Abstract
Due to the significant increase in antimicrobial resistance of Acinetobacter baumannii, immune system stimulation to block infection progression may be a therapeutic adjuvant to antimicrobial treatment. Lysophosphatidylcholine (LPC), a major component of phospholipids in eukaryotic cells, is involved in immune cell recruitment and modulation. The aim of this study was to show if LPC could be useful for treating infections caused by A. baumannii. A. baumannii ATCC 17978 was used in this study. Levels of serum LPC and levels of the inflammatory cytokines tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-1β, and IL-10 were determined by spectrophotometric assay and enzyme-linked immunosorbent assay (ELISA), respectively, using a murine peritoneal sepsis model in which mice were inoculated with 5.3 log CFU/ml of A. baumannii. The therapeutic efficacy of LPC against A. baumannii in murine peritoneal sepsis and pneumonia models was assessed for 48 h after bacterial infection. At early time points in the murine model of peritoneal sepsis caused by A. baumannii, LPC was depleted and was associated with an increase of inflammatory cytokine release. Preemptive therapy with LPC in murine peritoneal sepsis and pneumonia models markedly enhanced spleen and lung bacterial clearance and reduced the numbers of positive blood cultures and the mouse mortality rates. Moreover, treatment with LPC reduced proinflammatory cytokine production. These data demonstrate that LPC is efficacious as a preemptive treatment in experimental models of peritoneal sepsis and pneumonia caused by A. baumannii.
INTRODUCTION
Lysophosphatidylcholine (LPC) is a major component of phospholipids that is involved in the recruitment and stimulation of immune cells (1–3) and therefore may eliminate dead eukaryotic and prokaryotic cells during infection (4, 5). Recent attention has been drawn to the role of LPC as a chemotactic factor during bacterial infection (1, 5). In eukaryotic cells, LPC was released after activation of calcium-independent phospholipase A2 (iPLA2) and cytosolic phospholipase A2 (cPLA2) (4, 6, 7). The latter plays an important role in the cell damage induced by Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus (8–10).
Acinetobacter baumannii is a Gram-negative bacillus that is very important in the clinic due to an increase in the number of nosocomial infections and because of its resistance to most clinically available antimicrobials, which makes it difficult to manage A. baumannii infections. Infections caused by A. baumannii include the following, in order of prevalence and associated mortality: pneumonia, bacteremia, urinary tract infections, surgical wound infections, and meningitis (11). A. baumannii pneumonia and bacteremia are typically acquired in the hospital setting and are associated with significant mortality (12).
A. baumannii infection requires two steps. First, A. baumannii must adhere to the host cells and then penetrate these cells to join the surrounding tissue (13, 14). It has been shown that A. baumannii uses a common strategy to traverse the host cell: this microorganism induces the death of host cells in a caspase-3- and calpain calcium-dependent manner (14–16). The induction of death in host cells results in the release of LPC, which is an important factor for the stimulation of immune cells (1–3). Therefore, we hypothesized that LPC regulates the inflammatory cascade caused by A. baumannii and that, in this sense, it may be useful as an adjuvant in combination with antimicrobial agents for the treatment of infections by this pathogen. We showed that LPC pretreatment in murine models of peritoneal sepsis and pneumonia caused by A. baumannii reduced the bacterial loads and bacteremia and increased the mouse survival rates.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
A. baumannii ATCC 17978 was used in this study. The bacterial cells were grown in Luria-Bertani (LB) broth at 37°C with shaking. The growth of the bacterial cells was monitored for 4 or 24 h by determining the culture's optical density at 600 nm (OD4 h = 0.5 and OD24 h = 1.2).
In vitro susceptibility testing.
The MIC of LPC for ATCC 17978 was determined by a microdilution assay, using a standard inoculum of 1 × 105 to 5 × 105 CFU/ml, as previously described (16).
Animals.
Immunocompetent C57BL/6 female mice (16 to 18 g) were obtained from the University of Seville facility; they had a sanitary status of murine pathogen free and were assessed for genetic authenticity. Animals were housed in regulation cages, with food and water provided ad libitum. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals (17). The protocol was approved by the Committee on the Ethics of Animal Experiments of the University Hospital of Virgen del Rocío, Seville, Spain (03/2010). All surgery was performed under sodium thiopental anesthesia, and all efforts were made to minimize suffering.
A. baumannii peritoneal sepsis model.
A murine peritoneal sepsis model with A. baumannii ATCC 17978 was established by intraperitoneal (i.p.) inoculation of bacteria (18). Briefly, female C57BL/6 mice were inoculated with 0.5 ml of a bacterial suspension which had been incubated for 20 to 24 h in LB broth at 37°C and mixed at a 1:1 ratio with a saline solution containing 10% (wt/vol) porcine mucin (Sigma, Spain). The minimal lethal dose (MLD100), 50% lethal dose (LD50), and maximum tolerated dose (LD0) were determined by inoculating various groups of mice (6 mice per group) with decreasing amounts of A. baumannii ATCC 17978 inoculum, from 8.5 to 2.3 log CFU/ml, and monitoring the survival of the mice for 7 days. The LD0 and the LD50 were defined as the concentrations causing 0% and 50% mortality, respectively.
Cytokines and LPC assays.
Blood samples were collected from the periorbital plexuses of 40 anesthetized mice infected with ATCC 17978 at the MLD100, as previously described (19). The serum levels of tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), IL-1β, IL-10, and LPC were determined in mice at 0, 0.5, 1, 2, 4, 8, 12, and 24 h postinfection by using enzyme-linked immunosorbent assays (ELISAs) (eBioscience, San Diego, CA) and an LPC assay kit (Azwell, Japan).
In vivo LPC toxicity.
The Reed and Muench method (20) was used to determine toxicity. Mice were inoculated i.p. with a single dose of LPC (obtained from human brain; Sigma), starting at 10 mg/kg of body weight, in 0.5 ml 0.9% NaCl, and the solution was serially diluted until 50% mortality was reached; 6 mice were included in each group.
LPC pharmacokinetics.
Serum LPC levels were determined in healthy mice after a single i.p. administration of 25 mg/kg LPC. After 5, 15, 30, 60, and 120 min, blood was extracted from the periorbital plexuses of the anesthetized mice; three mice were used for each time point. The LPC levels were determined as described above. The maximum concentration in serum (Cmax; reported in micromolar units), the area under the concentration-time curve from time zero to infinity (AUC0–∞; reported in micromole-hours per liter), and the terminal half-life (t1/2; reported in hours) were calculated using a computer-assisted method (21).
LPC protective dose.
The protective dose for 50% of the population (PD50) was determined for LPC as described previously (20), with some modifications. Mice were infected i.p. with A. baumannii ATCC 17978 at the MLD100. Two hours later, the animals were treated i.p. with single increasing doses of LPC, starting from 10 mg/kg in 0.5 ml 0.9% NaCl and continuing until the LD0, and were observed for 2 days to measure the cumulative survival rates; 6 mice were used for each treatment group.
Therapeutic LPC effects in experimental animal models. (i) Peritoneal sepsis model.
Mice were inoculated i.p. with A. baumannii ATCC 17978 at the MLD100. One hour prior to inoculation, the animals were injected i.p. with a single dose of 25 mg/kg LPC diluted in 0.5 ml 0.9% NaCl. This dose corresponds to half the LD0 of LPC. Thirty mice were randomly assigned to two different therapeutic groups: a control group (without treatment) and a group receiving 25 mg/kg LPC. The mice were treated and monitored for mortality (%) for 48 h. After death or sacrifice of the mice at the end of the experimental period, aseptic thoracotomies were performed, and blood samples for qualitative blood cultures were obtained by cardiac puncture (data are reported as numbers [%] of positive cultures). The spleen and lungs were removed aseptically and homogenized (Stomacher 80; Tekmar Co., Cincinnati, OH) in 2 ml of sterile 0.9% NaCl. Tenfold dilutions of the homogenized spleen and lungs were plated onto sheep blood agar for quantitative cultures (data are reported in log10 CFU/g of spleen or lung).
(ii) Pneumonia model.
A previously described experimental murine pneumonia model (22) was used to evaluate the efficacy of LPC against A. baumannii ATCC 17978. Briefly, the mice were anesthetized by an i.p. injection of 5% (wt/vol) sodium thiopental (Braun Medical, Barcelona, Spain). They were suspended vertically, and the trachea of each was then cannulated with a blunt-tipped metal needle. The feel of the needle tip against the tracheal cartilage confirmed the intratracheal location. A microliter syringe (Hamilton Co., Reno, NV) was used for inoculation of 50 μl of a bacterial suspension (8 log CFU/ml) which had been grown for 24 h in LB broth at 37°C and mixed at a 1:1 ratio with a 0.9% NaCl solution containing 10% (wt/vol) porcine mucin. The mice remained in a vertical position for 3 min and then in a 30° position until awake. Preemptive LPC therapy was performed by using a single i.p. dose of 25 mg/kg LPC diluted in 0.5 ml 0.9% NaCl. For the analysis of LPC's therapeutic efficacy against infection, the animals were randomly assigned to two different therapeutic groups: a control group (without treatment) and a group receiving 25 mg/kg LPC. The mice were treated and monitored for mortality (%) for 48 h. After death or sacrifice of the mice at the end of the experimental period, aseptic thoracotomies were performed, and blood samples for qualitative blood culture were obtained by cardiac puncture (data are reported as numbers [%] of positive cultures). The lungs were aseptically removed and homogenized as described above for quantitative culture (data are reported in log10 CFU/g of lung).
Effects of LPC on serum cytokine levels in the A. baumannii peritoneal sepsis model.
The serum levels of TNF-α, IL-6, IL-1β, and IL-10 were determined for the preemptive LPC therapy group of the murine peritoneal sepsis model at 0, 0.5, 1, 2, 4, 8, and 12 h postinfection by using ELISA kits, as previously stated.
Statistical analysis.
Group data are presented as means ± standard errors of the means (SEM). For in vivo cytokine and LPC determinations, the Student t test was used to determine differences between means. For the peritoneal sepsis survival model, the Kaplan-Meier test was performed to determine the difference between mortality rates. For experimental models of peritoneal sepsis and pneumonia, differences between bacterial lung concentrations (means ± standard deviations, in log CFU/g of lung tissue) were analyzed by analysis of variance (ANOVA) and post hoc Dunnett's and Tukey's tests. Differences in mortality (%) and blood sterility (%) between groups were compared by the χ2 test. P values of <0.05 were considered significant. The SPSS (version 15.0) statistical package was used (SPSS Inc.).
RESULTS
MLD100, LD50, and LD0 of A. baumannii.
To determine the MLD100, LD50, and LD0 of A. baumannii ATCC 17978, the murine peritoneal sepsis model was used. Mortality was dependent on the concentration of bacteria in the inoculum (data not shown). The MLD100, LD50, and LD0 of strain ATCC 17978 were 3.2 log10 CFU/ml, 2.75 log10 CFU/ml, and 2.3 log10 CFU/ml, respectively.
Effects of A. baumannii on cytokine levels.
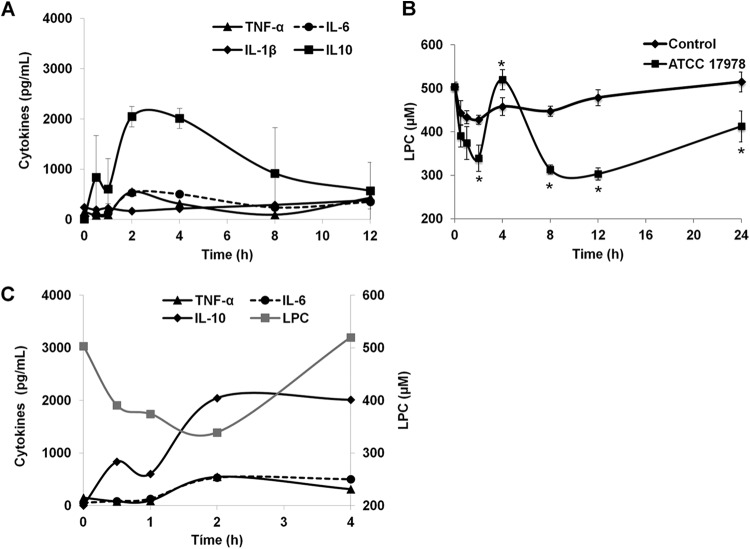
The effects of A. baumannii on serum cytokine levels were examined from 0 to 12 h after induction of the murine peritoneal sepsis model. At the early time points, A. baumannii infection induced large, transient changes in the cytokine levels. During the first 2 h after the mice were inoculated with bacteria, IL-10, TNF-α, and IL-6 levels reached their maximal serum levels (Fig. 1A). IL-1β levels were not affected during the first 2 h after bacterial infection, but these levels were increased 8 h after bacterial inoculation (Fig. 1A).
FIG 1.
Effects of A. baumannii on cytokine and LPC production in the murine peritoneal sepsis model. (A) Levels of TNF-α, IL-6, IL-1β, and IL-10 were determined from 0 to 12 h, using the sera of mice inoculated with A. baumannii ATCC 17978. (B) LPC production was determined from 0 to 24 h, using the sera of control mice and mice inoculated with A. baumannii ATCC 17978. (C) Patterns of LPC and cytokine production in the murine model of peritoneal sepsis caused by A. baumannii. *, P < 0.05 for comparison of control group and mice infected with ATCC 17978.
Effect of A. baumannii on the LPC level.
We next investigated whether these changes in cytokine levels were associated with changes in the level of LPC in serum. At early time points of the murine peritoneal sepsis model, the LPC levels in the infected mice decreased between 0 and 2 h (P = 0.02), coincidently with an increase of inflammatory cytokine levels (Fig. 1A), whereas the LPC levels were only slightly affected in the control mice (Fig. 1B). At 8, 12, and 24 h, a statistically significant decrease in LPC levels was observed in the infected mice in comparison with the control group (Fig. 1B).
LPC toxicity, protective dose, and pharmacokinetic studies.
The LD0 and LD50 values of LPC were 50 mg/kg and 100 mg/kg, respectively. Adverse effects were observed in a dose-dependent manner at doses of 50 mg/kg or higher, including transitory movement disorders and muscle spasms. The PD50 against A. baumannii ATCC 17978 was 25 mg/kg. After a single dose of LPC (25 mg/kg, i.p.), the pharmacokinetic parameters Cmax, t1/2, and AUC0–∞ were 73.17 μM, 0.48 h, and 29.25 μmol · h/liter, respectively. Serum LPC levels in healthy mice injected with LPC (25 mg/kg, i.p.) were increased 15% over the basal level 5 min after the dose (from 461.7 ± 17.24 μM to 534.87 ± 24.81 μM) (Fig. 2).
FIG 2.
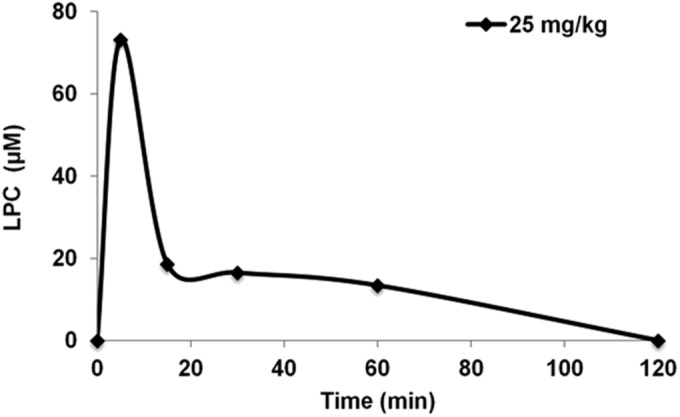
Serum LPC pharmacokinetics. LPC was administered to mice i.p. at a dose of 25 mg/kg, and the serum LPC concentrations were analyzed for 120 min.
LPC protects against A. baumannii peritoneal sepsis-induced infection and lethality.
We examined the effects of preemptive LPC therapy on bacterial tissue burdens and sterile blood cultures in the murine peritoneal sepsis model 2 days after bacterial inoculation of the mice. LPC reduced the lung and spleen bacterial concentrations by 2.37 and 3.9 log10 CFU/g, respectively, compared to the controls (P < 0.05). The numbers of sterile blood cultures and survivors were increased in the LPC-pretreated group compared to the control group (Table 1). Interestingly, the MIC of LPC against A. baumannii ATCC 17978 is >2 mg/ml, which is 5 × 104-fold higher than the LPC Cmax.
TABLE 1.
Therapeutic effect of LPC in murine peritoneal sepsis and pneumonia models of A. baumannii ATCC 17978 infection
| Infection model | Treatment group (n) | Survival (%) | Log10 CFU/g spleen (mean ± SEM) | Log10 CFU/g lung (mean ± SEM) | % sterile blood cultures |
|---|---|---|---|---|---|
| Pneumonia | Control (10) | 0 | ND | 9.45 ± 0.4 | 20 |
| 25 mg/kg LPC (15) | 68.75a | ND | 6.96 ± 0.67a | 44 | |
| Peritoneal sepsis | Control (13) | 0 | 9.72 ± 0.09 | 8.74 ± 0.18 | 0 |
| 25 mg/kg LPC (15) | 40 | 5.82 ± 0.76a | 6.37 ± 0.77a | 60a |
P < 0.05 with respect to the control group.
LPC protects against A. baumannii pneumonia-induced infection and lethality.
We examined the effects of preemptive LPC therapy on bacterial burdens and sterile blood cultures in the murine pneumonia model for 2 days after bacterial inoculation. LPC reduced the lung bacterial concentration by 2.49 log10 CFU/g compared to the control level (P < 0.05). The numbers of sterile blood cultures and survivors were increased in the LPC-treated group compared to the control group (Table 1).
Effect of LPC on cytokine production by A. baumannii.
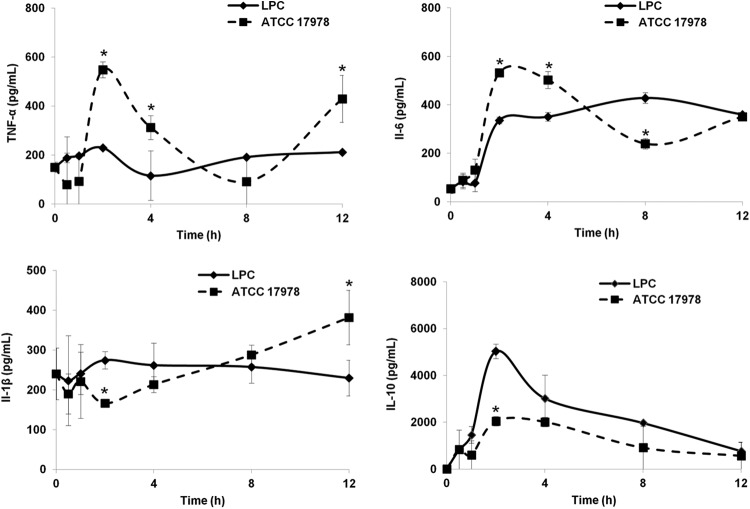
The effects of preemptive LPC therapy (25 mg/kg, i.p., 1 h prior to inoculation) on serum cytokine levels in the murine model of peritoneal sepsis caused by A. baumannii ATCC 17978 were examined from 0 to 12 h after inoculation (Fig. 3). At an early time point (4 h), LPC pretreatment induced decreases in the levels of the proinflammatory cytokines TNF-α and IL-6, whereas the level of the anti-inflammatory cytokine IL-10 was increased. The level of IL-1β, another proinflammatory cytokine, decreased in the group pretreated with LPC only at a late time point (12 h) in the experiment.
FIG 3.
Effects of LPC on cytokine production in a murine peritoneal sepsis model of A. baumannii infection. Mice received preemptive therapy with LPC and were then inoculated with ATCC 17978. The levels of TNF-α, IL-6, IL-1β, and IL-10 in the serum were determined from 0 to 12 h. Representative results are shown, and the data are presented as means ± SEM. *, P < 0.05 for comparison of ATCC 17978 and LPC.
DISCUSSION
The present study provides new data highlighting the role of LPC, which is known to be a chemotactic factor, in the regulation of pro- and anti-inflammatory cytokine production in vivo. Furthermore, this study evaluated the in vivo therapeutic efficacy of LPC against A. baumannii in the murine peritoneal sepsis and pneumonia models. Preemptive LPC therapy reduced the bacterial burdens in the lungs and spleen and increased the number of sterile blood cultures as well as the animal survival rate.
Interestingly, we found that serum LPC levels decreased during the first 2 h after infection of mice with A. baumannii, and this decrease was accompanied by increases in the levels of the proinflammatory cytokines TNF-α and IL-6. The decreased LPC concentration may reflect its enhanced and rapid conversion to lysophosphatidic acid (LPA) by a plasmatic lysophospholipase D (23). LPA is known to induce a multitude of cellular responses, including LPA-driven effects on proinflammatory cytokine production (24). Clinically, it was reported that plasma LPC was significantly decreased in septic patients (25, 26), and patients with unfavorable sepsis outcomes had significantly lower plasma LPC-phosphorylcholine levels than patients who survived a septic episode (25, 26). These clinical findings support the results of our study.
Preemptive LPC therapy with 25 mg/kg LPC effectively protected the mice from A. baumannii infections and effectively reduced the bacterial burdens in the organs and the positive blood culture rate. At the dose used in this study, the Cmax of LPC in the sera of the mice was 2- to 3-fold higher than the 10 to 30 μM required to induce the chemotaxis of immune cells (24, 26), and this treatment did not induce any apparent toxic effects in the mice. In the peritoneal sepsis model, we observed that 60% of the LPC-treated mice were negative for bacteremia and that 40% of the LPC-treated mice survived. We suggest that the death of the nonbacteremic mice was due to the inflammatory responses caused by A. baumannii and to unresolved infection in the organs, such as the spleen and lungs, even though the mice were treated with LPC. Figure 3 shows that the LPC-treated mice had significantly but not totally decreased the production of the proinflammatory cytokines. Clinically, the occurrence of pneumonia without bacteremia is frequent, and a large percentage of patients with this infection die during hospitalization (27).
LPC is a chemotactic factor that stimulates immune cells and regulates the balance between the release of pro- and anti-inflammatory cytokines. The beneficial effects of pretreating bacterial infections with LPC in the short term that we have shown here far exceed the potential effects of bacterial infection, as LPC could prevent the release of proinflammatory cytokines and increase the release of anti-inflammatory cytokines. The next issue that must be addressed is to determine whether multiple doses of LPC, given as treatment in combination with antimicrobials, will have a major impact on bacterial burdens and inflammatory responses, as a first step before evaluation in clinical studies. Moreover, in addition to this preclinical evaluation, we need to know which populations should be pretreated with LPC. Appropriate LPC clinical use should include patients at risk for severe A. baumannii infections, which have a high morbidity and mortality and usually are caused by multidrug-resistant strains, such as ventilator-associated pneumonia, bacteremia, and wound infections, among others (28). Additionally, the positive effect of LPC on bacterial burdens should be useful and may be used to complement treatment with antimicrobial agents for patients who are suddenly at risk for acquiring infection, such as patients who have extensive burns or trauma and patients admitted to an intensive care unit during an A. baumannii outbreak or epidemic.
In summary, the present study suggests the therapeutic application of LPC as a preemptive therapy for patients at risk of severe infections caused by A. baumannii.
ACKNOWLEDGMENTS
This study was supported by the Consejería de Innovación, Ciencia y Empresa (grant CTS 6317/11). Younes Smani was funded by the Ministerio de Economía y Competitividad, Instituto de Salud Carlos III, cofinanced by the European Development Regional Fund (“A way to achieve Europe”), and by the Spanish Network for Research in Infectious Diseases (grant REIPI RD12/00015/0001).
REFERENCES
- 1.Mesquita RD, Carneiro AB, Bafica A, Gazos-Lopes F, Takiya CM, Souto-Padron T, Vieira DP, Ferreira-Pereira A, Almeida IC, Figueiredo RT, Porto BN, Bozza MT, Graca-Souza AV, Lopes AH, Atella GC, Silva-Neto MA. 2008. Trypanosoma cruzi infection is enhanced by vector saliva through immunosuppressant mechanisms mediated by lysophosphatidylcholine. Infect Immun 76:5543–5552. doi: 10.1128/IAI.00683-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quinn MT, Parthasarathy S, Steinberg D. 1988. Lysophosphatidylcholine: a chemotactic factor for human monocytes and its potential role in atherogenesis. Proc Natl Acad Sci U S A 85:2805–2809. doi: 10.1073/pnas.85.8.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakai M, Miyazaki A, Hakamata H, Sasaki T, Yui S, Yamazaki M, Shichiri M, Horiuchi S. 1994. Lysophosphatidylcholine plays an essential role in the mitogenic effect of oxidized low density lipoprotein on murine macrophages. J Biol Chem 269:31430–31435. [PubMed] [Google Scholar]
- 4.Lauber K, Bohn E, Krober SM, Xiao YJ, Blumenthal SG, Lindemann RK, Marini P, Wiedig C, Zobywalski A, Baksh S, Xu Y, Autenrieth IB, Schulze-Osthoff K, Belka C, Stuhler G, Wesselborg S. 2003. Apoptotic cells induce migration of phagocytes via caspase-3-mediated release of a lipid attraction signal. Cell 113:717–730. doi: 10.1016/S0092-8674(03)00422-7. [DOI] [PubMed] [Google Scholar]
- 5.Yan JJ, Jung JS, Lee JE, Lee J, Huh SO, Kim HS, Jung KC, Cho JY, Nam JS, Suh HW, Kim YH, Song DK. 2004. Therapeutic effects of lysophosphatidylcholine in experimental sepsis. Nat Med 10:161–167. doi: 10.1038/nm989. [DOI] [PubMed] [Google Scholar]
- 6.Hayakawa M, Ishida N, Takeuchi K, Shibamoto S, Hori T, Oku N, Ito F, Tsujimoto M. 1993. Arachidonic acid-selective cytosolic phospholipase A2 is crucial in the cytotoxic action of tumor necrosis factor. J Biol Chem 268:11290–11295. [PubMed] [Google Scholar]
- 7.Voelkel-Johnson C, Thorne TE, Laster SM. 1996. Susceptibility to TNF in the presence of inhibitors of transcription or translation is dependent on the activity of cytosolic phospholipase A2 in human melanoma tumor cells. J Immunol 156:201–207. [PubMed] [Google Scholar]
- 8.Conde G, Garcia-Barreno P, Municio AM, Suarez A. 1981. In vitro and in vivo effect of Escherichia coli endotoxin on mitochondrial phospholipase A2 activity. FEBS Lett 127:115–120. doi: 10.1016/0014-5793(81)80355-9. [DOI] [PubMed] [Google Scholar]
- 9.Durkin JP, Shier WT. 1981. Staphylococcal delta toxin stimulates endogenous phospholipase A2 activity and prostaglandin synthesis in fibroblasts. Biochim Biophys Acta 663:467–479. doi: 10.1016/0005-2760(81)90175-2. [DOI] [PubMed] [Google Scholar]
- 10.Kirschnek S, Gulbins E. 2006. Phospholipase A2 functions in Pseudomonas aeruginosa-induced apoptosis. Infect Immun 74:850–860. doi: 10.1128/IAI.74.2.850-860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rodríguez-Baño J, Cisneros JM, Fernández-Cuenca F, Ribera A, Vila J, Pascual A, Martínez-Martínez L, Bou G, Pachón J. 2004. Clinical features and epidemiology of Acinetobacter baumannii colonization and infection in Spanish hospitals. Infect Control Hosp Epidemiol 25:819–824. doi: 10.1086/502302. [DOI] [PubMed] [Google Scholar]
- 12.Vila J, Pachón J. 2012. Therapeutic options for Acinetobacter baumannii infections: an update. Expert Opin Pharmacother 13:2319–2336. doi: 10.1517/14656566.2012.729820. [DOI] [PubMed] [Google Scholar]
- 13.Smani Y, Docobo-Pérez F, López-Rojas R, Domínguez-Herrera J, Ibáñez-Martínez J, Pachón J. 2012. Platelet-activating factor receptor initiates contact of Acinetobacter baumannii expressing phosphorylcholine with host cells. J Biol Chem 287:26901–26910. doi: 10.1074/jbc.M112.344556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smani Y, Docobo-Pérez F, McConnell MJ, Pachón J. 2011. Acinetobacter baumannii-induced lung cell death: role of inflammation, oxidative stress and cytosolic calcium. Microb Pathog 50:224–232. doi: 10.1016/j.micpath.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Choi CH, Lee EY, Lee YC, Park TI, Kim HJ, Hyun SH, Kim SA, Lee SK, Lee JC. 2005. Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol 7:1127–1138. doi: 10.1111/j.1462-5822.2005.00538.x. [DOI] [PubMed] [Google Scholar]
- 16.Smani Y, López-Rojas R, Domínguez-Herrera J, Docobo-Pérez F, Marti S, Vila J, Pachon J. 2012. In vitro and in vivo reduced fitness and virulence in ciprofloxacin-resistant Acinetobacter baumannii. Clin Microbiol Infect 18:E1–E4. doi: 10.1111/j.1469-0691.2011.03695.x. [DOI] [PubMed] [Google Scholar]
- 17.National Research Council. 2011. Guide for the care and use of laboratory animals, 8th ed National Academies Press, Washington, DC. [Google Scholar]
- 18.Smani Y, Domínguez-Herrera J, Pachón J. 2013. Association of the outer membrane protein Omp33 with fitness and virulence of Acinetobacter baumannii. J Infect Dis 208:1561–1570. doi: 10.1093/infdis/jit386. [DOI] [PubMed] [Google Scholar]
- 19.López-Rojas R, Docobo-Pérez F, Pachón-Ibáñez ME, de la Torre BG, Fernández-Reyes M, March C, Bengoechea JA, Andreu D, Rivas L, Pachón J. 2011. Efficacy of cecropin A-melittin peptides on a sepsis model of infection by pan-resistant Acinetobacter baumannii. Eur J Clin Microbiol Infect Dis 30:1391–1398. doi: 10.1007/s10096-011-1233-y. [DOI] [PubMed] [Google Scholar]
- 20.O'Reilly T, Cleeland R, Squires EL. 1996. Evaluation of antimicrobials in experimental animal infections, p 604–765. In Lorian V. (ed), Antibiotics in laboratory medicine. Williams and Wilkins, Baltimore, MD. [Google Scholar]
- 21.Usansky JL, Desai A, Tang-Liu D. 2012. PK functions for Microsoft Excel. Department of Pharmacokinetics and Drug Metabolism, Allergan, Irvine, CA: Accessed 1 July 2012. [Google Scholar]
- 22.Pachón-Ibáñez ME, Docobo-Pérez F, López-Rojas R, Domínguez-Herrera J, Jiménez-Mejias ME, Garcia-Curiel A, Pichardo C, Jiménez L, Pachón J. 2010. Efficacy of rifampin and its combinations with imipenem, sulbactam, and colistin in experimental models of infection caused by imipenem-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 54:1165–1172. doi: 10.1128/AAC.00367-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tokumura A. 2002. Physiological and pathophysiological roles of lysophosphatidic acids produced by secretory lysophospholipase D in body fluids. Biochim Biophys Acta 1582:18–25. doi: 10.1016/S1388-1981(02)00133-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhao Y, Natarajan V. 2013. Lysophosphatidic acid (LPA) and its receptors: role in airway inflammation and remodeling. Biochim Biophys Acta 1831:86–92. doi: 10.1016/j.bbalip.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cho WH, Park T, Park YY, Huh JW, Lim CM, Koh Y, Song DK, Hong SB. 2012. Clinical significance of enzymatic lysophosphatidylcholine (LPC) assay data in patients with sepsis. Eur J Clin Microbiol Infect Dis 31:1805–1810. doi: 10.1007/s10096-011-1505-6. [DOI] [PubMed] [Google Scholar]
- 26.Drobnik W, Liebisch G, Audebert FX, Frohlich D, Gluck T, Vogel P, Rothe G, Schmitz G. 2003. Plasma ceramide and lysophosphatidylcholine inversely correlate with mortality in sepsis patients. J Lipid Res 44:754–761. doi: 10.1194/jlr.M200401-JLR200. [DOI] [PubMed] [Google Scholar]
- 27.Garnacho J, Sole-Violan J, Sa-Borges M, Diaz E, Rello J. 2003. Clinical impact of pneumonia caused by Acinetobacter baumannii in intubated patients: a matched cohort study. Crit Care Med 31:2478–2482. doi: 10.1097/01.CCM.0000089936.09573.F3. [DOI] [PubMed] [Google Scholar]
- 28.McConnell MJ, Actis L, Pachón J. 2013. Acinetobacter baumannii: human infections, factors contributing to pathogenesis and animal models. FEMS Microbiol Rev 37:130–155. doi: 10.1111/j.1574-6976.2012.00344.x. [DOI] [PubMed] [Google Scholar]