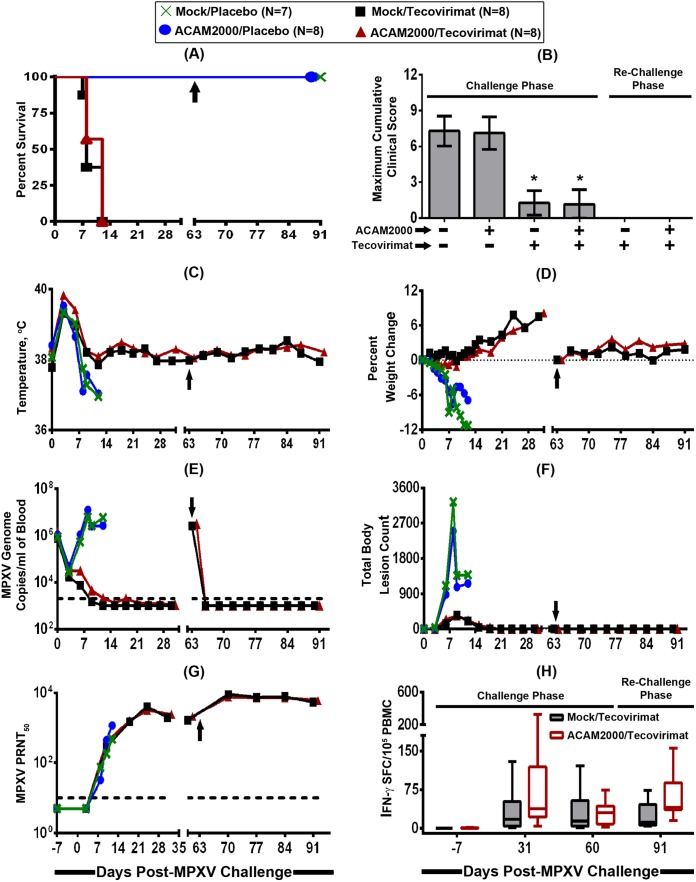
FIG 1.
Efficacy of smallpox antiviral tecovirimat given alone or in combination with smallpox vaccine ACAM2000 after MPXV exposure. Cynomolgus macaques (n = 7 or 8 animals/group) were intravenously infected with a target challenge dose of 5 × 107 PFU of MPXV Zaire-79 on study day 0 and vaccinated with vaccine diluent (mock vaccination) or ACAM2000 (one-time percutaneous scarification; 2.5 μl/2.5 × 105 to 12.5 × 105 PFU) on study day 3 and treated concurrently with a placebo or tecovirimat (10 mg/kg; oral gavage) once daily for 14 consecutive days (from study day 3 to day 16). The animals were then monitored for survival (A), clinical signs of disease (B), temperature (C) and weight (D) changes, viremia (E), body lesion formation (F), and development of humoral (G) and cellular (H) immune responses. Macaques that survived the initial MPXV challenge were rechallenged with a target challenge dose of 5 × 107 PFU of MPXV Zaire-79 2 months later (day 63; represented by an arrow) to assess for acquisition of immunological memory. Each curve in graphs B to G represents the arithmetic (B, C, D, and F) or geometric (E and G) mean value of the 7 or 8 animals in each treatment group. In order to avoid obscuring the mean data, the standard deviations are not shown (C to G). In panel H, the whiskers represent the minimum and the maximum values for the 7 or 8 animals in each treatment group. The dashed lines in panels E and G represent the lower limits of quantification for the assays. An asterisk (*) indicates statistical significance.
As shown in Fig. 1A, all tecovirimat-treated animals, irrespective of concomitant ACAM2000 vaccination (±ACAM2000), survived the initial MPXV challenge while all of the animals that were mock or ACAM2000 vaccinated and treated with a placebo succumbed to MPXV-induced disease by day 7 to 12 p.i. Unlike for tecovirimat treatment, there was no clearly evident ACAM2000 vaccination-induced efficacy, as there were no significant differences in survival rates between mock/placebo- and ACAM2000/placebo-treated groups and between mock/tecovirimat- and ACAM2000/tecovirimat-treated groups (P > 0.05; Sidak-adjusted Fisher exact test). Clinical signs of MPXV-induced disease were profound and severe in the mock/placebo-treated control animals, and the maximum clinical scores were not different from those seen in ACAM2000/placebo-treated animals (Fig. 1B) (P > 0.05; analysis of variance [ANOVA] with Tukey's multiple-comparison test). In contrast, all tecovirimat-treated (±ACAM2000) animals had significantly reduced clinical signs and were protected from severe MPXV disease. All of the macaques, irrespective of vaccination/treatment, experienced an elevation in body surface temperature during the acute-phase period between study days 3 and 6 (Fig. 1C). Following this acute-fever period, all tecovirimat-treated (±ACAM2000) animals maintained normal temperatures, while temperatures continued to dramatically decline below normal temperatures in the majority of placebo-treated (±ACAM2000) animals to as low as 94.5°F at the time of euthanasia for one animal. Similarly, most of the tecovirimat-treated (±ACAM2000) animals experienced a body weight gain, whereas all the placebo-treated (±ACAM2000) animals experienced a decline in body weight (Fig. 1D).
Compared to placebo-treated (±ACAM2000) animals, tecovirimat-treated (±ACAM2000) animals also controlled the initial infection by significantly reducing the maximum viral load, as assessed by number of MPXV genome copies in the blood (Fig. 1E) (P < 0.0001) and body pox lesion count (Fig. 1F) (P < 0.01). There were no significant differences in level of viremia or lesion count between mock/placebo- and ACAM2000/placebo-treated groups or between mock/tecovirimat- and ACAM2000/tecovirimat-treated groups (P > 0.05). However, there was a significant difference in median time to cessation of viremia (P = 0.0258: log rank test), but not lesions (P = 0.9237), between mock/tecovirimat- and ACAM2000/tecovirimat-treated groups (day 9 versus day 12, respectively, for viremia, while lesions were resolved by day 21 in both treatment groups). Logistical regression analysis showed a significant correlation between survival probability and both maximum viremia (P = 0.0101) and total body lesion count (P = 0.0071). The induction of MPXV-neutralizing antibodies was evident in all animals in all treatment groups (Fig. 1G), with the titers increasing dramatically as the infection progressed. It is notable that the neutralizing antibody titer continued to increase in surviving tecovirimat-treated animals after the death of the placebo-treated animals. However, at the time of death for the placebo-treated animals, there were no significant differences in PRNT50 level among all the treatment groups (P = 0.3967) and no significant correlation between survival probability and maximum PRNT50 level (P = 0.2171). This suggests that, due to the high magnitude of the MPXV challenge and established infection, the humoral immune response did not develop fast enough, even with ACAM2000 vaccination, to prevent animals from succumbing to systemic disease, unless the animals were treated with tecovirimat to keep virus levels in check and allow time for the development of the appropriate immune response. All animals treated with tecovirimat also demonstrated recall cellular responses on day 31, with similar responses observed between the tecovirimat-treated mock- and ACAM2000-vaccinated groups. Importantly, all tecovirimat-treated (±ACAM2000) animals showed complete resistance to a second lethal challenge at 2 months p.i., without showing any apparent outward clinical signs of MPXV disease, including pox lesions (Fig. 1A to H) (days 63 to 91). Following rechallenge, viral load was below the limit of detection (2,000 genome copies/ml) and both humoral and cellular anamnestic immune responses were evident and robust. Overall, the data from this study demonstrate that the postexposure administration of tecovirimat alone or in combination with ACAM2000 provides protection against a lethal MPXV challenge, even when treatment initiation is late enough that vaccination with ACAM2000 alone does not provide any protection from severe MPXV disease and mortality, and that the immunity acquired from surviving the initial exposure to MPXV due to treatment with tecovirimat is durable and effective against reexposure.
Tecovirimat treatment alone delayed up to 6 days after MPXV infection provides significant protection from mortality.
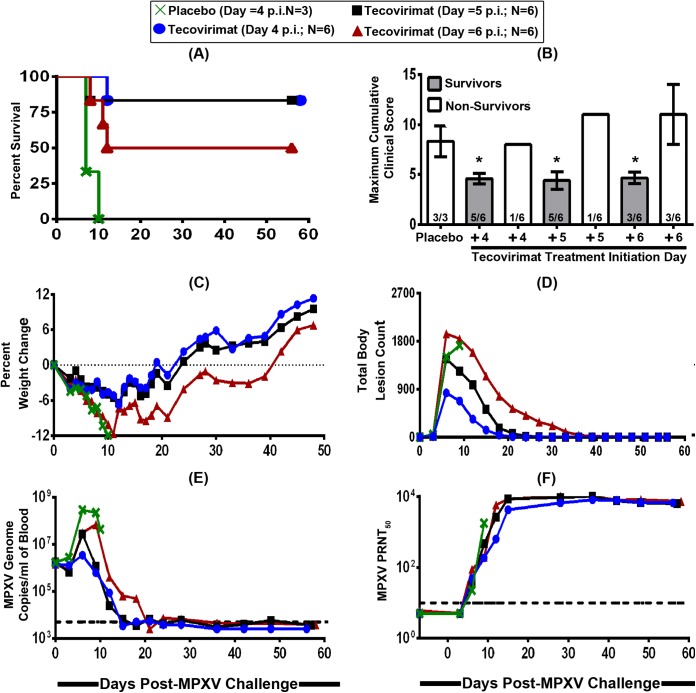
Given the lack of vaccine efficacy, even with administration early in the course of disease, in a separate study (Fig. 2), tecovirimat treatment initiation was delayed until after the animals had become both symptomatic and lesional (i.e., day 4 p.i.), but before they started to succumb to MPXV-induced disease (i.e., day 7 p.i.), in order to determine the time point postchallenge at which the drug failed to protect from morbidity and/or mortality. To do this, 21 male and female cynomolgus monkeys were assigned to four blinded groups and infected with MPXV as described above. The animals were then administered 14 once-daily treatments of a placebo (group 1; n = 3) or 10 mg/kg of tecovirimat beginning on day 4 (group 2; n = 6), day 5 (group 3; n = 6), or day 6 (group 4; n = 6) p.i. To ensure treatment blinding, group 2, 3, and 4 animals were administered a placebo on non-tecovirimat-dosing days. None of the animals (0/3) in the placebo-treated group survived the lethal MPXV infection, while 5 of 6 animals (83%) in the day 4 and 5 tecovirimat treatment groups and 3 of 6 animals (50%) in the day 6 treatment group survived (Fig. 2A). Survival was significantly increased (P < 0.05) in both the day 4 and the day 5 tecovirimat treatment groups. When all tecovirimat-treated animals were considered together (day 4, 5, and 6 treatment groups combined), survival was found to be 72%, which was statistically significantly better than that of the placebo-treated group (P < 0.05). In general, the frequency and severity of clinical signs of disease (Fig. 2B), weight loss (Fig. 2C), and trends in body lesion counts (Fig. 2D) and blood MPXV DNA levels (Fig. 2E) were all consistent with an earlier initiation of tecovirimat treatment resulting in a more robust antiviral efficacy against morbidity. That is, MPXV-induced disease manifestations were all lowest in the day 4 treatment group, followed successively by the day 5 and 6 treatment groups. The majority (7/8) of nonsurvivors generated low or undetectable levels of MPXV-neutralizing antibodies prior to succumbing to viral disease by day 12 p.i., while all of the survivors generated and maintained high levels (Fig. 2F), suggesting that the antiviral effect of tecovirimat does not interfere with the induction of a robust natural humoral immune response to the challenge virus.
FIG 2.
Effect of delayed tecovirimat treatment initiation on antiviral efficacy in cynomolgus macaques that are both symptomatic and lesional. Cynomolgus macaques (n = 3 to 6 animals/group) were intravenously infected with a target challenge dose of 5 × 107 PFU of MPXV Zaire-79 on study day 0 and treated with a placebo or tecovirimat (10 mg/kg; oral gavage) once daily for 14 consecutive days starting on study day 4, 5, or 6. The animals were then monitored for survival (A), clinical signs of disease (B), weight change (C), body lesion formation (D), viremia (E), and development of neutralizing humoral immune responses (F). Each curve in graphs B to F represents the arithmetic (B, C, and D) or geometric (E and F) mean value for the 3 to 6 animals in each treatment group. In order to avoid obscuring the mean data, the standard deviations are not shown (C to F). The dashed lines in panels E and F represent the lower limits of quantification for the assays. An asterisk (*) indicates statistical significance.
Both studies described above were conducted in accordance with the Animal Welfare Act (Public Law 99-198) and the Guide for the Care and Use of Laboratory Animals (Institute of Animal Resources, Commission on Life Sciences, National Research Council). The study protocols were reviewed and approved by the Institutional Animal Care and Use Committee of Southern Research Institute (Frederick, MD) prior to study initiation. In addition, given that MPXV is a select agent, both studies were conducted at Southern Research Institute (Frederick, MD) under conditions of animal biosafety level 3 (ABSL-3) containment.
Tecovirimat treatment should be incorporated into the CDC Smallpox Response Plan and Guidelines.
Overall, the results of the studies described above along with smallpox era observations implying the limited effectiveness of human vaccination beyond the first few days postexposure suggest that tecovirimat treatment (either alone or as an adjunct to vaccination), but not vaccination alone, should be considered the central policy basis of any smallpox response plan designed to protect humans suspected of exposure to variola virus following an accidental release, a terrorist attack, or a bio-warfare incident. Although vaccination administered following a systemic infection is unlikely to be therapeutically beneficial, vaccination is also unlikely to impact the efficacy of tecovirimat upon coadministration, given that all animals that were concomitantly treated with tecovirimat and ACAM2000 survived lethal MPXV infection and had levels of clinical signs of disease, viremia, pock lesions, and protective immunity similar to those in animals treated with tecovirimat alone.
ACKNOWLEDGMENTS
These studies were supported by Biomedical Advanced Research and Development Authority (BARDA) contract HHSO100201100023C (tecovirimat [±ACAM2000] study) and National Institutes of Health (NIH)/National Institute of Allergy and Infectious Diseases (NIAID) contract HHSN272200800041C (delayed-tecovirimat-treatment study). The following reagents were obtained for free through BEI Resources, NIAID, NIH: Monkeypox Virus Zaire 79 (V79-I-005) CDC, Animal Challenge Pool (NR-21738); Monkeypox Virus Hemagglutinin Gene-Specific Quantitative PCR Probe (NR-9347) and forward (NR-9348) and reverse (NR-9349) primers; and Plasmid Containing Hemagglutinin Gene from Monkeypox Virus, Zaire 79, Linearized (NR-4076).
A.B., K.M.H., D.E.H., and D.W.G. are employees of SIGA Technologies, Inc., and hold stock or equity interests in the company. J.T.P. and P.M.S. are current and former employees, respectively, of the organization (Southern Research Institute) that conducted both studies under paid contracts from SIGA Technologies, Inc.
REFERENCES
- 1.Fenner F, Henderson DA, Arita I, Jezek Z, Ladnyi ID. 1988. Smallpox and its eradication. World Health Organization, Geneva, Switzerland: http://whqlibdoc.who.int/smallpox/9241561106.pdf. [Google Scholar]
- 2.Keckler MS, Reynolds MG, Damon IK, Karem KL. 2013. The effects of post-exposure smallpox vaccination on clinical disease presentation: addressing the data gaps between historical epidemiology and modern surrogate model data. Vaccine 31:5192–5201. doi: 10.1016/j.vaccine.2013.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. 2014. Guidance for industry: product development under the Animal Rule. U.S. Food and Drug Administration, Silver Spring, MD: http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM399217.pdf. [Google Scholar]
- 4.Yang G, Pevear DC, Davies MH, Collett MS, Bailey T, Rippen S, Barone L, Burns C, Rhodes G, Tohan S, Huggins JW, Baker RO, Buller RL, Touchette E, Waller K, Schriewer J, Neyts J, DeClercq E, Jones K, Hruby D, Jordan R. 2005. An orally bioavailable antipoxvirus compound (ST-246) inhibits extracellular virus formation and protects mice from lethal orthopoxvirus challenge. J Virol 79:13139–13149. doi: 10.1128/JVI.79.20.13139-13149.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith SK, Olson VA, Karem KL, Jordan R, Hruby DE, Damon IK. 2009. In vitro efficacy of ST246 against smallpox and monkeypox. Antimicrob Agents Chemother 53:1007–1012. doi: 10.1128/AAC.01044-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berhanu A, King DS, Mosier S, Jordan R, Jones KF, Hruby DE, Grosenbach DW. 2009. ST-246 inhibits in vivo poxvirus dissemination, virus shedding, and systemic disease manifestation. Antimicrob Agents Chemother 53:4999–5009. doi: 10.1128/AAC.00678-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nalca A, Hatkin JM, Garza NL, Nichols DK, Norris SW, Hruby DE, Jordan R. 2008. Evaluation of orally delivered ST-246 as postexposure prophylactic and antiviral therapeutic in an aerosolized rabbitpox rabbit model. Antiviral Res 79:121–127. doi: 10.1016/j.antiviral.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Smith SK, Self J, Weiss S, Carroll D, Braden Z, Regnery RL, Davidson W, Jordan R, Hruby DE, Damon IK. 2011. Effective antiviral treatment of systemic orthopoxvirus disease: ST-246 treatment of prairie dogs infected with monkeypox virus. J Virol 85:9176–9187. doi: 10.1128/JVI.02173-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan R, Goff A, Frimm A, Corrado ML, Hensley LE, Byrd CM, Mucker E, Shamblin J, Bolken TC, Wlazlowski C, Johnson W, Chapman J, Twenhafel N, Tyavanagimatt S, Amantana A, Chinsangaram J, Hruby DE, Huggins J. 2009. ST-246 antiviral efficacy in a nonhuman primate monkeypox model: determination of the minimal effective dose and human dose justification. Antimicrob Agents Chemother 53:1817–1822. doi: 10.1128/AAC.01596-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mucker EM, Goff AJ, Shamblin JD, Grosenbach DW, Damon IK, Mehal JM, Holman RC, Carroll D, Gallardo N, Olson VA, Clemmons CJ, Hudson P, Hruby DE. 2013. Efficacy of tecovirimat (ST-246) in nonhuman primates infected with variola virus (smallpox). Antimicrob Agents Chemother 57:6246–6253. doi: 10.1128/AAC.00977-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. 2003. Smallpox response plan and guidelines (version 3.0). http://www.bt.cdc.gov/agent/smallpox/response-plan/.
- 12.Berhanu A, King DS, Mosier S, Jordan R, Jones KF, Hruby DE, Grosenbach DW. 2010. Impact of ST-246(R) on ACAM2000 smallpox vaccine reactogenicity, immunogenicity, and protective efficacy in immunodeficient mice. Vaccine 29:289–303. doi: 10.1016/j.vaccine.2010.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grosenbach DW, Jordan R, King DS, Berhanu A, Warren TK, Kirkwood-Watts DL, Tyavanagimatt S, Tan Y, Wilson RL, Jones KF, Hruby DE. 2008. Immune responses to the smallpox vaccine given in combination with ST-246, a small-molecule inhibitor of poxvirus dissemination. Vaccine 26:933–946. doi: 10.1016/j.vaccine.2007.11.095. [DOI] [PMC free article] [PubMed] [Google Scholar]