Abstract
Pseudomonas aeruginosa Liverpool epidemic strain (LES) infections in cystic fibrosis (CF) patients are associated with transmissibility and increased patient morbidity. This study was designed to assess the in vitro activities of cathelicidin LL-37 peptide (LL-37) and select cationic lipids against Pseudomonas aeruginosa LESB58 in CF sputum and in a setting mimicking the CF airway. We found that LL-37 naturally present in airway surface fluid and some nonpeptide cationic lipid molecules such as CSA-13, CSA-90, CSA-131, and D2S have significant, but broadly differing, bactericidal activities against P. aeruginosa LESB58. We observed strong inhibition of LL-37 bactericidal activity in the presence of purified bacteriophage Pf1, which is highly expressed by P. aeruginosa LES, but the activities of the cationic lipids CSA-13 and CSA-131 were not affected by this polyanionic virus. Additionally, CSA-13 and CSA-131 effectively prevent LESB58 biofilm formation, which is stimulated by Pf1 bacteriophage, DNA, or F-actin. CSA-13 and CSA-131 display strong antibacterial activities against different clinical strains of P. aeruginosa, and their activities against P. aeruginosa LESB58 and Xen5 strains were maintained in CF sputum. These data indicate that synthetic cationic lipids (mimics of natural antimicrobial peptides) are suitable for developing an effective treatment against CF lung P. aeruginosa infections, including those caused by LES strains.
INTRODUCTION
Pseudomonas aeruginosa chronic lung infections are the major cause of death in cystic fibrosis (CF) patients. The identification of the Liverpool epidemic strain (LES) in numerous children's CF centers challenged the previous belief that CF patients acquire only unique environmental strains of P. aeruginosa (1). LES infections were found to be associated with transmissibility (2), dominance over other P. aeruginosa populations in CF airways, increased patient morbidity (3), and frequent infections in parents of children with CF (4, 5). LES isolates display widely variable pathogenic characteristics (6). LES sequencing revealed that multiple inducible prophages with diverse infection properties have been conserved within the bacterial DNA (4, 7). When studied in a rat model of chronic lung infection, P. aeruginosa strains such as PAO1, PA14, and LESB58 demonstrated similar levels of in vivo growth but differences in virulence. These differences were further confirmed with biofilm and motility assays, where LESB58 produced more biofilm but had less capacity for motility than PAO1 and PA14 (8). Taking into consideration that LES isolates exhibit enhanced antimicrobial resistance, which is not fully understood but linked to specific mutations in efflux pump genes (9) and premature activation of quorum-sensing exoproducts (10), we hypothesized that cationic antibacterial peptides (CAPs) and their synthetic mimics, which use a nonspecific physicochemical mode of bacterial killing (11, 12), might represent promising new methods for eradicating this bacterium from lung infections. In a model of chronic P. aeruginosa pneumonia, 6 days of treatment with the cationic lipid squalamine (13) resulted in significant reductions in pulmonary bacterial count and pneumonia lesions with an efficacy comparable to colistin. Properties of squalamine and cationic antibacterial peptides were used in the development of a new class of synthetic antibacterial molecules, which includes cationic lipids such as ceragenins (14). Like natural antibacterial peptides (15, 16), synthetic cationic steroids (CSAs) are amphipathic (17, 18), relatively simple to prepare and purify, membrane active, and able to kill a broad range of Gram-positive and Gram-negative bacteria (11, 19). The ceragenin CSA-13 shows high biocompatibility in animal studies, supporting this compound's possible application in human treatment (20). In addition to its antibacterial activity, CSA-13 is able to inhibit host cell inflammatory responses to bacterial wall products (21).
In CF airways, P. aeruginosa predominantly adopts a pattern of biofilm growth that results in increased bacterial resistance to the host immune system and exogenous antibiotics and provides a niche for the generation of resistant organisms (22). The CF lung serves as a bacterial reservoir where P. aeruginosa is organized in a mucoid biofilm within the mucus. The current antibiotic therapy restricts but does not eradicate the bacteria. Therefore, new strategies to eliminate residential bacteria from the CF lung are needed (23). Natural polyelectrolytes such as DNA and F-actin have been shown to function as inducers and structural support systems of P. aeruginosa biofilm architecture (24). In addition, P. aeruginosa biofilm usually produces a large amount of a Pf1-like virus compared with phage production in planktonic P. aeruginosa cultures (25). This phage, Pf4, is densely produced in small colony variants of P. aeruginosa biofilm (26). An effective molecule designed to prevent and treat P. aeruginosa biofilm in CF lungs might target natural biopolymers involved in biofilm formation and/or effectively kill P. aeruginosa cells in a biofilm network. DNA/F-actin have previously been proposed as targets for treatment of P. aeruginosa chronic lung infections in CF patients (24).
We demonstrate that Pf1 bacteriophage significantly decreases the bactericidal activity of cathelicidin LL-37 peptide (LL-37) but does not interfere with antibacterial functions of synthetic cationic lipids such as CSA-13 and CSA-131. The ability of cationic lipids to prevent P. aeruginosa LESB58 biofilm formation and eradicate P. aeruginosa LESB58 bacteria from CF sputum in vitro indicates their potential to prevent and treat P. aeruginosa infections, including those caused by LES strains in CF airways.
MATERIALS AND METHODS
Materials.
Pseudomonas isolation agar (PIA), Luria-Bertani broth (LB), and tryptic soy broth (TSB) were purchased from Difco (Sparks, MD). LL-37 (LLGDFFRKSKEKIGKEFKRIVQRIKDFLRNLVPRTES) peptide was purchased from Peptide 2.0 (Chantilly, VA, USA) and Polish Peptide Laboratory (Łódź, Poland). P. aeruginosa strain Xen5, engineered through conjugation and transposition of a plasmid carrying transposon Tn5 luxCDABE, was purchased from Caliper Life Science Inc. (Alameda, CA, USA). Ceragenins were prepared as previously described (27) and were characterized by nuclear magnetic resonance and liquid chromatography/mass spectrometry. CF sputum samples were collected by spontaneous expectoration from patients attending the Adult Cystic Fibrosis Center, University of Pennsylvania Health System (institutional review board approval no. 803255). Informed consent was obtained from each patient before samples were collected. The CF sputum samples were collected from 6 subjects suffering from CF caused by homozygous or heterozygous mutations in the cystic fibrosis transmembrane conductance regulator (CFTR) gene (Table 1), with different degrees of obstruction as indicated by forced expiratory volume in 1 s (FEV1). DNA concentration in the samples, percentage of H2O, purulence, and pH were determined as described previously (28).
TABLE 1.
Demographics of patients that provided CF sputum samples used to assess LL-37, CSA-13, and CSA-131 activities against P. aeruginosa LESB58 and P. aeruginosa Xen5 (Fig. 1)a
| Patient no. | Age (yr) | Sexb | FEV1 (% of predicted) | Mutation | Sputum |
|||
|---|---|---|---|---|---|---|---|---|
| DNA (mg/g) | Percentage of H2O | Purulence | pH | |||||
| S1 | 55 | M | 96 | ΔF508/R117 H with 7T/9T | 0.66 | 97.85 | 4 | 7.04 |
| S2 | 27 | F | 84 | ΔF508 × 2 | 1.23 | 98.6 | 3 | 7.22 |
| S3 | 27 | M | 54 | ΔF508 × 2 | 7.95 | 96.5 | 4 | 6.71 |
| S4 | 39 | M | 78 | ΔF508/unknown | 1.82 | 96.8 | 3 | 6.86 |
| S5 | 24 | F | 24 | ΔF508/unknown | 1.98 | 93.3 | 5 | 6.95 |
| S6 | 23 | M | 85 | ΔF508 × 2 | 0.65 | 92.6 | 5 | 6.97 |
Patients included in this study were not treated either with Pulmozyme or with antibiotics. All patients have a homozygous or heterozygous mutation within the CFTR gene (ΔF508 is linked with deletion of the three nucleotides that comprise the codon for phenylalanine at position 508). A spirometry test to determine forced expiratory volume in 1 s (FEV1) was performed before sputum collection. After sample collection, the purulence index was determined visually (evaluation of color and apparent viscosity) by the same person. Values ranged from 1 for mucoid and fluid secretions to 5 for highly colored (green or yellow) and thick secretions. Intermediate values for secretions were defined as 2 for opaque and fluid, 3 for white and moderately thick, and 4 for poorly colored and thick.
M, male; F, female.
Antimicrobial testing. (i) MIC/MBC values.
Minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) were determined using bacteria at the logarithmic phase of growth. Antibacterial agents tobramycin (TOB), LL-37, CSA-13, and CSA-131 were tested against different clinical strains of P. aeruginosa isolated from CF sputum (∼105 CFU/ml). The MIC/MBC values were determined in LB using the microdilution method described by the reference Clinical and Laboratory Standards Institute (CLSI). Bacteria were incubated at 37°C for 18 to 24 h. The MIC values were determined versus bacterial concentrations of ∼5 × 105 CFU/ml, and the MBC was performed by plating each sample on cetrimide agar.
(ii) Killing assay.
Bacteria were grown overnight to reach a logarithmic phase of growth. Bacterial concentration was measured by spectrophotometry (optical density at 600 nm [OD600]) and confirmed by viable count on agar plates. To evaluate the antibacterial activities of tobramycin, LL-37, CSA-13, CSA-90, CSA-131, and D2S against P. aeruginosa LESB58 and different clinical strains of P. aeruginosa (n = 39), a bacterial killing assay was performed as described previously (21). In some experimental settings, Pf1 bacteriophage, DNA, or F-actin was added to incubation media. Bactericidal activities of LL-37, CSA-13, and CSA-131 against P. aeruginosa LESB58 and P. aeruginosa Xen5 were also tested with the addition of homogenized CF sputum in the presence of glass beads (50% dilution in phosphate-buffered saline [PBS]). The samples were treated with different concentrations of antibacterial agents (0 to 25 μg/ml). After a 1 h incubation at 37°C, the suspensions were placed on ice and diluted 10- to 1,000-fold. Ten-microliter aliquots of each dilution were spotted on cetrimide agar plates for ∼18 h at 37°C. The number of colonies at each dilution was counted the following morning. The CFU (CFU/milliliter) of the individual samples were determined from the dilution factor and were used to calculate the percentage of bacterial outgrowth.
(iii) Bacterial biofilm formation.
The biomass of biofilm formed from P. aeruginosa LESB58 bacteria suspended in LB (50%) with PBS by itself or containing polyelectrolytes such as DNA, F-actin, or Pf1 bacteriophage (0.1 to 1 mg/ml), developed in the presence of LL-37, CSA-13, and CSA-131, was assessed using crystal violet (CV) staining (0.1%) (29). Measurements of the chemiluminescence intensity of P. aeruginosa Xen5 biomass were performed as an additional determinant of biofilm viability. Chemiluminescence was evaluated using a Fuji Film LAS-300 system, and densitometry analysis was performed using Image Gauge (version 4.22) software (Fuji Photo Film Co., USA). In each experiment, an overnight culture of P. aeruginosa LESB58 or Xen5 in TSB was diluted to ∼105 CFU/ml, bacterial suspensions were placed in 96-well polystyrene plates, and a biofilm was allowed to form for 48 h. Bacteria adherent to the plate were considered a biofilm, and cells not adherent to the surface of the plate were considered planktonic and were washed out before CV staining/chemiluminescence measurements were performed.
(vi) Formation of DNA, F-actin, and Pf1 aggregates.
Dynamic light scattering (DLS) spectroscopy was used to assess the formation of Pf1, F-actin, and DNA aggregates in the presence of LL-37, CSA-13, and CSA-131. The average aggregate size (hydrodynamic diameter) was determined using a DynaPro 99 DLS instrument. The method measures the diffusion constant of the aggregates from the autocorrelation function of scattered light intensity. The diameter is calculated from the relation D = kT/6πη Rh, where D is the translational diffusion constant, k is Boltzmann's constant, T is temperature, η is the solvent viscosity, and Rh is the hydrodynamic radius. Briefly, the 100 μl samples of Pf1, F-actin (both at 0.1 mg/ml concentration), or DNA (0.3 mg/ml), suspended in isotonic (150 mM NaCl, 5 mM HEPES, 0.2 mM CaCl2, 0.12 mM MgCl2, pH 7.35) buffer, were placed in a cuvette and then incubated with different concentrations of LL-37, CSA-13, or CSA-131 (0.1 to 1,000 μg/ml) for 5 min before data acquisition.
(v) Atomic force microscopy.
Atomic force microscopy (AFM) images of LESB58 bacteria were generated in an air environment on a Dimension ICON (Bruker, Labsoft, Poland) operated in contact mode. All images shown are deflection images with scan rates of 1 Hz with a resolution of 512 pixels/line. The preparation of bacteria for analysis was performed as described previously (9). Briefly, for AFM evaluation of bacterial structure in control (nontreated) samples and in samples preincubated with LL-37, CSA-13, and CSA-131, a 10 μl drop of bacterial suspension was applied on cleaved mica (SPI Supplies, West Chester, PA).
Statistical analysis.
Data are reported as means ± standard deviations (SDs) from 3 to 6 experiments. Data analysis was performed using one-way analysis of variance (ANOVA) tests with a post hoc Bonferroni analysis test. Differences were considered statistically significant at a P value of <0.05.
RESULTS
Bactericidal activity against clinical strains of P. aeruginosa.
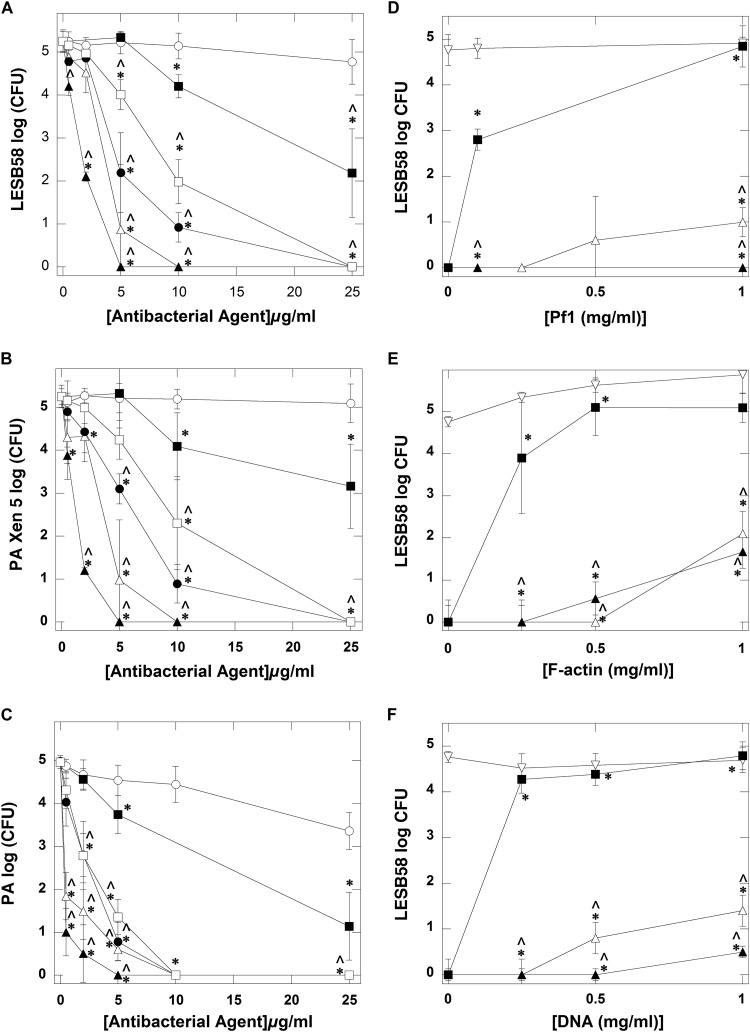
We evaluated the extent to which the activities of cationic antimicrobial agents such as TOB, LL-37, CSA-13, CSA-90, CSA-131, and D2S are compromised in the presence of negatively charged polyelectrolytes. To determine the bactericidal activities of LL-37 and cationic lipids, we used a conventional bacterial killing assay for P. aeruginosa strains. Susceptibility data from our study demonstrate that ceragenins (CSA-13, CSA-131, and CSA-90) have stronger bactericidal activities against P. aeruginosa LES58, Xen5, and other clinical strains than TOB and D2S do. Additionally, compared with LL-37, the ceragenins had significantly stronger activities against P. aeruginosa (Fig. 1A, B, and C), and CSA-131 displayed the strongest activity. The MIC/MBC values determined for TOB, LL-37, CSA-13, and CSA-131 against different strains of P. aeruginosa are shown in Table 2. CSA-13 and CSA-131 revealed potent antimicrobial activities against 40 tested strains, with MICs of 0.5 to 16 μg/ml. The MBC values for these ceragenins were 1 to 64 μg/ml, excluding one strain with an MBC of >256 µg/ml. The final two compounds, TOB and human cathelicidin LL-37, had MICs of 4 to 256 μg/ml and MBCs of 16 to 256 μg/ml. These data show that the activities of cationic lipids against highly resistant P. aeruginosa isolates and against the hypervirulent strain LESB58 (MIC and MBC of 4 μg/ml and 8 μg/ml, respectively) were much greater than the bactericidal activities of tobramycin and LL-37. This observation shows that cationic lipids are suitable for developing effective treatment against P. aeruginosa infections in the CF lung. Additionally, we investigated the bactericidal activity of LL-37 and cationic lipids against P. aeruginosa LES populations in the presence of Pf1 bacteriophage, F-actin, and DNA. As shown in Fig. 1D, Pf1 bacteriophage did not significantly affect the growth of LESB58 or the antibacterial activity of CSA-13 and CSA-131. However, the bactericidal activity of LL-37 is strongly inhibited by Pf1. Similar effects were produced by F-actin and DNA (Fig. 1E and F). Pf1, F-actin, and DNA inhibit the bactericidal activity of LL-37 more strongly than they inhibit ceragenins.
FIG 1.
Antibacterial activity of tobramycin (open circles), cathelicidin LL-37 peptide (filled squares), ceragenin CSA-13 (open triangles), CSA-90 (filled circles), CSA-131 (filled triangles), and D2S (open squares) against P. aeruginosa LESB58 (A), P. aeruginosa (PA) Xen5 (B), and five different clinical isolates of P. aeruginosa originating from cystic fibrosis sputum shown as average values (C). All results were compared to control. LESB58 bacterial growth (D through F) and bactericidal activity of LL-37 peptide (filled squares), CSA-13 (open triangles), and CSA-131 (filled triangles) in the presence of bacteriophage Pf1 (D), F-actin (E), and DNA (F). Open inverted triangles represent control group (LESB58 growth in the presence of Pf1, F-actin, or DNA [D, E, and F, respectively]). Error bars represent standard deviations from three to six measurements. * and ^ indicate statistical significance (P < 0.05) compared to control (logCFU with 0 μg/ml of tested antibacterial agent for A through C or LESB58 growth in the presence of Pf1, F-actin, or DNA for D through F) or samples treated with LL-37 peptide, respectively.
TABLE 2.
MICs and MBCs of tobramycin, LL-37, CSA-13, and CSA-131 against Pseudomonas aeruginosa
| Strain | MIC/MBC (μg/ml) for: |
|||
|---|---|---|---|---|
| TOB | LL-37 | CSA-13 | CSA-131 | |
| P. aeruginosaa | 128/>256 | >256 | 2/8 | 4/16 |
| P. aeruginosaa | 32/64 | >256 | 2/16 | 2/4 |
| P. aeruginosaa | >256 | >256 | 4/8 | 2/2 |
| P. aeruginosaa | 4/64 | >256 | 1/4 | 2/4 |
| P. aeruginosaa | 32/128 | >256 | 2/8 | 2/4 |
| P. aeruginosaa | 256/>256 | >256 | 4/16 | 4/4 |
| P. aeruginosaa | 128/256 | 256 | 8/8 | 2/2 |
| P. aeruginosaa | 16/32 | >256 | 1/8 | 4/4 |
| P. aeruginosaa | 64/>256 | >256 | 1/8 | 2/2 |
| P. aeruginosaa | 128/>256 | >256 | 0.5/4 | 2/4 |
| P. aeruginosaa | >256 | >256 | 1/1 | 0.5/4 |
| P. aeruginosaa | 256/>256 | >256 | 1/8 | 2/4 |
| P. aeruginosaa | 32/>256 | >256 | 16/64 | 4/4 |
| P. aeruginosaa | >256 | >256 | 4/8 | 2/4 |
| P. aeruginosaa | 256/>256 | 32/>256 | 1/4 | 4/16 |
| P. aeruginosaa | 256/>256 | >256 | 0.5/4 | 0.5/4 |
| P. aeruginosaa | 64/128 | >256 | 16/16 | 8/8 |
| P. aeruginosaa | 256/>256 | >256 | 8/16 | 4/4 |
| P. aeruginosaa | 16/16 | 64/256 | 2/8 | 2/4 |
| P. aeruginosaa | >256 | >256 | 4/16 | 4/8 |
| P. aeruginosaa | 4/>256 | >256 | 2/16 | 2/4 |
| P. aeruginosaa | 32/>256 | >256 | 16/16 | 4/16 |
| P. aeruginosaa | >256 | >256 | 1/2 | 2/4 |
| P. aeruginosaa | 32/64 | >256 | 4/8 | 4/4 |
| P. aeruginosaa | >256 | >256 | 8/16 | 4/8 |
| P. aeruginosaa | >256 | >256 | 8/8 | 4/4 |
| P. aeruginosaa | 32/>256 | >256 | 8/8 | 8/8 |
| P. aeruginosaa | 256/>256 | >256 | 8/16 | 4/4 |
| P. aeruginosaa | 256/>256 | >256 | 4/8 | 2/4 |
| P. aeruginosaa | >256 | >256 | 2/4 | 2/4 |
| P. aeruginosaa | 256/>256 | >256 | 2/8 | 2/4 |
| P. aeruginosaa | 64/128 | >256 | 8/8 | 2/8 |
| P. aeruginosaa | 256/>256 | >256 | 2/4 | 2/4 |
| P. aeruginosaa | 32/>256 | >256 | 4/4 | 2/16 |
| P. aeruginosaa | 32/64 | 128/256 | 2/8 | 4/4 |
| P. aeruginosaa | >256 | >256 | 1/4 | 0.5/2 |
| P. aeruginosaa | >256 | 32/64 | 2/8 | 2/8 |
| P. aeruginosaa | 256/>256 | >256 | 8/16 | 4/4 |
| P. aeruginosaa | 64/>256 | >256 | 16/32 | >256 |
| LESB58 | >256 | >256 | 4/8 | 4/8 |
| Xen5 | 64/128 | >256 | 8/8 | 8/8 |
Different clinical strains isolated from CF sputum.
LL-37, CSA-13, and CSA-131 activity against bacterial biofilm.
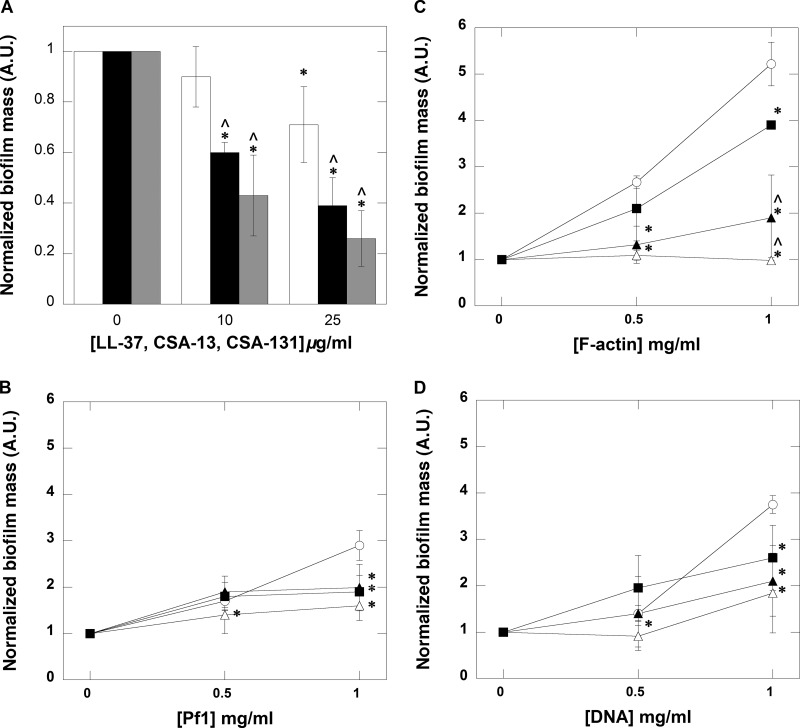
Previous studies demonstrated that ceragenins effectively kill bacteria in planktonic and biofilm settings. Here, we evaluated the ability to prevent biofilm growth with different concentrations of antibacterial agents. An LESB58 biofilm was formed for 48 h in 96-well polystyrene plates, and mass was assessed using CV staining. Figure 2A shows a decrease in biofilm mass after addition of LL-37, CSA-13, and CSA-131. These results show that the cationic lipids were able to prevent P. aeruginosa LESB58 biofilm formation more effectively than LL-37. Moreover, the antibiofilm activity of ceragenins was maintained in the presence of polyelectrolytes such as Pf1, F-actin, and DNA, each of which showed a strong ability to induce biofilm formation (Fig. 2B to D). Additionally, a decrease in chemiluminescence from a 48-h biofilm growth of P. aeruginosa Xen5 in the presence of CSA-13 and CSA-131 confirms their ability to decrease biofilm formation (data not shown).
FIG 2.
LL-37 (white column/filled squares), ceragenins CSA-13 (black column/open triangles), and CSA-131 (gray column/filled triangles) prevent (A) and decrease P. aeruginosa LESB58 biofilm formation induced with Pf1 bacteriophage, F-actin, or DNA (B, C, and D, respectively). In B through D, biofilm mass was formed in the presence of respective biopolymers. Control samples are indicated by open circles. Error bars represent standard deviations from three to five measurements. * and ^ indicate statistical significance (P < 0.05) compared to control or samples treated with LL-37 peptide, respectively.
LL-37, CSA-13, and CSA-131 bactericidal activities against P. aeruginosa LESB58 and P. aeruginosa Xen5 in CF sputum.
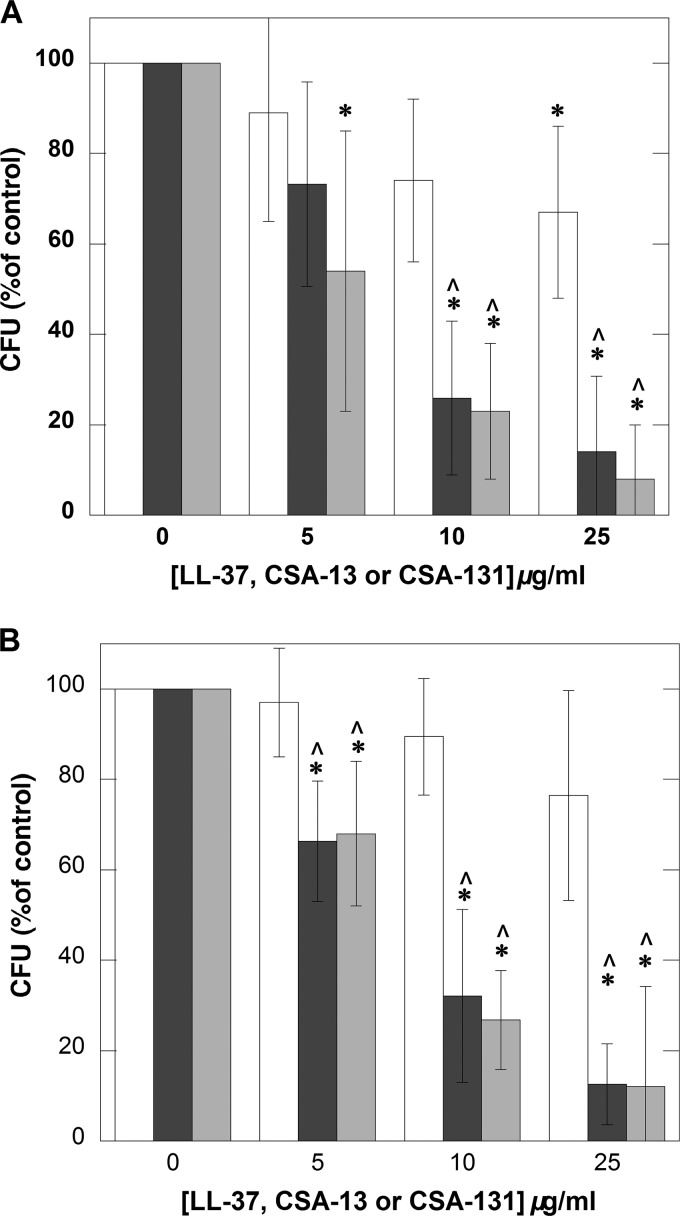
We compared the antibacterial activity of ceragenins against clinical strains of P. aeruginosa LESB58 and P. aeruginosa Xen5 to the antibacterial activity of LL-37 (Fig. 3A and B). This comparison, performed in diluted CF sputum samples, shows that ceragenins maintain stronger bactericidal activities in purulent sputum samples than peptide LL-37 does. Such a finding underlines their potential for future development of antibacterial agents that can effectively kill bacteria in CF airways.
FIG 3.
Outgrowth of P. aeruginosa LESB58 (A) and P. aeruginosa Xen5 (B) from CF sputum samples to which bacteria were added to achieve a final concentration of about 106 CFU/ml and 1 h incubation with and without different concentrations of LL-37 (white column), CSA-13 (black column), and CSA-131 (gray column) was performed. Error bars represent standard deviations from five measurements in which sputum samples collected from 5 different CF patients were used. * and ^ indicate statistical significance (P < 0.05) compared to control group (incubation without addition of tested agents) or samples treated with LL-37 peptide, respectively.
Aggregation of Pf1 bacteriophage, F-actin, and DNA in the presence of LL-37, CSA-13, and CSA-131.
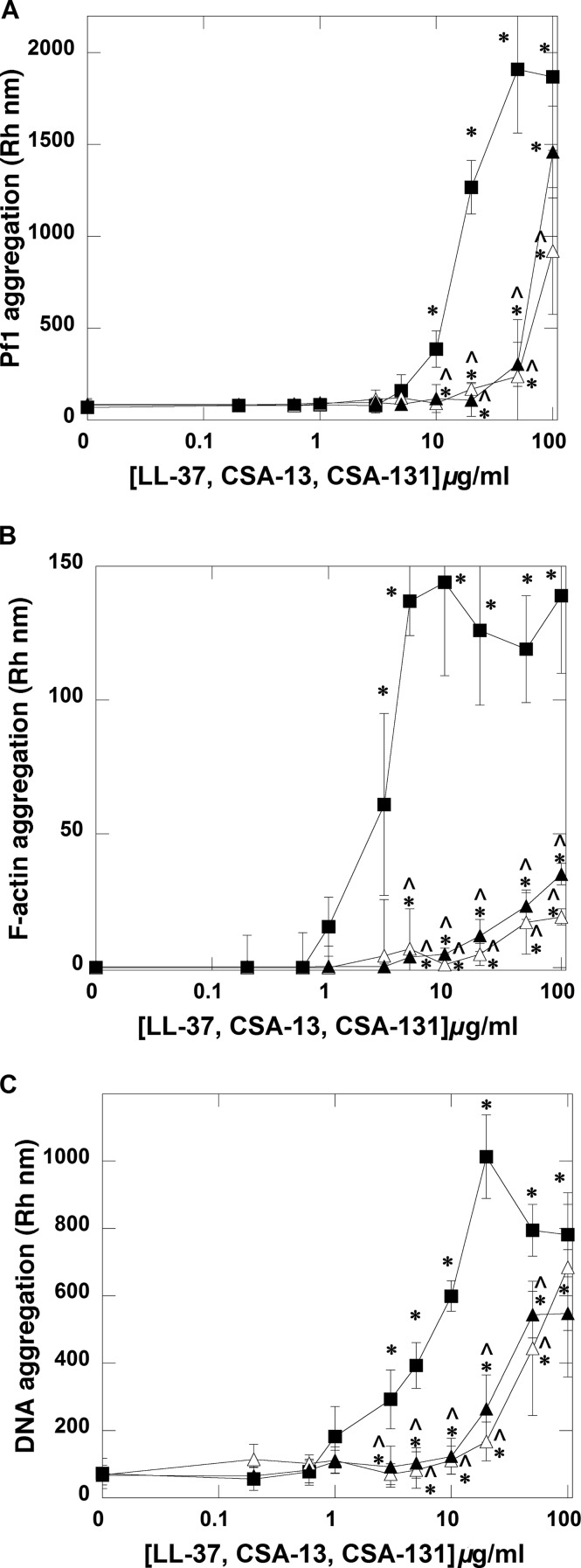
We detected the formation of Pf1, F-actin, and DNA aggregates in the presence of all tested antibacterial agents (Fig. 4A to C). However, with the same concentration of polyelectrolytes, the ability to form aggregates significantly differs between ceragenins and LL-37. More precisely, LL-37 has a much greater ability to bundle DNA, Pf1, and F-actin filaments than CSA-13 and CSA-131.
FIG 4.
Pf1 (A), F-actin (B), and DNA (C) aggregate formation in the presence of LL-37 peptide (filled squares), CSA-13 (open triangles), and CSA-131 (filled triangles). Error bars represent standard deviations from three to five measurements. * and ^ indicate statistical significance (P < 0.05) compared to control or samples treated with LL-37 peptide, respectively.
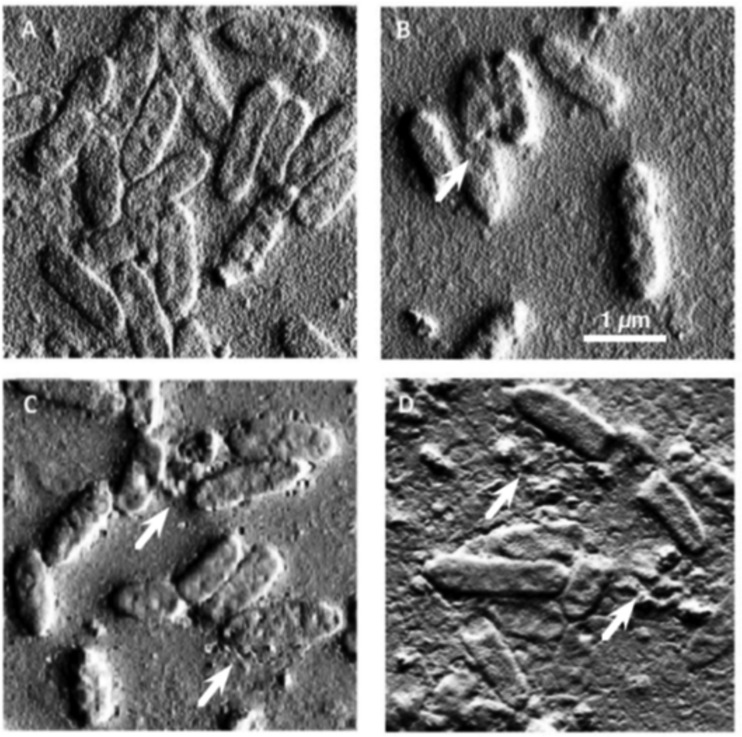
AFM.
Analysis of AFM images of Liverpool epidemic strain LESB58 (Fig. 5) exposed to LL-37, CSA-13, and CSA-131. Observed changes in surface morphology are indicative of bacterial membrane damage. These changes were very similar to previously observed PAO1 membrane damage under CSA-13 and LL-37 treatment (21).
FIG 5.
Atomic force microscope images of P. aeruginosa LESB58 bacteria before (A) and after treatment with LL-37 (B), CSA-13 (C), and CSA-131 (D). White arrows show the membrane damage occurring in samples treated with antibacterial agents. Data from one representative experiment performed in triplicate are shown.
DISCUSSION
Chronic airway infections resulting in lung damage are the major cause of shortened lifetime in patients suffering from cystic fibrosis. These infections in adult CF patients, predominantly caused by P. aeruginosa, are characterized by high rates of antibiotic resistance and a strong ability to form biofilm. Antibiotic resistance was especially observed in patients infected with highly virulent P. aeruginosa Liverpool epidemic strains (LES), which are known for long intracellular survival in bronchial epithelial cells of CF patients (6, 30). Analysis of the LESB58 genome revealed the presence of many large genomic islands, including five intact bacteriophages (LES prophages 2 to 6; one defective) and five nonphage islands (7). LESB58 is transmissible and is associated with increased morbidity (6). The aggressiveness of LES strains and their wide spectrum of antibiotic resistance underline the need to develop new treatment options, which require a better understanding of the mechanism leading to the high virulence of these strains. The capacity of human cathelicidin LL-37 to form aggregates with purified Pf1 phages, which results in inactivation of LL-37 bactericidal activity, provides a possible mechanism that might partially explain how LES escapes the natural defense of lung airways. This hypothesis is strongly supported by previous observations showing release of filamentous phages from LES cells and the presence of Pf1-like phages in CF sputum (25). Our study shows that phage molecules might bind and inactivate host antibacterial peptides. Stronger bactericidal activity of CSA-13 and CSA-131 than that of naturally present LL-37 against the LESB58 strain suggests that cationic lipids might be used to design a new treatment option that can potentially override the above mechanism of LES resistance. Net positive charge, charge distribution, and charge density of cationic lipids differ compared to those of the LL-37 peptide, which results in decreased charge-driven attractions between the cationic lipids tested here and negatively charged phage filaments (18, 31). One result of interactions between Pf1 anionic filaments and polycationic counterions might be an increase of elastic and viscous moduli due to network formation as phage filaments are bridged by LL-37 or other host defense molecules. Such phenomena might contribute to observed changes of the viscoelastic properties of CF sputum. Polymorphisms in regulatory genes are essential for phenotypic diversity among LES isolates, which in turn contribute to this strain's adaptability to various conditions in the CF lung (32).
In CF airways, the formation of biofilm represents a significant treatment challenge because despite aggressive antibiotic therapy and host immune response, P. aeruginosa is able to survive within the biofilm mass (24). Furthermore, LESB58 produces more biofilm than other P. aeruginosa strains (27). For this reason, our finding that Pf1 bacteriophage promotes formation of biofilm suggests that, similar to DNA and F-actin, phage filaments might be considered possible targets to inhibit this process. In addition, our study confirms a previous report that indicated a strong stimulatory effect on biofilm formation induced by negatively charged biopolymers (21, 24). All biopolymers used in our study, accumulated in CF sputum, stimulated biofilm formation that can be prevented by treatment with ceragenins. This observation agrees with a report that the production of Pf4 during growth of P. aeruginosa isolates in biofilm and under low oxygen conditions might signify either a protective or symbiotic role for Pf4 to allow P. aeruginosa growth under these conditions or else a mechanism for escape of Pf4 from a compromised host. A recent compelling study showed that Pf4 is required for P. aeruginosa biofilm formation and contributes to P. aeruginosa virulence in vivo (33). The potential of cationic lipids for treatment of lung P. aeruginosa infections is supported by data showing their high activity against different clinical isolates of P. aeruginosa obtained from CF sputum. At this point, we might speculate that the high activity of cationic lipids was observed due to their chemical nature and resistance to proteolysis, which very likely is responsible for the low antibacterial effects of LL-37. Additionally, our AFM observation of bacteria subjected to CSA-13 and CSA-131 treatment confirmed that the killing activity of LESB58 involves interaction with and damage of the bacterial surface.
These studies demonstrate that synthetic CSA-131 and CSA-13 offer an improvement over the natural LL-37 peptide in killing P. aeruginosa (notably the hypervirulent strain LESB58) and support the development and further study of ceragenins as novel treatment methods for CF lung infections caused by antibiotic-resistant and hypervirulent strains of P. aeruginosa.
ACKNOWLEDGMENTS
This work was supported by the National Science Center, Poland (grant UMO-2012/07/B/NZ6/03504 to R.B.), and the Leading National Research Centre (project 27/KNOW/2013).
We gratefully acknowledge the help of Marianne Ferrin and patients of the Adult Cystic Fibrosis Center of the University of Pennsylvania for providing sputum samples.
REFERENCES
- 1.Fothergill JL, Walshaw MJ, Winstanley C. 2012. Transmissible strains of Pseudomonas aeruginosa in cystic fibrosis lung infections. Eur Respir J 40:227–238. doi: 10.1183/09031936.00204411. [DOI] [PubMed] [Google Scholar]
- 2.Cheng K, Smyth RL, Govan JR, Doherty C, Winstanley C, Denning N, Heaf DP, van Saene H, Hart CA. 1996. Spread of beta-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 348:639–642. doi: 10.1016/S0140-6736(96)05169-0. [DOI] [PubMed] [Google Scholar]
- 3.Al-Aloul M, Crawley J, Winstanley C, Hart CA, Ledson MJ, Walshaw MJ. 2004. Increased morbidity associated with chronic infection by an epidemic Pseudomonas aeruginosa strain in CF patients. Thorax 59:334–336. doi: 10.1136/thx.2003.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.James CE, Fothergill JL, Kalwij H, Hall AJ, Cottell J, Brockhurst MA, Winstanley C. 2012. Differential infection properties of three inducible prophages from an epidemic strain of Pseudomonas aeruginosa. BMC Microbiol 12:216. doi: 10.1186/1471-2180-12-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCallum SJ, Gallagher MJ, Corkill JE, Hart CA, Ledson MJ, Walshaw MJ. 2002. Spread of an epidemic Pseudomonas aeruginosa strain from a patient with cystic fibrosis (CF) to non-CF relatives. Thorax 57:559–560. doi: 10.1136/thorax.57.6.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter ME, Fothergill JL, Walshaw MJ, Rajakumar K, Kadioglu A, Winstanley C. 2010. A subtype of a Pseudomonas aeruginosa cystic fibrosis epidemic strain exhibits enhanced virulence in a murine model of acute respiratory infection. J Infect Dis 202:935–942. doi: 10.1086/655781. [DOI] [PubMed] [Google Scholar]
- 7.Winstanley C, Langille MG, Fothergill JL, Kukavica-Ibrulj I, Paradis-Bleau C, Sanschagrin F, Thomson NR, Winsor GL, Quail MA, Lennard N, Bignell A, Clarke L, Seeger K, Saunders D, Harris D, Parkhill J, Hancock RE, Brinkman FS, Levesque RC. 2009. Newly introduced genomic prophage islands are critical determinants of in vivo competitiveness in the Liverpool epidemic strain of Pseudomonas aeruginosa. Genome Res 19:12–23. doi: 10.1101/gr.086082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kukavica-Ibrulj I, Bragonzi A, Paroni M, Winstanley C, Sanschagrin F, O'Toole GA, Levesque RC. 2008. In vivo growth of Pseudomonas aeruginosa strains PAO1 and PA14 and the hypervirulent strain LESB58 in a rat model of chronic lung infection. J Bacteriol 190:2804–2813. doi: 10.1128/JB.01572-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomas M, Doumith M, Warner M, Turton JF, Beceiro A, Bou G, Livermore DM, Woodford N. 2010. Efflux pumps, OprD porin, AmpC beta-lactamase, and multiresistance in Pseudomonas aeruginosa isolates from cystic fibrosis patients. Antimicrob Agents Chemother 54:2219–2224. doi: 10.1128/AAC.00816-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fothergill JL, Panagea S, Hart CA, Walshaw MJ, Pitt TL, Winstanley C. 2007. Widespread pyocyanin over-production among isolates of a cystic fibrosis epidemic strain. BMC Microbiol 7:45. doi: 10.1186/1471-2180-7-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lai XZ, Feng Y, Pollard J, Chin JN, Rybak MJ, Bucki R, Epand RF, Epand RM, Savage PB. 2008. Ceragenins: cholic acid-based mimics of antimicrobial peptides. Acc Chem Res 41:1233–1240. doi: 10.1021/ar700270t. [DOI] [PubMed] [Google Scholar]
- 12.Van Bambeke F, Mingeot-Leclercq MP, Struelens MJ, Tulkens PM. 2008. The bacterial envelope as a target for novel anti-MRSA antibiotics. Trends Pharmacol Sci 29:124–134. doi: 10.1016/j.tips.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 13.Moore KS, Wehrli S, Roder H, Rogers M, Forrest JN Jr, McCrimmon D, Zasloff M. 1993. Squalamine: an aminosterol antibiotic from the shark. Proc Natl Acad Sci U S A 90:1354–1358. doi: 10.1073/pnas.90.4.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hraiech S, Bregeon F, Brunel JM, Rolain JM, Lepidi H, Andrieu V, Raoult D, Papazian L, Roch A. 2012. Antibacterial efficacy of inhaled squalamine in a rat model of chronic Pseudomonas aeruginosa pneumonia. J Antimicrob Chemother 67:2452–2458. doi: 10.1093/jac/dks230. [DOI] [PubMed] [Google Scholar]
- 15.Bucki R, Pastore JJ, Randhawa P, Vegners R, Weiner DJ, Janmey PA. 2004. Antibacterial activities of rhodamine B-conjugated gelsolin-derived peptides compared to those of the antimicrobial peptides cathelicidin LL37, magainin II, and melittin. Antimicrob Agents Chemother 48:1526–1533. doi: 10.1128/AAC.48.5.1526-1533.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zanetti M, Gennaro R, Scocchi M, Skerlavaj B. 2000. Structure and biology of cathelicidins. Adv Exp Med Biol 479:203–218. doi: 10.1007/0-306-46831-X_17. [DOI] [PubMed] [Google Scholar]
- 17.Ding B, Taotofa U, Orsak T, Chadwell M, Savage PB. 2004. Synthesis and characterization of peptide-cationic steroid antibiotic conjugates. Org Lett 6:3433–3436. doi: 10.1021/ol048845t. [DOI] [PubMed] [Google Scholar]
- 18.Surel U, Niemirowicz K, Marzec M, Savage P, Bucki R. 2014. Ceragenins—a new weapon to fight multidrug resistant bacterial infections. Studia Medyczne 30:207–213. doi: 10.5114/ms.2014.45428. [DOI] [Google Scholar]
- 19.Epand RM, Epand RF, Savage PB. 2008. Ceragenins (cationic steroid compounds), a novel class of antimicrobial agents. Drug News Perspect 21:307–311. doi: 10.1358/dnp.2008.21.6.1246829. [DOI] [PubMed] [Google Scholar]
- 20.Saha S, Savage PB, Bal M. 2008. Enhancement of the efficacy of erythromycin in multiple antibiotic-resistant gram-negative bacterial pathogens. J Appl Microbiol 105:822–828. doi: 10.1111/j.1365-2672.2008.03820.x. [DOI] [PubMed] [Google Scholar]
- 21.Bucki R, Sostarecz AG, Byfield FJ, Savage PB, Janmey PA. 2007. Resistance of the antibacterial agent ceragenin CSA-13 to inactivation by DNA or F-actin and its activity in cystic fibrosis sputum. J Antimicrob Chemother 60:535–545. doi: 10.1093/jac/dkm218. [DOI] [PubMed] [Google Scholar]
- 22.Donlan RM, Costerton JW. 2002. Biofilms: survival mechanisms of clinically relevant microorganisms. Clin Microbiol Rev 15:167–193. doi: 10.1128/CMR.15.2.167-193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bjarnsholt T, Jensen PO, Fiandaca MJ, Pedersen J, Hansen CR, Andersen CB, Pressler T, Givskov M, Hoiby N. 2009. Pseudomonas aeruginosa biofilms in the respiratory tract of cystic fibrosis patients. Pediatr Pulmonol 44:547–558. doi: 10.1002/ppul.21011. [DOI] [PubMed] [Google Scholar]
- 24.Walker TS, Tomlin KL, Worthen GS, Poch KR, Lieber JG, Saavedra MT, Fessler MB, Malcolm KC, Vasil ML, Nick JA. 2005. Enhanced Pseudomonas aeruginosa biofilm development mediated by human neutrophils. Infect Immun 73:3693–3701. doi: 10.1128/IAI.73.6.3693-3701.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiteley M, Bangera MG, Bumgarner RE, Parsek MR, Teitzel GM, Lory S, Greenberg EP. 2001. Gene expression in Pseudomonas aeruginosa biofilms. Nature 413:860–864. doi: 10.1038/35101627. [DOI] [PubMed] [Google Scholar]
- 26.Webb JS, Lau M, Kjelleberg S. 2004. Bacteriophage and phenotypic variation in Pseudomonas aeruginosa biofilm development. J Bacteriol 186:8066–8073. doi: 10.1128/JB.186.23.8066-8073.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding B, Guan Q, Walsh JP, Boswell JS, Winter TW, Winter ES, Boyd SS, Li C, Savage PB. 2002. Correlation of the antibacterial activities of cationic peptide antibiotics and cationic steroid antibiotics. J Med Chem 45:663–669. doi: 10.1021/jm0105070. [DOI] [PubMed] [Google Scholar]
- 28.Deneuville E, Perrot-Minot C, Pennaforte F, Roussey M, Zahm JM, Clavel C, Puchelle E, de Bentzmann S. 1997. Revisited physicochemical and transport properties of respiratory mucus in genotyped cystic fibrosis patients. Am J Respir Crit Care Med 156:166–172. doi: 10.1164/ajrccm.156.1.9606123. [DOI] [PubMed] [Google Scholar]
- 29.Peeters E, Nelis HJ, Coenye T. 2008. Comparison of multiple methods for quantification of microbial biofilms grown in microtiter plates. J Microbiol Methods 72:157–165. doi: 10.1016/j.mimet.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 30.Frioni A, Conte MP, Cutone A, Longhi C, Musci G, di Patti MC, Natalizi T, Marazzato M, Lepanto MS, Puddu P, Paesano R, Valenti P, Berlutti F. 2014. Lactoferrin differently modulates the inflammatory response in epithelial models mimicking human inflammatory and infectious diseases. Biometals 27:843–856. doi: 10.1007/s10534-014-9740-9. [DOI] [PubMed] [Google Scholar]
- 31.Weiner DJ, Bucki R, Janmey PA. 2003. The antimicrobial activity of the cathelicidin LL37 is inhibited by F-actin bundles and restored by gelsolin. Am J Respir Cell Mol Biol 28:738–745. doi: 10.1165/rcmb.2002-0191OC. [DOI] [PubMed] [Google Scholar]
- 32.Jeukens J, Boyle B, Kukavica-Ibrulj I, Ouellet MM, Aaron SD, Charette SJ, Fothergill JL, Tucker NP, Winstanley C, Levesque RC. 2014. Comparative genomics of isolates of a Pseudomonas aeruginosa epidemic strain associated with chronic lung infections of cystic fibrosis patients. PLoS One 9:e87611. doi: 10.1371/journal.pone.0087611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rice SA, Tan CH, Mikkelsen PJ, Kung V, Woo J, Tay M, Hauser A, McDougald D, Webb JS, Kjelleberg S. 2009. The biofilm life cycle and virulence of Pseudomonas aeruginosa are dependent on a filamentous prophage. ISME J 3:271–282. doi: 10.1038/ismej.2008.109. [DOI] [PMC free article] [PubMed] [Google Scholar]