Abstract
Candida albicans is the most prevalent cause of fungemia worldwide. Its ability to develop resistance in patients receiving azole antifungal therapy is well documented. In a murine model of systemic infection, we show that ibuprofen potentiates fluconazole antifungal activity against a fluconazole-resistant strain, drastically reducing the fungal burden and morbidity. The therapeutic combination of fluconazole with ibuprofen may constitute a new approach for the management of antifungal therapeutics to reverse the resistance conferred by efflux pump overexpression.
TEXT
Candida invasive infections represent an increasing challenge for clinicians, with an assigned mortality rate of around 40% (1–3). The extensive use of fluconazole in both prophylaxis and therapy has resulted in the emergence of resistance (4–6).
Knowledge of the mechanisms underlying resistance is of crucial importance, since it might support approaches to achieve reversion, leading to the development of new therapeutic strategies. Alterations in the Candida albicans transcriptome induced by azole exposure have been extensively reported (5, 7, 8). However, few studies have tackled antifungal resistance reversion.
Previously, we showed that the antifungal resistance conferred by increased efflux activity, due to overexpression of the CDR1 and CDR2 genes, in Candida clinical strains can be reversed by ibuprofen (9, 10). This effect is also observed with other drugs, such as pyrazinamide (11) and amphotericin B (12). Ibuprofen is a nonsteroidal anti-inflammatory drug used for its antipyretic, analgesic, and anti-inflammatory effects.
In this study, we aimed to examine the in vivo reversion of fluconazole resistance by ibuprofen using a C. albicans systemic model of infection.
A C. albicans blood culture strain (CaS) susceptible to fluconazole, voriconazole, and posaconazole was used to induce an azole-resistant phenotype. For 60 days, a yeast suspension containing 106 cells in 10 ml of RPMI 1640 medium (Sigma) was incubated daily with fluconazole (Pfizer) at a final concentration of 16 μg/ml (the therapeutic serum level achieved during antifungal therapy) (13, 14). The MICs to azoles, fluconazole, voriconazole (Pfizer), and posaconazole (Schering-Plough) were determined for the parent strain and the successive fluconazole-exposed isolates, and the susceptibility profile was determined according to the CLSI M27-A3 protocol (15). For posaconazole, strains with an MIC of ≤0.06 μg/ml were considered susceptible (16). The repeated exposure of C. albicans to fluconazole resulted in the acquisition of an azole-cross-resistant phenotype (Table 1), achieved due to overexpression of efflux pump-encoding genes and the ERG11 gene (data not shown) displayed by the C. albicans resistant (CaR) strain.
TABLE 1.
In vitro development of azole resistance and its reversion by ibuprofen
| Strain | MIC (μg/ml)/phenotype fora: |
|||||
|---|---|---|---|---|---|---|
| FLC | FLC + Ibu | VRC | VRC + Ibu | PSC | PSC + Ibu | |
| CaS | 1/S | 1/S | 0.06/S | 0.06/S | 0.03/S | 0.06/S |
| CaR | >64/R | 2/S | >8/R | 0.06/S | >8/R | 0.125/R |
MICs to azoles, namely, fluconazole (FLC), voriconazole (VRC), and posaconazole (PSC), alone and in combination with subinhibitory concentration of ibuprofen (Ibu) (100 μg/ml). S, susceptible; R, resistant.
The azole-resistant phenotype of the CaR strain turned susceptible when the MICs to azoles were redetermined in the presence of 100 μg/ml ibuprofen, a concentration previously described to impair azole efflux (9, 10). These in vitro results demonstrate that ibuprofen potentiates azole fungistatic activity.
The pursuit of knowledge regarding the efflux pump mechanism in Candida arises from the homology between yeasts and human cells. In eukaryotic neoplasic cells, ATP-dependent drug efflux pumps, such as P-glycoprotein (P-gp), which is encoded by the MDR1 gene, are important mediators of resistance, contributing to the failure of cancer therapy. In the human kidney, ibuprofen can inhibit methotrexate efflux transporters (17). A similar effect has been described for FK506 (tacrolimus), a potent immunosuppressive agent that shows a synergistic effect when combined with antineoplasic agents on tumor cells, decreasing or even suppressing multidrug resistance by competing with cytotoxic drugs for the P-glycoprotein (18–20). Thus, a similar approach could be applied to C. albicans cells.
To investigate the potential clinical application of fluconazole and ibuprofen, in vivo experiments were conducted in a murine candidosis model approved by the Directorate General of Food and Veterinary Medicine of the European Union (authorization no. 6411).
Female specific-pathogen-free BALB/c mice (age, 6 to 8 weeks; weight, 17 to 20 g; Charles River Laboratories) were injected with 5 × 105 cells of the CaS or the CaR strain in 0.1 ml of sterile saline via the lateral tail vein (21). The fluconazole effective dose that reduced by 50% the pathological effects of intravenous (i.v.) C. albicans challenge relative to untreated mice (control) was defined as the 50% effective dose (ED50) (8). Therapy was initiated 3 h after the yeast challenge and was administered daily for a total of 3 days (8, 21). The mice were treated intraperitoneally with fluconazole (8 to 60 mg/kg of body weight/day) (8, 21), ibuprofen (10 or 20 mg/kg/day) (11, 22), or fluconazole (8 to 60 mg/kg of body weight/day) plus ibuprofen (10 or 20 mg/kg/day). The weight of each mouse was registered daily, and at day 4 postinfection, the mice were euthanized and the kidneys aseptically removed. The fungal burden was calculated as the number of CFU per gram of tissue, and the isolates were preserved for later MIC determination. For histological studies, the kidneys were processed for periodic acid-Schiff (PAS) staining.
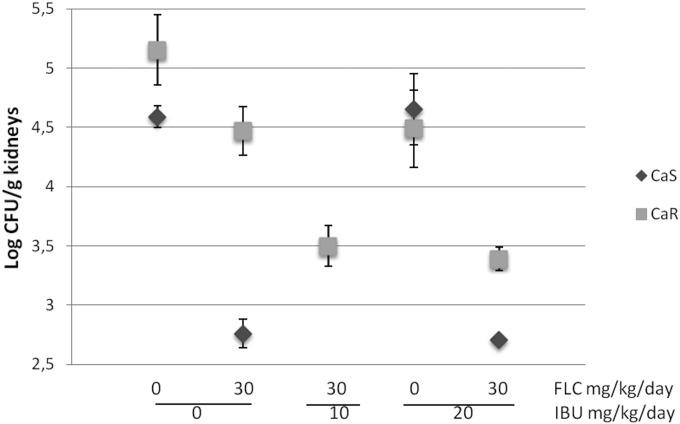
In mice infected with the CaS strain and treated with 30 mg/kg of body weight/day (ED50) of fluconazole, a significant reduction (P < 0.001) in yeast colonization in the kidney was found compared with that in the untreated mice (Fig. 1). In mice infected with the CaR strain, no significant reduction in fungal burden was achieved, even when treated with 60 mg/kg of fluconazole (data not shown). Mice infected with the CaS and CaR strains and treated with 10 or 20 mg/kg/day of ibuprofen did not show a reduction in fungal burden. However, when fluconazole was administered with ibuprofen, even at the lower concentration, a significant reduction in CaR fungal burden (P < 0.001) was observed (Fig. 1).
FIG 1.
In vivo antifungal potentiating effect between fluconazole and ibuprofen against C. albicans systemic infection. The log CFU per gram of kidney values are plotted as the mean and standard error. CaS, susceptible strain; CaR, resistant strain; FLC, fluconazole; Ibu, ibuprofen.
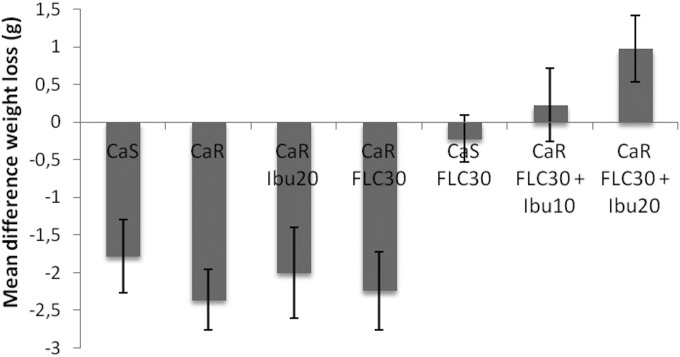
Mice infected with the CaR strain and treated with fluconazole plus ibuprofen showed the lowest weight loss (Fig. 2).
FIG 2.
Effect of the combined therapeutic fluconazole plus ibuprofen on mouse weight during systemic infection. Doses of drug are in milligrams per kilogram of body weight per day. Differences in weight loss between the first and the fourth day of infection are plotted as the mean values plus the respective standard errors. CaS, susceptible strain; CaR, resistant strain; FLC, fluconazole; Ibu, ibuprofen.
Interestingly, yeasts recovered from mouse kidneys retained their susceptibility profile, i.e., susceptible or resistant for the CaS or CaR strain, respectively.
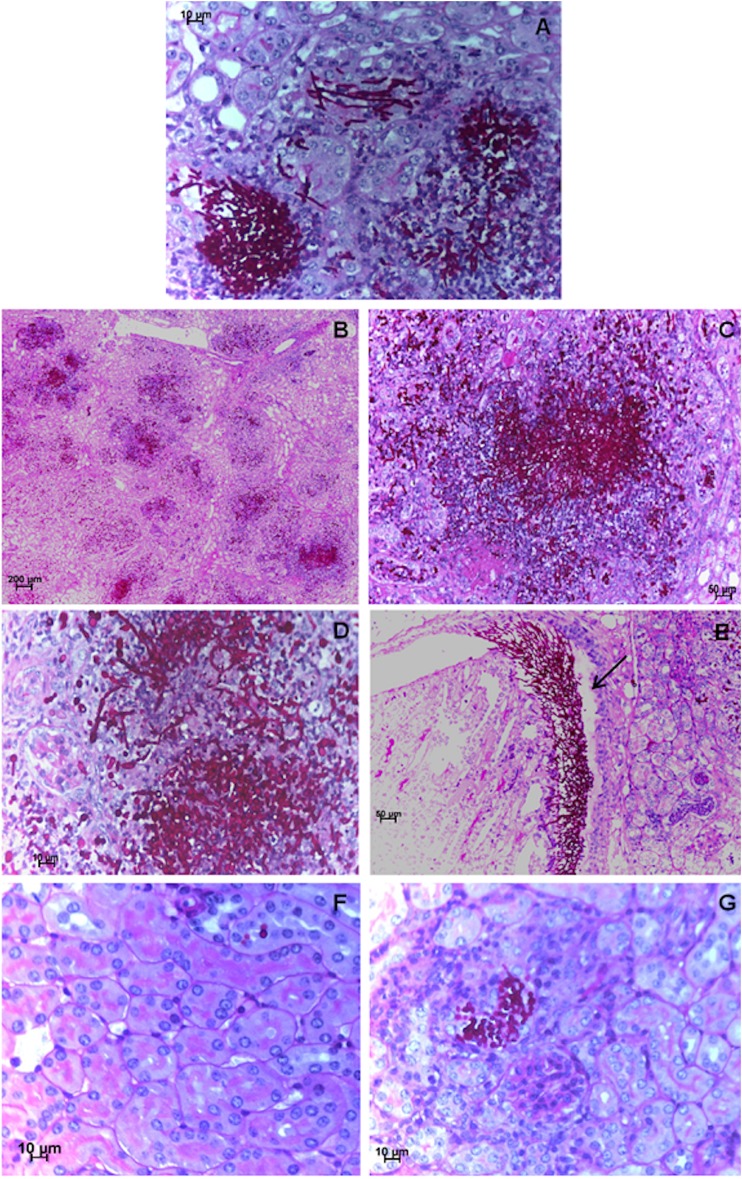
Histopathological sections of mouse kidneys confirmed the fungal burden quantitative analysis. Infection caused by CaR was evident in untreated and fluconazole-treated mice (Fig. 3A to E). At day four postinfection, untreated mice infected with the CaR strain revealed a dramatic increase in fungal colonization by different types of cells, yeasts and hypha, extensive tissue damage, and necrosis (Fig. 3A). An identical scenario was found in kidneys collected from mice treated with 30 mg/kg of fluconazole (Fig. 3B to E). In Fig. 3E, fungal cells were predominantly in the hyphal form and were apparently intact, forming a clear barrier to the progression of inflammatory leukocytes. Notably, in mice treated with fluconazole plus ibuprofen, the kidney tissue architecture was preserved, and the fungal cells were rare, all displaying a yeast form (Fig. 3F and G).
FIG 3.
C. albicans mouse kidney colonization. Representative example of kidney histology slides of PAS-stained paraffin sections of kidneys recovered from mice infected with a C. albicans-resistant (CaR) strain at day four postinfection and untreated (A) (×40 magnification), treated with 30 mg/kg/day of fluconazole (B to E) (×10, ×20, ×40 and ×20 magnification for the four panels, respectively), or treated with 30 mg/kg/day of fluconazole plus 20 mg/kg/day of ibuprofen (F and G) (×40 magnification). (E) Arrow shows a clear barrier to the progression of inflammatory leukocytes.
The phenotypic switching between yeast and hypha in C. albicans is often described as the major virulence factor, as hyphal formation is associated with elevated secretion of hydrolytic enzymes, direct tissue invasion, and adherence to host surfaces (23). Hyphal morphotypes are more invasive, and their extension is essential for dissemination and the subsequent events responsible for the gross damage of tissues, which are commonly observed in the kidneys of infected mice (24, 25).
Mice treated with fluconazole and ibuprofen experienced clearance of infection, recovery of body weight, and the conservation of tissue architecture, having scarce fungal cells in the kidneys, being predominantly yeast forms. The hypothesis that the presence of ibuprofen may target the regulation of the morphological switch from yeast to hypha thus deserves more attention and should be a subject of future in-depth research.
C. albicans cells recovered from mice treated with fluconazole plus ibuprofen still displayed a fluconazole-resistant phenotype. Consequently, we can conclude that the presence of ibuprofen is crucial and mandatory for the reversion of azole resistance. The in vivo assays clearly demonstrate that ibuprofen potentiates the antifungal activity of fluconazole and reduces the virulence of C. albicans. Since it is not immunosuppressive, its anti-inflammatory activity has advantages over FK506 (26). Further studies are being addressed to uncover the mechanism of ibuprofen on yeast cell physiology and to assess its influence on the dynamics of antifungal resistance induction and reversion. Ibuprofen in combination with fluconazole might play a relevant role in a therapeutic strategy for severe fungal infections.
ACKNOWLEDGMENTS
We thank Luisa Guardão for her assistance regarding animal experimentation and Isabel Santos for her excellent technical assistance.
This work was supported by the Fundação para a Ciência e a Tecnologia (FCT) under projects PTDC/DTP-EPI/1660/2012 and PTDC/EBB-BIO/119356/2010.
REFERENCES
- 1.Costa-de-Oliveira S, Pina-Vaz C, Mendonça D, Gonçalves Rodrigues A. 2008. A first Portuguese epidemiological survey of fungaemia in a university hospital. Eur J Clin Microbiol Infect Dis 27:365–374. doi: 10.1007/s10096-007-0448-4. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125:S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Arendrup MC, Dzajic E, Jensen RH, Johansen HK, Kjaeldgaard P, Knudsen JD, Kristensen L, Leitz C, Lemming LE, Nielsen L, Olesen B, Rosenvinge FS, Røder BL, Schønheyder HC. 2013. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect 19:E343–E353. doi: 10.1111/1469-0691.12212. [DOI] [PubMed] [Google Scholar]
- 4.Hof H. 2008. Is there a serious risk of resistance development to azoles among fungi due to the widespread use and long-term application of azole antifungals in medicine? Drug Resist Updat 11:25–31. doi: 10.1016/j.drup.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 5.Lepak A, Nett J, Lincoln L, Marchillo K, Andes D. 2006. Time course of microbiologic outcome and gene expression in Candida albicans during and following in vitro and in vivo exposure to fluconazole. Antimicrob Agents Chemother 50:1311–1319. doi: 10.1128/AAC.50.4.1311-1319.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Anderson JB. 2005. Evolution of antifungal-drug resistance: mechanisms and pathogen fitness. Nat Rev Microbiol 3:547–556. doi: 10.1038/nrmicro1179. [DOI] [PubMed] [Google Scholar]
- 7.Liu TT, Lee RE, Barker KS, Lee RE, Wei L, Homayouni R, Rogers PD. 2005. Genome-wide expression profiling of the response to azole, polyene, echinocandin, and pyrimidine antifungal agents in Candida albicans. Antimicrob Agents Chemother 49:2226–2236. doi: 10.1128/AAC.49.6.2226-2236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacCallum DM, Coste A, Ischer F, Jacobsen MD, Odds FC, Sanglard D. 2010. Genetic dissection of azole resistance mechanisms in Candida albicans and their validation in a mouse model of disseminated infection. Antimicrob Agents Chemother 54:1476–1483. doi: 10.1128/AAC.01645-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pina-Vaz C, Rodrigues AG, Costa-de-Oliveira S, Ricardo E, Mårdh PA. 2005. Potent synergic effect between ibuprofen and azoles on Candida resulting from blockade of efflux pumps as determined by FUN-1 staining and flow cytometry. J Antimicrob Chemother 56:678–685. doi: 10.1093/jac/dki264. [DOI] [PubMed] [Google Scholar]
- 10.Ricardo E, Costa-de-Oliveira S, Dias AS, Guerra J, Rodrigues AG, Pina-Vaz C. 2009. Ibuprofen reverts antifungal resistance on Candida albicans showing overexpression of CDR genes. FEMS Yeast Res 9:618–625. doi: 10.1111/j.1567-1364.2009.00504.x. [DOI] [PubMed] [Google Scholar]
- 11.Byrne ST, Denkin SM, Zhang Y. 2007. Aspirin and ibuprofen enhance pyrazinamide treatment of murine tuberculosis. J Antimicrob Chemother 59:313–316. doi: 10.1093/jac/dkl486. [DOI] [PubMed] [Google Scholar]
- 12.Venturini TP, Rossato L, Spader TB, Tronco-Alves GR, Azevedo MI, Weiler CB, Santurio JM, Alves SH. 2011. In vitro synergisms obtained by amphotericin B and voriconazole associated with non-antifungal agents against Fusarium spp. Diagn Microbiol Infect Dis 71:126–130. doi: 10.1016/j.diagmicrobio.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 13.Azanza JR, Garcia-Quetglas E, Sadaba B. 2007. Pharmacology of azoles. Rev Iberoam Micol 24:223–227. (In Spanish.) doi: 10.1016/S1130-1406(07)70047-5. [DOI] [PubMed] [Google Scholar]
- 14.Pinto e Silva AT, Costa-de-Oliveira S, Silva-Dias A, Pina-Vaz C, Rodrigues AG. 2009. Dynamics of in vitro acquisition of resistance by Candida parapsilosis to different azoles. FEMS Yeast Res 9:626–633. doi: 10.1111/j.1567-1364.2009.00508.x. [DOI] [PubMed] [Google Scholar]
- 15.CLSI. 2008. Reference method for broth dilution antifungal susceptibility testing of yeasts, 3rd ed CLSI document M27-A3. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 16.Pfaller MA, Diekema DJ. 2012. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol 50:2846–2856. doi: 10.1128/JCM.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El-Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. 2007. Interaction of nonsteroidal anti-inflammatory drugs with multidrug resistance protein (MRP) 2/ABCC2- and MRP4/ABCC4-mediated methotrexate transport. J Pharmacol Exp Ther 320:229–235. doi: 10.1124/jpet.106.110379. [DOI] [PubMed] [Google Scholar]
- 18.Cardenas ME, Cruz MC, Del Poeta M, Chung N, Perfect JR, Heitman J. 1999. Antifungal activities of antineoplastic agents: Saccharomyces cerevisiae as a model system to study drug action. Clin Microbiol Rev 12:583–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Farmer JD Jr, Lane WS, Friedman J, Weissman I, Schreiber SL. 1991. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 66:807–815. doi: 10.1016/0092-8674(91)90124-H. [DOI] [PubMed] [Google Scholar]
- 20.Arceci RJ, Stieglitz K, Bierer BE. 1992. Immunosuppressants FK506 and rapamycin function as reversal agents of the multidrug resistance phenotype. Blood 80:1528–1536. [PubMed] [Google Scholar]
- 21.Zhang YQ, Gamarra S, Garcia-Effron G, Park S, Perlin DS, Rao R. 2010. Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog 6:e1000939. doi: 10.1371/journal.ppat.1000939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cherif A, Khrouf N, Jabnoun S, Mokrani C, Amara MB, Guellouze N, Kacem S. 2008. Randomized pilot study comparing oral ibuprofen with intravenous ibuprofen in very low birth weight infants with patent ductus arteriosus. Pediatrics 122:e1256–e1261. doi: 10.1542/peds.2008-1780. [DOI] [PubMed] [Google Scholar]
- 23.Peters BM, Palmer GE, Nash AK, Lilly EA, Fidel PL Jr, Noverr MC. 2014. Fungal morphogenetic pathways are required for the hallmark inflammatory response during Candida albicans vaginitis. Infect Immun 82:532–543. doi: 10.1128/IAI.01417-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lionakis MS, Lim JK, Lee CC, Murphy PM. 2011. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun 3:180–199. doi: 10.1159/000321157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacobsen ID, Wilson D, Wachtler B, Brunke S, Naglik JR, Hube B. 2012. Candida albicans dimorphism as a therapeutic target. Expert Rev Anti Infect Ther 10:85–93. doi: 10.1586/eri.11.152. [DOI] [PubMed] [Google Scholar]
- 26.Rainsford KD. 2009. Ibuprofen: pharmacology, efficacy and safety. Inflammopharmacology 17:275–342. doi: 10.1007/s10787-009-0016-x. [DOI] [PubMed] [Google Scholar]