Abstract
Bedaquiline (Sirturo) and delamanid (Deltyba) have recently been approved by the regulatory authorities for treatment of multidrug-resistant tuberculosis (MDR-TB). Antimicrobial susceptibility testing is not established for either substance. On the basis of the use of the MGIT 960 system equipped with EpiCenter/TB eXiST, we determined a mean bedaquiline MIC for wild-type strains of 0.65 mg/liter (median, 0.4 mg/liter) and an epidemiological cutoff (ECOFF) of 1.6 mg/liter; for delamanid, a mean wild-type drug MIC of 0.013 mg/liter (median, 0.01 mg/liter) and an ECOFF of 0.04 mg/liter were determined.
TEXT
Globally, 3.5% of new and 20.5% of previously treated tuberculosis (TB) cases were estimated to have represented multidrug-resistant TB (MDR-TB) in 2013 (1). Bedaquiline (Sirturo [formerly known as TMC207 and R207910]; marketed by Janssen Therapeutics, Titusville, NJ, USA) is the lead compound of a series of recently discovered diarylquinolines, first described in 2005 (2). The U.S. Food and Drug Administration (FDA) approved bedaquiline for the treatment of adults with MDR-TB in 2012 (3). Because of the new mechanism of action of bedaquiline—the compound acts via inhibition of mycobacterial ATP synthase (AtpE)—it has been postulated that antimicrobial susceptibility testing (AST) is not needed in patients who have never received bedaquiline (4). However, cross-resistance between bedaquiline and the antimycobacterial drug clofazimine through overproduction of the MmpL5 efflux pump has recently been described (5, 6). Thus, resistance may develop independently of treatment with bedaquiline (2, 7). Delamanid (Deltyba [previously known as OPC-67683]; marketed by Otsuka Novel Products GmbH, Munich, Germany) was approved by the European Medicines Agency (EMA) in April 2014. The mechanism of action of delamanid is incompletely understood; delamanid is suggested to inhibit production of methoxymycolic acid and ketomycolic acid (8). Similarly to the related drug PA-824, delamanid is a prodrug requiring activation by the mycobacterial F420 system, including the nitroreductase Ddn (Rv3547) (8–10). Delamanid resistance is thought to arise from mutations in the mycobacterial F420 genes (ddn, fgd1, fbiA, fbiB, and fbiC) associated with the prodrug's activation (8, 11). The spontaneous rate of delamanid resistance has been reported to be as high as 6.44 × 10−6 to 4.19 × 10−5, emphasizing the need to protect delamanid with other active anti-TB drugs during therapy (9).
Initially, AST of bedaquiline was reported using radiometric Bactec 460TB (BD, Franklin Lakes, NJ, USA), production of which has since been discontinued (2). Reported MIC90s for delamanid range from 0.006 mg/liter to 0.05 mg/liter (depending on the test system) across Mycobacterium tuberculosis isolates (8, 9, 12). Ten years after the drugs' discoveries, established protocols for automated in vitro AST of bedaquiline and delamanid are still not available. To establish procedures for bedaquiline and delamanid AST, we used well-characterized, fully drug-susceptible clinical M. tuberculosis strains of bedaquiline and delamanid treatment-naive patients, MDR-TB strains, and subsequent isolates of a well-characterized extensively drug-resistant (XDR) strain (6). It has been speculated that the phylogenetic lineage of the M. tuberculosis complex may affect innate drug susceptibility (13). To assess the phylogenetic diversity of the set of strains studied, all M. tuberculosis strains included underwent genotypic characterization by mycobacterial interspersed repetitive-unit–variable-number tandem-repeat (MIRU-VNTR) analysis using a GenoScreen MIRU-VNTR typing kit (GenoScreen, Paris, France) according to the manufacturer's description. In order to determine the quality control (QC) MIC value, the pan-susceptible M. tuberculosis H37Rv reference strain was used. Details about the resistance patterns of the strains and genotypes are shown in Table S1 and S2 in the supplemental material. With the view to facilitating implementation in the routine laboratories, we used a semiautomated MGIT 960 system and EpiCenter software equipped with a TB eXiST module for quantitative drug susceptibility testing (14). The MGIT 960 platform is a fully automated system that uses a fluorescence-quenching-based oxygen sensor for growth detection. This system is widely used in routine laboratories for AST of M. tuberculosis. As bedaquiline and delamanid were not available to us as pure substances (supply of bedaquiline was denied; supply of delamanid would have been associated with unacceptable binding conditions), we decided to establish AST using tablet formulations. Based on the accompanying prescription information, the composition and drug content of the tablets were accessible. For bedaquiline, the tablet contained 100 mg active compound as well as colloidal anhydrous silica, croscarmellose sodium, hypromellose 2910, lactose monohydrate, magnesium stearate, corn starch, microcrystalline cellulose, and polysorbate 20 (15). For delamanid, one tablet contained 50 mg of active compound and, according to the summary of product characteristics as provided by the producer, hypromellose phthalate, povidone, all-rac-α-tocopherol, cellulose, microcrystalline, sodium starch glycolate (type A), carmellose calcium, colloidal hydrated silica, magnesium stearate, lactose monohydrate, hypromellose, macrogol 8000, titanium dioxide, talc, and yellow iron oxide (E172) (16). After the tablet was ground, the powder was dissolved in dimethyl sulfoxide (DMSO; Sigma D5879) and stored in small aliquots at −80°C. Test concentrations were obtained by serial 2-fold dilutions in DMSO. After thawing, stock solutions were used for same-day experiments. The stabilities of stock solutions for both drugs were assessed in parallel. For AST, MGIT tubes supplemented with 0.8 ml of oleic acid-albumin-dextrose-catalase (OADC) supplement (Becton Dickinson) were inoculated with 0.2 ml of the drug in DMSO solution and 0.5 ml of the test strain suspension (final DMSO concentration, 2.4%). For preparation of the drug-free-growth control tube, the organism suspension was diluted 1:100 with sterile saline solution, and then 0.5 ml was inoculated into the tube (for proportion testing) containing 2.4% (vol/vol) DMSO. The bacterial suspensions were prepared from MGIT subcultures. Results were interpreted as follows. At the time when the growth unit (GU) value for the drug-free control tube was >400, the strain was categorized as resistant (R) if the GU of the drug-containing tube was ≥100. If the GU value for the drug-containing tube was <100 at this time point, the strain was categorized as sensitive (S). The MIC of each strain was defined as the lowest drug concentration that was categorized as sensitive per the definition above. According to EUCAST (the European Committee on Antimicrobial Susceptibility Testing), the epidemiological cutoff (ECOFF) value is the MIC value identifying the upper limit for the wild-type population (17). The ECOFF can be estimated by visual inspection of a histographic population analysis of the tested strains (eyeball method) or calculated statistically (18, 19). We used visual inspection and a receiver operating characteristic (ROC) curve-based method to determine the ECOFF (20). Drug stability was tested in four series of 11 different drug concentrations in MGIT tubes using M. tuberculosis H37Rv as the test strain. For the first series, no preincubation was chosen. For the second series, MGIT tubes were preincubated without a bacterial inoculum for 1 week. For the third series, a preincubation time of 2 weeks was chosen. And for the fourth series, a preincubation time of 3 weeks was chosen. The results of all measurements were compared. No difference in susceptibility patterns for all series was detected for bedaquiline. For delamanid, MIC values 1 dilution higher were measured after 2 weeks and MIC values 2 dilutions higher were measured after 3 weeks, indicating a stability issue.
We tested 10 wild-type, fully drug-susceptible M. tuberculosis strains isolated between 2011 and 2014 from bedaquiline and delamanid treatment-naive patients. Due to the limited amount of bedaquiline and delamanid available, we chose 6 to 10 concentrations for AST (see Tables S1 and S2 in the supplemental material). For bedaquiline, the MIC arithmetic mean for the wild-type strain was 0.54 mg/liter and the median was 0.4 mg/liter. M. tuberculosis H37Rv had a drug MIC of 0.4 mg/liter. In an analysis of 12 MDR and pre-XDR isolates, the arithmetic mean of the drug MIC was 0.77 mg/liter and the median of the MIC was 0.8 mg/liter (P > 0.05 for fully drug-susceptible strains versus MDR strains [nonsignificant difference between medians]). The overall drug MIC arithmetic mean for the susceptible phenotype was 0.65 mg/liter; the overall median was 0.4 mg/liter. Two XDR isolates with a bedaquiline-associated resistance mutation (Rv0678 fMet1Ala) that also confers cross-resistance to clofazimine had a drug MIC of 6.4 mg/liter (6). Using the eyeball method for ECOFF determination, a value of 1.6 mg/liter can be supposed (Fig. 1A). This eyeball-derived ECOFF was confirmed by a ROC-based method at a >90% specificity level. The 10 strains from treatment-naive patients showed delamanid MIC values between 0.005 and 0.04 mg/liter. The delamanid MIC arithmetic mean for the wild-type strain was 0.016 mg/liter; the median was 0.01 mg/liter. M. tuberculosis H37Rv had a drug MIC of 0.01 mg/liter. The 12 MDR and pre-XDR strains had drug MIC values between 0.005 and 0.04 mg/liter. The overall drug MIC arithmetic mean for the susceptible phenotype was 0.013 mg/liter; the overall median was 0.01 mg/liter. A total of 3 XDR isolates from a patient with acquired delamanid resistance (case report in preparation) showed drug MIC values of >0.32 mg/liter (see Table S2). We propose an eyeball-derived ECOFF of 0.04 mg/liter (Fig. 1B). The ROC curve methodology could not be applied for delamanid, due to the lack of exact drug MIC values for the three resistotype isolates.
FIG 1.
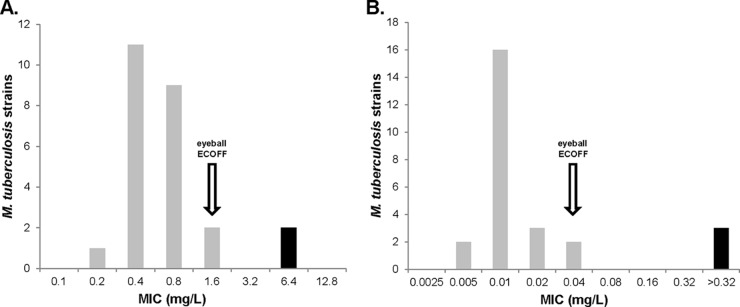
Distribution analysis of MIC values for bedaquiline (A) and delamanid (B). Proposed ECOFF values are marked with an arrow. Wild-type isolates are indicated by gray bars; resistant isolates are indicated by black bars.
The published MIC values for M. tuberculosis H37Rv (bedaquiline MIC, 0.03 mg/liter; delamanid MIC, 0.002 mg/liter) are considerably lower than those found in our study (2, 21). This probably reflects a systematic difference in methodology. Both bedaquiline and delamanid show extensive protein binding; i.e., pharmacokinetics/pharmacodynamics (PK/PD) data indicate a plasma protein-bound fraction of >99.9% (9, 22). It has been shown that the bedaquiline MIC increases in the presence of 5% bovine serum albumin (22). Previous studies determined drug susceptibility mostly in the absence of albumin (21). The albumin content in the MGIT 960 test tube resulting from addition of OADC (this study) or of MGIT growth supplement as supplied by BD was approximately 4% (wt/vol), comparable to the physiological plasma protein concentration. A challenge in AST for both substances is their poor solubility in water, a complication known for other antimycobacterial drugs such as ethionamide. Corresponding compounds have to be dissolved in DMSO as a solvent, and the growth control has to contain the same amount of DMSO to control for any possible effect on bacterial growth. In general, AST is done using pure substances as provided by the manufacturer. For this study, tablet formulations had to be used, because both producing companies denied the supply of the substances or were unwilling to provide the compound without extensive binding conditions for use and data publication. This is a policy not previously seen for new antimicrobials entering the market, as AST should be established and verified independently by expert laboratories (17, 23). The development and periodic revision of AST guidelines as part of drug development require close cooperation between academic experts, funding agencies, pharmaceutical companies, and regulatory authorities, as has occurred for antivirals in the past (24).
Our study had several limitations. Most notably, the limited amount of compound available precluded the analysis of a larger strain collection to more precisely determine the ECOFF. The proposed ECOFFs might change slightly with increasing sample size and a finer resolution of drug concentration scaling. In addition, given that bedaquiline and delamanid have entered the market only recently, M. tuberculosis isolates with acquired resistance are barely accessible. We established AST (see Tables S1 and S2 in the supplemental material) using a phylogenetically diverse strain set as shown by MIRU-VNTR analysis (see Fig. S1) in order to measure the variation in the “wild-type” MIC distribution and to maximize the chance of identifying genotypes that might be intrinsically resistant (13). Further studies evaluating in vitro laboratory drug MICs using pure compounds and PK/PD and clinical data from a large number of drug-susceptible and drug-resistant strains are required to define clinical breakpoints (17, 23).
Despite all these limitations, our report provides valid AST results. We propose ECOFF values based on population analysis and the eyeball method, which allow discrimination between wild-type and resistotype populations. Our study shows the feasibility of MGIT 960 equipped with TB eXiST for AST of bedaquiline and delamanid in the routine clinical laboratory.
Supplementary Material
ACKNOWLEDGMENTS
We thank the technicians in the mycobacteriology laboratory for invaluable help and Michael Hombach for critical reading of the manuscript.
This study was supported in part by the University of Zürich.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00614-15.
REFERENCES
- 1.World Health Organization. 2015. Global tuberculosis report 2014. World Health Organization, Geneva, Switzerland: http://www.who.int/tb/publications/global_report/en/ Accessed 9 May 2015. [Google Scholar]
- 2.Andries K, Verhasselt P, Guillemont J, Gohlmann HW, Neefs JM, Winkler H, Van Gestel J, Timmerman P, Zhu M, Lee E, Williams P, de Chaffoy D, Huitric E, Hoffner S, Cambau E, Truffot-Pernot C, Lounis N, Jarlier V. 2005. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- 3.Cox E, Laessig K. 2014. FDA approval of bedaquiline—the benefit-risk balance for drug-resistant tuberculosis. N Engl J Med 371:689–691. doi: 10.1056/NEJMp1314385. [DOI] [PubMed] [Google Scholar]
- 4.Segala E, Sougakoff W, Nevejans-Chauffour A, Jarlier V, Petrella S. 2012. New mutations in the mycobacterial ATP synthase: new insights into the binding of the diarylquinoline TMC207 to the ATP synthase C-ring structure. Antimicrob Agents Chemother 56:2326–2334. doi: 10.1128/AAC.06154-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andries K, Villellas C, Coeck N, Thys K, Gevers T, Vranckx L, Lounis N, de Jong BC, Koul A. 2014. Acquired resistance of Mycobacterium tuberculosis to bedaquiline. PLoS One 9:e102135. doi: 10.1371/journal.pone.0102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Somoskovi A, Bruderer V, Homke R, Bloemberg GV, Böttger EC. 2015. A mutation associated with clofazimine and bedaquiline cross-resistance in MDR-TB following bedaquiline treatment. Eur Respir J 45:554–557. doi: 10.1183/09031936.00142914. [DOI] [PubMed] [Google Scholar]
- 7.Tiberi S, De Lorenzo S, Centis R, Viggiani P, D'Ambrosio L, Migliori GB. 2014. Bedaquiline in MDR/XDR-TB cases: first experience on compassionate use. Eur Respir J 43:289–292. doi: 10.1183/09031936.00122313. [DOI] [PubMed] [Google Scholar]
- 8.Matsumoto M, Hashizume H, Tomishige T, Kawasaki M, Tsubouchi H, Sasaki H, Shimokawa Y, Komatsu M. 2006. OPC-67683, a nitro-dihydro-imidazooxazole derivative with promising action against tuberculosis in vitro and in mice. PLoS Med 3:e466. doi: 10.1371/journal.pmed.0030466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines Agency, Committee for Medicinal Products for Human Use. 2013. Assessment report Deltyba. European Medicines Agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002552/WC500166234.pdf.
- 10.Stover CK, Warrener P, VanDevanter DR, Sherman DR, Arain TM, Langhorne MH, Anderson SW, Towell JA, Yuan Y, McMurray DN, Kreiswirth BN, Barry CE, Baker WR. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- 11.Feuerriegel S, Köser CU, Bau D, Rusch-Gerdes S, Summers DK, Archer JA, Marti-Renom MA, Niemann S. 2011. Impact of Fgd1 and ddn diversity in Mycobacterium tuberculosis complex on in vitro susceptibility to PA-824. Antimicrob Agents Chemother 55:5718–5722. doi: 10.1128/AAC.05500-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diacon AH, Dawson R, Hanekom M, Narunsky K, Venter A, Hittel N, Geiter LJ, Wells CD, Paccaly AJ, Donald PR. 2011. Early bactericidal activity of delamanid (OPC-67683) in smear-positive pulmonary tuberculosis patients. Int J Tuberc Lung Dis 15:949–954. doi: 10.5588/ijtld.10.0616. [DOI] [PubMed] [Google Scholar]
- 13.Köser CU, Feuerriegel S, Summers DK, Archer JA, Niemann S. 2012. Importance of the genetic diversity within the Mycobacterium tuberculosis complex for the development of novel antibiotics and diagnostic tests of drug resistance. Antimicrob Agents Chemother 56:6080–6087. doi: 10.1128/AAC.01641-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Springer B, Lucke K, Calligaris-Maibach R, Ritter C, Böttger EC. 2009. Quantitative drug susceptibility testing of Mycobacterium tuberculosis by use of MGIT 960 and EpiCenter instrumentation. J Clin Microbiol 47:1773–1780. doi: 10.1128/JCM.02501-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Janssen Products. 2012. Highlights of prescribing information Sirturo (bedaquiline) tablets. Janssen Pharmaceutical Research and Development LLC, Titusville, NJ, USA: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/204384s000lbl.pdf. [Google Scholar]
- 16.Otsuka Novel Products GmbH. 2012. Annex I. Summary of product characteristics. Otsuka Novel Products GmbH, Munich, Germany: http://ec.europa.eu/health/documents/community-register/2014/20140428126881/anx_126881_en.pdf. [Google Scholar]
- 17.Ängeby K, Juréen P, Kahlmeter G, Hoffner SE, Schön T. 2012. Challenging a dogma: antimicrobial susceptibility testing breakpoints for Mycobacterium tuberculosis. Bull World Health Organ 90:693–698. doi: 10.2471/BLT.11.096644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterisation of bacterial wild-type MIC value distributions and the determination of epidemiological cut-off values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]
- 19.Turnidge J, Paterson DL. 2007. Setting and revising antibacterial susceptibility breakpoints. Clin Microbiol Rev 20:391–408. doi: 10.1128/CMR.00047-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Valsesia G, Hombach M, Maurer FP, Courvalin P, Roos M, Böttger EC. 2015. A statistical approach for determination of disk diffusion-based cutoff values for systematic characterization of wild-type and non-wild-type bacterial populations in antimicrobial susceptibility testing. J Clin Microbiol 53:1812–1822. doi: 10.1128/JCM.03506-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Upton AM, Cho S, Yang TJ, Kim Y, Wang Y, Lu Y, Wang B, Xu J, Mdluli K, Ma Z, Franzblau SG. 2015. In vitro and in vivo activities of the nitroimidazole TBA-354 against Mycobacterium tuberculosis. Antimicrob Agents Chemother 59:136–144. doi: 10.1128/AAC.03823-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rouan MC, Lounis N, Gevers T, Dillen L, Gilissen R, Raoof A, Andries K. 2012. Pharmacokinetics and pharmacodynamics of TMC207 and its N-desmethyl metabolite in a murine model of tuberculosis. Antimicrob Agents Chemother 56:1444–1451. doi: 10.1128/AAC.00720-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Böttger EC. 2011. The ins and outs of Mycobacterium tuberculosis drug susceptibility testing. Clin Microbiol Infect 17:1128–1134. doi: 10.1111/j.1469-0691.2011.03551.x. [DOI] [PubMed] [Google Scholar]
- 24.Köser CU, Javid B, Liddell K, Ellington MJ, Feuerriegel S, Niemann S, Brown NM, Burman WJ, Abubakar I, Ismail NA, Moore D, Peacock SJ, Török ME. 2015. Drug-resistance mechanisms and tuberculosis drugs. Lancet 385:305–307. doi: 10.1016/S0140-6736(14)62450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


