Abstract
Acanthamoeba cysts are resistant to extreme physical and chemical conditions. Autophagy is an essential pathway for encystation of Acanthamoeba cells. To evaluate the possibility of an autophagic Acanthamoeba encystation mechanism, we evaluated autophagy inhibitors, such as 3-methyladenine (3MA), LY294002, wortmannin, bafilomycin A, and chloroquine. Among these autophagy inhibitors, the use of 3MA and chloroquine showed a significant reduction in the encystation ratio in Acanthamoeba cells. Wortmannin also inhibited the formation of mature cysts, while LY294002 and bafilomycin A did not affect the encystation of Acanthamoeba cells. Transmission electron microscopy revealed that 3MA and wortmannin inhibited autophagy formation and that chloroquine interfered with the formation of autolysosomes. Inhibition of autophagy or autolysosome formation resulted in a significant block in the encystation in Acanthamoeba cells. Clinical treatment with 0.02% polyhexamethylene biguanide (PHMB) showed high cytopathic effects on Acanthamoeba trophozoites and cysts; however, it also revealed high cytopathic effects on human corneal epithelial cells. In this study, we investigated effects of the combination of a low (0.00125%) concentration of PHMB with each of the autophagy inhibitors 3MA, wortmannin, and chloroquine on Acanthamoeba and human corneal epithelial cells. These new combination treatments showed low cytopathic effects on human corneal cells and high cytopathic effects on Acanthamoeba cells. Taken together, these results provide fundamental information for optimizing the treatment of Acanthamoeba keratitis.
INTRODUCTION
Acanthamoeba trophozoites differentiate into cysts under conditions of physical stresses, starvation, and chemical exposure (1–3); the cysts are resistant to several chemical biocides (4). Resistance of the cysts to chemotherapeutic drugs makes the treatment of Acanthamoeba infections difficult (4, 5). Although polyhexamethylene biguanide (PHMB) (0.02%), alone or in combination with a diamidine (0.1% propamidine or 0.1% hexamidine), showed therapeutic efficacy against Acanthamoeba keratitis (6), PHMB was reported to be cytotoxic to human corneal epithelial (HCE) cells (7). Although therapy for Acanthamoeba keratitis needs better-acting drugs, due to the encystation of Acanthamoeba cells, cytotoxicity toward mammalian cells must also be considered.
Recent studies have demonstrated anticancer therapies that include autophagy targeting in different tumor cell lines (8, 9). One change in the cellular composition during the encystation of protozoa is the formation of an autophagy (10). The biological significance of autophagy associated with starvation, differentiation, development, and immunity has been previously demonstrated (10–13). In Acanthamoeba cells, several genes associated with autophagy, including those encoding Atg8, Atg8 isoform, Atg3, and Atg16L, were identified and their roles in the encystation of Acanthamoeba cells were studied (14–17). Autophagy is a major cellular pathway for the encystation of Acanthamoeba cells. The rapid degradation and recycling of cellular components by autophagy is an essential event during encystation. Autophagy is also essential for the differentiation of protozoan parasites. During the differentiation of Leishmania mexicana, an increase of autophagy and an increased level of protein degradation were observed (18). A mutation of the gene encoding Atg8 in Dictyostelium cells can make fruiting bodies that contain fewer, immature spores (19). Phosphoinositide 3-kinase inhibitors abolish proliferation, encystation, and the formation of autophagy in Entamoeba invadens (10).
The inhibition of autophagy formation suggests a new approach to screening therapeutic drugs for the encysting protozoan parasites. As there have been no studies published showing the effect of autophagy inhibitors on the encystation of Acanthamoeba cells, in this study, five autophagy inhibitors, i.e., 3-methyladenine (3MA), LY294002, wortmannin, bafilomycin A, and chloroquine, were investigated to determine the role of autophagy formation in the encystation of Acanthamoeba cells. Furthermore, we researched the cytopathic effects (CPE) of these inhibitors combined with PHMB on Acanthamoeba and human corneal cells. Autophagy inhibition increases cytotoxicity in combination with several antiamoebic drugs. Our results indicated the possibility of the use of autophagy inhibitors as new biocides to treat Acanthamoeba keratitis infections.
MATERIALS AND METHODS
Cultivation of Acanthamoeba cells and encystation.
Acanthamoeba castellanii Castellani was obtained from the American Type Culture Collection (ATCC 30011). Acanthamoeba trophozoites were cultured axenically in PYG medium (10 g proteose peptone–10 g yeast extract–10 ml 50% glucose–10 ml 0.5 M Na2HPO4–10 ml 0.5 M K2HPO4–970 ml distilled water [final pH adjusted to 6.5]) at 25°C in an incubator (Sanyo, San Diego, CA, USA). Encystation was induced in encystment medium (95 mM NaCl, 5 mM KCl, 8 mM MgSO4, 0.4 mM CaCl2, 1 mM NaHCO3, 20 mM Tris-HCl, pH 9.0) at 25°C for 3 days. The morphological changes of cells to cysts were observed under a light microscope.
Determination of the effects of autophagy inhibitors on encystation of Acanthamoeba cells.
Autophagy inhibitors provided by InvivoGen are listed in Table 1. To determine the effect of autophagy inhibitors on the encystation of A. castellanii, trophozoites (4 × 105 cells/well) were inoculated in 6-well plates containing 3 ml of encystment media. The autophagy inhibitors, i.e., 1 mM 3-methyladenine, 10 μM LY294002, 1 μM wortmannin, 0.1 μM bafilomycin A, and 100 μM chloroquine, were applied to the Acanthamoeba trophozoites, and the mixture was incubated at 25°C for 3 days. After incubation, the cells were observed under a microscope using trypan blue staining, and 0.5% sodium dodecyl sulfate was added for 10 min to solubilize trophozoites and immature cysts. The encystation ratios were calculated by counting cysts with a hemocytometer under a light microscope.
TABLE 1.
Autophagy inhibitorsa
| Classification | Name | Working concn |
|---|---|---|
| MAP kinase inhibitors | SP600125 | 10–50 μM |
| U0126 | 10–50 μM | |
| SB202190 | 1–20 μM | |
| SB203580 | 1–20 μM | |
| PI3K inhibitors | 3-Methyladenine* | 5 mM |
| LY294002* | 10–100 μM | |
| Wortmannin* | 0.1–10 μM | |
| Endosomal inhibitors | Bafilomycin A1* | 0.1–1 μM |
| Chloroquine* | 10 μM |
MAP, mitogen-activated protein. Each of the inhibitors used in the study is indicated with an asterisk.
Observation of the effects of autophagy inhibitors by TEM.
Transmission electron microscopy (TEM) was employed to visualize and quantify the effects of autophagy inhibitors on the encystation of Acanthamoeba cells. Trophozoites were grown at 25°C with or without 1 mM 3-methyladenine, 1 μM wortmannin, and 100 μM chloroquine in 6-well plates at a concentration of 4 × 105 cells/well for 3 days. After incubation, the cells were prefixed with 4% glutaraldehyde and postfixed with 1% osmium tetroxide. Fixed cells were dehydrated with ethyl alcohol and treated with propylene oxide-resin (1:1) overnight with continuous shaking. Samples were embedded in resin. Ultrathin sections were cut on a Reichert-Jung ultramicrotome, followed by staining with uranyl acetate and lead citrate. Sections were observed under a transmission electron microscope (H-7000; Hitachi, Tokyo, Japan).
CPE on human corneal cells and Acanthamoeba cells.
Human corneal epithelial (HCE) cells were cultured in endothelial cell growth medium kits (KGM BulletKit; Lonza, Portsmouth, NH, USA) in 5% CO2. HCE cells were seeded into 96-well culture plates at a density of 1 × 104 cells/well. At 24 h later, monolayered HCE cells were incubated with PHMB, PHMB with 3MA, PHMB with wortmannin, or PHMB with chloroquine at 37°C in 5% CO2 for 10 min to 5 h. Acanthamoeba trophozoites were seeded into 96-well culture plates at a density of 1 × 104 cells/well. At 24 h later, monolayered Acanthamoeba cells were incubated with PHMB, PHMB with 3MA, PHMB with wortmannin, or PHMB with chloroquine at 25°C for 2 h. Cytopathic effects (CPE) were assessed visually, after Giemsa staining and measurement of optical density at 590 nm, with 0.1 ml of cells solubilized in 5% SDS. Percent CPE was calculated according to the following formula (OD, optical density): % CPE = 100 − [(OD of experimental well − OD of Acanthamoeba alone)/OD of control cells] × 100. An assay was performed in triplicate.
Statistical analysis.
The data are expressed as means ± standard errors of the means (SEM) of the results of three independent experiments performed in triplicate. Statistical analysis was performed using Student's t test. A P value of <0.05 was considered statistically significant.
RESULTS
Autophagy inhibitors reduce the encystation ratio.
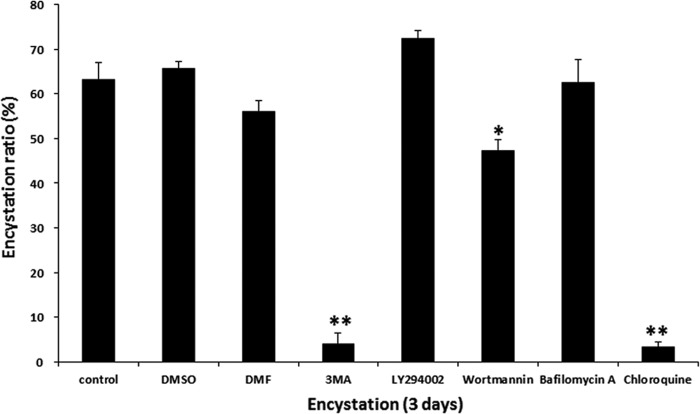
To investigate the effect of autophagy modulation on the process of encystation of Acanthamoeba cells, we evaluated the effect of autophagy inhibitors on encysting Acanthamoeba cells. Various autophagy inhibitors are listed in Table 1. We used five types, including 3-methyladenine (3MA), LY294002, wortmannin, bafilomycin A, and chloroquine. We first examined whether phosphoinositide 3-kinase (PI3K) inhibitors could reduce the encystation ratio of Acanthamoeba cells. Using SDS and trypan blue exclusion assay, 3MA and wortmannin both showed inhibition of mature cyst formation (Fig. 1). We also examined whether autolysosome formation inhibitors could inhibit the encystation of Acanthamoeba cells. Chloroquine showed inhibition of encystation (Fig. 1). However, LY294002 and bafilomycin A did not affect the encystation ratio in Acanthamoeba cells (Fig. 1).
FIG 1.
Effects of autophagy inhibitors on Acanthamoeba encystation. Trophozoites were incubated in encystment media with or without autophagy inhibitors, i.e., 1 mM 3-methyladenine (3MA), 10 μM LY294002, 1 μM wortmannin, 0.1 μM bafilomycin A, and 100 μM chloroquine, for 3 days. In the early stage of encystation (8 h after induction of encystation), 3MA and wortmannin reduced the Acanthamoeba encystation ratio. In the late stage of encystation (18 h after induction of encystation), chloroquine inhibited the encystation of Acanthamoeba cells. The data are expressed as means ± SEM of the results of three independent experiments, run in triplicate. Asterisks denote statistically significant (*, P < 0.05; **, P < 0.01) differences between control cells (including those subjected to dimethyl sulfoxide [DMSO] or dimethylformamide [DMF] treatment) and autophagy inhibitor-treated cells.
Transmission electron microscopy analysis of the encystation inhibition by autophagy modulation.
The effects of autophagy modulation on encysting Acanthamoeba cells were examined by transmission electron microscopy. The mature cysts of Acanthamoeba cells consisted of two layers and compacted cytoplasm (Fig. 2A). A portion of the encysting cells showed fully digested autolysosomes (Fig. 2A, left). However, wortmannin-treated amoebae showed that the mitochondria remained in the cytoplasm (Fig. 2B, arrows), suggesting that the PI3k inhibitor wortmannin blocked the formation of autophagy. Wortmannin-treated amoebae (Fig. 2B) had too many mitochondria compared with untreated amoebae (Fig. 2A). On the other hand, 3MA-treated amoebae showed many blebby structures in the membrane (Fig. 2C, left panel, arrows). Some amoebae showed destruction of membranes by 3MA (Fig. 2C, right panel). Autophagy-like structures were not found in 3MA-treated amoebae. In addition, chloroquine-treated amoebae revealed the presence of autophagy, including undigested organelles (Fig. 2D, squared areas). Chloroquine may prohibit the fusion of autophagy with lysosomes. Chloroquine-treated amoebae failed to form autolysosomes, and the autophagy did not result in the digestion of the contained organelles. Autophagy inhibitor 3MA may strongly affect the initiation stage of encystation in Acanthamoeba cells. 3MA-treated or chloroquine-treated trophozoites failed to transform into mature cysts. In addition, some of the wortmannin-treated cells did not form complete double walls during encystation. These results were summarized in Fig. 2E as a table.
FIG 2.
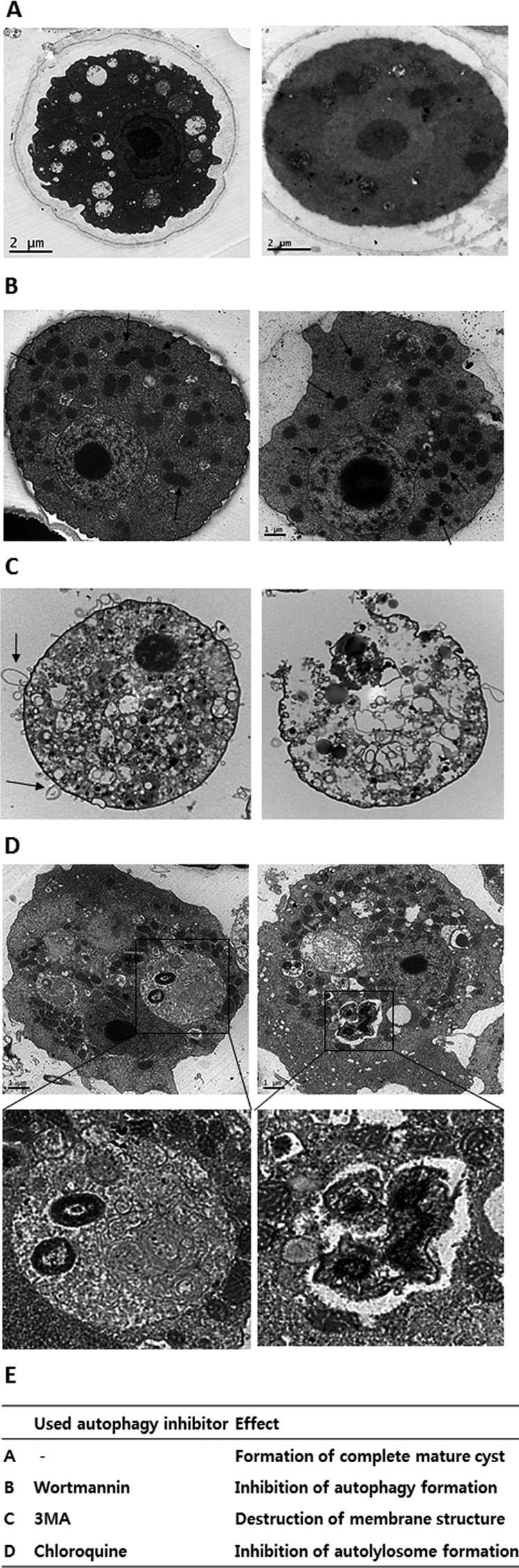
Observation of the effect of 3MA, wortmannin, and chloroquine by TEM. Untreated trophozoites were converted to mature cysts in encystment media for 3 days. (A) Cells treated with an autophagy inhibitor (1 μM wortmannin) could not make autophagy-like structures, and the mitochondria remained in the cytoplasm. (B) Cells treated with 1 mM 3MA failed to convert into mature cysts (arrows). (C) Some of the cells showed blebby structures in the membranes (arrows), and some of them showed broken membrane structures (right panel). (D) Cells treated with chloroquine (100 μM) showed autophagy-like structures that contained undigested organelles. The lower panels show magnified images of square areas of the upper panels. (E) Summary of the effects of the autophagy inhibitors used on encystation of Acanthamoeba cells.
Cytopathic effects of PHMB and autophagy inhibitors on human corneal cells.
In eye hospitals, PHMB is used for the treatment of patients with Acanthamoeba keratitis infections. To determine the cytotoxicity effects of PHMB on human corneal epithelial (HCE) cells, we treated HCE cells with 0.02% PHMB (Fig. 3). Treatment with 0.02% PHMB showed 46% lysis of the HCE cells within 10 min and 52% lysis of the HCE cells within 30 min. In a short time (30 min), 50% of the HCE cells were highly sensitive to PHMB. The membrane-damaging action of PHMB affected not only Acanthamoeba cells but also the HCE cells. Figure 4A shows that a significantly reduced (0.00125%) PHMB concentration did not affect the cytotoxicity of the HCE cells. The cytopathic effects of autophagy inhibitors, and of inhibitors in combination with PHMB, on the HCE cells were examined (Fig. 4B to D). Autophagy inhibitors reducing the encystation ratio of Acanthamoeba cells, i.e., 3MA, wortmannin, and chloroquine, did not show the cytopathic effect on the HCE cells. Their combinations did not increase the cytopathic effect compared to that seen with PHMB.
FIG 3.
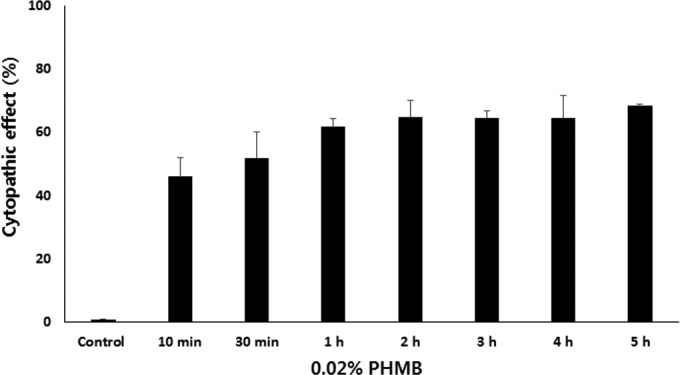
Cytopathic effects of PHMB on human corneal epithelial cells. Human corneal epithelial (HCE) cells were incubated with 0.02% PHMB for 10 min to 5 h. PHMB showed 46% lysis of the HCE cells within 10 min. After treatment of 0.02% PHMB for 2 h, over 60% of the HCE cells were lysed. The data are expressed as means ± SEM of the results of three independent experiments, run in triplicate.
FIG 4.
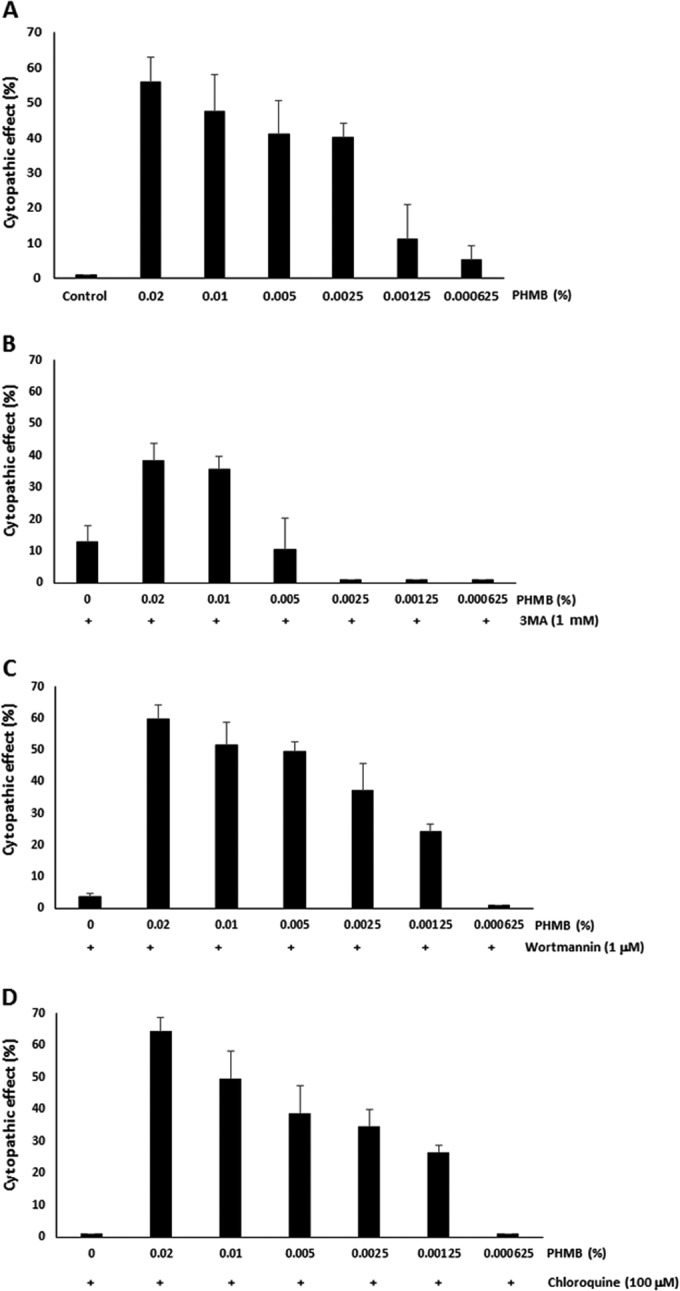
Cytopathic effects of the combinations with autophagy inhibitors on human corneal epithelial cells. To reduce the cytopathic effects of PHMB on the HCE cells, PHMB was diluted from 0.02% to 0.000625% and the diluted PHMB was used for incubation with HCE cells for 2 h. (A) The lowest concentrations of PHMB, 0.00125% and 0.000625%, did not show the cytopathic effects on the HCE cells. (B to D) The cytopathic effects of PHMB were not increased with the addition of 3MA, wortmannin, or chloroquine. The data are expressed as means ± SEM of the results of three independent experiments, performed in triplicate.
Cytopathic effects of new combinations on Acanthamoeba cells.
To increase the cytopathic effects of low concentrations of PHMB on Acanthamoeba cells, we added autophagy inhibitors to inhibit the encystation of Acanthamoeba cells. A new combination of 0.00125% PHMB with 1 mM 3MA showed higher cytopathic effects than PHMB alone (Fig. 5). When 0.00125% was used alone, 60% of the Acanthamoeba cells died within 2 h. However, PHMB in combination with 3MA increased the killing activity up to 80%. The other combinations, 0.00125% PHMB with 1 μM wortmannin and 0.00125% PHMB with 100 μM chloroquine, showed similar effects. These combinations showed levels of cytopathic effects similar to the levels seen with 0.02% PHMB treatment alone.
FIG 5.
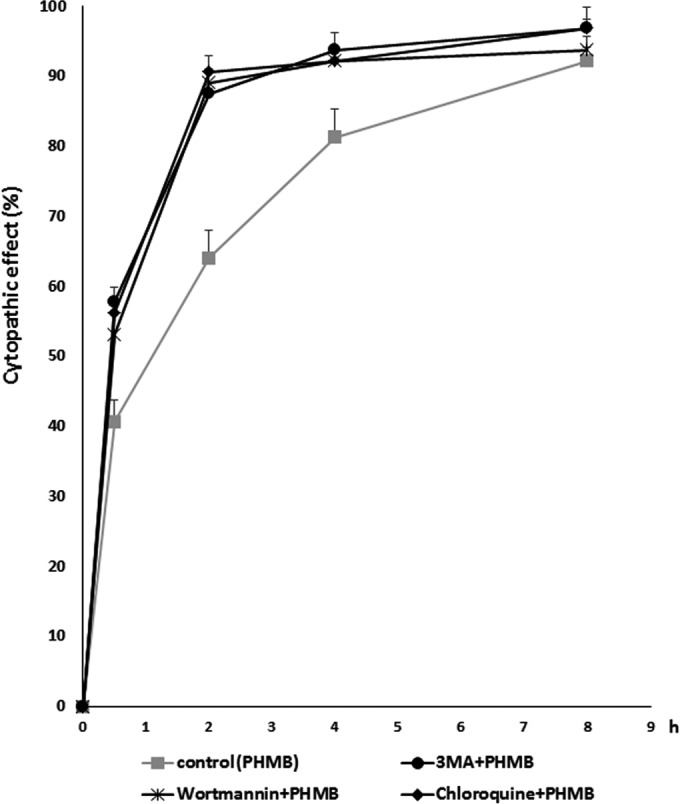
Cytopathic effects of the combinations with autophagy inhibitors on Acanthamoeba cells. Acanthamoeba cells was treated with the new combination of PHMB with autophagy inhibitors for 8 h. Within 2 h, 1 mM 3MA, 1 μM wortmannin, and 100 μM chloroquine increased the cytopathic effects of 0.00125% PHMB on Acanthamoeba cells. The data are expressed as means ± SEM of the results of three independent experiments, performed in triplicate.
DISCUSSION
The regulation of autophagy is associated with the control of cell growth, proliferation, survival, and death. In this study, we conducted experiments to explore if autophagy inhibitors help the encystation of Acanthamoeba cells. In addition, we tested autophagy inhibitors as possible candidates for treatment of Acanthamoeba keratitis infections. Groups of PI3K inhibitors, such as 3MA, wortmannin, and LY294002, have been used as autophagy inhibitors that inhibit PI3K activity (20). Inhibition of class III PI3K can block autophagy formation by inhibiting the recruitment of Atg proteins and LC3 proteins. In a later step of the autophagic process, lysosomes fuse with autophagic vacuoles and dispense their enzymes into the autophagic vacuoles digesting their contents. To inhibit the fusion of the lysosome with the autophagic vacuole, we added bafilomycin A and chloroquine. Bafilomycin A is a known inhibitor in the late phase of autophagy. Bafilomycin A prevents maturation of autophagic vacuoles by inhibiting fusion between the autophagy and lysosome (21). Bafilomycin A acts by inhibiting vacuolar H+ ATPase (V-ATPase). Chloroquine leads to the inhibition of lysosome-autophagy fusion and lysosomal protein degradation (22).
Previous reports have suggested that autophagy-related protein plays an important role during the encystation of Acanthamoeba cells (19, 23). In this study, we found that autophagy inhibitors 3MA and chloroquine significantly reduced the encystation ratio of Acanthamoeba cells. Wortmannin also inhibited the encystation of Acanthamoeba cells. Although trophozoites of Acanthamoeba are sensitive to most antiamoebic drugs, cysts are difficult to treat due to resistance to the drugs. This leads to the proposal that the inhibition of encystation under conditions of chemotherapeutic treatment could ensure a successful outcome in the treatment of amoebic infections if caught early enough.
Recent studies suggest a potential therapeutic application of autophagy inhibitors. The inhibition of autophagy with 3MA reduced calpain activation and virus replication (24) and induced apoptosis in colon cancer cells (25). Autophagy inhibitor 3-MA, alone or combined with doxorubicin, induced apoptosis in osteosarcoma (26). Chloroquine is widely used as an antimalarial drug that interferes with hemozoin formation (27). Currently, the most widely used autophagy inhibitor is chloroquine (23, 28). Chloroquine is a well-known antimalarial drug in clinical use (29).
Acanthamoeba keratitis infections are relatively rare; however, a quick diagnosis and an appropriate antiamoebic drug are required. At present, the most commonly used therapy for Acanthamoeba keratitis is PHMB treatment (30). Clinical treatment of PHMB affects Acanthamoeba trophozoites and cysts; however, it is also toxic to the epithelial cells of the cornea (31).
In this study, a novel approach to chemotherapy for Acanthamoeba keratitis at a low concentration of PHMB in combination with autophagy inhibitors was investigated. Acanthamoeba trophozoites and immature cysts are more sensitive to antibiotic therapy than mature cysts. Blocking encystation by autophagy inhibitors increased the effect of antiamoebic medicines (Fig. 5). Moreover, the combined use of a low concentration of an antiamoebic drug and autophagy inhibitors reduced the cytotoxic effects on corneal epithelial cells (Fig. 4). Medical applications of this combination could reduce toxic responses to corneal cells and enhance the therapeutic effects on Acanthamoeba cells. As mentioned above, chloroquine is widely used in clinical areas for treatment of malaria. The safety of chloroquine and its ability to inhibit the autolysosome formation make it a suitable starting point for the development of a new antiamoebic drug.
ACKNOWLEDGMENT
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science, and Technology (NRF-2013R1A1A4A01011147).
REFERENCES
- 1.Cordingley JS, Wills RA, Villemez CL. 1996. Osmolarity is an independent trigger of Acanthamoeba castellanii differentiation. J Cell Biochem 61:167–171. [DOI] [PubMed] [Google Scholar]
- 2.Byers TJ, Rudick VL, Rudick MJ. 1969. Cell size, macromolecule composition, nuclear number, oxygen consumption and cyst formation during two growth phases in unagitated cultures of Acanthamoeba castellanii. J Protozool 16:693–699. doi: 10.1111/j.1550-7408.1969.tb02329.x. [DOI] [PubMed] [Google Scholar]
- 3.Aqeel Y, Siddiqui R, Iftikhar H, Khan NA. 2013. The effect of different environmental conditions on the encystation of Acanthamoeba castellanii belonging to the T4 genotype. Exp Parasitol 135:30–35. doi: 10.1016/j.exppara.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 4.Coulon C, Collignon A, McDonnell G, Thomas V. 2010. Resistance of Acanthamoeba cysts to disinfection treatments used in health care settings. J Clin Microbiol 48:2689–2697. doi: 10.1128/JCM.00309-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bang S, Edell E, Eghrari AO, Gottsch JD. 2010. Treatment with voriconazole in 3 eyes with resistant Acanthamoeba keratitis. Am J Ophthalmol 149:66–69. doi: 10.1016/j.ajo.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Clarke B, Sinha A, Parmar DN, Sykakis E. 2012. Advances in the diagnosis and treatment of acanthamoeba keratitis. J Ophthalmol 2012:484892. doi: 10.1155/2012/484892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yanai R, Yamada N, Ueda K, Tajiri M, Matsumoto T, Kido K, Nakamura S, Saito F, Nishida T. 2006. Evaluation of povidone-iodine as a disinfectant solution for contact lenses: antimicrobial activity and cytotoxicity for corneal epithelial cells. Cont Lens Anterior Eye 29:85–91. doi: 10.1016/j.clae.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Maycotte P, Thorburn A. 2014. Targeting autophagy in breast cancer. World J Clin Oncol 5:224–240. doi: 10.5306/wjco.v5.i3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amaravadi R, Debnath J. 2014. Mouse models address key concerns regarding autophagy inhibition in cancer therapy. Cancer Discov 4:873–875. doi: 10.1158/2159-8290.CD-14-0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picazarri K, Nakada-Tsukui K, Nozaki T. 2008. Autophagy during proliferation and encystation in the protozoan parasite Entamoeba invadens. Infect Immun 76:278–288. doi: 10.1128/IAI.00636-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang WP, Klionsky DJ. 2002. Autophagy in yeast: a review of the molecular machinery. Cell Struct Funct 27:409–420. doi: 10.1247/csf.27.409. [DOI] [PubMed] [Google Scholar]
- 12.Pan H, Cai N, Li M, Liu GH, Izpisua Belmonte JC. 2013. Autophagic control of cell ‘stemness.’ EMBO Mol Med 5:327–331. doi: 10.1002/emmm.201201999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deretic V, Levine B. 2009. Autophagy, immunity, and microbial adaptations. Cell Host Microbe 5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon EK, Chung DI, Hong YC, Kong HH. 2009. Autophagy protein 8 mediating autophagosome in encysting Acanthamoeba. Mol Biochem Parasitol 168:43–48. doi: 10.1016/j.molbiopara.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Moon EK, Hong Y, Chung DI, Kong HH. 2013. Identification of atg8 isoform in encysting Acanthamoeba. Korean J Parasitol 51:497–502. doi: 10.3347/kjp.2013.51.5.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moon EK, Chung DI, Hong Y, Kong HH. 2011. Atg3-mediated lipidation of Atg8 is involved in encystation of Acanthamoeba. Korean J Parasitol 49:103–108. doi: 10.3347/kjp.2011.49.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Song SM, Han BI, Moon EK, Lee YR, Yu HS, Jha BK, Danne DB, Kong HH, Chung DI, Hong Y. 2012. Autophagy protein 16-mediated autophagy is required for the encystation of Acanthamoeba castellanii. Mol Biochem Parasitol 183:158–165. doi: 10.1016/j.molbiopara.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 18.Williams RA, Tetley L, Mottram JC, Coombs GH. 2006. Cysteine peptidases CPA and CPB are vital for autophagy and differentiation in Leishmania mexicana. Mol Microbiol 61:655–674. doi: 10.1111/j.1365-2958.2006.05274.x. [DOI] [PubMed] [Google Scholar]
- 19.Otto GP, Wu MY, Kazgan N, Anderson OR, Kessin RH. 2004. Dictyostelium macroautophagy mutants vary in the severity of their developmental defects. J Biol Chem 279:15621–15629. doi: 10.1074/jbc.M311139200. [DOI] [PubMed] [Google Scholar]
- 20.Petiot A, Ogier-Denis E, Blommaart EF, Meijer AJ, Codogno P. 2000. Distinct classes of phosphatidylinositol 3′-kinases are involved in signaling pathways that control macroautophagy in HT-29 cells. J Biol Chem 275:992–998. doi: 10.1074/jbc.275.2.992. [DOI] [PubMed] [Google Scholar]
- 21.Yamamoto A, Tagawa Y, Yoshimori T, Moriyama Y, Masaki R, Tashiro Y. 1998. Bafilomycin A1 prevents maturation of autophagic vacuoles by inhibiting fusion between autophagosomes and lysosomes in rat hepatoma cell line, H-4-II-E cells. Cell Struct Funct 23:33–42. doi: 10.1247/csf.23.33. [DOI] [PubMed] [Google Scholar]
- 22.Mizushima N, Yoshimori T, Levine B. 2010. Methods in mammalian autophagy research. Cell 140:313–326. doi: 10.1016/j.cell.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Firat E, Weyerbrock A, Gaedicke S, Grosu AL, Niedermann G. 2012. Chloroquine or chloroquine-PI3K/Akt pathway inhibitor combinations strongly promote γ-irradiation-induced cell death in primary stem-like glioma cells. PLoS One 7:e47357. doi: 10.1371/journal.pone.0047357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yoon SY, Ha YE, Choi JE, Ahn J, Lee H, Kim DH. 2009. Autophagy in coxsackievirus-infected neurons. Autophagy 5:388–389. doi: 10.4161/auto.5.3.7723. [DOI] [PubMed] [Google Scholar]
- 25.Coker-Gurkan A, Arisan ED, Obakan P, Guvenir E, Unsal NP. August 2014, posting date Inhibition of autophagy by 3-MA potentiates purvalanol-induced apoptosis in Bax deficient HCT 116 colon cancer cells. Exp Cell Res doi: 10.1016/j.yexcr.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 26.Zhao D, Yuan H, Yi F, Meng C, Zhu Q. 2014. Autophagy prevents doxorubicin-induced apoptosis in osteosarcoma. Mol Med Rep 9:1975–1981. doi: 10.3892/mmr.2014.2055. [DOI] [PubMed] [Google Scholar]
- 27.Ch'ng JH, Lee YQ, Gun SY, Chia WN, Chang ZW, Wong LK, Batty KT, Russell B, Nosten F, Renia L, Tan KS. 2014. Validation of a chloroquine-induced cell death mechanism for clinical use against malaria. Cell Death Dis 5:e1305. doi: 10.1038/cddis.2014.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sotelo J, Briceño E, López-González MA. 2006. Adding chloroquine to conventional treatment for glioblastoma multiforme: a randomized, double-blind, placebo-controlled trial. Ann Intern Med 144:337–343. doi: 10.7326/0003-4819-144-5-200603070-00008. [DOI] [PubMed] [Google Scholar]
- 29.Foley M, Tilley L. 1998. Quinoline antimalarials: mechanisms of action and resistance and prospects for new agents. Pharmacol Ther 79:55–87. doi: 10.1016/S0163-7258(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 30.Larkin DF, Kilvington S, Dart JK. 1992. Treatment of Acanthamoeba keratitis with polyhexamethylene biguanide. Ophthalmology 99:185–191. doi: 10.1016/S0161-6420(92)31994-3. [DOI] [PubMed] [Google Scholar]
- 31.Rusciano G, Capriglione P, Pesce G, Del Prete S, Cennamo G, Di Cave D, Cerulli L, Sasso A. 2013. Raman microspectroscopy analysis in the treatment of Acanthamoeba keratitis. PLoS One 8:e72127. doi: 10.1371/journal.pone.0072127. [DOI] [PMC free article] [PubMed] [Google Scholar]