Abstract
Sensititre YeastOne (SYO) is an affordable alternative to the Clinical and Laboratory Standards Institute (CLSI) reference method for antifungal susceptibility testing. In this study, the MICs of yeast isolates from 1,214 bloodstream infection episodes, generated by SYO during hospital laboratory activity (January 2005 to December 2013), were reanalyzed using current CLSI clinical breakpoints/epidemiological cutoff values to assign susceptibility (or the wild-type [WT] phenotype) to systemic antifungal agents. Excluding Candida albicans (57.4% of all isolates [n = 1,250]), the most predominant species were Candida parapsilosis complex (20.9%), Candida tropicalis (8.2%), Candida glabrata (6.4%), Candida guilliermondii (1.6%), and Candida krusei (1.3%). Among the non-Candida species (1.9%), 7 were Cryptococcus neoformans and 17 were other species, mainly Rhodotorula species. Over 97% of Candida isolates were susceptible (WT phenotype) to amphotericin B and flucytosine. Rates of susceptibility (WT phenotype) to fluconazole, itraconazole, and voriconazole were 98.7% in C. albicans, 92.3% in the C. parapsilosis complex, 96.1% in C. tropicalis, 92.5% in C. glabrata, 100% in C. guilliermondii, and 100% (excluding fluconazole) in C. krusei. The fluconazole-resistant isolates consisted of 6 C. parapsilosis complex isolates, 3 C. glabrata isolates, 2 C. albicans isolates, 2 C. tropicalis isolates, and 1 Candida lusitaniae isolate. Of the non-Candida isolates, 2 C. neoformans isolates had the non-WT phenotype for susceptibility to fluconazole, whereas Rhodotorula isolates had elevated azole MICs. Overall, 99.7% to 99.8% of Candida isolates were susceptible (WT phenotype) to echinocandins, but 3 isolates were nonsusceptible (either intermediate or resistant) to caspofungin (C. albicans, C. guilliermondii, and C. krusei), anidulafungin (C. albicans and C. guilliermondii), and micafungin (C. albicans). However, when the intrinsically resistant non-Candida isolates were included, the rate of echinocandin nonsusceptibility reached 1.8%. In summary, the SYO method proved to be able to detect yeast species showing antifungal resistance or reduced susceptibility.
INTRODUCTION
Almost all of the classes of antifungal agents available to date, such as polyenes, azoles, flucytosine, and echinocandins, are systemically active against Candida or non-Candida yeasts causing bloodstream infections (BSIs) (1–4). Nevertheless, the expanding use of newer (e.g., caspofungin or posaconazole) and older (e.g., fluconazole) antifungal agents for prophylactic or empirical purposes (5, 6) has led to and in part has driven the changing epidemiology of fungemia (7–10) and the emergence of fungal pathogens with decreased susceptibility or resistance to currently prescribed antifungals (11, 12). It is noteworthy that while Candida albicans is the most frequently encountered species in most hospital settings worldwide (13), non-albicans Candida species (i.e., Candida glabrata, Candida parapsilosis, Candida tropicalis, Candida krusei, etc.) were recently shown to be the cause of two-thirds of all cases of candidemia in a population-based laboratory study (14). Additionally, more than half of Candida isolates found to be resistant to one of two antifungal classes (i.e., azoles and echinocandins) were C. glabrata, with 8 of 9 isolates being resistant to both an echinocandin and fluconazole (14). It is also notable that in about 62% of candidemia episodes studied over a 10-year period at Duke University Hospital, patients who failed to respond or responded only initially to an echinocandin therapy were infected with C. glabrata isolates for which the MICs indicated echinocandin resistance and which harbored FKS mutations (15).
In keeping with the need for reproducible and clinically relevant fungal susceptibility testing, the Sensititre YeastOne (SYO; Thermo Fisher Scientific, MA) colorimetric plate was marketed to provide an easy and affordable alternative to the Clinical and Laboratory Standards Institute (CLSI) or the European Committee on Antimicrobial Susceptibility Testing (EUCAST) standard broth microdilution methods (16, 17). It now represents, to our knowledge, a suitable method for the routine testing of the susceptibilities of clinical Candida isolates to amphotericin B, flucytosine, fluconazole, itraconazole, posaconazole, voriconazole, and the three echinocandins, particularly when it is used on a large scale (18; see also reference 19). Using 24-h MIC results obtained by SYO, Huang et al. assessed the in vitro antifungal susceptibility profiles of 474 blood Candida isolates by applying the newly revised CLSI clinical breakpoints (CBPs) or, in the absence of CBPs, epidemiological cutoff values (ECVs) for nine antifungal agents (20). Based on data from a prospective candidemia study, van Hal et al. were able to support the revised fluconazole CBP for C. albicans by use of the MICs that were obtained using the SYO method (21).
In the present study, we carried out a retrospective analysis of antifungal MIC data generated by the SYO system during a 9-year hospital laboratory activity with regard to fungal BSIs. Thus, the original MICs of 1,250 isolates of Candida and non-Candida species from 1,214 infectious episodes were reanalyzed by adopting the current interpretive criteria to determine the rates of antifungal resistance and to detect emerging resistance among the isolates. Furthermore, isolates of Candida species showing elevated echinocandin MICs were molecularly characterized to define the mechanisms of echinocandin resistance.
MATERIALS AND METHODS
Data collection.
A total of 1,214 BSI episodes due to Candida or non-Candida species were diagnosed in 1,214 patients during the years from 2005 to 2013 and identified through a search of the clinical microbiology laboratory information system at the Università Cattolica del Sacro Cuore (UCSC), a large institution comprising a 1,200-bed tertiary-level hospital in Rome, Italy. Episodes in which more than one fungal species were detected were considered polyfungal BSIs, whereas episodes occurring in patients whose blood samples for culture for analysis of the incident episode (i.e., the first blood culture positive for a fungal species) were collected >48 h after hospital admission were considered hospital-onset BSIs (HO-BSIs). Outpatient-acquired BSIs were episodes detected ≤48 h after hospital admission. As no multiple episodes of fungemia in the same patient (defined as episodes due to the same fungal species that occurred at least >21 days after the incident episode) were diagnosed, all the first episodes of fungemia diagnosed during the study period were included in the study. Data were reported into a customized database created for the inclusion of patient identifiers, hospital wards or outpatient services/departments, dates of BSI onset, and the species and antifungal susceptibility patterns of the yeast isolates from the BSI patients (n = 1,250 isolates, including those recovered from episodes with a single [n = 1,214] or mixed [n = 36] fungal etiology). Additionally, data concerning the dosage and duration of any antifungal treatment, primary disease, source of fungal infection, and clinical outcome were retrieved from the patients' hospital charts (only for patients infected with isolates nonsusceptible [including susceptible dose dependent/intermediate and resistant] to antifungals), whereas data on hospital antifungal consumption (in defined daily doses [DDDs] per year) were available from the UCSC pharmacy database. The study did not require oversight by the institutional ethics committee because of its descriptive nature.
Species identification and antifungal susceptibility testing.
Yeast organisms were isolated, after growth on Difco Candida bromcresol green (BCG) agar plates, from cultures of patient blood, which was collected as part of normal clinical practice and processed using a Bactec (BD Diagnostic Systems, Sparks, MD) or BacT/Alert (bioMérieux, Marcy l'Etoile, France) system. Isolates were identified to the species level by standard methods, such as morphology on cornmeal-Tween 80 agar, growth at 45°C (for C. albicans/C. dubliniensis), and/or yeast assimilation/enzymatic tests using Vitek 2 and RapID Yeast Plus identification systems (22) or, since 2010, by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry (23), supplemented by molecular identification, as needed (24). This was the case for isolates yielding inconclusive phenotypic profiles or insufficient mass spectra. Antifungal susceptibility testing was performed as part of routine patient care, and colorimetric MIC endpoints were determined visually, after 24 of incubation at 35°C in a non-CO2 atmosphere, using the SYO panel (progressively upgraded until it included all 10 antifungal agents available in 2009 [the SYO-10 version]) for a total of 1,250 (100% tested with amphotericin B, flucytosine, fluconazole, itraconazole, and voriconazole), 1,059 (84.7% tested with caspofungin), 908 (72.6% tested with posaconazole), and 740 (59.2% tested with anidulafungin and micafungin) isolates, according to the manufacturer's instructions. In cases in which a prolonged incubation of SYO plates was required (e.g., for cryptococcal isolates), visual readings of MICs was performed regardless of colorimetric changes. The concentrations of the antifungals in version SYO-10 ranged from 0.12 to 8 μg/ml for amphotericin B, 0.06 to 64 μg/ml for flucytosine, 0.015 to 8 μg/ml for anidulafungin, 0.008 to 8 μg/ml for caspofungin, micafungin, voriconazole, and posaconazole, 0.12 to 256 μg/ml for fluconazole, and 0.015 to 16 μg/ml for itraconazole. As the ranges for amphotericin B, flucytosine, fluconazole, and itraconazole were different from those for the previous SYO versions (SYO-06, SYO-07, SYO-8) used throughout the study period (see Table S1 in the supplemental material), MIC values of 0.008 to 0.12 μg/ml for amphotericin B and of 0.03 to 0.12 μg/ml for fluconazole were reported as ≤0.12 μg/ml, MIC values of 0.03 to 0.06 μg/ml for flucytosine were reported as ≤0.06 μg/ml, and MIC values of 0.008 to 0.015 μg/ml for itraconazole were reported as ≤0.015 μg/ml.
Data analysis.
The interpretive antifungal MIC breakpoints were the species-specific CBPs of fluconazole, voriconazole, and echinocandins (25–27), which were recently revised by the CLSI (28) to identify resistant strains of the 5 most common species of Candida (C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei); exceptions were the species C. krusei, for which all isolates are defined to be intrinsically resistant to fluconazole, and the voriconazole and C. glabrata combination, for which no CBPs were assigned by the CLSI (26, 29). The CLSI resistance breakpoint for fluconazole was defined as an MIC of >4 μg/ml against C. albicans, C. parapsilosis, and C. tropicalis and an MIC of >32 μg/ml against C. glabrata; the CLSI resistance breakpoint for voriconazole was defined as an MIC of >0.5 μg/ml against C. albicans, C. parapsilosis, and C. tropicalis and an MIC of >1 μg/ml against C. krusei. The CLSI resistance breakpoint for anidulafungin, caspofungin, and micafungin was defined as an MIC of >0.5 μg/ml against C. albicans, C. tropicalis, and C. krusei and an MIC of >4 μg/ml against C. parapsilosis; the CLSI resistance breakpoint both for anidulafungin and caspofungin and for micafungin was defined as an MIC of >0.25 μg/ml and >0.12 μg/ml, respectively, against C. glabrata. In lieu of CBPs, the ECV of >0.5 μg/ml was used to identify isolates of C. glabrata nonsusceptible (i.e., isolates with the non-wild-type [non-WT] phenotype) to voriconazole; ECVs of >0.06 μg/ml, >0.25 μg/ml, >0.12 μg/ml, >2 μg/ml, and >0.5 μg/ml were used to identify isolates of C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, and C. krusei, respectively, nonsusceptible (non-WT phenotype) to posaconazole (29). ECVs were also used to identify isolates of C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, and C. krusei nonsusceptible (non-WT phenotype) to amphotericin B (>2 μg/ml for all) and flucytosine (>0.5 μg/ml for of C. albicans, C. parapsilosis, C. tropicalis, and C. glabrata and >32 μg/ml for C. krusei), as well as those of other Candida species, such as Candida guilliermondii (>2 μg/ml and >1 μg/ml) and Candida lusitaniae (>2 μg/ml and 0.5 μg/ml) (29). For C. guilliermondii and the echinocandins, the CLSI resistance breakpoint of >4 μg/ml (28, 29) was used. Also, ECVs for triazoles and echinocandins were used to identify nonsusceptible (non-WT phenotype) isolates of C. guilliermondii (only for triazoles), C. lusitaniae, and other Candida species, such as Candida dubliniensis, Candida kefyr, and Candida pelliculosa (29). Among non-Candida yeasts, we used ECVs only for Cryptococcus neoformans and fluconazole (16 μg/ml), itraconazole (1 μg/ml), posaconazole (0.5 μg/ml), and voriconazole (0.25 μg/ml), as reported elsewhere (30); Rhodotorula species, C. neoformans, and Trichosporon asahii were considered intrinsically resistant to echinocandins. Rates of resistance were not calculated for the remaining species and antifungal compound combinations.
All Candida isolates with MICs for anidulafungin, caspofungin, and/or micafungin greater than the CBPs or ECVs were investigated for the presence or absence of a mutation in the hot spot (HS) regions of the FKS gene, as previously described (see reference 31 and references therein). This gene encodes the target enzyme (glycan synthase) for echinocandins (32).
Statistics.
All incidence rates were calculated using as the denominator the summed numbers of inpatient days of the UCSC hospital during the study period and are presented per 1,000 inpatient days (33). Categorical variables were analyzed using the chi-square test or Fisher's exact test, and continuous variables were analyzed by the Mann-Whitney U test. Significance was set as a P value of <0.05 (two-tailed). All analyses were done using STATA software (version 11.1; StataCorp, College Station, TX).
RESULTS AND DISCUSSION
Table 1 shows the distribution of species for the BSI episodes caused by 1,250 yeasts during the study period (January 2005 to December 2013). Among the isolates, 1,226 were Candida species and 24 were non-Candida species (7 C. neoformans isolates and 17 isolates of other species). As expected, Candida species accounted for 98.1% of the BSI isolates and C. albicans was the predominant species (n = 718 isolates, 57.4%), followed by the C. parapsilosis complex (n = 262, 20.9%), C. tropicalis (n = 102, 8.2%), C. glabrata (n = 80, 6.4%), C. guilliermondii (n = 20, 1.6%), and C. krusei (n = 16, 1.3%); miscellaneous species of Candida (n = 28, 2.2%) included C. lusitaniae (n = 9, 0.7%) and 10 other infrequent species (n = 19, 1.5%). Non-Candida yeasts accounted for 1.9% of all BSI isolates, and these were dominated by Rhodotorula species (Rhodotorula mucilaginosa, Rhodotorula glutinis, and Rhodotorula dairenensis; 9 isolates) and C. neoformans, which together accounted for 1.3% of all BSI isolates and 66.6% of all non-Candida yeasts. Overall, we recorded 1,214 first episodes of BSI, among which 1,183 were diagnosed in patients admitted to medical wards (n = 580, 47.8%), surgical wards (n = 335, 27.6%), the intensive care unit (ICU; n = 166, 13.7%), and oncology or hematology ward (n = 102, 8.4%) at the time of blood sample collection; the remaining 31 (2.5%) BSI episodes were acquired when the patients were outpatients (Table 1). Compared with the other Candida species, C. albicans and the C. parapsilosis complex were more likely to infect patients with hematological diseases and/or malignancies (P < 0.001), whereas C. albicans, the C. parapsilosis complex, and C. guilliermondii were more likely to infect ICU patients (P = 0.024, P = 0.004, and P = 0.014, respectively). As calculated from the total number of inpatient days (n = 3,574,148), the overall incidence rate was 0.33/1,000 inpatient days; the highest incidence was observed in ICU patients (0.61/1,000 inpatient days), followed by medical patients (0.42/1,000 inpatient days), malignancy patients (0.29/1,000 inpatient days), and surgical patients (0.21/1,000 inpatient days). Also, the overall incidence rates per 1,000 inpatient days were calculated for C. albicans, the C. parapsilosis complex, C. tropicalis, C. glabrata, C. guilliermondii, and C. krusei (Table 1).
TABLE 1.
Characteristics of BSI episodes by causative organism over a 9-year study perioda
| Characteristic | Result for the following yeast species (no. of episodes): |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| All BSIs (n = 1,214) | C. albicans (n = 692) | C. parapsilosis (n = 248) | C. tropicalis (n = 92) | C. glabrata (n = 69) | C. guilliermondii (n = 18) | C. krusei (n = 13) | Otherb (n = 47) | Multiple speciesc (n = 35) | |
| Median (IQRd) age (yr) | 72 (56–82) | 74 (59.5–82) | 65.5 (49–81) | 75 (61–83.5) | 71 (61–79) | 48 (4–71) | 67 (54–78) | 59 (41–79) | 76 (68–86) |
| No. (%) of male patients | 661 (54.4) | 364 (52.6) | 132 (53.2) | 54 (58.7) | 38 (55.1) | 11 (61.1) | 8 (61.5) | 32 (68.1) | 22 (31.0) |
| No. (%) of patients with BSI in the following yr: | |||||||||
| 2005 | 94 (7.7) | 41 (43.6) | 28 (29.8) | 9 (9.6) | 6 (6.4) | 1 (1.1) | 3 (3.2) | 3 (3.2) | 2 (2.1) |
| 2006 | 115 (9.5) | 70 (60.9) | 17 (14.8) | 9 (7.8) | 12 (10.4) | 0 (0.0) | 2 (1.7) | 3 (2.6) | 3 (2.6) |
| 2007 | 118 (9.7) | 71 (60.2) | 26 (22.0) | 5 (4.2) | 5 (4.2) | 1 (0.8) | 1 (0.8) | 7 (5.9) | 2 (1.7) |
| 2008 | 129 (10.6) | 71 (55.0) | 19 (14.7) | 7 (5.4) | 9 (7.0) | 6 (4.6) | 2 (1.5) | 6 (4.6) | 9 (7.0) |
| 2009 | 153 (12.6) | 89 (58.2) | 28 (18.3) | 16 (10.4) | 8 (5.2) | 3 (2.0) | 0 (0.0) | 1 (0.6) | 8 (5.2) |
| 2010 | 145 (11.9) | 87 (60.0) | 31 (21.4) | 8 (5.5) | 7 (4.8) | 2 (1.4) | 1 (0.7) | 4 (2.7) | 5 (3.4) |
| 2011 | 155 (12.7) | 90 (58.1) | 30 (19.3) | 13 (8.4) | 4 (2.6) | 3 (1.9) | 1 (0.6) | 11 (7.1) | 3 (1.9) |
| 2012 | 175 (14.4) | 111 (63.4) | 36 (20.6) | 11 (6.3) | 10 (5.7) | 0 (0.0) | 0 (0.0) | 7 (4.0) | 0 (0.0) |
| 2013 | 130 (10.7) | 62 (47.7) | 33 (25.4) | 14 (10.8) | 8 (6.1) | 2 (1.5) | 3 (2.3) | 5 (3.8) | 3 (2.3) |
| Incidence rate of BSIe | 0.33 | 0.23 | 0.08 | 0.03 | 0.025 | 0.007 | 0.005 | ND | ND |
| No. (%) of patients in the following categoryf: | |||||||||
| Medical | 580 (47.8) | 329 (56.7) | 123 (21.2) | 47 (8.1) | 30 (5.2) | 7 (1.2) | 4 (0.7) | 22 (3.8) | 18 (3.1) |
| Surgical | 335 (27.6) | 205 (61.2) | 63 (18.8) | 23 (6.9) | 21 (6.3) | 3 (0.9) | 1 (0.3) | 10 (3.0) | 9 (2.7) |
| ICU | 166 (13.7) | 108 (65.1) | 20 (12.0) | 12 (7.2) | 8 (4.8) | 6 (3.6) | 2 (1.2) | 4 (2.4) | 6 (3.6) |
| Oncology-hematology | 102 (8.4) | 34 (33.3) | 37 (36.3) | 7 (6.9) | 9 (8.8) | 1 (1.0) | 5 (4.9) | 8 (7.8) | 1 (1.0) |
| Outpatient settingg | 31 (2.5) | 16 (51.6) | 5 (31.2) | 3 (9.7) | 1 (3.2) | 1 (3.2) | 1 (3.2) | 3 (9.7) | 1 (3.2) |
| Median (IQR) time (days) to BSI onseth | 25 (11–42) | 28 (16–45) | 25.5 (14–42.5) | 20 (9–38) | 24 (10–40) | 22.5 (13–31) | 9 (4–20) | 21 (9–30) | 26 (11–44) |
From a total of 1,214 episodes of bloodstream infection (BSI) identified between January 2005 and December 2013, 35 episodes had a polyfungal etiology (see footnote c of Table 1 for details about the infecting species).
Other Candida and non-Candida species included isolates of C. lusitaniae (n = 9), Cryptococcus neoformans (n = 7), Blastoschizomyces capitatus (n = 5), Rhodotorula mucilaginosa (n = 6), C. famata (n = 3), C. dubliniensis (n = 2), C. lipolytica (n = 2), C. rugosa (n = 2), Candida utilis (n = 2), and 1 isolate each of Candida intermedia, C. kefyr, C. lambica, C. norvegensis, C. pelliculosa, Rhodotorula dairenensis, Rhodotorula glutinis, Saccharomyces cerevisiae, and Trichosporon asahii.
Multiple species included 71 isolates of C. albicans (n = 9) plus C. glabrata (n = 9), C. albicans (n = 9) plus C. parapsilosis (n = 9), C. albicans (n = 5) plus C. tropicalis (n = 5), C. albicans (n = 2) plus C. krusei (n = 2), C. albicans (n = 1) plus C. guilliermondii (n = 1), B. capitatus (n = 1) plus C. tropicalis (n = 1), C. famata (n = 1) plus C. parapsilosis (n = 1), C. glabrata (n = 1) plus C. parapsilosis (n = 1), C. glabrata (n = 1) plus C. parapsilosis (n = 1) plus C. tropicalis (n = 1), C. guilliermondii (n = 1) plus C. parapsilosis (n = 1), C. krusei (n = 1) plus C. tropicalis (n = 1), C. pelliculosa (n = 1) plus C. tropicalis (n = 1), C. rugosa (n = 1) plus C. tropicalis (n = 1), and R. mucilaginosa (n = 1) plus C. parapsilosis (n = 1).
IQR, interquartile range.
The incidence rate was calculated for unique BSI isolates of yeast species and is presented per 1,000 inpatient days. ND, not determined.
ICU, intensive care unit. The oncology-hematology category includes patients hospitalized in the oncology or hematology ward.
This also includes patients who visited the emergency department during the study period.
The time indicates the interval elapsing from the day of the patient's admission to the day that the first blood culture was found to be positive for that patient.
Among the 1,183 HO-BSI patients, the median time from the time of admission to the time of detection of the first positive blood culture was 25 days (interquartile range [IQR], 11 to 42 days), with C. krusei BSIs being diagnosed the earliest (9 days; IQR, 4 to 20 days; P < 0.001) and C. albicans or C. tropicalis BSIs being diagnosed the latest (28 days [IQR, 16 to 45 days; P < 0.001] and 20 days [IQR, 9 to 38 days; P = 0.02], respectively) (Table 1). The number of total BSIs averaged ∼135 per year, with no discernible trends in either the number of infections or the species distribution per year being found (P > 0.05). The median age of all BSI patients (72 years) did not differ significantly with respect to whether the causative species was C. albicans, C. parapsilosis, C. tropicalis, C. glabrata, or C. krusei, with the exception of patients infected with C. guilliermondii, who were aged 48 years (P < 0.001) (Table 1). Polyfungal BSIs occurred in 35 patients (2.8%), of which 34 were infected by 2 species and 1 was infected by 3 species (C. glabrata, a C. parapsilosis complex isolate, and C. tropicalis) (Table 1). In 26 (74.3%) of these patients, C. albicans was isolated in combination with another yeast, among which C. glabrata and the C. parapsilosis complex accounted for 9 episodes each. Other mixed BSIs involved species like Blastoschizomyces capitatus, Candida famata, C. pelliculosa, Candida rugosa, and R. mucilaginosa, which are not commonly isolated worldwide (34, 35), although these species must be regarded as emerging causes of fungemia (36). It is noteworthy that C. parapsilosis was isolated together with C. famata in one case and with C. guilliermondii in another case. In fact, less prevalent Candida species are difficult to differentiate from each another with many identification systems that are currently used in clinical laboratories (37), except for the newly introduced MALDI-TOF mass spectrometry (23), and polyfungal fungemias also fail to be detected using a combination of conventional identification methods, like the ID 32C system plus CHROMagar (38).
Excluding C. albicans, the rank order of the six most frequently encountered Candida species in the present study was C. parapsilosis complex > C. tropicalis > C. glabrata > C. guilliermondii > C. krusei > C. lusitaniae (frequency range, 20.9 to 0.7%). As in other European countries (38), the C. parapsilosis complex was the most common of the non-albicans Candida species, but this is in apparent contrast to the findings of fungemia surveillances recently conducted in the United States (35, 39). In one of these studies, C. parapsilosis was found to be the most prevalent species in 4 of 24 medical centers surveyed, whereas C. krusei ranked second or third in prevalence in seven of these centers (39). Thus, it is not surprising that C. guilliermondii (accounting for 18 single-species infections and 2 mixed infections) was fourth in rank order among the non-albicans Candida species in our study. Likewise, the C. parapsilosis complex, C. tropicalis, and C. glabrata were the first three species to be identified as causes of invasive candidiasis among 1,072 isolates from a 3-year national surveillance in China (40).
Table 2 shows the results of testing of the in vitro susceptibilities of BSI isolates to nine antifungal agents, as routinely performed using the SYO method. Although such testing was done by common laboratory personnel, quality control procedures were performed each time that a new SYO panel batch was used during the study period, and the MICs for control strains (C. krusei ATCC 6258 and C. parapsilosis ATCC 22019) were within the acceptable range for the antifungals tested in each run (data not shown). As shown in Table 2, the MICs for the 1,250 yeast isolates were not always determined for all antifungals, since the number of antifungals in the SYO panels increased over time, i.e., from 6 in 2005 (version SYO-06) to 10 in 2009 (version SYO-10). Although ketoconazole has been available since the SYO-06 version, the MICs of this nonsystemic antifungal agent were disregarded in the present analysis.
TABLE 2.
In vitro susceptibilities of yeast BSI isolates tested against nine antifungals by SYO method
| Species | Antifungal agent | No. of isolates tested | MIC (μg/ml)a |
No. (%) of isolates in the indicated susceptibility category by CBPb |
No. (%) of isolates by ECVb |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | S | S-DD | I | R | Wild type | Non-wild type | |||
| C. albicans | Amphotericin B | 718 | ≤0.12 to 1 | 0.12 | 0.5 | 718 (100) | 0 (0.0) | ||||
| Flucytosine | 718 | ≤0.06 to 8 | ≤0.06 | 0.12 | 714 (99.4) | 4 (0.6) | |||||
| Fluconazole | 718 | ≤0.12 to 16 | 0.25 | 0.5 | 716 (99.7) | 0 (0.0) | 2 (0.3) | ||||
| Itraconazole | 718 | ≤0.015 to 1 | 0.03 | 0.06 | 713 (99.3) | 5 (0.7) | |||||
| Voriconazole | 718 | ≤0.008 to 0.5 | 0.008 | 0.008 | 716 (99.7) | 2 (0.3) | 0 (0.0) | ||||
| Posaconazole | 530 | ≤0.008 to 1 | 0.008 | 0.03 | 526 (99.2) | 4 (0.8) | |||||
| Caspofungin | 619 | ≤0.008 to 4 | 0.03 | 0.06 | 618 (99.8) | 0 (0.0) | 1 (0.2) | ||||
| Anidulafungin | 431 | ≤0.015 to 1 | ≤0.015 | 0.03 | 430 (99.8) | 0 (0.0) | 1 (0.2) | ||||
| Micafungin | 431 | ≤0.008 to 1 | ≤0.008 | 0.015 | 430 (99.8) | 0 (0.0) | 1 (0.2) | ||||
| C. parapsilosis complexc | Amphotericin B | 262 | ≤0.12 to 2 | 0.25 | 0.5 | 262 (100) | 0 (0.0) | ||||
| Flucytosine | 262 | ≤0.06 to ≥64 | 0.06 | 0.12 | 257 (98.1) | 5 (1.9) | |||||
| Fluconazole | 262 | ≤0.12 to 16 | 0.5 | 2 | 244 (93.1) | 12 (4.6) | 6 (2.3) | ||||
| Itraconazole | 262 | ≤0.015 to 0.5 | 0.03 | 0.12 | 262 (100) | 0 (0.0) | |||||
| Voriconazole | 262 | ≤0.008 to 0.25 | 0.015 | 0.03 | 260 (99.2) | 2 (0.8) | 0 (0.0) | ||||
| Posaconazole | 186 | ≤0.008 to 0.25 | 0.03 | 0.06 | 186 (100) | 0 (0.0) | |||||
| Caspofungin | 218 | 0.03 to 2 | 0.25 | 0.5 | 218 (100) | 0 (0.0) | 0 (0.0) | ||||
| Anidulafungin | 156 | ≤0.015 to 2 | 0.5 | 1 | 156 (100) | 0 (0.0) | 0 (0.0) | ||||
| Micafungin | 156 | 0.03 to 2 | 0.5 | 1 | 156 (100) | 0 (0.0) | 0 (0.0) | ||||
| C. tropicalis | Amphotericin B | 102 | ≤0.12 to 1 | 0.25 | 0.5 | 102 (100) | 0 (0.0) | ||||
| Flucytosine | 102 | ≤0.06 to ≥64 | 0.06 | 32 | 83 (81.4) | 19 (18.6) | |||||
| Fluconazole | 102 | ≤0.12 to 16 | 0.25 | 0.5 | 99 (97.0) | 1 (1.0) | 2 (2.0) | ||||
| Itraconazole | 102 | ≤0.015 to 0.5 | 0.12 | 0.25 | 102 (100) | 0 (0.0) | |||||
| Voriconazole | 102 | ≤0.008 to 0.25 | 0.03 | 0.06 | 101 (99.0) | 1 (1.0) | 0 (0.0) | ||||
| Posaconazole | 75 | ≤0.008 to 0.5 | 0.06 | 0.25 | 63 (84.0) | 12 (16.0) | |||||
| Caspofungin | 84 | 0.015 to 0.12 | 0.03 | 0.06 | 84 (100) | 0 (0.0) | 0 (0.0) | ||||
| Anidulafungin | 64 | ≤0.015 to 0.12 | 0.015 | 0.03 | 64 (100) | 0 (0.0) | 0 (0.0) | ||||
| Micafungin | 64 | 0.015 to 0.06 | 0.03 | 0.03 | 64 (100) | 0 (0.0) | 0 (0.0) | ||||
| C. glabrata | Amphotericin B | 80 | ≤0.12 to 1 | ≤0.12 | 0.5 | 80 (100) | 0 (0.0) | ||||
| Flucytosine | 80 | ≤0.06 | ≤0.06 | ≤0.06 | 80 (100) | 0 (0.0) | |||||
| Fluconazole | 80 | 0.25 to ≥256 | 8 | 16 | 77 (96.3) | 3 (3.7) | |||||
| Itraconazole | 80 | ≤0.015 to ≥16 | 0.5 | 1 | 77 (96.3) | 3 (3.7) | |||||
| Voriconazole | 80 | ≤0.008 to ≥8 | 0.12 | 0.5 | 77 (96.3) | 3 (3.7) | |||||
| Posaconazole | 53 | 0.25 to ≥8 | 0.5 | 2 | 50 (94.3) | 3 (5.7) | |||||
| Caspofungin | 62 | 0.03 to 0.25 | 0.06 | 0.12 | 62 (100) | 0 (0.0) | 0 (0.0) | ||||
| Anidulafungin | 42 | ≤0.015 to 0.12 | ≤0.015 | 0.03 | 42 (100) | 0 (0.0) | 0 (0.0) | ||||
| Micafungin | 42 | ≤0.008 to 0.03 | 0.015 | 0.015 | 42 (100) | 0 (0.0) | 0 (0.0) | ||||
| C. guilliermondii | Amphotericin B | 20 | ≤0.12 to 0.5 | 0.12 | 0.25 | 20 (100) | 0 (0.0) | ||||
| Flucytosine | 20 | ≤0.06 to ≥64 | 0.06 | 64 | 14 (70.0) | 6 (30.0) | |||||
| Fluconazole | 20 | 0.5 to 8 | 2 | 8 | 20 (100) | 0 (0.0) | |||||
| Itraconazole | 20 | 0.03 to 0.5 | 0.12 | 0.5 | 20 (100) | 0 (0.0) | |||||
| Voriconazole | 20 | ≤0.008 to 0.12 | 0.03 | 0.12 | 20 (100) | 0 (0.0) | |||||
| Posaconazole | 19 | 0.03 to 0.5 | 0.12 | 0.25 | 19 (100) | 0 (0.0) | |||||
| Caspofungin | 19 | 0.06 to 4 | 0.25 | 1 | 18 (94.7) | 1 (5.3) | 0 (0.0) | ||||
| Anidulafungin | 13 | 0.12 to 1 | 0.5 | 1 | 13 (100) | 0 (0.0) | 0 (0.0) | ||||
| Micafungin | 13 | 0.06 to 0.5 | 0.25 | 0.5 | 13 (100) | 0 (0.0) | 0 (0.0) | ||||
| C. krusei | Amphotericin B | 16 | ≤0.12 to 1 | 0.03 | 0.5 | 16 (100) | 0 (0.0) | ||||
| Flucytosine | 16 | 1 to 16 | 4 | 16 | 16 (100) | 0 (0.0) | |||||
| Fluconazole | 16 | 16 to 64 | 64 | 64 | 16 (100) | 0 (0.0) | |||||
| Itraconazole | 16 | 0.06 to 0.5 | 0.25 | 0.5 | 16 (100) | 0 (0.0) | |||||
| Voriconazole | 16 | 0.03 to 0.5 | 0.12 | 0.5 | 16 (100) | 0 (0.0) | 0 (0.0) | ||||
| Posaconazole | 10 | 0.06 to 0.25 | 0.25 | 0.25 | 10 (100) | 0 (0.0) | |||||
| Caspofungin | 11 | 0.12 to 2 | 0.25 | 0.25 | 10 (90.9) | 0 (0.0) | 1 (9.1) | ||||
| Anidulafungin | 7 | ≤0.015 to 0.5 | ND | ND | 6 (85.7) | 1 (14.3) | 0 (0.0) | ||||
| Micafungin | 7 | 0.06 to 0.5 | ND | ND | 6 (85.7) | 1 (14.3) | 0 (0.0) | ||||
| C. lusitaniae | Amphotericin B | 9 | 0.03 to 0.5 | ND | ND | 9 (100) | 0 (0.0) | ||||
| Flucytosine | 9 | ≤0.06 to 1 | ND | ND | 9 (100) | 0 (0.0) | |||||
| Fluconazole | 9 | 0.25 to 4 | ND | ND | 8 (88.9) | 1 (11.1) | |||||
| Itraconazole | 9 | ≤0.015 to 0.12 | ND | ND | 9 (100) | 0 (0.0) | |||||
| Voriconazole | 9 | ≤0.008 to 0.03 | ND | ND | 9 (100) | 0 (0.0) | |||||
| Posaconazole | 6 | ≤0.008 to 0.03 | ND | ND | 6 (100) | 0 (0.0) | |||||
| Caspofungin | 8 | 0.03 to 0.25 | ND | ND | 8 (100) | 0 (0.0) | |||||
| Anidulafungin | 3 | 0.03 to 0.12 | ND | ND | 3 (100) | 0 (0.0) | |||||
| Micafungin | 3 | 0.03 to 0.06 | ND | ND | 3 (100) | 0 (0.0) | |||||
| C. neoformans | Amphotericin B | 7 | ≤0.12 to 0.5 | ND | ND | ||||||
| Flucytosine | 7 | 4 to 32 | ND | ND | |||||||
| Fluconazole | 7 | 4 to 64 | ND | ND | 5 (71.4) | 2 (28.6) | |||||
| Itraconazole | 7 | 0.03 to 0.25 | ND | ND | 7 (100) | 0 (0.0) | |||||
| Voriconazole | 7 | 0.06 to 0.25 | ND | ND | 7 (100) | 0 (0.0) | |||||
| Posaconazole | 4 | 0.03 to 0.5 | ND | ND | 4 (100) | 0 (0.0) | |||||
| Caspofungin | 6 | ≥8 | ND | ND | |||||||
| Anidulafungin | 4 | ≥8 | ND | ND | |||||||
| Micafungin | 4 | ≥8 | ND | ND | |||||||
| Other yeastsd | Amphotericin B | 36 | ≤0.12 to 2 | 0.25 | 1 | ||||||
| Flucytosine | 36 | ≤0.06 to 16 | 0.06 | 4 | |||||||
| Fluconazole | 36 | 0.12 to 128 | 4 | 128 | |||||||
| Itraconazole | 36 | ≤0.015 to 2 | 0.12 | 0.5 | |||||||
| Voriconazole | 36 | ≤0.008 to 2 | 0.06 | 0.5 | |||||||
| Posaconazole | 25 | 0.015 to 4 | 0.25 | 1 | |||||||
| Caspofungin | 32 | 0.03 to ≥8 | 2 | ≥8 | |||||||
| Anidulafungin | 20 | ≤0.015 to ≥8 | 0.5 | ≥8 | |||||||
| Micafungin | 20 | ≤0.008 to ≥8 | 0.5 | ≥8 | |||||||
MICs are reported as the range, MIC50, and MIC90. The MIC50s and MIC90s were calculated only for those species with at least 10 isolates tested. ND, not determined.
Clinical breakpoints (CBPs) for susceptible (S), susceptible dose dependent (S-DD), intermediate (I), and resistant (R) were those of the CLSI (28, 29). In the absence of CBPs for amphotericin B, flucytosine, itraconazole, and posaconazole and the five most common species of Candida (C. albicans, C. glabrata, C. parapsilosis, C. tropicalis, and C. krusei), as for the voriconazole and C. glabrata combination, for which no CBPs were assigned by the CLSI (26, 29), isolates were classified as having the WT and non-WT drug susceptibility phenotypes according to the epidemiological cutoff values (ECVs) recently proposed by CLSI (29). In lieu of CBPs, ECVs were also used for the amphotericin B, flucytosine, triazole, and echinocandin antifungal agents to identify isolates of C. guilliermondii with the non-WT phenotype (excluding echinocandins), C. lusitaniae, and other listed Candida species, such as C. dubliniensis, C. kefyr, and C. pelliculosa (see footnote d below) (29). Among the non-Candida yeasts (see footnote d below), ECVs were used only for Cryptococcus neoformans, as specified in the text.
Includes two isolates that were identified as C. orthopsilosis since their isolation from the respective patients' blood cultures in 2010 and 2011, which was subsequent to the MALDI-TOF mass spectrometry implementation in the clinical microbiology laboratory. These isolates were classified as resistant to fluconazole according to the C. parapsilosis species-specific CBP mentioned in footnote b above, or as having the non-WT phenotype for susceptibility to fluconazole according to the established ECV (>2 μg/ml) (29). Two of the remaining four fluconazole-resistant isolates initially designated to be C. parapsilosis species complex were analyzed using MALDI-TOF mass spectrometry at the time of the present study and were identified as C. parapsilosis sensu stricto.
Other Candida and non-Candida species included isolates of Blastoschizomyces capitatus (n = 6), Rhodotorula mucilaginosa (n = 7), C. famata (n = 4), C. rugosa (n = 3), C. dubliniensis (n = 2), C. lipolytica (n = 2), C. pelliculosa (n = 2), Candida utilis (n = 2), and 1 isolate each of C. intermedia, C. kefyr, C. lambica, C. norvegensis, Rhodotorula dairenensis, Rhodotorula glutinis, Saccharomyces cerevisiae, and Trichosporon asahii (see also Table S2 in the supplemental material).
Among 1,209 isolates of common and less common Candida species (including 9 isolates of C. lusitaniae and 2 isolates of C. dubliniensis), over 97% were of the WT phenotype for amphotericin B and flucytosine susceptibility; no isolates had amphotericin B MICs above the ECV, whereas 34 isolates across C. albicans (4/718 isolates, 0.6%), the C. parapsilosis complex (5/262 isolates, 1.9%), C. tropicalis (19/102 isolates, 18.6%), and C. guilliermondii (6/20 isolates, 30%) were found to have the non-WT phenotype for flucytosine susceptibility. The remaining 17 isolates belonged to those Candida species (e.g., C. kefyr, C. pelliculosa) for which amphotericin B or flucytosine ECVs were not defined (29). Tentative ECVs for the SYO method were recently proposed, and the median values obtained by the five approaches employed for flucytosine and C. albicans, C. parapsilosis, and C. tropicalis were almost identical to those obtained with the CLSI method (41). For these species, the MIC ranges of flucytosine obtained in the present study were similar to those obtained in two earlier surveys, both of which used the SYO method (20, 40), but a higher proportion of our C. parapsilosis complex or C. tropicalis isolates exhibited flucytosine MICs greater than the CLSI ECVs.
With regard to C. albicans, 2 (0.3%) isolates were resistant to fluconazole and 2 (0.3%) isolates were susceptible dose dependent to voriconazole, whereas 5 (0.7%) isolates and 4 (0.8%) isolates had the non-WT phenotype for itraconazole and posaconazole susceptibility, respectively. With regard to the C. parapsilosis complex, 6 (2.3%) isolates were resistant and 12 (4.6%) isolates were susceptible dose dependent to fluconazole and 2 (0.8%) isolates were susceptible dose dependent to voriconazole; no isolates with itraconazole or posaconazole MICs greater than the ECVs were found. With regard to C. tropicalis, 2 (2.0%) isolates and 1 (1.0%) isolate were resistant and susceptible dose dependent to fluconazole, respectively, and 1 (1.0%) isolate was susceptible dose dependent to voriconazole, whereas 12 (16%) isolates had the non-WT phenotype for posaconazole susceptibility. With regard to C. glabrata, 3 (3.7%) isolates were resistant to fluconazole, 3 (3.7%) isolates had the non-WT phenotype for itraconazole or voriconazole susceptibility, and 3 (5.7%) had the non-WT phenotype for posaconazole susceptibility. All C. krusei isolates in this study were susceptible to voriconazole and had the WT phenotype for itraconazole and posaconazole susceptibility. The isolates of C. neoformans (the most represented among the non-Candida species studied) showed high MIC values only to fluconazole, with 2 (28.6%) of 7 isolates classified as having the non-WT phenotype for susceptibility to this antifungal agent.
Among the 11 remaining Candida species studied, 1 (11.1%) isolate of C. lusitaniae had the non-WT phenotype for fluconazole susceptibility, whereas 2 isolates of C. pelliculosa and 1 isolate of C. kefyr had fluconazole MICs that were below the ECVs established for this antifungal agent (see Table S2 in the supplemental material). In contrast, fluconazole MICs were consistently ≥2 μg/ml for C. famata (3 of 4 isolates), C. rugosa (2 of 3 isolates), Candida lipolytica (1 of 2 isolates), Candida lambica (1 isolate), and Candida norvegensis (1 isolate) (see Table S2 in the supplemental material). Otherwise, lower MICs of itraconazole and voriconazole were seen for C. famata (0.25 and ≤0.12 μg/ml, respectively), C. rugosa (0.06 and ≤0.06 μg/ml, respectively), C. lipolytica (0.25 and ≤0.12 μg/ml, respectively), C. lambica (0.12 and 0.03 μg/ml, respectively), and C. norvegensis (0.25 and 0.12 μg/ml, respectively); similarly, the MICs of posaconazole, when tested, were 0.12 μg/ml for C. famata (1 isolate), 0.25 μg/ml and 0.5 μg/ml for C. lipolytica (2 isolates), and 0.25 μg/ml for C. norvegensis (1 isolate) (see Table S2 in the supplemental material).
Among the Candida isolates tested (1,024 isolates for caspofungin and 718 isolates for both anidulafungin and micafungin across C. albicans, C. parapsilosis complex, C. tropicalis, C. glabrata, C. guilliermondii, C. krusei, C. lusitaniae, C. dubliniensis, C. kefyr, and C. pelliculosa isolates), susceptibility to echinocandins was very high. Despite this, the rates at which isolates were nonsusceptible (either intermediate or resistant) to echinocandins were 0.2% (1/619) for C. albicans, 5.3% (1/19) for C. guilliermondii, and 9.1% (1/11) for C. krusei (only to caspofungin), but no resistance was found among C. glabrata and C. tropicalis isolates. The C. albicans isolate was found to harbor a point mutation (S645F) in HS1 of fks1, whereas the C. guilliermondii isolate (except for a constitutive polymorphism) and the C. krusei isolate were wild type for the fks gene; of note, the C. guilliermondii isolate showed an intermediate phenotype for susceptibility to caspofungin and anidulafungin (Table 3). It was noticed that adoption of the revised CLSI CBPs for caspofungin may overstate the rates at which isolates are nonsusceptible (especially intermediate) to caspofungin among C. glabrata and C. krusei isolates (18), and the interlaboratory variability in caspofungin MICs for C. albicans, C. glabrata, C. tropicalis, and C. krusei may considerably limit the use of both the CLSI and EUCAST reference methods (42). Thus, while clinical microbiology laboratories should use micafungin or anidulafungin as a surrogate marker to predict caspofungin susceptibility (43, 44), the use of SYO assays was recently advised for hospitals that routinely perform echinocandin susceptibility testing of bloodstream isolates (18). This advice was provided to overcome the variability in caspofungin MICs that occurs when Candida species are tested by the reference methods. To support this concept, we observed low variability among the caspofungin MICs obtained for isolates of the most common Candida species, even through the testing performed with different SYO batches throughout the study period (see Fig. S1 in the supplemental material).
TABLE 3.
Characteristics of BSI episodes caused by Candida isolates shown to be fluconazole resistant or echinocandin nonsusceptible in vitroh
| Date of BSI (day/mo/yr) | Candida species | Underlying condition(s) | Antifungal therapy | Duration (days)a | Resistance (non-WT phenotype) profile of the isolateb | MIC of the isolate (μg/ml)c |
Source control (hour timing)d | Prior antifungal treatment | Outcomee | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FLC | ITC | VRC | POS | CAS | ANF | MCF | |||||||||
| 14/11/2005 | C. lusitaniae | Gastric lymphoma | FLC | 7 | FLC | 4 | 0.12 | 0.03 | ND | Yes (72) | Azoles (FLC) | Deceased | |||
| 16/12/2005 | C. parapsilosis complexf | Acute myeloid leukemia | AMB | 16 | FLC | 8 | 0.12 | 0.12 | ND | Yes (96) | Azoles (ITC) | Alive | |||
| 20/6/2006 | C. parapsilosis complexf | Small intestine syndrome | AMB | 24 | FLC | 16 | 0.5 | 0.25 | ND | Yes (72) | Azoles (FLC) | Alive | |||
| 27/9/2006 | C. albicans | Cholecystitis | FLC | 10 | FLC, ITC | 16 | 1 | 0.5 | ND | No | No | Deceased | |||
| 6/6/2007 | C. parapsilosis complexf | Hematopoietic stem cell transplantation | AMB | 22 | FLC | 8 | 0.25 | 0.12 | ND | Yes (48) | AMB and azoles (ITC, FLC) | Alive | |||
| 29/8/2007 | C. parapsilosis complexf | Anorexia, peripheral nervous system involvement | FLC | 15 | FLC | 8 | 0.12 | 0.12 | ND | Yes (48) | No | Alive | |||
| 7/4/2008 | C. glabrata | Staphylococcal prosthetic valve endocarditis | CAS | 35 | FLC, ITC, VRC, POS | 256 | >16 | 4 | 8 | No | Azoles (FLC) | Alive | |||
| 16/4/2008 | C. glabrata | Chronic lymphoid leukemia, COPD | CAS | 5 | FLC, ITC, VRC, POS | 128 | 16 | 4 | 8 | No | Azoles (FLC) | Deceased | |||
| 13/7/2009 | C. tropicalis | Hydrocephalus | VRC | 21 | FLC, POS | 16 | 0.25 | 0.25 | 0.25 | Yes (48) | Azoles (FLC) | Alive | |||
| 13/8/2009 | C. tropicalis | Glioblastoma | VRC | 5 | FLC, POS | 8 | 0.12 | 0.12 | 0.25 | Yes (72) | No | Deceased | |||
| 13/5/2010 | C. albicans | Uncontrolled diabetes | ANF | 19 | FLC, ITC, POS | 16 | 1 | 0.12 | 1 | No | Azoles (FLC) | Alive | |||
| 22/12/2010 | C. orthopsilosisg | Pharyngeal cancer | CAS | 31 | FLC, VRC | 8 | 0.12 | 0.25 | 0.06 | Yes (48) | Azoles (FLC) and candins (CAS) | Alive | |||
| 10/10/2011 | C. orthopsilosisg | Renal transplantation | ANF | 18 | FLC, VRC | 8 | 0.12 | 0.25 | 0.12 | Yes (24) | Azoles (FLC) | Alive | |||
| 8/1/2013 | C. glabrata | Thyroiditis | CAS | 4 | FLC, ITC, VRC, POS | 256 | 16 | 4 | >8 | No | Azoles (FLC) | Deceased | |||
| 21/4/2008 | C. albicans | Systemic lupus erythematosus | FLC | 23 | CAS | 4 | 1 | 1 | No | Candins (CAS) | Alive | ||||
| 6/10/2008 | C. guilliermondii | Preterm newborn | AMB | 17 | CAS | 4 | 4 | 2 | Yes (48) | No | Alive | ||||
| 3/8/2010 | C. krusei | Acute lymphoblastic leukemia | AMB | 4 | CAS | 1 | 0.25 | 0.12 | Yes (72) | Candins (CAS) | Deceased | ||||
All patients who died within 7 days after infection were not treated appropriately with respect to the duration of antifungal therapy.
Resistant isolates (with the non-WT phenotype) include isolates from normally susceptible Candida species that showed antifungal MICs above the CBPs or ECVs used in this study (see the text for details). All 16 C. krusei isolates studied (MIC range, 16 to 64 μg/ml) were considered intrinsically fluconazole resistant and also are not listed. Nonsusceptible includes either intermediate or resistant to echinocandins.
The MICs of the following antifungal agents were determined: fluconazole, itraconazole; voriconazole, posaconazole, amphotericin B, caspofungin, anidulafungin, and micafungin. Boldface denotes intermediate susceptibility. The isolates of C. albicans and C. guilliermondii (both with caspofungin MICs of 4 μg/ml) were tested for susceptibility to anidulafungin and micafungin only later, because the two echinocandins were not available at the date of their isolation (the year 2008).
Adequate source control was defined as removal of any preexisting central vein catheters or other fluid collections thought to be the source of Candida infection within 48 h of the onset of BSI, as determined by the positivity of the patient's blood culture(s) (47).
Deaths were recorded at ≤8 days (for 5 patients) and 11 days (for 1 patient) after the first positive blood culture, whereas survival at 30 and 45 days was recorded for the remaining patients.
Isolates from the C. parapsilosis species complex could be not differentiated as C. parapsilosis sensu stricto, Candida metapsilosis, or C. orthopsilosis, because MALDI-TOF mass spectrometry was not available in the clinical microbiology laboratory until the end of 2009.
Two isolates were identified as C. orthopsilosis by MALDI-TOF mass spectrometry. These isolates were classified as WT for susceptibility to itraconazole (MIC, 0.12 μg/ml) using the ECV of ≤0.5 established for C. parapsilosis sensu lato (29).
Abbreviations: FLC, fluconazole; ITC, itraconazole; VRC; voriconazole; POS, posaconazole; AMB, amphotericin B; CAS, caspofungin; ANF, anidulafungin; MCF, micafungin; COPD, chronic obstructive pulmonary disease; ND, not determined.
The percentages of resistance reported in our study are similar to those reported from two recent Spanish studies (38, 45), showing that resistance to echinocandins is not emerging like it is in other geographical areas, such as the United States (15, 35). In addition, it is notable that antifungal susceptibility testing in those studies was performed by using EUCAST and CLSI reference procedures, with comparable results being obtained between the two methods (45), and it is notable that our findings are also similar to those reported after analyzing yeast isolates collected from all over the world (SENTRY Program 2010-2011), using CLSI broth microdilution methods (30). In a study by Pfaller et al., decreased susceptibility to posaconazole was prominently (>5%) observed in 8.3% of the isolates of C. albicans (ECV, 0.06 μg/ml) and 7.1% of the isolates of C. krusei (ECV, 0.5 μg/ml) that were obtained from European laboratories (30). Interestingly, in that study (30) the C. krusei isolates for which posaconazole MICs were >0.5 μg/ml (non-WT phenotype) yet which had the WT phenotype for voriconazole susceptibility are reminiscent of C. tropicalis isolates for which posaconazole MICs were >0.12 μg/ml (non-WT phenotype) yet were classified as having the WT phenotype for voriconazole susceptibility in the present study. This provides further support for the concept that posaconazole ECVs for C. krusei and other common species of Candida may be set too low, perhaps because the ECVs were derived from MIC distributions which were obtained from a single laboratory (30). However, ECVs for MIC distributions originating from ≥6 laboratories for posaconazole remained substantially unchanged for eight species of Candida, including C. albicans, C. tropicalis, and C. krusei (46).
Overall (only Candida species), the rate of susceptibility was 97.5% (1,196/1,226 isolates) for fluconazole and 99.7% (1,032/1,035 isolates) for caspofungin. Among the fluconazole-resistant isolates, 16 isolates were C. krusei and the remaining 14 isolates were the C. parapsilosis complex (6 isolates, including 2 Candida orthopsilosis isolates), C. glabrata (3 isolates), C. albicans (2 isolates), C. tropicalis (2 isolates), and C. lusitaniae (1 isolate) (Table 3). Five isolates (3 C. glabrata and 2 C. orthopsilosis isolates) were resistant (non-WT phenotype) to fluconazole and voriconazole, and 3 isolates (all C. glabrata) were resistant (non-WT phenotype) to the other three azoles. Two C. albicans isolates were cross-resistant to fluconazole and itraconazole, and 1 C. albicans isolate and 2 C. tropicalis isolates exhibited a non-WT phenotype for posaconazole susceptibility. Overall (all isolates), the rate of resistance to fluconazole and echinocandin antifungals was 3.9%, as reflected by the number of BSI episodes caused by species with decreased susceptibility to fluconazole or by intrinsically resistant species, such as C. neoformans, Rhodotorula spp., or Trichosporon asahii. Even though these species are regarded to be rare pathogens, they merit particular attention because their challenging intrinsic susceptibility pattern often leads to delayed appropriate antifungal treatment (4).
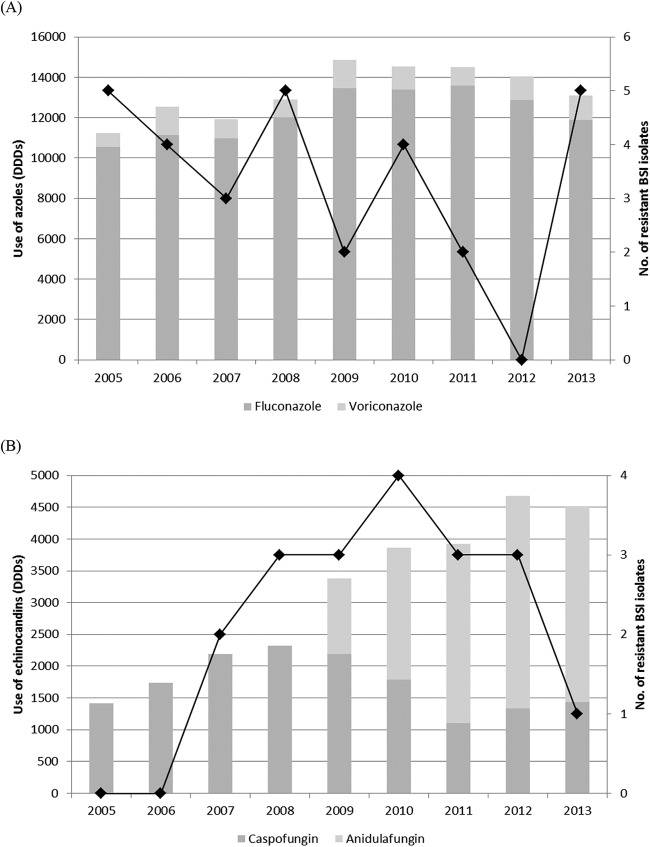
Table 3 also shows the characteristics of patients with BSIs caused by Candida isolates found to be nonsusceptible to an azole(s) or echinocandin(s) in vitro. Among 14 patients infected with fluconazole-resistant Candida species, 13 (92.8%) were adults (age range, 22 to 91 years) and 8 (57.1%) were male. One of three patients with a BSI caused by an echinocandin-resistant Candida species was a newborn who was infected with C. guilliermondii. Excluding the last patient and 3 other patients (1 infected with C. albicans, 1 infected with the C. parapsilosis complex, and 1 infected with C. tropicalis), all the remaining patients had experienced prior exposure to azoles (n = 9, 64.2%) or echinocandins (n = 2, 14.2%) alone; 1 patient (infected with C. orthopsilosis) had previously been treated with either an azole or an echinocandin antifungal agent, and another patient (infected with an isolate of the C. parapsilosis complex) had previously been treated with either amphotericin B or azoles (both fluconazole and itraconazole). Six of 17 patients died, and in 5 of these patients the death occurred ≤8 days after initiation of antifungal therapy. Three of 14 patients infected with fluconazole-resistant isolates were treated with fluconazole, and 2 of them (i.e., 1 with a C. lusitaniae BSI and 1 with a C. albicans BSI) died after only 7 and 10 days of antifungal therapy, respectively; the third patient (with a C. parapsilosis complex BSI) survived after 15 days of antifungal therapy. The patients infected with echinocandin-nonsusceptible isolates were treated with fluconazole (1 patient) and amphotericin B (2 patients), but in one of them (i.e., the patient infected with C. krusei), the amphotericin B therapy was administered only for 4 days because the death occurred early. Furthermore, 6 of 17 patients had not received adequate control of the source infection, and half of these patients did not survive, according to previously published observations (47). The 17 BSI episodes described in Table 3 were distributed uniformly over the time period from 14 November 2005 to 8 January 2013, with 3 episodes (in the years 2005 and 2008) to 1 episodes (in the years 2006, 2007, 2009, and 2010) occurring per year (Fig. 1). This was despite the persistently high rate of fluconazole consumption during the study period (10,542 DDDs in 2005 to 11,889 DDDs in 2013); in contrast, the hospital use of echinocandins had greatly increased during the same 9-year period, ranging from 1,414 DDDs (only caspofungin) in 2005 to 4,522 DDDs (both caspofungin and anidulafungin) in 2013. Of note, the echinocandin DDD ratio, which was 1.8 for caspofungin/anidulafungin in 2009, was noticed to reverse in favor of anidulafungin in 2010 and to reach values of 2.5 in 2011, which remained stable until 2013 (Fig. 1). No DDDs of micafungin were shown because this echinocandin was not included in the formulary of the hospital.
FIG 1.
Trends of azole (A) and echinocandin (B) consumption (in DDDs) in the UCSC hospital over the study period (2005 to 2013). The overall distribution of BSI episodes caused by Candida and non-Candida isolates with intrinsic or acquired fluconazole (A) or echinocandin (B) resistance in the same years is denoted by a black line.
A limitation of the present study is that no comparisons with the CLSI broth microdilution methods were made, but previous studies have documented that antifungal MICs generated by the SYO are in good essential agreement with those obtained by the CLSI methodology, from which SYO is adapted (48, 49). However, the categorical agreement may be lower, especially for some fungal species-antifungal drug combinations (19). We applied the CLSI CBPs where applicable, yet we were aware that the SYO method should really be employed to screen fungal isolates showing high MICs of antifungal agents. In this context, ECVs for Candida species based on the SYO method have been set up, but though they are within 1 2-fold dilution of those determined by the CLSI reference method (41, 50), they need to be further validated for routine use.
In conclusion, the present study shows that the development of secondary antifungal resistance among common Candida species is not a growing threat in our hospital but that the emergence of Candida or non-Candida species with intrinsically reduced susceptibility or resistance needs to be continuously monitored. This emphasizes the necessity to perform locally relevant epidemiological studies as well as antifungal susceptibility studies, which in turn will reinforce the role of the clinical microbiology laboratory in assisting clinicians with the treatment of invasive fungal infections.
Supplementary Material
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00285-15.
REFERENCES
- 1.Pappas PG, Kauffman CA, Andes D, Benjamin DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg BJ, Ostrosky-Zeichner L, Reboli AC, Rex JH, Walsh TJ, Sobel JD, Infectious Diseases Society of America. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 48:503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S, Bille J, Castagnola E, Cuenca-Estrella M, Donnelly JP, Groll AH, Herbrecht R, Hope WW, Jensen HE, Lass-Flörl C, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Viscoli C, Ullmann AJ, ESCMID Fungal Infection Study Group . 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect 18(Suppl 7):S19–S37. doi: 10.1111/1469-0691.12039. [DOI] [PubMed] [Google Scholar]
- 3.Ullmann AJ, Akova M, Herbrecht R, Viscoli C, Arendrup MC, Arikan-Akdagli S, Bassetti M, Bille J, Calandra T, Castagnola E, Cornely OA, Donnelly JP, Garbino J, Groll AH, Hope WW, Jensen HE, Kullberg BJ, Lass-Flörl C, Lortholary O, Meersseman W, Petrikkos G, Richardson MD, Roilides E, Verweij PE, Cuenca-Estrella M, ESCMID Fungal Infection Study Group . 2012. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: adults with haematological malignancies and after haematopoietic stem cell transplantation (HCT). Clin Microbiol Infect 18(Suppl 7):S53–S67. doi: 10.1111/1469-0691.12041. [DOI] [PubMed] [Google Scholar]
- 4.Arendrup MC, Boekhout T, Akova M, Meis JF, Cornely OA, Lortholary O, European Society of Clinical Microbiology and Infectious Diseases Fungal Infection Study Group, European Confederation of Medical Mycology. 2014. ESCMID and ECMM joint clinical guidelines for the diagnosis and management of rare invasive yeast infections. Clin Microbiol Infect 20(Suppl 3):S76–S98. doi: 10.1111/1469-0691.12360. [DOI] [PubMed] [Google Scholar]
- 5.Türel O. 2011. Newer antifungal agents. Expert Rev Anti Infect Ther 9:325–338. doi: 10.1586/eri.10.163. [DOI] [PubMed] [Google Scholar]
- 6.Fera MT, La Camera E, De Sarro A. 2009. New triazoles and echinocandins: mode of action, in vitro activity and mechanisms of resistance. Expert Rev Anti Infect Ther 7:981–998. doi: 10.1586/eri.09.67. [DOI] [PubMed] [Google Scholar]
- 7.Tumbarello M, Sanguinetti M, Trecarichi EM, La Sorda M, Rossi M, de Carolis E, de Gaetano Donati K, Fadda G, Cauda R, Posteraro B. 2008. Fungaemia caused by Candida glabrata with reduced susceptibility to fluconazole due to altered gene expression: risk factors, antifungal treatment and outcome. J Antimicrob Chemother 62:1379–1385. doi: 10.1093/jac/dkn381. [DOI] [PubMed] [Google Scholar]
- 8.Lortholary O, Desnos-Ollivier M, Sitbon K, Fontanet A, Bretagne S, Dromer F, French Mycosis Study Group. 2011. Recent exposure to caspofungin or fluconazole influences the epidemiology of candidemia: a prospective multicenter study involving 2,441 patients. Antimicrob Agents Chemother 55:532–538. doi: 10.1128/AAC.01128-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah DN, Yau R, Lasco TM, Weston J, Salazar M, Palmer HR, Garey KW. 2012. Impact of prior inappropriate fluconazole dosing on isolation of fluconazole-nonsusceptible Candida species in hospitalized patients with candidemia. Antimicrob Agents Chemother 56:3239–3243. doi: 10.1128/AAC.00019-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arendrup MC, Dzajic E, Jensen RH, Johansen HK, Kjaeldgaard P, Knudsen JD, Kristensen L, Leitz C, Lemming LE, Nielsen L, Olesen B, Rosenvinge FS, Røder BL, Schønheyder HC. 2013. Epidemiological changes with potential implication for antifungal prescription recommendations for fungaemia: data from a nationwide fungaemia surveillance programme. Clin Microbiol Infect 19:E343–E353. doi: 10.1111/1469-0691.12212. [DOI] [PubMed] [Google Scholar]
- 11.Arendrup MC. 2014. Update on antifungal resistance in Aspergillus and Candida. Clin Microbiol Infect 20(Suppl 6):S42–S48. doi: 10.1111/1469-0691.12513. [DOI] [PubMed] [Google Scholar]
- 12.Cuenca-Estrella M. 2014. Antifungal drug resistance mechanisms in pathogenic fungi: from bench to bedside. Clin Microbiol Infect 20(Suppl 6):S54–S59. doi: 10.1111/1469-0691.12495. [DOI] [PubMed] [Google Scholar]
- 13.Guinea J. 2014. Global trends in the distribution of Candida species causing candidemia. Clin Microbiol Infect 20(Suppl 6):S5–S10. doi: 10.1111/1469-0691.12539. [DOI] [PubMed] [Google Scholar]
- 14.Cleveland AA, Farley MM, Harrison LH, Stein B, Hollick R, Lockhart SR, Magill SS, Derado G, Park BJ, Chiller TM. 2012. Changes in incidence and antifungal drug resistance in candidemia: results from population-based laboratory surveillance in Atlanta and Baltimore, 2008-2011. Clin Infect Dis 55:1352–1361. doi: 10.1093/cid/cis697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alexander BD, Johnson MD, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. 2013. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis 56:1724–1732. doi: 10.1093/cid/cit136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cuenca-Estrella M, Rodriguez-Tudela JL. 2010. The current role of the reference procedures by CLSI and EUCAST in the detection of resistance to antifungal agents in vitro. Expert Rev Anti Infect Ther 8:267–276. doi: 10.1586/eri.10.2. [DOI] [PubMed] [Google Scholar]
- 17.Pfaller MA. 2012. Antifungal drug resistance: mechanisms, epidemiology, and consequences for treatment. Am J Med 125(1 Suppl):S3–S13. doi: 10.1016/j.amjmed.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Eschenauer GA, Nguyen MH, Shoham S, Vazquez JA, Morris AJ, Pasculle WA, Kubin CJ, Klinker KP, Carver PL, Hanson KE, Chen S, Lam SW, Potoski BA, Clarke LG, Shields RK, Clancy CJ. 2014. Real-world experience with echinocandin MICs against Candida species in a multicenter study of hospitals that routinely perform susceptibility testing of bloodstream isolates. Antimicrob Agents Chemother 58:1897–1906. doi: 10.1128/AAC.02163-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Posteraro B, Sanguinetti M. 2014. The future of fungal susceptibility testing. Future Microbiol 9:947–967. doi: 10.2217/fmb.14.55. [DOI] [PubMed] [Google Scholar]
- 20.Huang YT, Liu CY, Liao CH, Chung KP, Sheng WH, Hsueh PR. 2014. Antifungal susceptibilities of Candida isolates causing bloodstream infections at a medical center in Taiwan, 2009-2010. Antimicrob Agents Chemother 58:3814–3819. doi: 10.1128/AAC.01035-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Hal SJ, Chen SC, Sorrell TC, Ellis DH, Slavin M, Marriott DM. 2014. Support for the EUCAST and revised CLSI fluconazole clinical breakpoints by Sensititre® YeastOne® for Candida albicans: a prospective observational cohort study. J Antimicrob Chemother 69:2210–2214. doi: 10.1093/jac/dku124. [DOI] [PubMed] [Google Scholar]
- 22.Sanguinetti M, Porta R, Sali M, La Sorda M, Pecorini G, Fadda G, Posteraro B. 2007. Evaluation of VITEK 2 and RapID yeast plus systems for yeast species identification: experience at a large clinical microbiology laboratory. J Clin Microbiol 45:1343–1346. doi: 10.1128/JCM.02469-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Posteraro B, De Carolis E, Vella A, Sanguinetti M. 2013. MALDI-TOF mass spectrometry in the clinical mycology laboratory: identification of fungi and beyond. Expert Rev Proteomics 10:151–164. doi: 10.1586/epr.13.8. [DOI] [PubMed] [Google Scholar]
- 24.Pfaller MA, Woosley LN, Messer SA, Jones RN, Castanheira M. 2012. Significance of molecular identification and antifungal susceptibility of clinically significant yeasts and moulds in a global antifungal surveillance programme. Mycopathologia 174:259–271. doi: 10.1007/s11046-012-9551-x. [DOI] [PubMed] [Google Scholar]
- 25.Pfaller MA, Andes D, Diekema DJ, Espinel-Ingroff A, Sheehan D, CLSI Subcommittee for Antifungal Susceptibility Testing. 2010. Wild-type MIC distributions, epidemiological cutoff values and species-specific clinical breakpoints for fluconazole and Candida: time for harmonization of CLSI and EUCAST broth microdilution methods. Drug Resist Updat 13:180–195. doi: 10.1016/j.drup.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Pfaller MA, Andes D, Arendrup MC, Diekema DJ, Espinel-Ingroff A, Alexander BD, Brown SD, Chaturvedi V, Fowler CL, Ghannoum MA, Johnson EM, Knapp CC, Motyl MR, Ostrosky-Zeichner L, Walsh TJ. 2011. Clinical breakpoints for voriconazole and Candida spp. revisited: review of microbiologic, molecular, pharmacodynamic, and clinical data as they pertain to the development of species-specific interpretive criteria. Diagn Microbiol Infect Dis 70:330–343. doi: 10.1016/j.diagmicrobio.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Pfaller MA, Diekema DJ, Andes D, Arendrup MC, Brown SD, Lockhart SR, Motyl M, Perlin DS, CLSI Subcommittee for Antifungal Testing. 2011. Clinical breakpoints for the echinocandins and Candida revisited: integration of molecular, clinical, and microbiological data to arrive at species-specific interpretive criteria. Drug Resist Updat 14:164–176. doi: 10.1016/j.drup.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Clinical and Laboratory Standards Institute. 2012. Reference method for broth dilution antifungal susceptibility testing of yeasts; fourth informational supplement. CLSI document M27-S4. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 29.Pfaller MA, Diekema DJ. 2012. Progress in antifungal susceptibility testing of Candida spp. by use of Clinical and Laboratory Standards Institute broth microdilution methods, 2010 to 2012. J Clin Microbiol 50:2846–2856. doi: 10.1128/JCM.00937-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pfaller MA, Messer SA, Woosley LN, Jones RN, Castanheira M. 2013. Echinocandin and triazole antifungal susceptibility profiles for clinical opportunistic yeast and mold isolates collected from 2010 to 2011: application of new CLSI clinical breakpoints and epidemiological cutoff values for characterization of geographic and temporal trends of antifungal resistance. J Clin Microbiol 51:2571–2581. doi: 10.1128/JCM.00308-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katiyar SK, Alastruey-Izquierdo A, Healey KR, Johnson ME, Perlin DS, Edlind TD. 2012. Fks1 and Fks2 are functionally redundant but differentially regulated in Candida glabrata: implications for echinocandin resistance. Antimicrob Agents Chemother 56:6304–6309. doi: 10.1128/AAC.00813-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perlin DS. 2011. Current perspectives on echinocandin class drugs. Future Microbiol 6:441–457. doi: 10.2217/fmb.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai CC, Chu CC, Wang CY, Tsai HY, Cheng A, Lee YC, Huang YT, Liao CH, Hsueh PR. 2012. Association between incidence of candidaemia and consumption of antifungal agents at a medical centre in Taiwan. Int J Antimicrob Agents 40:349–353. doi: 10.1016/j.ijantimicag.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 34.Beyda ND, Chuang SH, Alam MJ, Shah DN, Ng TM, McCaskey L, Garey KW. 2013. Treatment of Candida famata bloodstream infections: case series and review of the literature. J Antimicrob Chemother 68:438–443. doi: 10.1093/jac/dks388. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto E, Boyken L, Tendolkar S, McDanel J, Castanheira M, Pfaller M, Diekema D. 2014. Candidemia surveillance in Iowa: emergence of echinocandin resistance. Diagn Microbiol Infect Dis 79:205–208. doi: 10.1016/j.diagmicrobio.2014.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Miceli MH, Díaz JA, Lee SA. 2011. Emerging opportunistic yeast infections. Lancet Infect Dis 11:142–151. doi: 10.1016/S1473-3099(10)70218-8. [DOI] [PubMed] [Google Scholar]
- 37.Castanheira M, Woosley LN, Diekema DJ, Jones RN, Pfaller MA. 2013. Candida guilliermondii and other species of Candida misidentified as Candida famata: assessment by Vitek 2, DNA sequencing analysis, and matrix-assisted laser desorption ionization–time of flight mass spectrometry in two global antifungal surveillance programs. J Clin Microbiol 51:117–124. doi: 10.1128/JCM.01686-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcos-Zambrano LJ, Escribano P, Sánchez C, Muñoz P, Bouza E, Guinea J. 2014. Antifungal resistance to fluconazole and echinocandins is not emerging in yeast isolates causing fungemia in a Spanish tertiary care center. Antimicrob Agents Chemother 58:4565–4572. doi: 10.1128/AAC.02670-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfaller MA, Andes DR, Diekema DJ, Horn DL, Reboli AC, Rotstein C, Franks B, Azie NE. 2014. Epidemiology and outcomes of invasive candidiasis due to non-albicans species of Candida in 2,496 patients: data from the Prospective Antifungal Therapy (PATH) registry 2004-2008. PLoS One 9:e101510. doi: 10.1371/journal.pone.0101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao M, Fan X, Chen SC, Wang H, Sun ZY, Liao K, Chen SL, Yan Y, Kang M, Hu ZD, Chu YZ, Hu TS, Ni YX, Zou GL, Kong F, Xu YC. 2015. Antifungal susceptibilities of Candida glabrata species complex, Candida krusei, Candida parapsilosis species complex and Candida tropicalis causing invasive candidiasis in China: 3 year national surveillance. J Antimicrob Chemother 70:802–810. doi: 10.1093/jac/dku460. [DOI] [PubMed] [Google Scholar]
- 41.Cantón E, Pemán J, Hervás D, Iñiguez C, Navarro D, Echeverría J, Martínez-Alarcón J, Fontanals D, Gomila-Sard B, Buendía B, Torroba L, Ayats J, Bratos A, Sánchez-Reus F, Fernández-Natal I, FUNGEMYCA Study Group. 2012. Comparison of three statistical methods for establishing tentative wild-type population and epidemiological cutoff values for echinocandins, amphotericin B, flucytosine, and six Candida species as determined by the colorimetric Sensititre YeastOne method. J Clin Microbiol 50:3921–3926. doi: 10.1128/JCM.01730-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Espinel-Ingroff A, Arendrup MC, Pfaller MA, Bonfietti LX, Bustamante B, Canton E, Chryssanthou E, Cuenca-Estrella M, Dannaoui E, Fothergill A, Fuller J, Gaustad P, Gonzalez GM, Guarro J, Lass-Flörl C, Lockhart SR, Meis JF, Moore CB, Ostrosky-Zeichner L, Pelaez T, Pukinskas SR, St-Germain G, Szeszs MW, Turnidge J. 2013. Interlaboratory variability of caspofungin MICs for Candida spp. using CLSI and EUCAST methods: should the clinical laboratory be testing this agent? Antimicrob Agents Chemother 57:5836–5842. doi: 10.1128/AAC.01519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pfaller MA, Messer SA, Diekema DJ, Jones RN, Castanheira M. 2014. Use of micafungin as a surrogate marker to predict susceptibility and resistance to caspofungin among 3,764 clinical isolates of Candida by use of CLSI methods and interpretive criteria. J Clin Microbiol 52:108–114. doi: 10.1128/JCM.02481-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfaller MA, Diekema DJ, Jones RN, Castanheira M. 2014. Use of anidulafungin as a surrogate marker to predict susceptibility and resistance to caspofungin among 4,290 clinical isolates of Candida by using CLSI methods and interpretive criteria. J Clin Microbiol 52:3223–3229. doi: 10.1128/JCM.00782-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guinea J, Zaragoza Ó, Escribano P, Martín-Mazuelos E, Pemán J, Sánchez-Reus F, Cuenca-Estrella M, CANDIPOP Project, GEIH-GEMICOMED (SEIMC), REIPI. 2014. Molecular identification and antifungal susceptibility of yeast isolates causing fungemia collected in a population-based study in Spain in 2010 and 2011. Antimicrob Agents Chemother 58:1529–1537. doi: 10.1128/AAC.02155-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Espinel-Ingroff A, Pfaller MA, Bustamante B, Canton E, Fothergill A, Fuller J, Gonzalez GM, Lass-Flörl C, Lockhart SR, Martin-Mazuelos E, Meis JF, Melhem MS, Ostrosky-Zeichner L, Pelaez T, Szeszs MW, St-Germain G, Bonfietti LX, Guarro J, Turnidge J. 2014. Multilaboratory study of epidemiological cutoff values for detection of resistance in eight Candida species to fluconazole, posaconazole, and voriconazole. Antimicrob Agents Chemother 58:2006–2012. doi: 10.1128/AAC.02615-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bassetti M, Righi E, Ansaldi F, Merelli M, Trucchi C, De Pascale G, Diaz-Martin A, Luzzati R, Rosin C, Lagunes L, Trecarichi EM, Sanguinetti M, Posteraro B, Garnacho-Montero J, Sartor A, Rello J, Rocca GD, Antonelli M, Tumbarello M. 2014. A multicenter study of septic shock due to candidemia: outcomes and predictors of mortality. Intensive Care Med 40:839–845. doi: 10.1007/s00134-014-3310-z. [DOI] [PubMed] [Google Scholar]
- 48.Cuenca-Estrella M, Gomez-Lopez A, Alastruey-Izquierdo A, Bernal-Martinez L, Cuesta I, Buitrago MJ, Rodriguez-Tudela JL. 2010. Comparison of the Vitek 2 antifungal susceptibility system with the Clinical and Laboratory Standards Institute (CLSI) and European Committee on Antimicrobial Susceptibility Testing (EUCAST) broth microdilution reference methods and with the Sensititre YeastOne and Etest techniques for in vitro detection of antifungal resistance in yeast isolates. J Clin Microbiol 48:1782–1786. doi: 10.1128/JCM.02316-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pfaller MA, Chaturvedi V, Diekema DJ, Ghannoum MA, Holliday NM, Killian SB, Knapp CC, Messer SA, Miskou A, Ramani R. 2012. Comparison of the Sensititre YeastOne colorimetric antifungal panel with CLSI microdilution for antifungal susceptibility testing of the echinocandins against Candida spp., using new clinical breakpoints and epidemiological cutoff values. Diagn Microbiol Infect Dis 73:365–368. doi: 10.1016/j.diagmicrobio.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 50.Cantón E, Pemán J, Iñiguez C, Hervás D, Lopez-Hontangas JL, Pina-Vaz C, Camarena JJ, Campos-Herrero I, García-García I, García-Tapia AM, Guna R, Merino P, Pérez del Molino L, Rubio C, Suárez A, FUNGEMYCA Study Group . 2013. Epidemiological cutoff values for fluconazole, itraconazole, posaconazole, and voriconazole for six Candida species as determined by the colorimetric Sensititre YeastOne method. J Clin Microbiol 51:2691–2695. doi: 10.1128/JCM.01230-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.