Abstract
Paracoccidioidomycosis (PCM) is a public health concern in Latin America and South America that when not correctly treated can lead to patient death. In this study, the influence of melanin produced by Paracoccidioides spp. on the effects of treatment with antimicrobial photodynamic inhibition (aPI) and antifungal drugs was evaluated. aPI was performed using toluidine blue (TBO) as a photosensitizer and a 630-nm light-emitting diode (LED) light. The antifungals tested were itraconazole and amphotericin B. We evaluated the effects of each approach, aPI or antifungals, against nonmelanized and melanized yeast cells by performing susceptibility tests and by quantifying oxidative and nitrosative bursts during the experiments. aPI reduced nonmelanized cells by 3.0 log units and melanized cells by 1.3 log units. The results showed that melanization protects the fungal cell, probably by acting as a scavenger of nitric oxide and reactive oxygen species, but not of peroxynitrite. Melanin also increased the MICs of itraconazole and amphotericin B, and the drugs were fungicidal for nonmelanized and fungistatic for melanized yeast cells. Our study shows that melanin production by Paracoccidioides yeast cells serves a protective function during aPI and treatment with itraconazole and amphotericin B. The results suggest that melanin binds to the drugs, changing their antifungal activities, and also acts as a scavenger of reactive oxygen species and nitric oxide, but not of peroxynitrite, indicating that peroxynitrite is the main radical that is responsible for fungal death after aPI.
INTRODUCTION
Paracoccidioidomycosis (PCM) is a systemic disease endemic to Latin America and South America that can be fatal if not treated (1). In Brazil, PCM is a serious health problem and is the most important cause of death among the systemic mycoses in immunocompetent patients (1–3). The etiological agents of PCM, dimorphic fungi that present a filamentous form at 25°C and yeast forms at 37°C, belong to the Paracoccidioides species complex, which includes four known phylogenetic lineages, Pb01-like (or Paracoccidioides lutzii), S1, PS2, and PS3 (Paracoccidioides brasiliensis) (4–7). Thermal dimorphism is a survival strategy to resist the host environment (5, 7). It includes secretion of proteases and melanin synthesis as putative virulence factors contributing to the protection of yeast cells from environmental stressors (8).
Currently, there has been much discussion about the role of melanin as a putative virulence factor of several fungal species, such as Histoplasma capsulatum, Sporothrix schenckii, and Cryptococcus neoformans (8–11). Melanin is a multifunctional polymer synthesized from 1,8-dihydroxynaphthalene (DHN) by the pentakide pathway or formed when cells grow in the presence of phenolic compounds, such as l-3,4-dihydroxyphenylalanine (l-DOPA) (12). Typically, it is a brown to black pigment formed through the activity of the phenoloxidase enzyme (laccase) on phenolic compounds (8, 13). Its influence on the biology of fungal cells is better known in Cryptococcus spp., where previous studies have demonstrated that melanized Cryptococcus cells are less susceptible to oxidative damage and phagocytosis by macrophages (10, 13). In Paracoccidioides spp., Gomez et al. (14) showed that both conidia and yeasts can synthesize melanin, a characteristic that was also associated with a reduction in phagocytosis and increasing survival of yeasts within macrophages (10). The ability of the fungus to produce this pigment in host tissues has been reported (10, 14).
The treatment of PCM is usually long term (15), and there are few drugs available, the most common being a combination of sulfamethoxazole-trimethoprim, amphotericin B (AMB), and azoles (mainly itraconazole [ITC]). However, none of these is able to prevent clinical failure or relapse (1, 15). In this context, the search for alternative treatment is important, and antimicrobial photodynamic inhibition (aPI) may be a promising option, particularly to treat skin lesions and oral mucosa.
aPI refers to a treatment that combines a photosensitizer (PS) and a light source to induce the production of harmful biological species that cause cell death (16, 17). The production of these biological effectors occurs in the presence of molecular oxygen and involves two types of reaction, named type I and type II. In the type I reaction, after the transfer of an electron from the PS to biological molecules, the production of different reactive oxygen species (ROS) and reactive nitrogen species (RNS), such as superoxide anion, hydrogen peroxide, and peroxynitrite (ONOO), occurs. In the type II reaction, there is transfer of energy from the PS to molecular oxygen, forming an oxygen singlet, which is the main mediator of cell injury after aPI (17–20).
The effectiveness of photodynamic inhibition as a fungicidal and bactericidal agent has been demonstrated by different authors (17, 19, 21–23), although little is known about aPI, its mechanism of action against dimorphic fungi, and the influence of melanin on the effects of aPI. Considering that melanin has antioxidant properties, the aim of this study was to evaluate the effects of aPI and of classical antifungal drugs in nonmelanized and melanized Paracoccidioides sp. yeast cells to verify metabolic activity curves and the production of oxygen and nitrogen species.
MATERIALS AND METHODS
Fungal isolates.
A set of 17 Paracoccidioides species strains were selected for in vitro aPI tests (Table 1). The yeast form of the fungi was maintained in yeast-peptone-dextrose (YPD) medium (1% yeast extract, 2% peptone, and 2% dextrose) at 35 to 37°C for 7 days (24). Additionally, C. neoformans (ATCC 62066) and Candida albicans (ATCC 18804) reference strains were grown on Sabouraud dextrose medium (Prodimol Biotecnologia, Belo Horizonte, Minas Gerais [MG], Brazil) at 37°C for 48 h prior to the tests. All strains were from the Laboratório de Biologia de Microrganismos and Laboratório de Micologia of Universidade Federal de Minas Gerais, Belo Horizonte, Brazil.
TABLE 1.
Paracoccidioides species isolates used in this work
| Isolate | Origin | City and/or country | Phylogenetic species |
|---|---|---|---|
| Ed01 | Clinical | Goiás, Brazil | Pb01-like or P. lutzii |
| 1578 | Clinical | Goiás, Brazil | Pb01-like or P. lutzii |
| Pb01a (ATCC MYA-826) | Clinical | Goiás, Brazil | Pb01-like or P. lutzii |
| Pb4 | Chronic PCM | São Paulo, Brazil | PS2 |
| Pb2 | Chronic PCM | Venezuela | PS2 |
| Pb03b | Chronic PCM | São Paulo, Brazil | PS2 |
| B339 (ATCC 32069) | Chronic PCM | São Paulo, Brazil | S1 |
| Uterus | Chronic PCM | Argentina | S1 |
| Pb05 | Chronic PCM | Paraná, Brazil | S1 |
| Penguin | Penguin feces | Uruguay | S1 |
| Tatu | Armadillo | Pará, Brazil | S1 |
| Mg5 | Acute PCM | Paraná, Brazil | S1 |
| Pb8 | Chronic PCM | São Paulo, Brazil | S1 |
| Pb18b | Chronic PCM | São Paulo, Brazil | S1 |
| EPM83 | Unknown | Colombia | PS3 |
P. lutzii isolate used in the genome-sequencing project (Broad Institute, MIT, and Harvard).
P. brasiliensis isolates used in the genome-sequencing project (Broad Institute, MIT, and Harvard).
Study design.
The strains used were chosen randomly, regardless of whether melanin production by Paracoccidioides spp. could change the efficacy of aPI, as well as of antifungal drugs. In addition, using the same method of sample selection, we also investigated the likely mechanism of this alteration. Both forms of reference strain PbB339 (ATCC 32069) were employed to analyze the influence of oxidative and nitrosative bursts. The nonmelanized and melanized isolates Pb 05, Pb 08, and Pb 18 were chosen for susceptibility tests, metabolic activity curves, and oxidative and nitrosative burst assays after treatment with antifungal drugs.
Melanization of fungal cells in YPD medium supplemented with l-DOPA.
Paracoccidioides isolates were grown in YPD medium supplemented with 1 mM l-DOPA (Sigma-Aldrich) at 37°C for 15 days (25). All isolates were observed under light microscopy to evaluate the presence of melanin pigment.
Phenoloxidase (laccase) activity.
Phenoloxidase activity was evaluated according to the protocol established by Da Silva et al. (26) with a few modifications. C. neoformans (ATCC 62066), a melanin producer, and C. albicans (ATCC 18804), tested as a nonproducer, were used as controls. All yeast cells tested (controls and Paracoccidioides isolates) were first grown in McVeigh-Morton (McVM) broth supplemented with glucose (27) for 48 h with rotary shaking at 120 rpm. Next, the cells were washed three times with McVM broth without glucose and suspended in the same medium, followed by an incubation period of 48 h with agitation at 120 rpm. Then, cells from each species were harvested by centrifugation for 10 min at 7,826 × g and washed with sterile phosphate-buffered saline (PBS). Lysis buffer (200 mM Tris-HCl, 250 mM NaCl, 25 mM EDTA [pH 8.0], and 0.5% [wt/vol] SDS) was added to 108 yeast cells/ml, and the suspensions were incubated for 1 h at 54°C. The yeast cells were washed with 50 mM Na2HPO4 buffer and centrifuged at 7,826 × g. The pellet was suspended in Na2HPO4 buffer supplemented with l-DOPA at a final concentration of 1 mM, followed by incubation for 2 h in a rotary shaker at 37°C. These suspensions were centrifuged at 7,826 × g, and absorbance of the supernatant was read at 480 nm in a spectrophotometer (μQuant; Biotek, USA). A 50 mM Na2HPO4 solution supplemented with 1 mM l-DOPA was used as the blank control. The enzyme units were calculated according the following formula: (absorbance at 480 nm [test] − absorbance at 480 nm [control])/108 yeast cells/ml (units per 108 yeast cells per milliliter). One enzyme unit was defined as the amount that causes a change in absorbance of 0.001 at 480 nm (28).
Preparation of the inocula.
The fungal cells were grown in YPD medium for 7 days at 35 to 37°C in the presence or absence of l-DOPA and suspended in PBS to achieve 1 × 107 viable cells/ml. The final concentrations of inocula for susceptibility tests and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) assays were 0.5 × 105 to 1 × 105 yeast cells/ml for the antifungal tests and 1 × 106 cells/ml for the aPI tests (19, 24).
Photosensitizer and light source.
Toluidine blue (TBO) (Sigma, St. Louis, MO, USA) was used as a photosensitizer at concentrations of 70 mg/liter, 40 mg/liter, and 10 mg/liter. A light-emitting diode (LED) (Fisioled; MMoptics, São Paulo, Brazil) was used as a source of monochromatic light (19). The energy doses tested were 30 J/cm2, 60 J/cm2, and 90 J/cm2.
Selecting the optimal conditions for performance of in vitro aPI with Paracoccidioides spp.
The nonmelanized P. brasiliensis isolate PbB339 (ATCC 32069) was chosen for the standardization and determination of optimal conditions for aPI, followed by application of better parameters to the other 16 isolates. All of the tests were performed in duplicate in sterile 96-well flat-bottom black microplates (Optiplate-96 Black; PerkinElmer, Waltham, MA, USA) with a maximum suspension volume of 200 μl (19). For the determination of the optimal TBO concentrations and LED light dose, the yeast suspensions were divided into eight independent groups, as shown in Table 2. After these procedures, 100-μl samples of the suspensions were subcultured in petri dishes with brain heart infusion (BHI) medium supplemented with 4% (vol/vol) fetal calf serum and 5% (vol/vol) spent P. brasiliensis PbB339 culture medium and incubated at 35 to 37°C for 15 days, at which time the viable colonies were counted.
TABLE 2.
Groups and conditions used for determination of the optimal TBO concentration and LED light dose for in vitro antimicrobial photodynamic inhibition of Paracoccidioides spp.
| Group | Conditions |
|---|---|
| TBO concna | |
| Control groups | |
| C1 | Untreated |
| C2 | Irradiated by LED with a light dose of 90 J/cm2 without a photosensitizer |
| C3 | Exposed to TBO at a concn of 10 mg/liter without light irradiation |
| C4 | Exposed to TBO at a concn of 40 mg/liter without light irradiation |
| C5 | Exposed to TBO at a concn of 70 mg/liter without light irradiation |
| Treatment groups | |
| T1 | Exposed to TBO at a concn of 10 mg/liter followed by LED irradiation with a light dose of 90 J/cm2 |
| T2 | Exposed to TBO at a concn of 40 mg/liter followed by LED irradiation with a light dose of 90 J/cm2 |
| T3 | Exposed to TBO at a concn of 70 mg/liter followed by LED irradiation with a light dose of 90 J/cm2 |
| LED light dosea | |
| Control groups | |
| C1 | Untreated |
| C2 | Exposed to TBO at a concn of 10 mg/liter without light emission |
| C3 | Irradiated with LED at a dose of 30 J/cm2 without photosensitizer exposure |
| C4 | Irradiated with LED at a dose of 60 J/cm2 without photosensitizer exposure |
| C5 | Irradiated with LED at a dose of 90 J/cm2 without photosensitizer exposure |
| Treatment groups | |
| T1 | Exposed to TBO at a concn of 10 mg/liter and then LED irradiated with a light dose of 30 J/cm2 |
| T2 | Exposed to TBO at a concn of 10 mg/liter and then LED irradiated with a light dose of 60 J/cm2 |
| T3 | Exposed to TBO at a concn of 10 mg/liter and then LED irradiated with a light dose of 90 J/cm2 |
Five-minute preincubation of the fungal conidia with TBO was performed in the dark.
Susceptibilities of nonmelanized and melanized Paracoccidioides spp. to aPI.
The optimal conditions for performing in vitro aPI of Paracoccidioides spp. were further applied to all Paracoccidioides spp. in nonmelanized and melanized forms. To evaluate the reversibility of the protection conferred by melanin, melanized Paracoccidioides sp. cells that had recovered after aPI were cultivated on YPD medium without l-DOPA and subjected to a second aPI treatment. The growth control was the average number of colonies from all the isolates that had not undergone aPI (19, 25).
Evaluation of the susceptibility of Paracoccidioides spp. to itraconazole and amphotericin B.
The MICs for ITC and AMB were determined for Pb 05, Pb 08, and Pb 18 in the melanized yeast form by a previously described microdilution method according to CLSI guidelines with a few modifications (24). Standard RPMI 1640 medium was buffered with 0.165 M morpholinepropanesulfonic acid (MOPS) at 34.54 g per liter and supplement with l-DOPA at a concentration of 1 mM. Antifungal drugs were dissolved in 100% dimethyl sulfoxide (Synth) and prepared in a stock solution at 5,000 mg/liter. The itraconazole concentrations ranged from 0.0039 to 12.0 mg/liter, and the amphotericin B concentrations ranged from 0.0039 to 14.0 mg/liter. All experiments were performed in duplicate using 96 flat-bottom microdilution wells. For each test plate, two drug-free controls were included, one with medium alone (sterile control) and the other with 100 μl of medium plus 100 μl of inoculum suspension (growth control). The plates were incubated at 37°C and read visually and by MTT (Sigma-Aldrich) assay after 15 days of incubation (19). Finally, to evaluate whether melanin could reduce the drug concentrations in the solution, synthetic melanin (Sigma-Aldrich) at a concentration of 20 mg/liter was added to solutions of itraconazole and amphotericin B and incubated for 2 h at room temperature, followed by centrifugation (29). Then, the susceptibility test and reading were performed according to the protocol described above.
Metabolic activity assay.
After treatment with itraconazole and amphotericin B (at their MICs), 100-μl samples of nonmelanized and melanized yeast suspensions were transferred to 96-well microdilution plates with 100 μl of RPMI 1640 medium buffered with 0.165 M MOPS at 34.54 g per liter (24). The microdilution plates were incubated for 0, 6, 12, 24, 48, 72, 96, 120, 144, and 168 h (19, 24). Then, 0.005 mg/liter of MTT (Sigma-Aldrich) solution was added to each well, followed by incubation at 37°C for 4 h, removal of a 100-μl aliquot, and addition of 100 μl of dimethyl sulfoxide (19). Readings were performed at 490 nm in a spectrophotometer (μQuant; Biotek, USA).
Analysis of oxygen and nitrogen production after aPI and after treatment with itraconazole and amphotericin B.
The levels of reactive oxygen and nitrogen species were measured in the samples treated with aPI (under optimal conditions) and with ITC and AMB (at their MICs). The samples were treated with 10 μM 4-amino-5-methylamino-2′,7′-difluorofluorescein (DAF-FM) (Invitrogen) to quantify the presence of nitric oxide (NO), with 50 μM 2′,7′ dichlorodihydrofluorescein diacetate (H2DCFDA) (Invitrogen) to quantify ROS, and with 25 μM dihydrorhodamine 123 (DHR 123) (Invitrogen) to quantify ONOO (19) for 30 min at 37°C. For all of the analyses, fluorescence was determined in a fluorimeter (Synergy 2; Biotek, USA) at a wavelength of 485 nm and measuring emissions at 530 nm. The data are expressed as the means ± standard errors of the mean (SEM) for fold increases in fluorescence over the control.
Analysis of the influence of oxygen and nitrogen species on aPI.
The PbB339 isolate (nonmelanized and melanized) was used to evaluate the roles of oxidative and nitrosative stresses on fungal death after aPI, according to the protocol of Baltazar et al. (19) with modifications. While performing the treatment, 20-tetrakis-(4-sulfonatophenyl)-porphyrinato iron(III) chloride (Fetpps) (100 μM; Merck) was used as an ONOO scavenger, 4,5-dihydroxy-1,3-benzenedisulfonic acid disodium hydrate (Tiron) (50 mM; Sigma-Aldrich) was used as a superoxide anion scavenger, and Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) (50 mM; Sigma-Aldrich) was used as a nitric oxide synthase (NOS) inhibitor. Fungal viability was measured by counting CFU after plating 100 μl of 103 cells diluted in BHI medium supplemented with 4% (vol/vol) fetal calf serum and 5% (vol/vol) spent PbB339 culture medium and incubating them at 37°C for 15 days.
Analysis of the absorbance spectrum of melanin.
To evaluate whether melanin is able to interact with itraconazole, amphotericin B, TBO, and TBO treated with LED at 60 J/cm2, we made different suspensions of each substance with the same concentration of melanin (20 mg/liter). Next, we evaluated the absorbance spectrum of each suspension using a spectrophotometer (Multiskan Spectrum; Thermo Scientific, USA). The readings were performed from 200 nm to 900 nm in a 96-well flat-bottom UV microdilution plate.
Data analysis.
Statistical analyses were performed using one-way analysis of variance (ANOVA) and Kruskal-Wallis and Newman-Keuls multiple-comparison tests. A P value of <0.05 was considered significant.
RESULTS
Evaluation of phenoloxidase (laccase) activity.
Isolates of Paracoccidioides spp. cultured on YPD medium supplemented with l-DOPA showed darkly pigmented yeast cells (Fig. 1A). In contrast, no pigmentation was observed when fungal cells were cultured on YPD medium without l-DOPA (Fig. 1B). All the evaluated isolates showed phenoloxidase activity significantly higher than that of C. albicans and similar to that of C. neoformans cells (P < 0.0001) (Fig. 1C).
FIG 1.
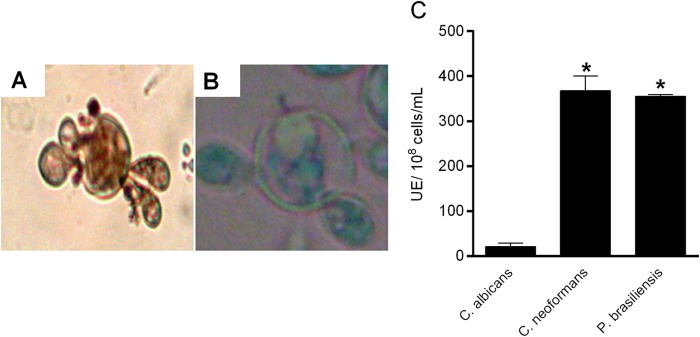
Production of melanin by yeast cells of Paracoccidioides spp. and analysis of phenoloxidase activity. (A and B) Light microscopy of ATCC 32069 growth on YPD medium supplemented with l-DOPA (A) and on YPD medium without l-DOPA (B). Original magnification, ×400. (C) Phenoloxidase activity of melanized Paracoccidioides spp. compared with C. albicans and C. neoformans. UE, units of enzyme. *, statistically significant difference (P < 0.0001). The error bars indicate SEM.
Effect of photodynamic inhibition of Paracoccidioides isolates.
In all experiments, aPI using higher concentrations of TBO and doses of light efficiently decreased the number of viable colonies compared to experimental controls (P < 0.0001) (Fig. 2A and B). A TBO dose of 40 mg/liter and an LED dose of 60 J/cm2 were selected as experimental conditions to test the other 16 Paracoccidioides isolates. aPI of nonmelanized and melanized cells under optimal conditions resulted in reductions of 3.0 log units and 1.3 log units, respectively, in fungal viability compared to the growth control (Fig. 2C). Melanized yeast cells recovered from aPI and subjected to a second round of aPI treatment showed a complete loss of viability (data not shown).
FIG 2.
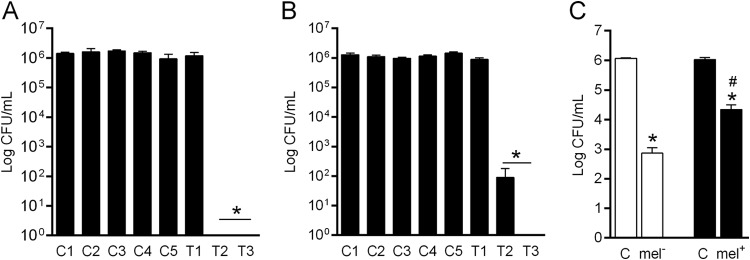
Photodynamic inhibition of Paracoccidioides spp. (A) Determination of the optimal concentration of TBO. C1, control; C2, 90 J/cm2 LED; C3, 10 mg/liter TBO; C4, 40 mg/liter TBO; C5, 70 mg/liter TBO; T1, 10 mg/liter TBO plus 90 J/cm2 LED; T2, 40 mg/liter TBO plus 90 J/cm2 LED; T3, 70 mg/liter TBO plus 90 J/cm2 LED. (B) Determination of the optimal energy density of LED at 630 nm. C1, control; C2, 40 mg/liter TBO; C3, 30 J/cm2 LED; C4, 60 J/cm2 LED; C5, 90 J/cm2 LED; T1, 40 mg/liter TBO plus 30 J/cm2 LED; T2, 40 mg/liter TBO plus 60 J/cm2 LED; T3, 40 mg/liter TBO plus 90 J/cm2 LED. (C) Numbers of nonmelanized and melanized Paracoccidioides species strains after aPI. *, statistically significant difference in comparison with the control for each aPI treatment (P < 0.0001); #, statistically significant difference in comparison to nonmelanized cells subjected to aPI (P < 0.05). C, growth control; mel−, nonmelanized cells; mel+, melanized cells. The error bars indicate SEM.
Analysis of the influence of oxidative and nitrosative bursts on aPI of nonmelanized and melanized cells.
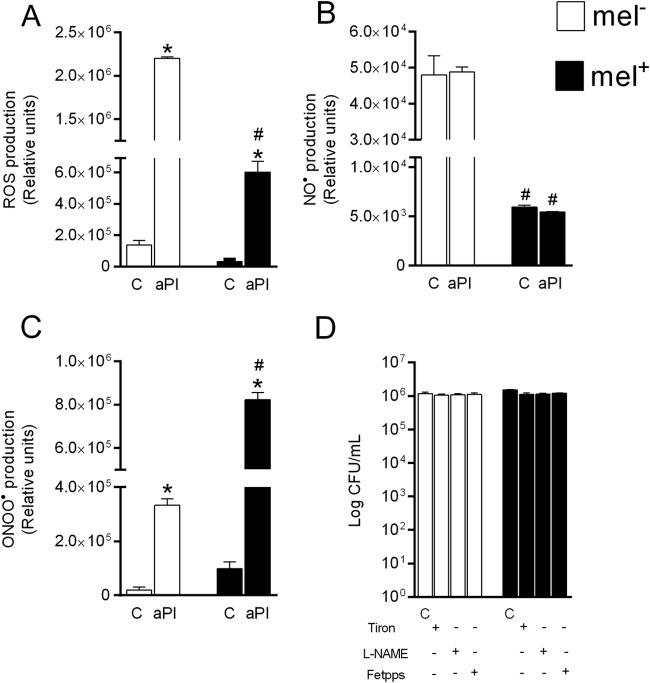
aPI increased the levels of ROS and ONOO, but not of NO, compared to the controls (Fig. 3A, B, and C). Interestingly, nonmelanized cells had significantly higher (P < 0.0001) levels of ROS and NO but significantly lower (P < 0.0001) levels of ONOO than melanized cells (Fig. 3A, B, and C). When Tiron, l-NAME, and Fetpps were used during aPI, we noted an increase in fungal viability under both non-melanin-producing and melanin-producing conditions (Fig. 3D).
FIG 3.
Analysis of the generation of reactive oxygen and nitrogen species by nonmelanized and melanized Paracoccidioides yeast cells after aPI. (A to C) Production of ROS (A), NO (B), and ONOO (C). (D) Numbers of CFU/ml after treatment with Tiron (ROS scavenger), l-NAME (NOS inhibitor), and Fetpps (ONOO). *, statistically significant difference in comparison to the control for each aPI treatment (P < 0.0001); #, statistically significant difference in comparison to nonmelanized cells subjected to aPI (P < 0.0001). C, growth control; mel−, nonmelanized cells; mel+, melanized cells. The error bars indicate SEM.
Antifungal activities of itraconazole and amphotericin B against melanized Paracoccidioides spp.
The MIC values for itraconazole and amphotericin B against nonmelanized and melanized yeast cells of three P. brasiliensis isolates are summarized in Table 3. Interestingly, the susceptibility of melanized cells to both antifungals tested was drastically reduced. Overall, MICs for itraconazole were increased by 2 to 800 times when melanized yeasts were tested, and although smaller, the increase for amphotericin B varied from 2 to 14 times. The addition of synthetic melanin to the MIC assays caused a slight increase in the MIC values (data not shown).
TABLE 3.
MICs of itraconazole and amphotericin B against nonmelanized and melanized P. brasiliensis yeasts
| Isolate | MICa (mg/liter) |
|||
|---|---|---|---|---|
| Itraconazole |
Amphotericin B |
|||
| Mel− | Mel+ | Mel− | Mel+ | |
| Pb 18 | 0.0039 | 0.125 | 1.0 | 2.0 |
| Pb 05 | 0.015 | 12.0 | 1.0 | 14.0 |
| Pb 08 | 0.062 | 0.125 | 1.0 | 4.0 |
MICs for nonmelanized (Mel−) cells were demonstrated by Cruz et al. (24) according to a modified CLSI protocol. Mel+, melanized cells cultured in l-DOPA medium.
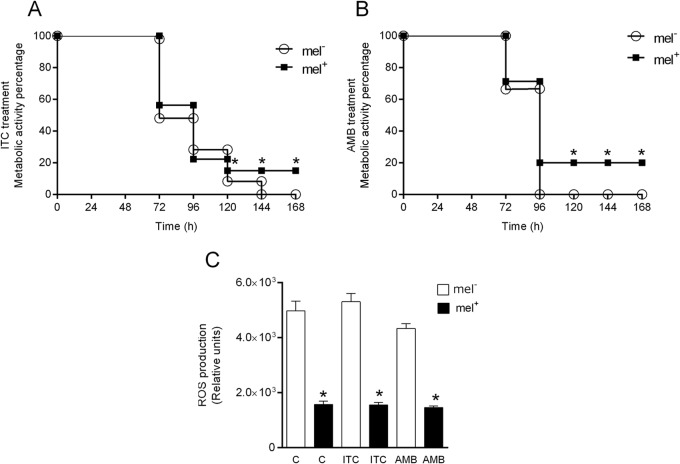
Influence of melanin on metabolic activity and oxidative and nitrosative bursts after treatment with itraconazole and amphotericin B.
Based on the higher MIC values of the antifungals for melanized yeasts, we evaluated whether melanization would alter metabolic activity and ROS production in the presence of antifungals. Treatment with ITC inhibited 100% of the metabolic activity of nonmelanized cells after 144 h of treatment; however, melanized cells showed inhibition of only 85% during the same period (Fig. 4A). Treatment with AMB inhibited 100% of the metabolic activity of nonmelanized cells after 96 h of treatment, and the melanized cells showed inhibition of 80% during the same period (Fig. 4B). It was interesting to observe that lower ROS levels were produced by melanized Paracoccidioides sp. yeast cells (Fig. 4C), although no differences were observed between treated and untreated yeasts.
FIG 4.
MTT assay and ROS production after treatment with itraconazole and amphotericin B. (A and B) Metabolic activities of nonmelanized and melanized yeast cells treated with itraconazole (A) and amphotericin B (B) at the MIC. (C) ROS production after treatment with itraconazole and amphotericin B at the MIC. *, statistically significant difference in comparison to nonmelanized cells (P < 0.0001). mel−, nonmelanized cells; mel+, melanized cells. The error bars indicate SEM.
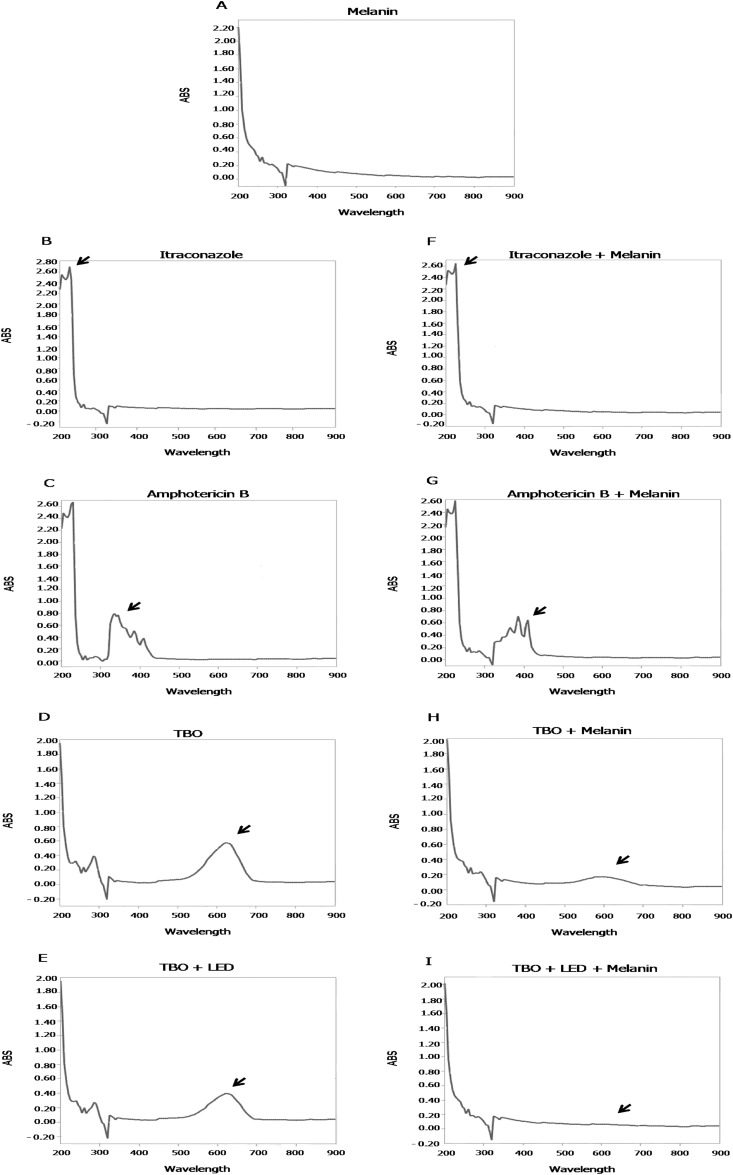
Analysis of the absorbance spectrum.
Melanin changes the profile of the absorbance spectra of ITC, AMB, and TBO. When ITC was incubated with melanin, a slight change was observed in the absorbance peak at 200 nm (±10 nm) (Fig. 5B and F). In a similar manner, when melanin was incubated with AMB, there was an alteration in the absorbance peaks at 310 nm (±10 nm) and 400 nm (±10 nm) (Fig. 5C and G). Finally, melanin also changed the absorbance peak of TBO, and it was smaller than that of the control (TBO alone). Further, a new absorbance peak was observed at 200 nm (Fig. 5D and H). Although TBO treated with an LED showed peak absorbance at 600 nm (±10 nm), when the solution was treated in the presence of melanin, this absorbance peak at 600 nm (±10 nm) was not observed (Fig. 5E and I).
FIG 5.
Analysis of the absorbance (ABS) spectra of melanin and different substances. Shown are the absorbance spectra of melanin (A), itraconazole (B), amphotericin B (C), TBO (D), TBO treated with an LED dose of 60 J/cm2 (E), itraconazole and melanin (F), amphotericin B and melanin (G), TBO and melanin (H), andTBO and melanin treated with an LED dose of 60 J/cm2 (I).
DISCUSSION
Melanin is considered a virulence factor of some pathogenic fungi based on its antiphagocytic and antioxidant properties, which are important for protecting fungal cells from different environmental stressors (30, 31). All the Paracoccidioides species isolates used in this study demonstrated the ability to produce melanin on YPD medium supplemented with l-DOPA, showing brown-pigmented colonies and darkly pigmented yeast cells under optical microscopy. Additionally, all the Paracoccidioides species isolates exhibited phenoloxidase activity higher (P < 0.0001) than that of C. albicans and similar to that of C. neoformans, a fungus known to be a melanin producer (32). This is the first evidence of phenoloxidase activity in a set of Paracoccidioides spp., including isolates from the four known phylogenetic lineages, Pb01-like (or P. lutzii) (4, 6), S1, PS2, and PS3 (P. brasiliensis) (4, 7). The correlation between phenoloxidase activity and melanin production is well established, because the enzyme catalyzes the synthesis of melanin when fungal cells are grown in the presence of phenolic compounds (8, 31).
In the present study, we demonstrated that aPI can reduce the viability of Paracoccidioides sp. yeast cells, corroborating the study of Almeida et al. (23). The analysis of ROS and RNS generated after aPI reinforces the statement that, independent of the microorganism, photodynamic inhibition reduces cell viability in a nonspecific way, decreasing the possibility of selecting strains resistant to aPI (16, 17, 19). Nonmelanized yeast cells were more susceptible to aPI than melanized cells, suggesting that melanin can change the efficacy of aPI (33). The low levels of ROS and NO presented by melanized cells corroborate previous studies that demonstrated that melanin could act as a free-radical scavenger protecting Paracoccidioides sp. yeast cells from death from ROS and RNS (such as NO) (11). However, this observation was not true for ONOO, which indicates that melanin is unable to scavenge the radical, which is a powerful reactive species that could drastically decrease fungal viability (19, 34–36). Interestingly, melanin changed the absorbance peak of TBO, which leads to loss of resonance with LED light, reducing the amount of activated TBO and consequently decreasing the production of ROS and RNS after aPI.
Our MIC data show that melanized yeasts are less susceptible to the drugs AMB and ITC, confirming data from previous studies with amphotericin B and caspofungin against melanized C. neoformans (26, 29, 36). However, melanin was not efficient in protecting C. neoformans from fluconazole and itraconazole (29). In contrast, we demonstrated that melanized Paracoccidioides yeast cells show lower susceptibility to itraconazole. Da Silva et al. (26) did not find differences in MIC values between melanized and nonmelanized Paracoccidioides sp. yeasts. Further, our results confirm previous studies that have shown that synthetic melanin reduces the fungicidal activity of AMB, and also of ITC, which is new data (26, 29). Even though the addition of synthetic melanin resulted in a slight increase in the MIC values, these results were important to confirm its capacity to change the MIC values of the drugs tested. Scanning electronic microscopy showed that melanized Paracoccidioides sp. yeasts have electron-dense granules both on the surface and in the cytoplasm, a characteristic that is associated with the tight space between melanin granules observed at the cell wall, suggesting that the entry of drugs into the cytoplasm may be altered in these cells (8, 26, 31, 37). The levels of ROS and RNS indicated that melanized cells contained lower levels of free radicals than nonmelanized cells, confirming that melanin may function as a ROS scavenger generated by drug action (31, 38, 39). The roles of oxidative and nitrosative bursts caused by itraconazole in Cryptococcus gattii were previously demonstrated (38, 39) and also help to explain the role of melanin in protecting Paracoccidioides spp. from the antifungals.
Melanin is a molecule that presents many free carboxyl groups (—COOH) in its structure, which permits stable binding to different compounds (31, 40, 41). This characteristic explains the alterations in the absorbance spectra of drugs in the presence of melanin, a result that shows that melanin interacts with antifungal drugs. Some authors have shown that melanin can change the antimicrobial activity of a drug (31, 37, 40–42), a fact that was also observed in the present study, because melanized Paracoccidioides sp. cells showed lower susceptibility to ITC and AMB. The ability of melanin to bind to AMB has been demonstrated by other authors; however, the present study shows a small modification in the ITC spectrum (29, 37). Even though van Duin et al. (29) did not find a difference in the elemental composition of C:N:O after incubation of itraconazole with melanin, our results demonstrated the possibility that there is an alteration of the drug when incubated with melanin. Because this is a slight modification, we believe that the main mechanism by which melanin reduces ITC activity is by physically blocking entry of the drug into the yeast. In contrast, the prominent changes observed in the AMB spectrum suggest that melanin, in addition to blocking drug penetration and scavenging ROS and RNS, binds to the antifungal and may reduce its affinity for the ergosterol membrane.
In conclusion, our study demonstrates that melanin produced by Paracoccidioides sp. yeast cells likely acts as a protective factor during aPI and also during treatment with ITC and AMB. The melanin produced by Paracoccidioides spp. altered the efficacy of aPI and the efficacy of classical antifungal drugs in vitro, providing more resistance to treatment. Furthermore, our results showed that melanin functioned as a scavenger of ROS and NO, but not of ONOO, suggesting that ONOO may be the main radical responsible for fungal death after aPI. In addition, our results showed that melanin can interact with ITC, AMB, and TBO and, consequently, change their antifungal activities.
ACKNOWLEDGMENTS
This study was supported by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
We thank Gilvânia Ferreira Silva Santos for technical support. The manuscript was edited for English language by American Journal Experts.
REFERENCES
- 1.Prado M, Silva MB, Laurenti R, Travassos LR, Taborda CP. 2009. Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz 104:513–521. doi: 10.1590/S0074-02762009000300019. [DOI] [PubMed] [Google Scholar]
- 2.Shikanai-Yasuda MA, Telles Filho FDQ, Mendes RP, Colombo AL, Moretti ML. 2006. Guidelines in paracoccidioidomycosis. Rev Soc Bras Med Trop 39:297–310. (In Portuguese.) doi: 10.1590/S0037-86822006000300017. [DOI] [PubMed] [Google Scholar]
- 3.Shankar J, Restrepo A, Clemons KV, Stevens DA. 2011. Hormones and the resistance of women to paracoccidioidomycosis. Clin Microbiol Rev 24:296–313. doi: 10.1128/CMR.00062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teixeira MM, Theodoro RC, de Carvalho MJ, Fernandes L, Paes HC, Hahn RC, Mendoza L, Bagagli E, San-Blas G, Felipe MS. 2009. Phylogenetic analysis reveals a high level of speciation in the Paracoccidioides genus. Mol Phylogenet Evol 52:273–283. doi: 10.1016/j.ympev.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Parente JA, Borges CL, Bailao AM, Felipe MS, Pereira M, de Almeida Soares CM. 2008. Comparison of transcription of multiple genes during mycelia transition to yeast cells of Paracoccidioides brasiliensis reveals insights to fungal differentiation and pathogenesis. Mycopathologia 165:259–273. doi: 10.1007/s11046-007-9078-8. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira MDM, Theodoro RC, Oliveira FF, Machado GC, Hahn RC, Bagagli E, San-Blas G, Soares Felipe MS. 2014. Paracoccidioides lutzii sp. nov.: biological and clinical implications. Med Mycol 52:19–28. doi: 10.3109/13693786.2013.794311. [DOI] [PubMed] [Google Scholar]
- 7.Matute DR, McEwen JG, Puccia R, Montes BA, San-Blas G, Bagagli E, Rauscher JT, Restrepo A, Morais F, Nino-Vega G, Taylor JW. 2006. Cryptic speciation and recombination in the fungus Paracoccidioides brasiliensis as revealed by gene genealogies. Mol Biol Evol 23:65–73. doi: 10.1093/molbev/msj008. [DOI] [PubMed] [Google Scholar]
- 8.Taborda CP, da Silva MB, Nosanchuk JD, Travassos LR. 2008. Melanin as a virulence factor of Paracoccidioides brasiliensis and other dimorphic pathogenic fungi: a minireview. Mycopathologia 165:331–339. doi: 10.1007/s11046-007-9061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nosanchuk JD, Gomez BL, Youngchim S, Diez S, Aisen P, Zancope-Oliveira RM, Restrepo A, Casadevall A, Hamilton AJ. 2002. Histoplasma capsulatum synthesizes melanin-like pigments in vitro and during mammalian infection. Infect Immun 70:5124–5131. doi: 10.1128/IAI.70.9.5124-5131.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mednick AJ, Nosanchuk JD, Casadevall A. 2005. Melanization of Cryptococcus neoformans affects lung inflammatory responses during cryptococcal infection. Infect Immun 73:2012–2019. doi: 10.1128/IAI.73.4.2012-2019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silva MB, Thomaz L, Marques AF, Svidzinski AE, Nosanchuk JD, Casadevall A, Travassos LR, Taborda CP. 2009. Resistance of melanized yeast cells of Paracoccidioides brasiliensis to antimicrobial oxidants and inhibition of phagocytosis using carbohydrates and monoclonal antibody to CD18. Mem Inst Oswaldo Cruz 104:644–648. doi: 10.1590/S0074-02762009000400019. [DOI] [PubMed] [Google Scholar]
- 12.Gomez BL, Nosanchuk JD. 2003. Melanin and fungi. Curr Opin Infect Dis 16:91–96. doi: 10.1097/00001432-200304000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Casadevall A, Rosas AL, Nosanchuk JD. 2000. Melanin and virulence in Cryptococcus neoformans. Curr Opin Microbiol 3:354–358. doi: 10.1016/S1369-5274(00)00103-X. [DOI] [PubMed] [Google Scholar]
- 14.Gomez BL, Nosanchuk JD, Diez S, Youngchim S, Aisen P, Cano LE, Restrepo A, Casadevall A, Hamilton AJ. 2001. Detection of melanin-like pigments in the dimorphic fungal pathogen Paracoccidioides brasiliensis in vitro and during infection. Infect Immun 69:5760–5767. doi: 10.1128/IAI.69.9.5760-5767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.San-Blas G, Nino-Vega G. 2008. Paracoccidioides brasiliensis: chemical and molecular tools for research on cell walls, antifungals, diagnosis, taxonomy. Mycopathologia 165:183–195. doi: 10.1007/s11046-007-9040-9. [DOI] [PubMed] [Google Scholar]
- 16.Calzavara-Pinton P, Rossi MT, Sala R, Venturini M. 2012. Photodynamic antifungal chemotherapy. Photochem Photobiol 88:512–522. doi: 10.1111/j.1751-1097.2012.01107.x. [DOI] [PubMed] [Google Scholar]
- 17.Baltazar L, Ray A, Santos D, Cisalpino P, Friedman A, Nosanchuk JD. 2015. Antimicrobial photodynamic therapy: an effective alternative approach to control fungal infections. Front Microbiol 6:202. doi: 10.3389/fmicb.2015.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamblin MR, Hasan T. 2004. Photodynamic therapy: a new antimicrobial approach to infectious disease? Photochem Photobiol Sci 3:436–450. doi: 10.1039/b311900a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baltazar LDM, Soares BM, Carneiro HC, Avila TV, Gouveia LF, Souza DG, Ferreira MV, Pinotti M, Santos DDA, Cisalpino PS. 2013. Photodynamic inhibition of Trichophyton rubrum: in vitro activity and the role of oxidative and nitrosative bursts in fungal death. J Antimicrob Chemother 68:354–361. doi: 10.1093/jac/dks414. [DOI] [PubMed] [Google Scholar]
- 20.Plaetzer K, Krammer B, Berlanda J, Berr F, Kiesslich T. 2009. Photophysics and photochemistry of photodynamic therapy: fundamental aspects. Lasers Med Sci 24:259–268. doi: 10.1007/s10103-008-0539-1. [DOI] [PubMed] [Google Scholar]
- 21.Ferro S, Ricchelli F, Mancini G, Tognon G, Jori G. 2006. Inactivation of methicillin-resistant Staphylococcus aureus (MRSA) by liposome-delivered photosensitising agents. J Photochem Photobiol B 83:98–104. doi: 10.1016/j.jphotobiol.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 22.Peloi LS, Soares RR, Biondo CE, Souza VR, Hioka N, Kimura E. 2008. Photodynamic effect of light-emitting diode light on cell growth inhibition induced by methylene blue. J Biosci 33:231–237. doi: 10.1007/s12038-008-0040-9. [DOI] [PubMed] [Google Scholar]
- 23.Almeida LM, Zanoelo FF, Castro KP, Borissevitch IE, Soares CM, Goncalves PJ. 2012. Cell survival and altered gene expression following photodynamic inactivation of Paracoccidioides brasiliensis. Photochem Photobiol 88:992–1000. doi: 10.1111/j.1751-1097.2012.01112.x. [DOI] [PubMed] [Google Scholar]
- 24.Cruz RC, Werneck SM, Oliveira CS, Santos PC, Soares BM, Santos DA, Cisalpino PS. 2013. Influence of different media, incubation times, and temperatures for determining the MICs of seven antifungal agents against Paracoccidioides brasiliensis by microdilution. J Clin Microbiol 51:436–443. doi: 10.1128/JCM.02231-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenman HC, Mues M, Weber SE, Frases S, Chaskes S, Gerfen G, Casadevall A. 2007. Cryptococcus neoformans laccase catalyses melanin synthesis from both D- and L-DOPA. Microbiology 153:3954–3962. doi: 10.1099/mic.0.2007/011049-0. [DOI] [PubMed] [Google Scholar]
- 26.Da Silva MB, Marques AF, Nosanchuk JD, Casadevall A, Travassos LR, Taborda CP. 2006. Melanin in the dimorphic fungal pathogen Paracoccidioides brasiliensis: effects on phagocytosis, intracellular resistance and drug susceptibility. Microbes Infect 8:197–205. doi: 10.1016/j.micinf.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 27.Restrepo A, Jimenez BE. 1980. Growth of Paracoccidioides brasiliensis yeast phase in a chemically defined culture medium. J Clin Microbiol 12:279–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vidotto V, Defina N, Pugliese A, Aoki S, Nakamura K, Takeo K. 2002. Effect of different K+ concentrations on Cryptococcus neoformans phenoloxidase activity. Mycopathologia 156:171–176. [DOI] [PubMed] [Google Scholar]
- 29.van Duin D, Casadevall A, Nosanchuk JD. 2002. Melanization of Cryptococcus neoformans and Histoplasma capsulatum reduces their susceptibilities to amphotericin B and caspofungin. Antimicrob Agents Chemother 46:3394–3400. doi: 10.1128/AAC.46.11.3394-3400.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunha MM, Franzen AJ, Seabra SH, Herbst MH, Vugman NV, Borba LP, de Souza W, Rozental S. 2010. Melanin in Fonsecaea pedrosoi: a trap for oxidative radicals. BMC Microbiol 10:80. doi: 10.1186/1471-2180-10-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nosanchuk JD, Casadevall A. 2003. The contribution of melanin to microbial pathogenesis. Cell Microbiol 5:203–223. doi: 10.1046/j.1462-5814.2003.00268.x. [DOI] [PubMed] [Google Scholar]
- 32.Williamson PR, Wakamatsu K, Ito S. 1998. Melanin biosynthesis in Cryptococcus neoformans. J Bacteriol 180:1570–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prates RA, Fuchs BB, Mizuno K, Naqvi Q, Kato IT, Ribeiro MS, Mylonakis E, Tegos GP, Hamblin MR. 2013. Effect of virulence factors on the photodynamic inactivation of Cryptococcus neoformans. PLoS One 8:e54387. doi: 10.1371/journal.pone.0054387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radi R. 2013. Peroxynitrite, a stealthy biological oxidant. J Biol Chem 288:26464–26472. doi: 10.1074/jbc.R113.472936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gebicka L, Didik J. 2010. Oxidative stress induced by peroxynitrite. Postepy Biochem 56:103–106. (In Polish.) [PubMed] [Google Scholar]
- 36.Wang Y, Casadevall A. 1994. Growth of Cryptococcus neoformans in the presence of L-dopa decreases its susceptibility to amphotericin B. Antimicrob Agents Chemother 38:2648–2650. doi: 10.1128/AAC.38.11.2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nosanchuk JD, Casadevall A. 2006. Impact of melanin on microbial virulence and clinical resistance to antimicrobial compounds. Antimicrob Agents Chemother 50:3519–3528. doi: 10.1128/AAC.00545-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sangalli-Leite F, Scorzoni L, Mesa-Arango AC, Casas C, Herrero E, Gianinni MJ, Rodriguez-Tudela JL, Cuenca-Estrella M, Zaragoza O. 2011. Amphotericin B mediates killing in Cryptococcus neoformans through the induction of a strong oxidative burst. Microbes Infect 13:457–467. doi: 10.1016/j.micinf.2011.01.015. [DOI] [PubMed] [Google Scholar]
- 39.Ferreira GF, Baltazar LDM, Santos JR, Monteiro AS, Fraga LA, Resende-Stoianoff MA, Santos DA. 2013. The role of oxidative and nitrosative bursts caused by azoles and amphotericin B against the fungal pathogen Cryptococcus gattii. J Antimicrob Chemother 68:1801–1811. doi: 10.1093/jac/dkt114. [DOI] [PubMed] [Google Scholar]
- 40.Fukuda M, Sasaki K. 1990. Changes in the antibacterial activity of melanin-bound drugs. Ophthalmic Res 22:123–127. doi: 10.1159/000267011. [DOI] [PubMed] [Google Scholar]
- 41.Larsson BS. 1993. Interaction between chemicals and melanin. Pigment Cell Res 6:127–133. doi: 10.1111/j.1600-0749.1993.tb00591.x. [DOI] [PubMed] [Google Scholar]
- 42.Kaliszan R, Kaliszan A, Wainer IW. 1993. Prediction of drug binding to melanin using a melanin-based high-performance liquid chromatographic stationary phase and chemometric analysis of the chromatographic data. J Chromatogr 615:281–288. doi: 10.1016/0378-4347(93)80342-2. [DOI] [PubMed] [Google Scholar]