Abstract
Artemisinin-derived monomers and dimers inhibit human cytomegalovirus (CMV) replication in human foreskin fibroblasts (HFFs). The monomer artesunate (AS) inhibits CMV at micromolar concentrations, while dimers inhibit CMV replication at nanomolar concentrations, without increased toxicity in HFFs. We report on the variable anti-CMV activity of AS compared to the consistent and reproducible CMV inhibition by dimer 606 and ganciclovir (GCV). Investigation of this phenomenon revealed that the anti-CMV activity of AS correlated with HFFs synchronized to the G0/G1 stage of the cell cycle. In contact-inhibited serum-starved HFFs or cells arrested at early/late G1 with specific checkpoint regulators, AS and dimer 606 efficiently inhibited CMV replication. However, in cycling HFFs, in which CMV replication was productive, virus inhibition by AS was significantly reduced, but inhibition by dimer 606 and GCV was maintained. Cell cycle analysis in noninfected HFFs revealed that AS induced early G1 arrest, while dimer 606 partially blocked cell cycle progression. In infected HFFs, AS and dimer 606 prevented the progression of cell cycle toward the G1/S checkpoint. AS reduced the expression of cyclin-dependent kinases (CDK) 2, 4, and 6 in noninfected cycling HFFs, while the effect of dimer 606 on these CDKs was moderate. Neither compound affected CDK expression in noninfected contact-inhibited HFFs. In CMV-infected cells, AS activity correlated with reduced CDK2 levels. CMV inhibition by AS and dimer 606 also correlated with hypophosphorylation (activity) of the retinoblastoma protein (pRb). AS activity was strongly associated with pRb hypophosphorylation, while its reduced anti-CMV activity was marked by pRb phosphorylation. Roscovitine, a CDK2 inhibitor, antagonized the anti-CMV activities of AS and dimer 606. These data suggest that cell cycle modulation through CDKs and pRb might play a role in the anti-CMV activities of artemisinins. Proteins involved in this modulation may be identified and targeted for CMV inhibition.
INTRODUCTION
Artemisinins, drugs of choice for malaria therapy, inhibit human cytomegalovirus (CMV) replication (1–4). Artesunate (AS) and the parent compound artemisinin inhibit CMV replication in vitro and in vivo. Variable responses to AS in case reports of CMV-infected patients have been attributed to the dosing regimen, duration of therapy, or tissue penetration of AS (5–8). In our effort to improve and uncover the anti-CMV activities of artemisinins, we reported on in vitro highly selective inhibition of CMV replication with artemisinin-derived dimers, significantly more than with their monomeric counterparts, without increasing toxicity in human foreskin fibroblasts (HFFs) (3, 9). Although similar effects on CMV replication were observed between monomers and dimers (timing of CMV inhibition, effects on DNA replication, and virus yield), dimers inhibited CMV at nanomolar concentrations and had a high slope of the dose-response curve, a measure of cooperativity in binding of multiple ligands to linked binding sites. Monomers inhibited CMV at micromolar concentrations and had a slope of 1 (similar to the slope of ganciclovir [GCV]).
We report on inconsistent anti-CMV activity of AS in HFFs, while artemisinin-derived dimer 606 and GCV maintained consistent CMV inhibition. Our data suggest that the underlying mechanism of this phenomenon may be a result of cell cycle modulation by artemisinins. CMV infection induces G1/S arrest in HFFs (10–12), hyperphosphorylation of the retinoblastoma protein (pRb), and increased transcriptional activity of E2F1. In noncycling arrested cells, CMV alters the cell cycle toward a more favorable S-stage-like environment, while in actively dividing cells, viral immediate early (IE) gene expression is delayed until the cells reach the next G1 stage (13, 14). We describe the cell cycle activities of artemisinins and their correlates with CMV inhibition.
MATERIALS AND METHODS
Compounds.
The synthesis of the highly stable C-10-carba trioxane dimer alcohol (molecular weight, 606) from artemisinin has been reported (15). AS cannot form a dimer, but chemical synthesis resulted in several artemisinin-derived dimers, including dimer 606. Studies describing the anti-CMV activity of AS and dimer 606 have shown that the latter was significantly more active than AS, much more than two units of monomers combined (9, 16, 17). The compounds were dissolved in dimethyl sulfoxide (DMSO), and stocks of 10 mM were stored at −80°C. GCV, mimosine (for induction of late G1 arrest), lovastatin (for induction of early G1 arrest), staurosporine (a positive control for apoptosis), and roscovitine (a cyclin-dependent kinase 2 [CDK2] inhibitor) were purchased from Sigma Chemicals (St. Louis, MO). The concentrations of AS and dimer 606 resulting in full CMV inhibition were 30 and 0.3 μM, respectively, and used in all experiments (3). The concentration of each compound was calculated and adjusted by volume such that it was constant throughout the experiment.
Viruses.
The pp28-luciferase Towne CMV strain was constructed as previously described (18). Briefly, the recombinant virus expresses luciferase under the control of the UL99 (pp28) late promoter 48 to 72 h postinfection (hpi). Luciferase expression from this promoter is almost completely inhibited in the presence of viral DNA polymerase inhibitors such as GCV and foscarnet (18). Luciferase activity is highly correlated with plaque reduction assay (18). The Towne CMV strain (ATCC, VR-977) was used for plaque reduction, DNA replication, apoptosis, and cell cycle assays.
Cell culture, virus infection, and antiviral assays.
HFFs (passage 12 to 16; ATCC, CRL-2088) were grown in Dulbecco's modified Eagle medium (DMEM) containing 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA) in a 5% CO2 incubator at 37°C and used for infection with pp28-luciferase Towne CMV strain. Human colorectal carcinoma cell line HCT116 (ATCC, CCL-247) cells were maintained in DMEM containing 10% FBS.
To determine the effect of cell density/cycling on the anti-CMV activity of artemisinins, HFFs were plated at 0.5 × 106, 1 × 106, 2 × 106, or 3 × 106 cells per 96-well plate 24 h prior to infection. Infection was carried out at a multiplicity of infection of 1 PFU/cell (MOI = 1), unless otherwise noted. Following 90 min of adsorption, medium containing virus was removed and replaced by DMEM with 4% FBS (Gibco) containing antiviral compounds. Infected treated HFFs were collected at 72 hpi, and pp28 activity was quantified by a luciferase assay kit (Promega, Madison, WI) on the GloMax-Multi+ detection system (Promega) according to the manufacturer's instructions. In second-cycle replication assays, supernatants were collected from the tested conditions of the first cycle and used for infection of fresh HFFs in 96-well plates. Luciferase activity was measured 72 h after the second-cycle infection. To better delineate the cell cycle stage of artemisinin activity, HFFs were arrested at G0 by serum-free DMEM, at early G1 by lovastatin (10 μM), or at late G1 by mimosine (400 μM). The medium was washed, and infection was performed at MOI = 1 in DMEM (Gibco). Following 90 min of adsorption, medium containing virus was removed and replaced by DMEM with 4% FBS and the desired artemisinins. Luciferase activity was quantified at 72 hpi.
A plaque reduction assay was performed in HFFs. One day prior to infection, HFFs were seeded at 3 × 106 or 1 × 106 cells per 24-well plate. The Towne CMV strain was diluted in DMEM to a desired titer that gave ∼50 plaques/well and added to each well in quadruplicate. Plates were incubated for 90 min with shaking every 10 min; thereafter, compounds were added and replenished every 3 days. After a 10-day incubation, cells were stained with crystal violet, and plaques were counted at ×40 magnification.
CMV DNA replication in cells was quantified using a real-time PCR of the highly conserved US17 as previously described (18, 19).
Selection of drug-resistant viral mutants.
A multistep resistance selection protocol was used in which the pp28-luciferase Towne CMV strain was serially passaged in the presence of increasing drug concentrations (20). HFFs (0.5 × 106/T-25 flask) were grown overnight and infected with the pp28-luciferase Towne CMV strain (MOI = 0.025) using serum-free medium. After 90 min of adsorption, cells were washed with PBS, and media containing the appropriate drugs (in DMEM with 4% FBS) were added (dimer 606 at 40 nM, AS at 2 μM, and GCV at 0.5 μM) to the flasks. A control flask containing infected cells was incubated in the absence of any drug (DMEM with 4% FBS). After 5 to 7 days, when a cytopathic effect (CPE) was visible, the supernatants were collected, and cells were scraped and kept at −80°C for determination of virus replication by luciferase assay. Supernatants collected from each flask were spun down at 3,000 rpm for 5 min, and cell-free supernatants (5% to 10% supernatants) were used to infect fresh HFFs treated with 2× drug concentration. This procedure was repeated 5 times.
Flow cytometry.
Apoptosis was determined in noninfected and CMV-infected cells. HFFs (1 × 106) were seeded in 12-well plates and cultured for 4 days with or without compounds before flow cytometry. CMV-infected HFFs (Towne CMV strain, MOI = 1 PFU/cell) were treated with artemisinins, and an apoptosis assay was performed at 4 days postinfection. Cells were collected and stained with the annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit (Sigma) and analyzed on an LSR II flow cytometer (BD Biosciences, San Jose, CA). Staurosporine (4 μM) was used as positive control for apoptosis in HFFs. Since artemisinins were reported to induce apoptosis in cancer cells, HCT116 colon carcinoma cells were also treated with artemisinins for 48 h, and an apoptosis assay was performed as a control. Percentage of apoptosis was calculated as the number of cells counted in the lower-right quadrant divided by the total number of cells.
To identify the cell cycle stage affected by AS and dimer 606, noninfected HFFs were serum starved for 72 h and then released in DMEM with 0% FBS (control for arrest in G0), 10% FBS (control for cell cycle progression), or 10% FBS plus artemisinins for 24 h. To determine the ability of artemisinins to affect cell cycle progression after induction of late G1 arrest, HFFs were treated with 400 μM mimosine for 48 h, after which, cells remained arrested in late G1 with DMEM containing 0% FBS (a control for mimosine late G1 arrest) or were released with 10% FBS (a control for cell cycle progression) or 10% FBS plus the indicated compounds for 24 h. To determine the ability of artemisinins to affect cell cycle progression after induction of early G1 arrest, HFFs were treated with 10 μM lovastatin for 24 h and then either remained arrested with DMEM–0% FBS (a control for lovastatin early G1 arrest) or were released with 10% FBS (a control for cell cycle progression) or 10% FBS plus indicated compounds for 24 h. In a subset of experiments, artemisinins were added 24 or 12 h before release from mimosine or lovastatin arrest, respectively. Then, HFFs were treated with DMEM containing 0% FBS, 10% FBS, or 10% FBS with the indicated compounds for 24 h. To determine the effects of artemisinins on cell cycle progression in CMV-infected HFFs, 1.0 × 106 cells were serum starved, followed by infection at MOI = 1 and treatment with AS, dimer 606, or GCV. Cells were harvested for cell cycle analysis at 72 hpi as described for noninfected cells. Data analysis was performed using FlowJo software (Tree Star, USA). The percentages of cells in G1, S, and G2/M stages appear in the cell cycle box of each condition.
SDS-PAGE and immunoblot analysis.
Cell lysates were quantified for total protein content using a Pierce bicinchoninic acid (BCA) protein assay kit (Thermo-Fisher Scientific, Waltham, MA). Equivalent amounts of proteins were mixed with equal volumes of sample buffer (125 mM Tris-HCl [pH 6.8], 4% SDS, 20% glycerol, 5% β-mercaptoethanol) and boiled at 100°C for 10 min. Denatured proteins were resolved in Tris-glycine polyacrylamide gels (10% to 12%) and transferred to polyvinylidene difluoride membranes (Bio-Rad Laboratories, Hercules, CA) by electroblotting (21). Membranes were incubated in blocking solution (5% nonfat dry milk or bovine serum albumin [BSA] and 0.1% Tween 20 in PBS [PBST]) for 1 h and washed three times with PBST, and incubated primary antibodies were diluted in 5% milk or BSA (for pRb) at 4°C overnight. The membranes were washed with PBST, followed by incubation with horseradish peroxidase (HRP)-conjugated secondary antibodies in 5% milk or BSA for 1 h at room temperature. After three washing steps with PBST, protein bands were visualized by chemiluminescence using SuperSignal West Pico reagent (Pierce Chemical, Rockford, IL). The following antibodies were used: mouse monoclonal anti-pp65 antibody (Vector Laboratories, Burlingame, CA); rabbit polyclonal anti-phospho Rb antibody (Ser 807/811) (Cell Signaling Technology, Beverly, MA); rabbit anti-CDK2, CDK4, and CDK6 polyclonal antibodies (Santa Cruz Biotechnology, Santa Cruz, CA); mouse anti-β-actin monoclonal antibody (Santa Cruz Biotechnology); HRP-conjugated goat anti-rabbit IgG antibody (Cell Signaling Technology); and HRP-conjugated sheep anti-mouse IgG (GE Healthcare, Waukesha, WI).
Drug combination and analysis.
Drug combinations were performed as previously reported (22). Briefly, 2 × 106 HFFs were seeded in a 96-well plate and infected with the pp28-luciferase Towne CMV strain at MOI = 1. First, a dose-response curve was generated for each drug individually to determine its 50% effective concentration (EC50). Then, the drugs were combined at twice their EC50, diluted in DMEM with 4% FBS followed by serial dilution, and added together after infection. Luciferase activities of the combination and each drug individually were quantified at 72 hpi. The Bliss model was used to calculate the effect of each drug combination on pp28-luciferase activity (22). In this model, drug combination represents the product of two probabilistically independent events as described in the following equation (23):
where D is the drug concentration, m is the slope, and EC50 is the effective concentration resulting in 50% virus inhibition. The combined effect of two inhibitors (FU, fractional unaffected) is computed as the product of individual effects of the two inhibitors FU1 and FU2. If the ratio of observed fold inhibition divided by the expected fold inhibition is >1, the compounds are synergistic. If the ratio is <1, the combination is considered antagonistic, and if it equals 1, the combination is additive.
Statistical analysis.
A Student t test was performed using SigmaPlot (Systat software, San Jose, CA) and GraphPad Prism (GraphPad software, La Jolla, CA) software. P values of <0.05 were considered significant.
RESULTS
Inconsistent anti-CMV activity of AS is not a result of selection of resistant viral mutants.
Our ongoing studies revealed that while GCV and dimer 606 maintained consistent CMV inhibition, the activity of AS against CMV was inconsistent. Loss of AS activity was not a result of advanced cell passage, selection of AS-resistant viral mutants, or compound instability. The same AS was effective against malaria parasites (unpublished data), and replenishing AS after 1 or 2 days did not improve its anti-CMV activity. Selection of drug-resistant CMV strains by serial passaging of supernatants in the presence of increasing drug concentrations revealed that neither AS nor dimer 606 selected for resistant CMV mutants. No viral progeny were detected in supernatants collected from infected cells exposed to higher drug concentrations. GCV treatment resulted in the selection of a GCV-resistant CMV strain at 16 μM GCV, evident by a C607Y substitution in UL97 and confirmed by sequence analysis (24).
The anti-CMV activity of AS correlates with confluence of HFFs.
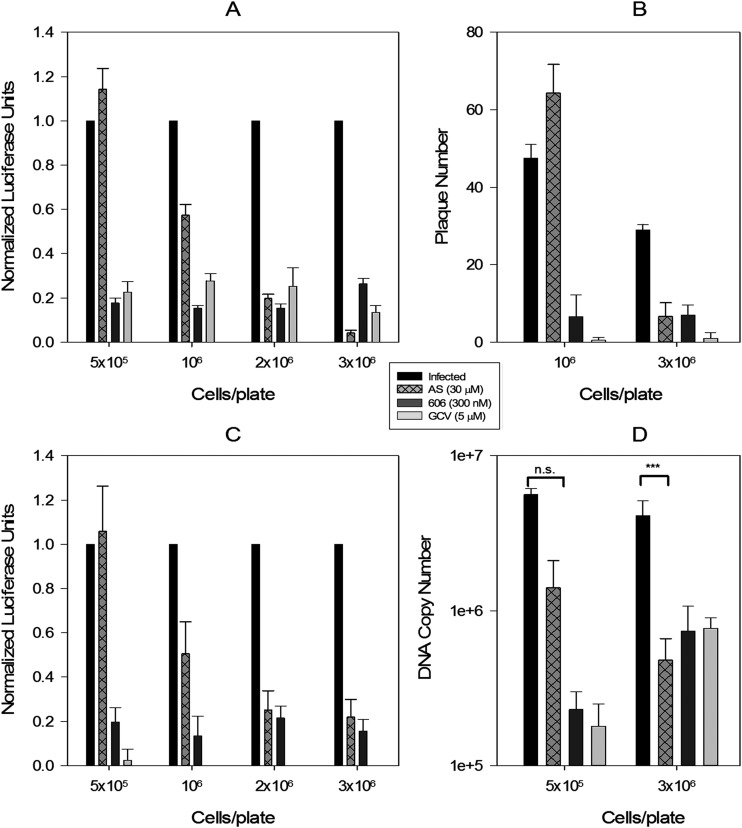
The contribution of cell density/contact inhibition to the anti-CMV activity of artemisinins was tested. Virus inhibition by AS depended on cell confluence at the initiation of the assay (Fig. 1A and B). Increasing cell confluence provided a more conducive environment for AS activity than a lower cell density. Activity dependence on cell density was specific to AS and was not observed for dimer 606 or GCV. Results of the pp28-luciferase assay (Fig. 1A) were confirmed by a plaque reduction assay (Fig. 1B), second-cycle luciferase expression (Fig. 1C), and DNA replication at different cell densities (Fig. 1D). In confluent HFFs (at the time of infection), AS inhibited CMV. In subconfluent cells, AS lost its anti-CMV activity.
FIG 1.
CMV inhibition by AS depends on cell confluence at the time of infection. (A) HFFs were seeded in 96-well plates at 0.5 × 106, 1 × 106, 2 × 106, and 3 × 106 cells/plate, infected with the pp28-luciferase Towne CMV strain at MOI = 1 after 24 h, and treated with the indicated compounds. Luciferase activity was measured at 72 hpi and normalized to activity measured in infected nontreated HFFs. Data represent means ± SE of four independent experiments. Differences in luciferase activity between infected untreated and infected AS-treated cells were statistically significant for 3 × 106 and 2 × 106 cells/plate (unpaired t test, P = 0.0008 and 0.0003, respectively) and nonsignificant for 1 × 106 and 0.5 × 106 cells/plate (P = 0.17 and 0.3, respectively). (B) HFFs were seeded in 24-well plates at 1 × 106 and 3 × 106 cells/plate, infected with 50 PFU/well of Towne CMV strain, and treated with the indicated compounds every 3 days for 10 days. Plaques were stained with crystal violet and counted. Data shown are the average of four wells ± SE for a representative experiment from two independent experiments. The difference in plaque number between infected low-density and high-density HFFs was not significant (unpaired t test, P = 0.5), but the difference in infected AS-treated cells was significant between the different cell densities (unpaired t test, P = 0.0002). (C) Supernatants from wells shown in panel A were used to infect HFFs (1 × 106 cells in a 96-well plate), and luciferase activity was measured at 72 hpi and normalized to activity measured in supernatants from infected untreated HFFs. (D) HFFs were seeded in 96-well plates (0.5 × 106, 3 × 106 cells/plate), infected with the Towne CMV strain at MOI = 1, and treated with the indicated compounds. At 48 hpi, DNA was isolated from the cells, and viral DNA copies were quantified by real-time PCR of the US17 gene. The differences in viral DNA replication between the infected untreated and AS-treated cells were statistically significant for 3 × 106 cells/plate (unpaired t test, P = 0.0002) and nonsignificant for 0.5 × 106 cells/plate (P = 0.3).
CMV inhibition by AS depends on a synchronous population of HFFs.
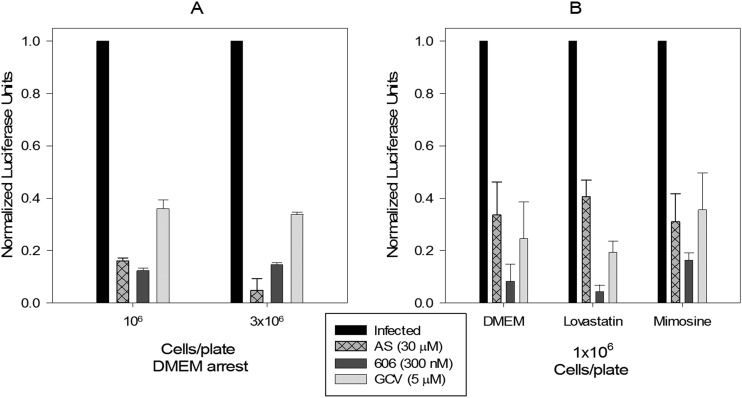
Induction of G0 arrest with serum starvation using either low or high cell density resulted in CMV inhibition with AS (Fig. 2A), suggesting that the cell cycle stage rather than cell density determined AS activity against CMV. Even in low-density HFFs, cell synchronization at G0 with serum starvation, late G1 with mimosine, or early G1 with lovastatin resulted in effective CMV inhibition with AS (Fig. 2B), indicating that a homogeneous cell population is an important factor in the anti-CMV activity of AS. Unlike in infected HFFs, in HCT116, both AS and 606 were more active in inhibiting cell proliferation as cell density decreased (see Fig. S1A in the supplemental material). Thus, differences between monomers and dimers were more distinguishable and significant in CMV-infected primary HFFs.
FIG 2.
Effect of cell cycle checkpoints on the anti-CMV activity of artemisinins. (A) HFFs were seeded at 1 × 106 or 3 × 106 cells/96-well plate, and G0 arrest was induced with serum starvation in DMEM for 24 h followed by infection with the pp28-luciferase Towne CMV strain (MOI = 1) and treatment with the indicated compounds. Virus replication was quantified by luciferase assay at 72 hpi. Data represent means ± SE of three independent experiments. An unpaired t test comparing pp28-luciferase expression in infected AS-treated 1 × 106 and 3 × 106 HFFs was not significant (P = 0.23). (B) 1 × 106 cells were seeded in a 96-well plate, and cell cycle arrest was induced with DMEM, 10 μM lovastatin, or 400 μM mimosine for 24 h. Cells were then infected and treated with the indicated compounds. Data represent means ± SE from three independent experiments.
Effect of artemisinins on apoptosis and cell cycle progression in primary HFFs.
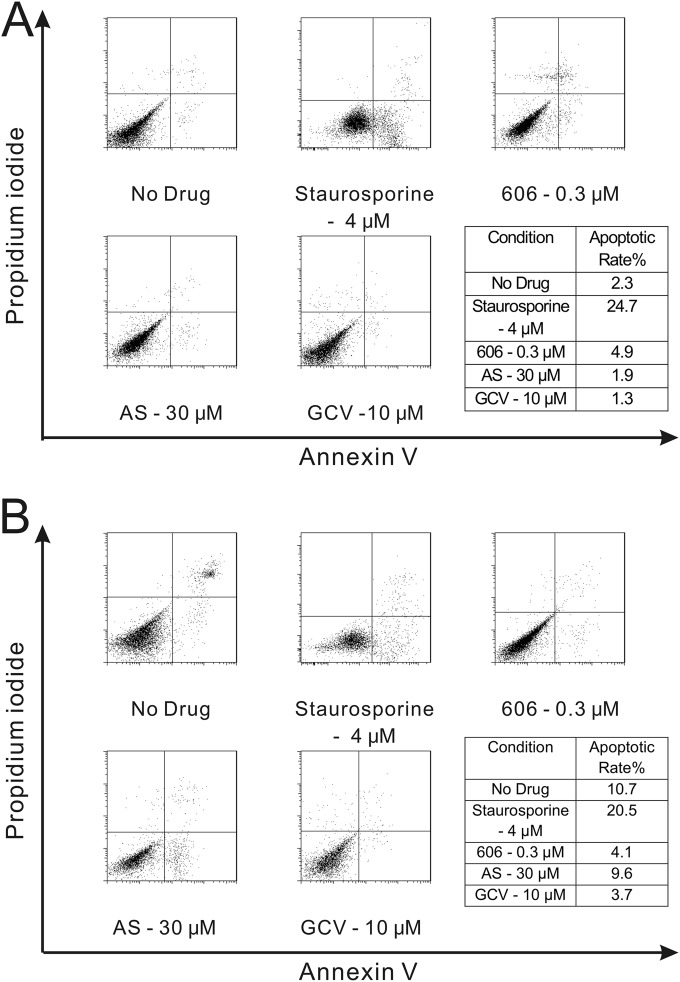
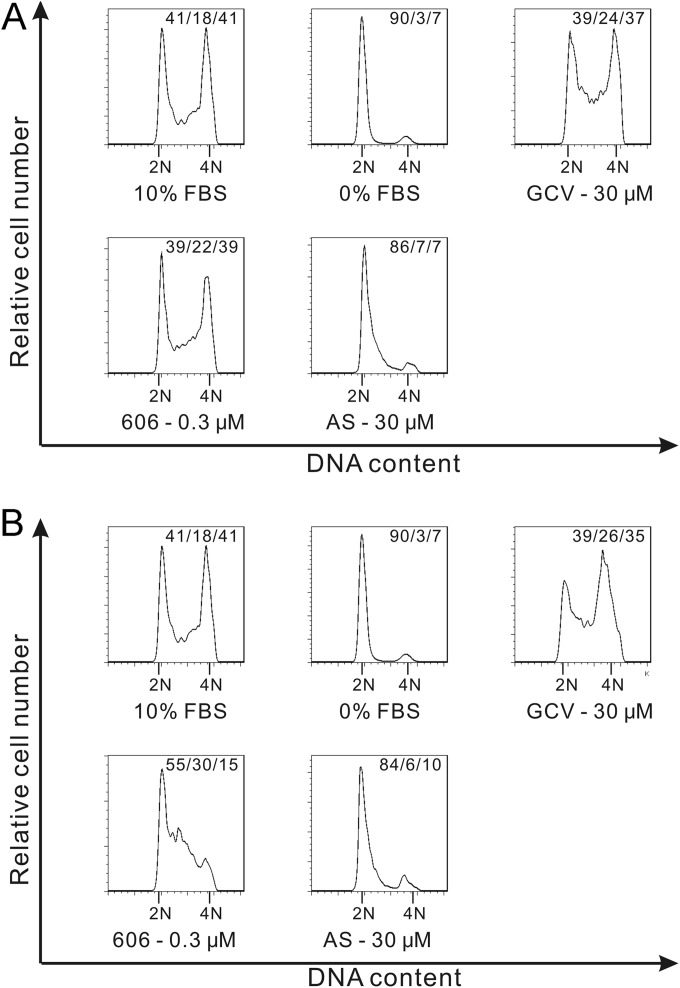
Since artemisinins were reported to inhibit cancer cell proliferation through induction of cell cycle arrest and apoptosis (25), their effects on these pathways were tested in primary HFFs. In noninfected or CMV-infected HFFs, artemisinins did not induce apoptosis (Fig. 3A and B), but staurosporine (positive control) did. In contrast, in HCT116, treatment with artemisinins induced apoptosis (see Fig. S1B in the supplemental material). Artemisinins induced cell cycle arrest in noninfected HFFs. When AS was added after cells were released from serum starvation (G0), it blocked cell cycle progression from the G1 to S phase, while dimer 606 only partially blocked this progression (Fig. 4A). When artemisinins were added 24 h before release from serum starvation, both AS and dimer 606 could arrest the cell cycle, although again the effect of AS was stronger. With AS, 84% of cells were at the G0/G1 stage, 6% were at the S stage, and 10% were at the G2/M stage; while with dimer 606, the fractions of cells in the respective stages were 55%, 30%, and 15% (Fig. 4B). These results indicated that both AS and dimer 606 were able to block cell cycle progression through different modes of action.
FIG 3.
Artemisinins do not induce apoptosis in HFFs. (A) 1 × 106 HFFs were seeded in 12-well plates and treated with the indicated concentrations of compounds for 96 h, dual stained by annexin V and propidium iodide (PI), and analyzed by flow cytometry. Staurosporine was used as positive control (4 μM for 2 h). Apoptotic cells (annexin V positive) appear in the lower-right quadrant of each data plot. The percentage of cells in the upper-right quadrants (late apoptosis) and lower-left quadrants (alive cells) did not change significantly among the different conditions. (B) 1 × 106 HFFs were seeded in 12-well plates, infected with the pp28-luciferase Towne CMV strain (MOI = 1) and treated with the indicated concentrations of compounds for 96 h. Staurosporine was used as positive control (4 μM for 2 h). The apoptotic rate was determined using flow cytometry by dual staining with annexin V-FITC antibody and PI.
FIG 4.
Effect of artemisinins on cell cycle progression in HFFs. Artemisinins were added after release from serum starvation (A) or 24 h before release from serum starvation (B). Cell cycle analysis was performed 24 h after release from serum starvation. (A) 1 × 106 HFFs were seeded in 12-well plates, starved in nonserum DMEM for 72 h, and then treated with DMEM containing 0% FBS, 10% FBS, or 10% FBS plus the indicated compounds for 24 h. Following PI DNA staining, cells were analyzed by flow cytometry. Data shown are representative of results from three independent experiments. (B) 1 × 106 HFFs were seeded in 12-well plates and starved in nonserum DMEM for 48 h, followed by treatment with nonserum DMEM containing the indicated compounds for 24 h, after which media were replaced by DMEM containing 0% FBS, 10% FBS, or 10% FBS plus the indicated compounds. Following PI DNA staining, cells were analyzed by flow cytometry.
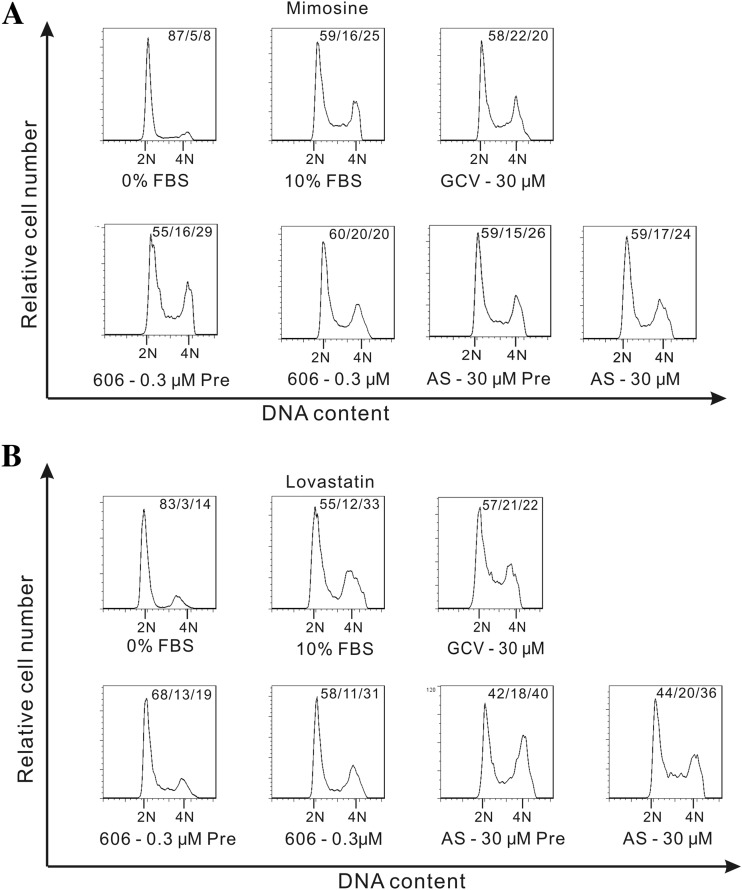
Artemisinins arrest cell cycle at early or late G1.
To further identify the stage of cell cycle affected by artemisinins, mimosine and lovastatin, which arrest cells at the late G1/S or early G1 checkpoints, respectively, were used (Fig. 5). HFFs were treated with artemisinins either before or after removal of the respective checkpoint compound. Following removal of mimosine (cells synchronized at G1/S), neither AS nor dimer 606 could arrest cell cycle progression (Fig. 5A; see also the Fig. S2 scheme in the supplemental material), suggesting that they induced cell cycle arrest before the G1/S checkpoint. Similarly, when added to HFFs 24 h before mimosine removal, cell cycle progression was not inhibited by AS or dimer 606 (Fig. 5A). AS could not arrest cell cycle progression in cells that were at the checkpoint induced by lovastatin or mimosine arrest (Fig. 5A and B), indicating that it blocked the cell cycle at early G1, before the stages induced by these compounds (see the Fig. S2 scheme). Addition of dimer 606 12 h before release from lovastatin blocked cell cycle progression, but treatment after release from lovastatin did not (Fig. 5B), suggesting that dimer 606 modulated the cell cycle at a stage after lovastatin and before mimosine (see Fig. S2 for model). Taken together, compared to AS, dimer 606 had moderate effects on cell cycle arrest and affected cell cycle at a later stage during G1.
FIG 5.
Effect of checkpoint agents on cell cycle progression with artemisinins. Cell cycle arrest was induced with mimosine (late G1/S) or lovastatin (early G1). (A) HFFs were treated with DMEM containing 10% FBS and mimosine (400 μM) for 48 h, after which mimosine was removed, and cells were treated with DMEM containing 0% FBS (G1/S mimosine checkpoint, control), 10% FBS, or 10% FBS with the indicated compounds for 24 h. Following PI DNA staining, the cells were analyzed by flow cytometry. Artemisinins were also added 24 h before release from mimosine arrest (Pre). Then, HFFs were treated with DMEM containing 0% FBS, 10% FBS, or 10% FBS with the indicated compounds for 24 h. (B) HFFs were treated with DMEM containing 10% FBS and lovastatin (10 μM) for 24 h, after which lovastatin was removed, and cells were treated with DMEM containing 0% FBS (early G1 lovastatin checkpoint, control), 10% FBS, or 10% FBS with the indicated compounds for 24 h. Following PI DNA staining, cells were analyzed by flow cytometry. Artemisinins were also added 12 h before release from lovastatin arrest (Pre). Then, HFFs were treated with DMEM containing 0% FBS, 10% FBS, or 10% FBS with the indicated compounds for 24 h.
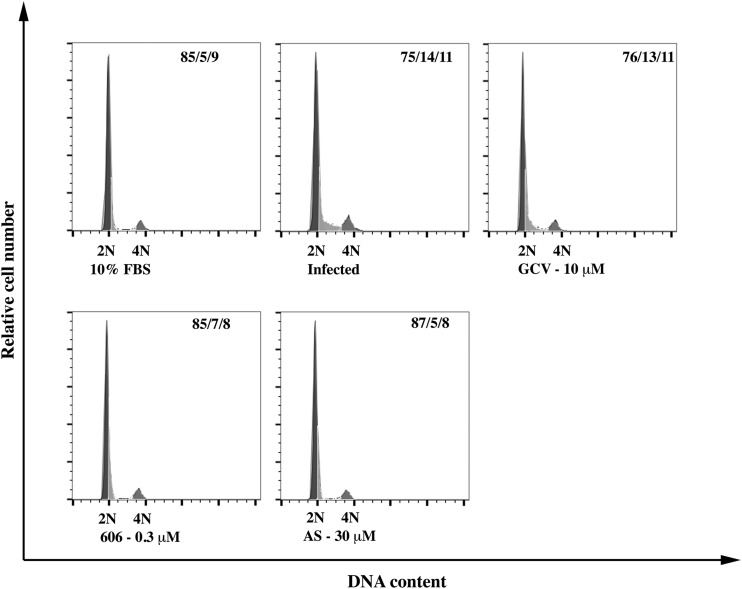
AS and dimer 606 modulate cell cycle progression in CMV-infected HFFs.
In HFFs arrested by serum starvation and infected with CMV, cell cycle progressed to the G1/S boundary. Treatment with AS and dimer 606 prevented the progression from G1 to G1/S. These effects were not observed with GCV (Fig. 6).
FIG 6.
Artemisinins modulate cell cycle progression in CMV-infected cells. 1 × 106 HFFs were seeded in 12-well plates and serum starved for 72 h. Cells were infected with the pp28-luciferase Towne CMV strain (MOI = 3) followed by drug treatment in DMEM with 4% FBS. Noninfected cells were released in DMEM with 10% FBS as control. Cells were harvested at 72 hpi, PI stained, and analyzed by flow cytometry.
Expression of cell cycle regulators correlates with CMV inhibition by artemisinins.
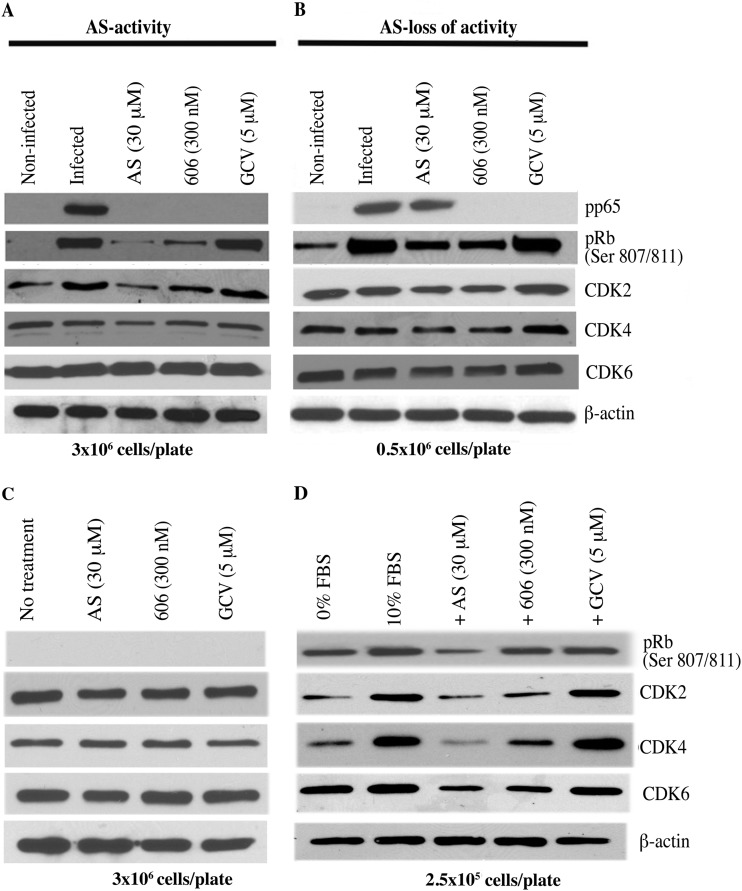
Our results suggested that cell cycle regulators might contribute to the anti-CMV activities of artemisinins. The expression of major cell cycle proteins was measured in CMV-infected HFFs in which CMV was either inhibited (CMV pp65 significantly reduced) or not inhibited (pp65 not reduced) by AS (Fig. 7). CMV infection induced CDK2 and the phosphorylation of pRb (Ser 807/811) in both low- and high-density cells (Fig. 7A and B). The expression of pRb and CDK2 was significantly reduced in AS-treated HFFs in which AS was CMV inhibitory, compared to that in HFFs in which AS lost its activity (Fig. 7A and B). A lack of CMV inhibition by AS correlated with higher levels of phosphorylated pRb and CDK2 (Fig. 7B). Treatment with dimer 606 resulted in mild reductions in pRb and CDK2, which were similar between high- and low-density cells (Fig. 7A and B). The effects of artemisinins on expression of CDK4 and CDK6 were determined. In AS-treated CMV-infected HFFs, CDK4 expression was mildly reduced. Dimer 606 did not change CDK4 expression. Levels of CDK6 were not affected by either AS or dimer 606 in infected cells irrespective of cell density (Fig. 7A and B). Thus, the anti-CMV activity of AS was associated with reductions in pRb and CDK2 but not in CDK4 or CDK6.
FIG 7.
Inhibition of CMV replication with artemisinins correlates with pRb expression. AS, dimer 606, and GCV were added to 96-well plates after infection with the pp28-luciferase Towne CMV strain, and cell lysates were collected for Western blotting at 72 hpi. CMV replication was measured by CMV pp65 expression. Seeding density was 3 × 106/plate (A) or 0.5 × 106 cells/plate (B). (C) Artemisinins modulated CDK expression depending on the growth phase of HFFs. Contact-inhibited HFFs (3 × 106 per 96-well plate) were treated with AS, dimer 606, and GCV for 72 h, and cell lysates were collected for Western blots. (D) HFFs (2.5 × 105 per 96-well plate) were serum starved for 72 h, followed by release in DMEM containing 0% FBS or 10% FBS along with AS, dimer 606, and GCV. Cell lysates were collected at 72 h for Western blots. Representative data from two independent experiments are shown. Protein expression was quantified using ImageJ.
To determine whether the above-described changes in cell cycle regulators were induced by CMV infection or an intrinsic property of artemisinins in primary HFFs, the expression of CDKs was measured in noninfected HFFs (Fig. 7C and D). In contact-inhibited HFFs, neither AS nor dimer 606 decreased the expression of any of the tested CDKs (Fig. 7C). However, in growing noninfected HFFs, the expression of all tested CDKs was reduced, more by AS than by dimer 606 (Fig. 7D). In contact-inhibited cells, the levels of CDKs were only reduced in the setting of infection (Fig. 7A and C), pointing to compound activity induced in CMV-infected cells. The effect of AS on expression of CDKs was similar to DMEM with 0% FBS, indicating strong cell cycle arrest.
CMV infection and AS treatment resulted in virus inhibition or lack of virus inhibition. In contact-inhibited HFFs in which the majority of cells were in the G0/G1 stage, CMV would attempt to carry the cells to the G1/S stage, but AS-induced arrest of a homogeneous population at G1 would prevent transit to G1/S, as required by CMV. In contrast, in a heterogeneous population of cycling cells that were infected by CMV, AS-induced arrest was only effective in a minor population of cells, and therefore its anti-CMV activity was reduced (Fig. 7A and B). Since dimer 606 partially blocked cell cycle progression, its CMV inhibition may not have been dependent on cell density, although some effects were observed on expression of pRb and CDK2.
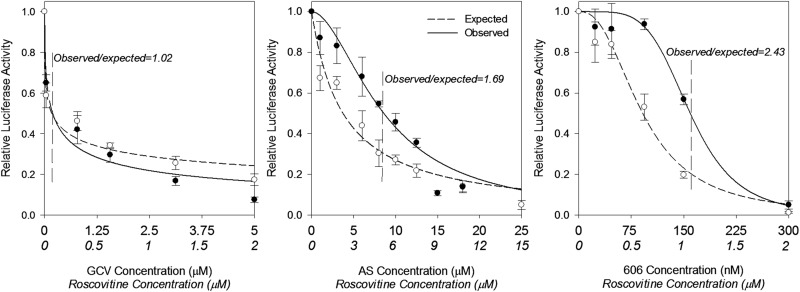
Drug combination with a CDK2 inhibitor suggests that artemisinins act through a mechanism that involves CDK2 in infected cells.
Since artemisinins inhibited cell cycle progression in HFFs and CDK2 expression in CMV-infected HFFs, we tested whether their activities could be mediated through modulation of CDK activity by using roscovitine (a CDK2 inhibitor). The EC50s of AS and roscovitine were 12.2 and 5.9 μM, respectively. The combination of roscovitine and AS or dimer 606 revealed an antagonistic effect on CMV replication (Fig. 8A and B), indicating a potential shared pathway. GCV did not exhibit any effects on pRb/CDK expression and showed an additive effect with roscovitine (Fig. 7A and B and 8C), confirming that the changes in the profile of cell cycle proteins were specific to artemisinins.
FIG 8.
Antagonistic effect of roscovitine and artemisinins in CMV inhibition. Drug combination was analyzed using the Bliss model as previously described (18). HFFs were seeded in 96-well plates, infected with the pp28-lucifersse Towne CMV strain, and treated with AS, dimer 606, roscovitine, or their combination. The concentration of each compound in the combination was started at twice the EC50, followed by serial dilutions. Luciferase activity was measured in cell lysates collected at 72 hpi. Data represent the means ± SE from three independent experiments. Roscovitine concentrations appear in italics. The Bliss coefficient (observed/expected fold inhibition around the EC50) is shown for each combination.
DISCUSSION
Uncovering the mechanisms of CMV inhibition by artemisinin-derived monomers and dimers is important, as they may lead to novel strategies for virus suppression. The monomeric artemisinins, AS and artemether, have shown good activity against plasmodium parasites and have become the standard of care for malaria. Other intracellular pathogens that infect a broad range of host cells, including viruses, parasites (Toxoplasma), and protozoa (Leishmania), are inhibited by artemisinins (26, 27).
We previously reported on the anti-CMV activities of artemisinin-derived monomers and dimers and their synergistic activity with GCV (9, 16, 17). Dimers demonstrated a higher selectivity index and slope of the dose-response curve than those of monomers (22).
The current findings support mechanistic differences between artemisinin monomers and dimers. The inconsistent anti-CMV activity of AS along with the inability to select for resistant viral mutants in the presence of increasing concentrations of AS or dimer 606 suggested that artemisinins may inhibit CMV by targeting cellular function(s) required for CMV replication. While selection of resistant CMV mutants may require prolonged drug exposure and may eventually emerge, no resistant viral mutants were observed in our studies.
Our data reveal that artemisinins modulate cell cycle progression in HFFs, that AS arrests cell cycle at an early G1 stage, and that dimer 606 partially blocks cell cycle at a later stage. The anti-CMV activity of AS correlates with the cell cycle stage and is markedly reduced in a heterogeneous/cycling cell population (Fig. 4, 5, and 7). In contrast to cell cycle arrest and induction of apoptosis in cancer cells (see Fig. S1B in the supplemental material), artemisinins did not induce apoptosis in noninfected or infected HFFs (Fig. 3A and B) (28–30). Monomeric and dimeric artemisinins were reported to induce G1 arrest and apoptosis in cancer cell lines (25, 29), activities that correlated with changes in the expression of genes involved in cell proliferation (31). In a panel of 55 cancer cell lines, the cytotoxicity of AS resulted in induction of G0/G1 arrest and changes in expression of cell cycle regulatory proteins (32). In contrast to our findings in primary HFFs, studies in cancer cells and our results in HCT116 (see Fig. S1A in the supplemental material) showed greater inhibition of cancer cell proliferation by AS and dimer 606 at lower cell density, when a higher percentage of cells is in S stage. These data suggest that the observed variability in the effects of artemisinins on cell cycle and apoptosis in normal versus cancer cells may reflect differences in the cellular environment.
The pRb protein plays a major role in controlling the cell cycle. Progression through G1 into S stage is associated with increased pRb phosphorylation, and hypophosphorylated pRb induces cell cycle arrest. The phosphorylation of pRb and the subsequent progression through the cell cycle depend on functional CDKs (33). CDK activity was reported to occur at multiple steps during human CMV infection (34), and roscovitine inhibited CMV replication at early and late stages, reminiscent of the reported activities of artemisinins (16). CMV inhibition with AS in confluent HFFs correlated with hypophosphorylated (active) pRb, while CMV inhibition in subconfluent cells was lost, and pRB remained in hyperphosphorylated form (Fig. 7A and B). Infection of cycling cells likely resulted in low percentages of cells reaching the cell cycle stage at which AS could induce arrest and inhibit CMV (Fig. 7B). Thus, AS-induced arrest was ineffective in infected cells of a growing population. Dimer 606 also reduced pRb phosphorylation, but the effect was modest compared to that of AS, reflecting the later stage of cell cycle affected by dimer 606 and the incomplete arrest induced by it.
CDK2 expression was also reduced in infected HFFs inhibited by AS, but there were no significant changes in CDK4 or CDK6 expression (Fig. 7A and B). The fact that the expressions of CDKs 2, 4, and 6 were unchanged in noninfected artemisinin-treated contact-inhibited HFFs suggests that the anti-CMV activity of artemisinins depends on modulation of the cellular response to CMV infection, resulting in downregulation of CDK2 and hypophosphorylation of pRb. This is corroborated by the antagonism of AS/dimer 606 and roscovitine in CMV inhibition (Fig. 8). Roscovitine can inhibit the kinase activity of additional CDKs that play a role in CMV replication (35). Future studies will address the role of artemisinins in modulating these proteins as a strategy for CMV inhibition.
In summary, CMV inhibition with artemisinins depends on the outcome of cell cycle modulation by infection and drug treatment through mechanisms involving pRb. Understanding the mechanisms may play an important role in considering future drug designs for CMV therapy.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by NIH 1R01AI093701 and March of Dimes 6-FY11-268 (R.A.-B.) and by NIH AI 34885 (G.H.P.).
We thank Tricia Nilles and Richard L. Blosser for assistance with flow cytometry.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00262-15.
REFERENCES
- 1.Kaptein SJ, Efferth T, Leis M, Rechter S, Auerochs S, Kalmer M, Bruggeman CA, Vink C, Stamminger T, Marschall M. 2006. The anti-malaria drug artesunate inhibits replication of cytomegalovirus in vitro and in vivo. Antiviral Res 69:60–69. doi: 10.1016/j.antiviral.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Efferth T, Marschall M, Wang X, Huong SM, Hauber I, Olbrich A, Kronschnabl M, Stamminger T, Huang ES. 2002. Antiviral activity of artesunate towards wild-type, recombinant, and ganciclovir-resistant human cytomegaloviruses. J Mol Med 80:233–242. doi: 10.1007/s00109-001-0300-8. [DOI] [PubMed] [Google Scholar]
- 3.Arav-Boger R, He R, Chiou CJ, Liu J, Woodard L, Rosenthal A, Jones-Brando L, Forman M, Posner GH. 2010. Artemisinin-derived dimers have greatly improved anti-cytomegalovirus activity compared to artemisinin monomers. PLoS One 5:e10370. doi: 10.1371/journal.pone.0010370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flobinus A, Taudon N, Desbordes M, Labrosse B, Simon F, Mazeron MC, Schnepf N. 2014. Stability and antiviral activity against human cytomegalovirus of artemisinin derivatives. J Antimicrob Chemother 69:34–40. doi: 10.1093/jac/dkt346. [DOI] [PubMed] [Google Scholar]
- 5.Gantt S, Huang ML, Magaret A, Bunts L, Selke S, Wald A, Rosenthal PJ, Dorsey G, Casper C. 2013. An artesunate-containing antimalarial treatment regimen did not suppress cytomegalovirus viremia. J Clin Virol 58:276–278. doi: 10.1016/j.jcv.2013.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapira MY, Resnick IB, Chou S, Neumann AU, Lurain NS, Stamminger T, Caplan O, Saleh N, Efferth T, Marschall M, Wolf DG. 2008. Artesunate as a potent antiviral agent in a patient with late drug-resistant cytomegalovirus infection after hematopoietic stem cell transplantation. Clin Infect Dis 46:1455–1457. doi: 10.1086/587106. [DOI] [PubMed] [Google Scholar]
- 7.Germi R, Mariette C, Alain S, Lupo J, Thiebaut A, Brion JP, Epaulard O, Saint RC, Malvezzi P, Morand P. 2014. Success and failure of artesunate treatment in five transplant recipients with disease caused by drug-resistant cytomegalovirus. Antiviral Res 101:57–61. doi: 10.1016/j.antiviral.2013.10.014. [DOI] [PubMed] [Google Scholar]
- 8.Lau PK, Woods ML, Ratanjee SK, John GT. 2011. Artesunate is ineffective in controlling valganciclovir-resistant cytomegalovirus infection. Clin Infect Dis 52:279. doi: 10.1093/cid/ciq050. [DOI] [PubMed] [Google Scholar]
- 9.He R, Mott BT, Rosenthal AS, Genna DT, Posner GH, Arav-Boger R. 2011. An artemisinin-derived dimer has highly potent anti-cytomegalovirus (CMV) and anti-cancer activities. PLoS One 6:e24334. doi: 10.1371/journal.pone.0024334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bresnahan WA, Boldogh I, Thompson EA, Albrecht T. 1996. Human cytomegalovirus inhibits cellular DNA synthesis and arrests productively infected cells in late G1. Virology 224:150–160. doi: 10.1006/viro.1996.0516. [DOI] [PubMed] [Google Scholar]
- 11.Dittmer D, Mocarski ES. 1997. Human cytomegalovirus infection inhibits G1/S transition. J Virol 71:1629–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jault FM, Jault JM, Ruchti F, Fortunato EA, Clark C, Corbeil J, Richman DD, Spector DH. 1995. Cytomegalovirus infection induces high levels of cyclins, phosphorylated Rb, and p53, leading to cell cycle arrest. J Virol 69:6697–6704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu M, Shenk T. 1996. Human cytomegalovirus infection inhibits cell cycle progression at multiple points, including the transition from G1 to S. J Virol 70:8850–8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvant BS, Fortunato EA, Spector DH. 1998. Cell cycle dysregulation by human cytomegalovirus: influence of the cell cycle phase at the time of infection and effects on cyclin transcription. J Virol 72:3729–3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Posner GH, Paik IH, Sur S, McRiner AJ, Borstnik K, Xie S, Shapiro TA. 2003. Orally active, antimalarial, anticancer, artemisinin-derived trioxane dimers with high stability and efficacy. J Med Chem 46:1060–1065. doi: 10.1021/jm020461q. [DOI] [PubMed] [Google Scholar]
- 16.He R, Park K, Cai H, Kapoor A, Forman M, Mott B, Posner GH, Arav-Boger R. 2012. Artemisinin-derived dimer diphenyl phosphate is an irreversible inhibitor of human cytomegalovirus replication. Antimicrob Agents Chemother 56:3508–3515. doi: 10.1128/AAC.00519-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.He R, Forman M, Mott BT, Venkatadri R, Posner GH, Arav-Boger R. 2013. The unique and highly-selective anti-cytomegalovirus activities of artemisinin-derived dimer diphenyl phosphate stem from combination of dimer unit and a diphenyl phosphate moiety. Antimicrob Agents Chemother 57:4208–4217. doi: 10.1128/AAC.00893-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He R, Sandford G, Hayward GS, Burns WH, Posner GH, Forman M, Arav-Boger R. 2011. Recombinant luciferase-expressing human cytomegalovirus (CMV) for evaluation of CMV inhibitors. Virol J 8:40. doi: 10.1186/1743-422X-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka Y, Kanda Y, Kami M, Mori S, Hamaki T, Kusumi E, Miyakoshi S, Nannya Y, Chiba S, Arai Y, Mitani K, Hirai H, Mutou Y. 2002. Monitoring cytomegalovirus infection by antigenemia assay and two distinct plasma real-time PCR methods after hematopoietic stem cell transplantation. Bone Marrow Transplant 30:315–319. doi: 10.1038/sj.bmt.1703661. [DOI] [PubMed] [Google Scholar]
- 20.Chou S, Marousek GI, Senters AE, Davis MG, Biron KK. 2004. Mutations in the human cytomegalovirus UL27 gene that confer resistance to maribavir. J Virol 78:7124–7130. doi: 10.1128/JVI.78.13.7124-7130.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sambrook J, Fritsch E, Maniatis T. 1989. Molecular cloning: a laboratory manual, 2nd ed Cold Spring Harbor Laboratory Press, Plainview, NY. [Google Scholar]
- 22.Cai H, Kapoor A, He R, Venkatadri R, Forman M, Posner GH, Arav-Boger R. 2014. In vitro combination of anti-cytomegalovirus compounds acting through different targets: role of the slope parameter and insights into mechanisms of action. Antimicrob Agents Chemother 58:986–994. doi: 10.1128/AAC.01972-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jilek BL, Zarr M, Sampah ME, Rabi SA, Bullen CK, Lai J, Shen L, Siliciano RF. 2012. A quantitative basis for antiretroviral therapy for HIV-1 infection. Nat Med 18:446–451. doi: 10.1038/nm.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lurain NS, Chou S. 2010. Antiviral drug resistance of human cytomegalovirus. Clin Microbiol Rev 23:689–712. doi: 10.1128/CMR.00009-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Shan F, Wu JM, Wu GS, Ding J, Xiao D, Yang WY, Atassi G, Leonce S, Caignard DH, Renard P. 2001. Novel antitumor artemisinin derivatives targeting G1 phase of the cell cycle. Bioorg Med Chem Lett 11:5–8. doi: 10.1016/S0960-894X(00)00578-3. [DOI] [PubMed] [Google Scholar]
- 26.Gomes TC, de Andrade Junior HF, Lescano SA, Amato-Neto V. 2012. In vitro action of antiparasitic drugs, especially artesunate, against Toxoplasma gondii. Rev Soc Bras Med Trop 45:485–490. doi: 10.1590/S0037-86822012000400014. [DOI] [PubMed] [Google Scholar]
- 27.Sen R, Ganguly S, Saha P, Chatterjee M. 2010. Efficacy of artemisinin in experimental visceral leishmaniasis. Int J Antimicrob Agents 36:43–49. doi: 10.1016/j.ijantimicag.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 28.Firestone GL, Sundar SN. 2009. Anticancer activities of artemisinin and its bioactive derivatives. Expert Rev Mol Med 11:e32. doi: 10.1017/S1462399409001239. [DOI] [PubMed] [Google Scholar]
- 29.Alagbala AA, McRiner AJ, Borstnik K, Labonte T, Chang W, D'Angelo JG, Posner GH, Foster BA. 2006. Biological mechanisms of action of novel C-10 non-acetal trioxane dimers in prostate cancer cell lines. J Med Chem 49:7836–7842. doi: 10.1021/jm060803i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Efferth T. 2007. Willmar Schwabe Award 2006: antiplasmodial and antitumor activity of artemisinin–from bench to bedside. Planta Med 73:299–309. doi: 10.1055/s-2007-967138. [DOI] [PubMed] [Google Scholar]
- 31.Efferth T, Olbrich A, Bauer R. 2002. mRNA expression profiles for the response of human tumor cell lines to the antimalarial drugs artesunate, arteether, and artemether. Biochem Pharmacol 64:617–623. doi: 10.1016/S0006-2952(02)01221-2. [DOI] [PubMed] [Google Scholar]
- 32.Efferth T, Sauerbrey A, Olbrich A, Gebhart E, Rauch P, Weber HO, Hengstler JG, Halatsch ME, Volm M, Tew KD, Ross DD, Funk JO. 2003. Molecular modes of action of artesunate in tumor cell lines. Mol Pharmacol 64:382–394. doi: 10.1124/mol.64.2.382. [DOI] [PubMed] [Google Scholar]
- 33.Kato J, Matsushime H, Hiebert SW, Ewen ME, Sherr CJ. 1993. Direct binding of cyclin D to the retinoblastoma gene product (pRb) and pRb phosphorylation by the cyclin D-dependent kinase CDK4. Genes Dev 7:331–342. doi: 10.1101/gad.7.3.331. [DOI] [PubMed] [Google Scholar]
- 34.Sanchez V, Spector DH. 2006. Cyclin-dependent kinase activity is required for efficient expression and posttranslational modification of human cytomegalovirus proteins and for production of extracellular particles. J Virol 80:5886–5896. doi: 10.1128/JVI.02656-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kapasi AJ, Spector DH. 2008. Inhibition of the cyclin-dependent kinases at the beginning of human cytomegalovirus infection specifically alters the levels and localization of the RNA polymerase II carboxyl-terminal domain kinases cdk9 and cdk7 at the viral transcriptosome. J Virol 82:394–407. doi: 10.1128/JVI.01681-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.