Abstract
Emerging antimicrobial resistance in members of the Bacteroides fragilis group is a concern in clinical medicine. Although metronidazole and carbapenem resistance have been reported in Bacteroides thetaiotaomicron, a member of the B. fragilis group, they have not, to the best of our knowledge, been reported together in the same B. thetaiotaomicron isolate. Herein, we report isolation of piperacillin-tazobactam-, metronidazole-, clindamycin-, ertapenem-, and meropenem-resistant B. thetaiotaomicron from a patient with postoperative intra-abdominal abscess and empyema. Whole-genome sequencing demonstrated the presence of nimD with at least a portion of IS1169 upstream, a second putative nim gene, two β-lactamase genes (one of which has not been previously reported), two tetX genes, tetQ, ermF, two cat genes, and a number of efflux pumps. This report highlights emerging antimicrobial resistance in B. thetaiotaomicron and the importance of identification and antimicrobial susceptibility testing of selected anaerobic bacteria.
INTRODUCTION
Increasing antimicrobial resistance has been reported in members of the Bacteroides fragilis group. Carbapenem resistance is rare and has been associated with expression of a metallo-β-lactamase encoded by cfiA, although other mechanisms have been reported. Metronidazole resistance is also rare, and though its mechanisms are incompletely delineated, it has been associated with plasmid-borne and chromosomal nim genes as well as efflux pumps (1). Some resistance genes may be present but not expressed unless activated by upstream insertion sequence (IS) elements.
In 2013, the first metronidazole-nonsusceptible Bacteroides thetaiotaomicron isolate was reported from Turkey; the isolate was imipenem susceptible (2). Here, we report a case of carbapenem- and metronidazole-resistant B. thetaiotaomicron in a patient with postoperative intra-abdominal abscess and empyema. Whole-genome sequencing was used to identify putative resistance genes.
(These data were presented in part at the 54th Interscience Conference on Antimicrobial Agents and Chemotherapy, Washington, DC, 5 to 9 September 2014.)
CASE REPORT
A 43-year-old man with a 3-year history of recurrent, medically intractable sigmoid diverticulitis underwent elective laparoscopic sigmoid resection at an outside institution. His postoperative course was complicated by abdominal pain and fevers. Computed tomography revealed intra-abdominal collections requiring placement of multiple percutaneous drains. Cultures from the intra-abdominal collections yielded polymicrobial growth of Escherichia coli (susceptible to meropenem, piperacillin-tazobactam, and cephalosporins, and resistant to fluoroquinolones and aminoglycosides), B. fragilis group (β-lactamase positive), and Candida albicans. To evaluate for possible colonic leakage, an exploratory laparotomy and intraoperative colonoscopy were performed on postoperative day 5. Turbid-appearing fluid was observed in the pelvis proximal to the left colon, but no frank leak was identified. The patient received intravenous (i.v.) meropenem, vancomycin, and fluconazole. Due to persistent fever and abdominal pain, repeat exploratory laparotomy and peritoneal lavage were performed with resection of the colorectal anastomosis and creation of an end colostomy. Because of continued fever and leukocytosis (19 × 109 cells/liter), the patient was transferred to the Mayo Clinic (Rochester, MN). He lived in Iowa, where he worked as a manager for a gelatin factory. He had lived in China for 2 years and had not received medical care there.
His antibiotics were initially transitioned to i.v. piperacillin-tazobactam, i.v. vancomycin, and fluconazole. All blood cultures were negative. Sinograms of the intra-abdominal drains confirmed appropriate drain placement and control of his abdominal fluid collections. A drain in a left upper quadrant intra-abdominal collection had been transpleurally placed, resulting in the development of a large left pleural effusion. Pleural fluid cultures obtained via thoracentesis grew B. thetaiotaomicron and C. albicans. The B. thetaiotaomicron isolate was β-lactamase positive. Susceptibility testing using gradient antibiotic strips (Etest; bioMérieux, Durham, NC) showed the isolate to be resistant (Clinical and Laboratory Standards Institute breakpoints [3]) to piperacillin-tazobactam (MIC, >256 and 4 μg/ml, respectively), metronidazole (MIC, 32 μg/ml), clindamycin (MIC, >256 μg/ml), ertapenem (MIC, ≥16 μg/ml), and meropenem (MIC, >32 μg/ml), and susceptible (U.S. Food and Drug Administration breakpoint [4]) to tigecycline (MIC, 4 μg/ml). An ultrasound-guided left pleural pigtail catheter was placed, and thrombolytic therapy was instilled to optimize pleural fluid drainage. The patient's antimicrobial regimen was transitioned to i.v. tigecycline, and fluconazole was continued. He improved clinically with resolution of fever and normalization of his peripheral white blood cell count. Subsequent imaging showed resolution of the fluid collections in the left upper quadrant and improvement in the left pleural effusion. The drains were removed. Because of intractable nausea and other gastrointestinal side effects, tigecycline and fluconazole were discontinued, and he was followed clinically over the next few months without disease recurrence.
MATERIALS AND METHODS
Whole-genome sequencing.
The B. thetaiotaomicron isolate (IDRL-10114) was suspended in 1× Tris-EDTA buffer and treated with 5 mg/ml lysozyme at 37°C for 3 h. Genomic DNA was extracted using a Maxwell 16 tissue DNA purification cartridge (Promega, Madison, WI) and purified by column purification using the Genomic DNA Clean & Concentrator-10 kit (Zymo Research Corp., Irvine, CA).
Illumina next generation sequencing was performed on paired-end and Nextera mate-pair libraries using a MiSeq platform (Illumina, Inc., San Diego, CA) with a 600-cycle kit, resulting in an average coverage of 85×. Sequencing reads were processed for library adapter removal and filtering using Trimmomatic 0.32 (5) with the following parameters: ILLUMINACLIP:adapter.fasta:2:30:10 LEADING:3 TRAILING:3 MAXINFO:220:0.1 MINLEN:70. Assembly was generated with SPAdes 3.1.1 (6). Resistance genes were searched against ResFams 1.2 (7) using HMMER 3.1b2 (8). Insertion sequence elements were searched using the ISfinder tool (9).
Mayo Clinic B. thetaiotaomicron antibiogram.
An antibiogram was generated for all B. thetaiotaomicron isolates subjected to antimicrobial susceptibility testing at the Mayo Clinic between November 2011 and December 2014. Susceptibility testing was performed using antibiotic gradient strips; results were interpreted using Clinical and Laboratory Standards Institute guidelines (3).
RESULTS
Whole-genome sequencing analysis.
Results of the analysis of whole-genome sequence data for resistance genes using ResFams 1.2 demonstrated a number of efflux pumps, two tetX genes, tetQ, ermF, mef, and two cat genes (Table 1). Two β-lactamase genes were detected, one of which was 98.8% identical to a β-lactamase gene previously reported in B. thetaiotaomicron VPI 5482 (10) and was not associated with an upstream insertion sequence. The second β-lactamase gene was novel. IS612B was located upstream of it, suggesting a possible role in expression.
TABLE 1.
Antimicrobial resistance genes identified by whole-genome sequencing in B. thetaiotaomicron
| Resistance mechanism(s) | Function | NCBI accession no. | Nucleotide identity |
|---|---|---|---|
| ABC transporter, ATP-binding protein | Efflux pump | AE015928.1 | B. thetaiotaomicron VPI 5482, complete genome, ABC transporter, ATP-binding protein 1814/1824 (99.5%) |
| RND family efflux transporter, MFP subunit (2) | Efflux pump | AE015928.1 | B. thetaiotaomicron VPI 5482, complete genome 1081/1083 (99.8%) and 1216/1236 (98.4%) |
| AcrB/AcrD/AcrF family efflux transporter | Efflux pump | AE015928.1 | B. thetaiotaomicron VPI 5482, complete genome 2975/3033 (98.1%) |
| Efflux pump | Efflux pump | AE015928.1 | B. thetaiotaomicron VPI 5482, complete genome 3036/3105 (97.8%) |
| β-Lactamasea | β-Lactamase | AE015928.1 | B. thetaiotaomicron VPI 5482, complete genome β-lactamase 871/882 (98.8%) |
| cat (2) | Chloramphenicol-inactivation enzyme | AE015928.1 | B. thetaiotaomicron VPI 5482, complete genome 618/624 (99.0%) and 628/645 (97.3%) |
| nim-like | 5-Nitroimidazole resistance | AE015928.1 | B. thetaiotaomicron VPI 5482, complete genome 468/480 (97.5%) |
| ABC transporter, ATP-binding protein | Efflux pump | CP002530.1 | Bacteroides salanitronis DSM 18170 1332/1664 (80.0%) |
| Putative class A β-lactamase (2 identical copies)b | Novel class A β-lactamase | KP233892 | This report |
| ermF 23S rRNA methyltransferase | Clindamycin resistance | AJ311171.1 | B. thetaiotaomicron transposon CTnDOT ermF region 800/801 (99.9%) |
| mef | Macrolide efflux | KJ816753, CP003351, CP000673.1, AY355407 | B. fragilis strain HMW 615 transposon CTnHyb 1209/1218 (99.3%); Enterococcus faecium Aus0004 1198/1218 (98.4%); Clostridium kluyveri NBRC 12016 1178/1209 (97.4%); Streptococcus sp. “group G” strain 02M157741 macrolide-efflux protein (mef) gene 1163/1218 (95.5%) |
| tetX (2) | Tetracycline-inactivation enzyme | AJ311171.1 | B. thetaiotaomicron transposon CTnDOT 1077/1077 (100%) and 1129/1167 (96.7%) |
| tetQ | Tetracycline resistance | CP008741, CP003274.1, AP006841.1, AY515263 | Bacteroides dorei 1926/1926 (100%); Alistipes finegoldii DSM 17242 1926/1926 (100%); B. fragilis YCH46 DNA, complete genome 1926/1926 (100%); B. fragilis conjugative transposon CTn341 1926/1926 (100%) |
| nimDc | 5-Nitroimidazole resistance | X76949 | B. fragilis 491/495 (99.2%) |
Insertion element necessary for upregulation not detected.
IS612B located upstream, indicating a potential role in expression.
IS1169 found upstream of nimD as reported in X76949, but an incomplete sequence was obtained for IS1169.
ResFams 1.2 does not include nim genes. A separate analysis for nim genes showed the presence of a plasmid-borne nimD with at least a portion of IS1169 (the contig terminated within the insertion sequence) inserted upstream, as previously reported in B. fragilis (11). There was a second nim-like element, which was 97.5% identical to that previously reported in B. thetaiotaomicron VPI 5482. cfiA was not detected.
Most of the detected resistance genes were highly similar to those reported in the complete genome sequence of B. thetaiotaomicron VPI 5482 (10). However, there were several not reported in B. thetaiotaomicron VPI 5482, including that for the novel putative β-lactamase, as well as an efflux pump gene, ermF (with IS612B upstream), two tetX genes, tetQ, nimD, and mef. ermF and one of the tetX genes have been previously reported on a B. thetaiotaomicron transposon (CTnDOT), tetQ has been previously reported on a B. fragilis conjugative transposon (CTn341), and mef has been previously reported on a B. fragilis transposon (CTnHyb). ermF, the two tetX genes, and mef were located in proximity to one another, possibly as part of a transposon (Fig. 1). In addition to IS1169 and IS612B, ISBthe3 and ISBthe4 were detected.
FIG 1.
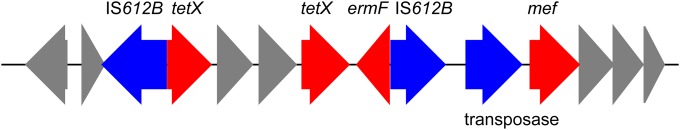
Arrangement of the genetic region harboring ermF and tetX. The red arrows represent resistance genes, the blue arrows are mobile/transposable elements, and the gray arrows represent other genes/open reading frames (ORFs).
Susceptibility of Mayo Clinic B. thetaiotaomicron isolates.
From November 2011 to December 2014, 65 B. thetaiotaomicron isolates were tested for antimicrobial susceptibility (Table 2). With the exception of the isolate described herein, all were metronidazole susceptible; however, 17% were ertapenem nonsusceptible, 23% were piperacillin-tazobactam nonsusceptible, and 94% were clindamycin nonsusceptible.
TABLE 2.
Antimicrobial susceptibility of 65 B. thetaiotaomicron isolates (Mayo Clinic, Rochester, MN), November 2011 to December 2014a
| Antimicrobial agent | No. susceptibleb (%) | No. intermediateb (%) | No. resistantb (%) |
|---|---|---|---|
| Ertapenem | 54 (83) | 1 (2) | 10 (15) |
| Clindamycin | 4 (6) | 11 (17) | 50 (77) |
| Metronidazole | 64 (99) | 0 (0) | 1 (2) |
| Piperacillin-tazobactam | 50 (77) | 3 (5) | 12 (19) |
Data courtesy of Nicolynn C. Cole.
Clinical and Laboratory Standards Institute guidelines applied (3).
DISCUSSION
Anaerobic bacteria, including B. fragilis group members, are a normal part of the human intestinal flora and can be important pathogens in intra-abdominal and postoperative surgical infections. Among anaerobic flora within intra-abdominal infections, B. fragilis is most commonly identified, followed by B. thetaiotaomicron (12). There has been increasing antimicrobial resistance reported in the B. fragilis group in recent years, with resistance to β-lactam antibiotics via β-lactamase production becoming progressively more common (13). Clinical B. thetaiotaomicron isolates from a Turkish study showed resistance to ampicillin, piperacillin, clindamycin, and on occasion chloramphenicol; however, all isolates were susceptible to metronidazole and imipenem (14). Because of emerging resistance, metronidazole, carbapenems, and β-lactam–β-lactamase inhibitor combinations, such as piperacillin-tazobactam, have been increasingly used to treat B. fragilis group infections. This report cautions that today, these agents should not be considered to be universally active.
The first metronidazole-nonsusceptible B. thetaiotaomicron isolate was from a pancreatic cancer patient in Turkey who developed B. thetaiotaomicron bacteremia. The isolate had a metronidazole MIC of 16 μg/ml, corresponding to intermediate based on Clinical and Laboratory Standards Institute criteria and resistant based on European Committee on Antimicrobial Susceptibility Testing criteria (2). This isolate was, however, susceptible to carbapenems and ampicillin-sulbactam. We believe that the case reported herein is the first case of concurrent metronidazole and carbapenem resistance in an isolate of B. thetaiotaomicron as well as the first case of fully metronidazole-resistant B. thetaiotaomicron.
Metronidazole resistance in the B. fragilis group has been associated with acquisition of nim genes (nimA to nimH and nimJ) located on the chromosome or on plasmids (15). The B. thetaiotaomicron isolate from the aforementioned case carried nimE (2). The presence of nim may not correlate with clinically relevant metronidazole resistance, as the gene may not be expressed or may be expressed at low levels unless activated by upstream insertion sequence elements (16, 17). The function of Nim proteins is controversial. Also, in a recent study, no correlation was found between levels of Nim and levels of metronidazole resistance (18). Whole-genome sequencing showed that our isolate had nimD with at least a portion of IS1169 inserted upstream, suggesting that this may have been the mechanism underlying metronidazole resistance. A second nim gene similar to that reported in B. thetaiotaomicron VPI 5482 (10) was also present.
Carbapenem resistance in B. fragilis has been attributed to metallo-β-lactamase production, first described in 1986 (19). The associated gene is cfiA; however, not all B. fragilis strains carrying this gene express phenotypic carbapenem resistance (20), and carbapenem resistance has been reported in B. fragilis group members in the absence of cfiA. High-level cfiA β-lactamase-associated carbapenem resistance appears to be associated with the presence of an insertion element immediately upstream of cfiA that provides an efficient promoter (21). As mentioned, cfiA is not the only mechanism of carbapenem resistance in the B. fragilis group; Fernández-Canigia et al., for example, detected cfiA in only 8 out of 23 B. fragilis group isolates with decreased susceptibility to carbapenems, all of which were B. fragilis species (22). In their study, the mechanism of carbapenem resistance in B. thetaiotaomicron and Bacteroides ovatus was undefined (22). Other mechanisms of carbapenem nonsusceptibility include altered penicillin-binding proteins, efflux, and, hypothetically, outer membrane porin protein mutations (16). cfiA was not detected in our isolate. Snydman et al. reported three carbapenem-resistant B. thetaiotaomicron isolates, none of which tested positive for cfiA using PCR (12). Interestingly, multiple efflux pumps were detected in our isolate, suggesting a possible contribution of efflux to carbapenem nonsusceptibility. The function of the novel β-lactamase reported here remains unknown; the IS element IS612/IS612B located upstream of the novel putative β-lactamase has been found upstream of other β-lactamases (23).
A national survey of antibiotic susceptibility of 363 clinical B. fragilis group isolates collected from 2006 through 2009 from 17 centers in Argentina showed that among the 198 B. fragilis isolates tested, 1.5% and 2.4% were resistant to imipenem and ertapenem, respectively, and that among the 69 isolates of B. thetaiotaomicron and B. ovatus tested, 1.4% and 4.1% were resistant to imipenem and ertapenem, respectively; no metronidazole resistance was detected (22). Among 66 Bacteroides isolates collected between 2003 and 2008 from a Turkish hospital, 5 were resistant to meropenem, of which 4 were also resistant to imipenem; no metronidazole resistance was detected (20). Roh et al. reported four meropenem-resistant B. fragilis isolates recovered between 1997 and 2004 from a Korean hospital (23). A recent report from Russia describes a metronidazole- and imipenem-resistant B. fragilis isolate as well as an imipenem-resistant but metronidazole-susceptible B. fragilis strain, the latter apparently isolated from two patients, suggesting possible person-to-person transmission (24).
Limited in vitro data suggest high rates of susceptibility of the B. fragilis group to tigecycline (25). Our patient's isolate was susceptible to tigecycline, albeit with an MIC of 4 μg/ml (at the U.S. Food and Drug Administration susceptibility breakpoint), and exhibited the presence of two tetX genes. Although he did not tolerate a prolonged course of tigecycline, effective drainage of his intra-abdominal and pleural fluid collections resulted in a successful clinical outcome.
We and others have used whole-genome sequencing in an attempt to characterize resistance mechanisms in B. fragilis group members (15, 26). A recent report described an imipenem-, metronidazole-, piperacillin-tazobactam-, and clindamycin-resistant Bacteroides genome species strain related to B. fragilis; whole-genome sequencing detected several antimicrobial resistance genes in this isolate, including cfiA13 (with an upstream insertion sequence), cfxA, two putative nim genes, ermF, tetQ, and efflux-associated genes (26). Another recent report described whole-genome sequencing of five meropenem-nonsusceptible B. fragilis strains, three of which were nonsusceptible to metronidazole (15). cfiA was detected in all five, and nim genes were detected in the metronidazole-nonsusceptible isolates; tetQ, bexB, ermF, linAn2, and mefEn2 were variously detected (15). Whole-genome sequencing currently has limitations for detecting antimicrobial resistance genes in the B. fragilis group. Assembly of complete, finished genomes, which is ideally needed, is hindered by the frequent presence of multiple IS elements. Annotation is also problematic. A more complete understanding of the genetic basis of antimicrobial resistance in this group of organisms is needed to develop tools to use genetic data to infer susceptibility or resistance to antimicrobial agents. Sydenham et al. (15) used a combination of the ResFinder database (27) and a custom BLAST database to survey for resistance genes in their B. fragilis strains. Since these tools failed to detect the β-lactamases of our isolate, we instead chose to use the hidden Markov model-based ResFams (7), which, while more challenging to use, found the known β-lactamase and other resistance genes and even predicted a novel β-lactamase gene.
The emergence of antimicrobial resistance in B. fragilis group members raises concern for horizontal gene transfer in the human intestinal tract. The source of our patient's resistant isolate/resistance genes is unknown but might hypothetically be endogenous gene transfer/selection, travel-related transmission, or nosocomial/procedural transmission. Although theoretical, the findings presented and discussed herein raise the possibility that, as with carbapenem-resistant Enterobacteriaceae, resistant B. fragilis group species may be nosocomially transmitted (24).
Carbapenems and metronidazole were once considered to be generally active against B. fragilis group organisms. The Clinical and Laboratory Standards Institute guidelines provide two strategies for clinical microbiology laboratories to perform susceptibility testing on anaerobic bacteria, routine or periodic (i.e., intermittent) surveillance testing (28); the former should be the preferred strategy for clinically significant isolates of B. thetaiotaomicron because, as highlighted by our antibiogram data, carbapenem resistance is not uncommon in this species. Fortunately, with the advent of matrix-assisted laser desorption ionization–time of flight mass spectrometry, identification of the individual members of the B. fragilis group to the species level has been simplified (29), although misidentifications of individual species have been reported (26).
Metronidazole- and carbapenem-resistant B. thetaiotaomicron poses a therapeutic challenge for severe infections involving this organism. Our case along with other recent reports of metronidazole and carbapenem resistance in the non-B. thetaiotaomicron B. fragilis group (15, 24, 26) suggests that carbapenems and metronidazole, once considered to be universally active against B. fragilis group organisms, cannot be assumed to be active today, emphasizing the need for routine identification and antimicrobial susceptibility testing of selected anaerobic bacteria.
ACKNOWLEDGMENTS
This work was supported in part by the National Cancer Institute of the National Institutes of Health under grant R01 CA179243. R.P. was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under grant R01 AR056647 and the National Institute of Allergy and Infectious Diseases under grant R01 AI091594. P.R.J. was supported by the Mayo-Illinois Strategic Alliance for Technology Based Healthcare.
The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Special thanks go to Nicholas Chia and Daniel R. Gustafson for their technical assistance.
REFERENCES
- 1.Pumbwe L, Wareham DW, Aduse-Opoku J, Brazier JS, Wexler HM. 2007. Genetic analysis of mechanisms of multidrug resistance in a clinical isolate of Bacteroides fragilis. Clin Microbiol Infect 13:183–189. doi: 10.1111/j.1469-0691.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 2.Toprak Ulger N, Sayin E, Soyad A, Dane F, Soyletir G. 2013. The first metronidazole-resistant Bacteroides species isolated at Marmara University Hospital: Bacteroides thetaiotaomicron. Mikrobiyol Bul 47:717–721. (In Turkish.) doi: 10.5578/mb.5064. [DOI] [PubMed] [Google Scholar]
- 3.Clinical and Laboratory Standards Institute. 2015. Performance standards for antimicrobial susceptibility testing; 25th informational supplement. CLSI M100-S25. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 4.Wyeth Pharmaceuticals, Inc. 2014. Tygacil-tigecycline injection, powder, lyophilized, for solution. Wyeth Pharmaceuticals, Inc., Philadelphia, PA: http://labeling.pfizer.com/ShowLabeling.aspx?id=491 Accessed 22 April 2015. [Google Scholar]
- 5.Bolger AM, Lohse M, Usadel B. 2014. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gibson MK, Forsberg KJ, Dantas G. 2015. Improved annotation of antibiotic resistance determinants reveals microbial resistomes cluster by ecology. ISME J 9:207–216. doi: 10.1038/ismej.2014.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eddy SR. 2011. Accelerated profile HMM searches. PLoS Comput Biol 7:e1002195. doi: 10.1371/journal.pcbi.1002195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siguier P, Perochon J, Lestrade L, Mahillon J, Chandler M. 2006. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res 34:D32-6. doi: 10.1093/nar/gkj014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu J, Bjursell MK, Himrod J, Deng S, Carmichael LK, Chiang HC, Hooper LV, Gordon JI. 2003. A genomic view of the human-Bacteroides thetaiotaomicron symbiosis. Science 299:2074–2076. doi: 10.1126/science.1080029. [DOI] [PubMed] [Google Scholar]
- 11.Trinh S, Haggoud A, Reysset G, Sebald M. 1995. Plasmids pIP419 and pIP421 from Bacteroides: 5-nitroimidazole resistance genes and their upstream insertion sequence elements. Microbiology 141:927–935. [DOI] [PubMed] [Google Scholar]
- 12.Snydman DR, Jacobus NV, McDermott LA, Golan Y, Goldstein EJ, Harrell L, Jenkins S, Newton D, Pierson C, Rosenblatt J, Venezia R, Gorbach SL, Queenan AM, Hecht DW. 2011. Update on resistance of Bacteroides fragilis group and related species with special attention to carbapenems 2006-2009. Anaerobe 17:147–151. doi: 10.1016/j.anaerobe.2011.05.014. [DOI] [PubMed] [Google Scholar]
- 13.Eitel Z, Soki J, Urban E, Nagy E. 2013. The prevalence of antibiotic resistance genes in Bacteroides fragilis group strains isolated in different European countries. Anaerobe 21:43–49. doi: 10.1016/j.anaerobe.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Ulger Toprak N, Celik C, Cakici O, Soyletir G. 2004. Antimicrobial susceptibilities of Bacteroides fragilis and Bacteroides thetaiotaomicron strains isolated from clinical specimens and human intestinal microbiota. Anaerobe 10:255–259. doi: 10.1016/j.anaerobe.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Sydenham TV, Soki J, Hasman H, Wang M, Justesen US. 2015. Identification of antimicrobial resistance genes in multidrug-resistant clinical Bacteroides fragilis isolates by whole genome shotgun sequencing. Anaerobe 31:59–64. doi: 10.1016/j.anaerobe.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Wexler HM. 2007. Bacteroides: the good, the bad, and the nitty-gritty. Clin Microbiol Rev 20:593–621. doi: 10.1128/CMR.00008-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haggoud A, Reysset G, Azeddoug H, Sebald M. 1994. Nucleotide sequence analysis of two 5-nitroimidazole resistance determinants from Bacteroides strains and of a new insertion sequence upstream of the two genes. Antimicrob Agents Chemother 38:1047–1051. doi: 10.1128/AAC.38.5.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leitsch D, Soki J, Kolarich D, Urban E, Nagy E. 2014. A study on Nim expression in Bacteroides fragilis. Microbiology 160:616–622. doi: 10.1099/mic.0.074807-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuchural GJ Jr, Malamy MH, Tally FP. 1986. Beta-lactamase-mediated imipenem resistance in Bacteroides fragilis. Antimicrob Agents Chemother 30:645–648. doi: 10.1128/AAC.30.5.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Toprak NU, Uzunkaya OD, Soki J, Soyletir G. 2012. Susceptibility profiles and resistance genes for carbapenems (cfiA) and metronidazole (nim) among Bacteroides species in a Turkish university hospital. Anaerobe 18:169–171. doi: 10.1016/j.anaerobe.2011.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Yamazoe K, Kato N, Kato H, Tanaka K, Katagiri Y, Watanabe K. 1999. Distribution of the cfiA gene among Bacteroides fragilis strains in Japan and relatedness of cfiA to imipenem resistance. Antimicrob Agents Chemother 43:2808–2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Canigia L, Litterio M, Legaria MC, Castello L, Predari SC, Di Martino A, Rossetti A, Rollet R, Carloni G, Bianchini H, Cejas D, Radice M, Gutkind G. 2012. First national survey of antibiotic susceptibility of the Bacteroides fragilis group: emerging resistance to carbapenems in Argentina. Antimicrob Agents Chemother 56:1309–1314. doi: 10.1128/AAC.05622-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roh KH, Kim S, Kim CK, Yum JH, Kim MS, Yong D, Jeong SH, Lee K, Kim JM, Chong Y. 2010. New cfiA variant and novel insertion sequence elements in carbapenem-resistant Bacteroides fragilis isolates from Korea. Diagn Microbiol Infect Dis 66:343–348. doi: 10.1016/j.diagmicrobio.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 24.Shilnikova II, Dmitrieva NV. 2015. Evaluation of antibiotic susceptibility of Bacteroides, Prevotella and Fusobacterium species isolated from patients of the N. N. Blokhin Cancer Research Center, Moscow, Russia. Anaerobe 31:15–18. doi: 10.1016/j.anaerobe.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 25.Petersen PJ, Ruzin A, Tuckman M, Jones CH. 2010. In vitro activity of tigecycline against patient isolates collected during phase 3 clinical trials for diabetic foot infections. Diagn Microbiol Infect Dis 66:407–418. doi: 10.1016/j.diagmicrobio.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Salipante SJ, Kalapila A, Pottinger PS, Hoogestraat DR, Cummings L, Duchin JS, Sengupta DJ, Pergam SA, Cookson BT, Butler-Wu SM. 2015. Characterization of a multidrug-resistant, novel Bacteroides genomospecies. Emerg Infect Dis 21:95–98. doi: 10.3201/eid2101.140662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. 2012. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 67:2640–2644. doi: 10.1093/jac/dks261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2012. Methods for antimicrobial susceptibility testing of anaerobic bacteria; approved standard—8th ed. CLSI document M11-A8. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Schmitt BH, Cunningham SA, Dailey AL, Gustafson DR, Patel R. 2013. Identification of anaerobic bacteria by Bruker Biotyper matrix-assisted laser desorption ionization-time of flight mass spectrometry with on-plate formic acid preparation. J Clin Microbiol 51:782–786. doi: 10.1128/JCM.02420-12. [DOI] [PMC free article] [PubMed] [Google Scholar]


