Abstract
BMS-663068 is a prodrug of BMS-626529, a first-in-class attachment inhibitor that binds directly to HIV-1 gp120, preventing initial viral attachment and entry into host CD4+ T cells. This open-label, multiple-dose, four-sequence, crossover study addressed potential two-way drug-drug interactions following coadministration of BMS-663068 (BMS-626529 is a CYP3A4 substrate), atazanavir (ATV), and ritonavir (RTV) (ATV and RTV are CYP3A4 inhibitors). Thirty-six healthy subjects were randomized 1:1:1:1 to receive one of four treatment sequences with three consecutive treatments: BMS-663068 at 600 mg twice daily (BID), BMS-663068 at 600 mg BID plus RTV at 100 mg once daily (QD), ATV at 300 mg QD plus RTV at 100 mg QD (RTV-boosted ATV [ATV/r]), or BMS-663068 at 600 mg BID plus ATV at 300 mg QD plus RTV at 100 mg QD. Compared with the results obtained by administration of BMS-663068 alone, coadministration of BMS-663068 with ATV/r increased the BMS-626529 maximum concentration in plasma (Cmax) and the area under the concentration-time curve in one dosing interval (AUCtau) by 68% and 54%, respectively. Similarly, coadministration of BMS-663068 with RTV increased the BMS-626529 Cmax and AUCtau by 53% and 45%, respectively. Compared with the results obtained by administration of ATV/r alone, ATV and RTV systemic exposures remained similar following coadministration of BMS-663068 with ATV/r. BMS-663068 was generally well tolerated, and there were no adverse events (AEs) leading to discontinuation, serious AEs, or deaths. Moderate increases in BMS-626529 systemic exposure were observed following coadministration of BMS-663068 with ATV/r or RTV. However, the addition of ATV to BMS-663068 plus RTV did not further increase BMS-626529 systemic exposure. ATV and RTV exposures remained similar following coadministration of BMS-663068 with either ATV/r or RTV. BMS-663068 was generally well tolerated alone or in combination with either RTV or ATV/r.
INTRODUCTION
There is an ongoing need for new classes of antiretroviral agents that can provide potent and durable activity against HIV-1, primarily due to the development of resistance to existing compounds and the need for improved safety and tolerability profiles compared with those of current treatments (1, 2). Some HIV-positive patients, particularly treatment-experienced patients, may have limited treatment options owing to the presence of viral mutations causing reduced antiretroviral drug susceptibility, the emergence of drug toxicities from long-term antiretroviral therapy, and contraindications from the need to manage concurrent infections. To manage the complex and highly individualized requirements of treatment-experienced patients, antiretrovirals with activity against drug-resistant virus and limited or manageable drug-drug interactions (DDIs) are needed so they can be used effectively in combination with other antiretroviral agents or concomitant medications (3, 4).
BMS-663068 is a prodrug that is metabolized to the active moiety BMS-626529, a first-in-class, potent HIV-1 attachment inhibitor that prevents the initial interaction between virus and host cell by binding to the viral envelope protein gp120 (5, 6). This blocks attachment of the virus to the CD4 receptor of CD4+ T cells (6). By targeting the initial step in viral attachment and entry, BMS-626529 is not affected by coreceptor tropism and is therefore active against CCR5-tropic, CXCR4-tropic, and dual CCR5- and CXCR4-tropic (R5X4) strains of HIV-1 (6–10). Preliminary in vitro data show that HIV-1 isolates are generally susceptible to BMS-626529 irrespective of subtype, with the exception of subtype AE and, possibly, group O (8, 9). BMS-626529 also has a unique resistance profile with no in vitro cross-resistance to other classes of antiretrovirals (7, 8). In an ongoing phase IIb study, BMS-663068 combined with tenofovir disoproxil fumarate (TDF) and raltegravir (RAL) demonstrated efficacy comparable to that of ritonavir (RTV)-boosted atazanavir (ATV) (ATV/r) also combined with TDF and RAL. BMS-663068 was generally well tolerated, and through week 24, there were no BMS-663068-related serious adverse events (SAEs) or adverse events (AEs) leading to discontinuation (11).
BMS-663068 is delivered as an extended-release formulation, converted to BMS-626529 by alkaline phosphatase in the gastrointestinal lumen, and then rapidly absorbed due to its efficient membrane permeability (5). The active moiety circulating in plasma, BMS-626529, is predominantly metabolized by an esterase-mediated hydrolysis pathway with contributions from a cytochrome P450 (CYP) 3A (CYP3A)-mediated oxidative pathway. In vitro studies indicate that neither BMS-663068 nor BMS-626529 inhibits or induces CYP enzymes or is a strong inhibitor of drug transporters, including P-glycoprotein (P-gp) (Bristol-Myers Squibb, unpublished data).
Ritonavir-boosted protease inhibitors (PIs), including ATV/r, are important components of many antiretroviral treatment regimens in both treatment-naive and treatment-experienced patients (3, 4). As both ATV and RTV are potent inhibitors of CYP3A4 (12, 13), it was anticipated that coadministration of ATV and RTV with BMS-663068 could result in greater systemic exposures of BMS-626529. This is supported by results from early-phase pharmacokinetic (PK) studies (14–16) that have shown that RTV moderately increases BMS-626529 systemic exposure. In contrast, as neither BMS-663068 nor BMS-626529 inhibits CYP3A4 in vitro, coadministration of BMS-663068 with RTV and/or ATV is not expected to affect RTV or ATV exposures.
The objectives of this study were to address the potential DDIs following coadministration of BMS-663068, ATV, and RTV and assess the safety and tolerability of these agents in combination in healthy subjects.
(This work was presented in part at the 20th Conference on Retroviruses and Opportunistic Infections, Atlanta, GA, 3 to 6 March 2013 [17].)
MATERIALS AND METHODS
Trial design.
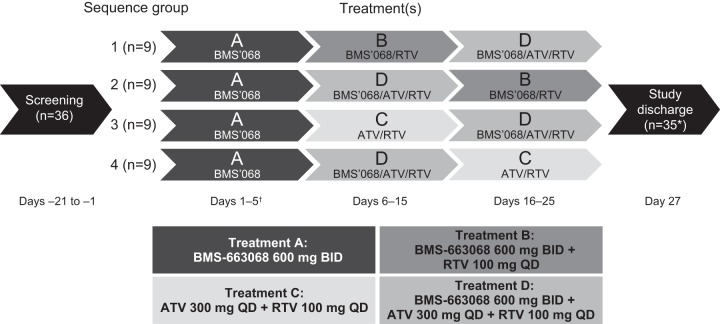
Study AI438012 was a randomized, open-label, multiple-dose, four-sequence, crossover, two-way drug-interaction study in healthy subjects. The study was conducted at a single clinical site with a planned total sample size of at least 32 evaluable subjects (8 subjects per treatment sequence). Subjects underwent screening evaluations to determine eligibility within 21 days prior to starting study treatment. Subjects were admitted to the clinical facility on day 1, prior to dosing, and were confined to the clinical facility until study discharge on day 27.
The study comprised four treatments: BMS-663068 at 600 mg twice daily (BID) (treatment A), BMS-663068 at 600 mg BID plus RTV at 100 mg once daily (QD) (treatment B), ATV at 300 mg QD plus RTV at 100 mg QD (treatment C), or BMS-663068 at 600 mg BID plus ATV at 300 mg QD plus RTV at 100 mg QD (treatment D). Subjects were randomized 1:1:1:1 to receive three consecutive treatments according to one of the four treatment sequences (Fig. 1).
FIG 1.
Design of study AI438012. *, one subject receiving treatment sequence A-D-B did not complete the study due to poor compliance/noncompliance; †, only the a.m. dose was given on day 5.
In the first treatment period, all subjects received treatment A for 4 days and a single morning dose on day 5. In the second treatment period, subjects received treatment B, C, or D for 10 days. In the final treatment period, subjects received one of the three remaining treatments (treatment B, C, or D) for 10 days (the treatment sequence is shown in Fig. 1). There were no washout intervals between treatment periods. Randomization was carried out using a computer-generated randomization scheme.
All doses were administered under fed conditions. The morning dose was administered following a standard breakfast (approximately 423 kcal) (18), and the evening dose was administered following an evening snack (approximately 300 to 350 kcal).
Plasma samples for analysis of BMS-626529 were obtained predose and at 1, 2, 3, 4, 6, 8, and 12 h postdose on days 5, 15, and 25. Plasma samples for analysis of ATV and RTV were obtained predose and at 1, 2, 3, 4, 6, 8, 12, and 24 h postdose on days 15 and 25. Physical examinations, measurement of vital signs, 12-lead electrocardiograms (ECGs), and clinical laboratory evaluations were performed at selected times throughout the study, and subjects were closely monitored for AEs and SAEs throughout the study.
Written informed consent was obtained from all subjects, and the study was conducted in accordance with good clinical practice, as defined by the International Conference on Harmonization. The protocol, amendments, and subject informed consent were approved by the Institutional Review Board/Independent Ethics Committee of Integreview Ltd. (Austin, TX, USA) prior to initiation of the study.
Subjects.
Eligible subjects were healthy adults (age range, 18 to 49 years) with a body mass index of 18 to 32 kg/m2 who were deemed to be in good health on the basis of their medical history, physical examination, 12-lead ECG, and clinical laboratory assessments. Subjects were excluded from participation if they were pregnant or breast-feeding or had any significant acute or chronic medical illness.
Study objectives.
The primary objectives of the study were to estimate the effects of two-way drug interactions on the PKs of BMS-626529 and ATV, comparing the coadministration of BMS-663068 at 600 mg BID, ATV at 300 mg QD, and RTV at 100 mg QD with the coadministration of BMS-663068 at 600 mg BID and RTV at 100 mg QD and comparing the coadministration of BMS-663068 at 600 mg BID, ATV at 300 mg QD, and RTV at 100 mg QD with the coadministration of ATV at 300 mg QD and RTV at 100 mg QD, and to estimate the effects of ATV/r on the PKs of BMS-626529 following the coadministration of BMS-663068 at 600 mg BID, ATV at 300 mg QD, and RTV at 100 mg QD compared with the PKs of BMS-663068 at 600 mg BID alone in healthy subjects. The secondary objectives were to estimate the effects of RTV on the PKs of BMS-626529 when administered as BMS-663068 at 600 mg BID and to compare the maximum concentration in plasma (Cmax) and the area under the concentration-time curve (AUC) in one dosing interval (AUCtau) of RTV when RTV at 100 mg QD was coadministered with BMS-663068 at 600 mg BID with the Cmax and AUCtau of RTV when RTV was administered with ATV at 300 mg QD. In addition, the safety and tolerability of BMS-663068 at 600 mg BID either alone or in combination with ATV at 300 mg QD, RTV at 100 mg QD, or ATV/r at 300/100 mg QD were assessed.
Assessments. (i) Bioanalytical methods.
Following extraction from human K2EDTA-anticoagulated plasma, BMS-626529 concentrations were determined using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) assay at Intertek Pharmaceuticals Services LCMS (El Dorado Hills, CA, USA). ATV and RTV concentrations were determined by LC-MS/MS at Tandem Labs (Trenton, NJ, USA).
The lower limit of quantification (LLOQ) and the upper limit of quantification (ULOQ) for the analysis of BMS-626529 and RTV in human plasma samples were established to be 5 ng/ml and 5,000 ng/ml, respectively. The LLOQ and ULOQ for the analysis of ATV were established to be 10 ng/ml and 10,000 ng/ml, respectively. The within-run and between-run precisions for quantification of BMS-626529 were ≤9.3% and ≤5.1%, respectively; those for quantification of RTV were ≤6.2% and ≤4.7%, respectively; and those for quantification of ATV were ≤4.1% and ≤5.5%, respectively.
(ii) PK analysis.
Multiple-dose PK parameters for BMS-626529, ATV, and RTV were derived from plasma concentration-versus-time data and included the following: Cmax, time to Cmax (Tmax), AUCtau (time zero to 12 h postdose for BMS-626529 and time zero to 24 h postdose for ATV and RTV), plasma concentration at 12 h postdose (C12) for BMS-626529, and plasma concentration at 24 h postdose (C24) for ATV and RTV.
Individual PK parameter values were derived by noncompartmental methods, using Kinetica software (version 5.0; Thermo Electron Corporation, Pittsburgh, PA, USA). Actual sampling times were used for PK calculations, and nominal times were used for the generation of mean plasma concentration-time plots and summaries. AUCtau was calculated using mixed log-linear trapezoidal summations.
(iii) Statistical analysis.
Statistical analyses were carried out using SAS/STAT software (version 8.2; SAS Institute Inc., Cary, NC, USA). Summary statistics are provided by treatment for BMS-626529, RTV, and ATV PK parameters.
For each within-subject comparison, a linear mixed-effect model was used to analyze log-transformed data, with treatment, period, and sequence being fixed effects and measurements within each subject being repeated measurements. Point estimates and 90% confidence intervals (CIs) for differences on the log scale were exponentiated to obtain estimates for ratios of the geometric means on the original scale. The model assumptions and potential confounding factors were examined. No adjustment for multiplicity was made in constructing the CIs. For comparison of treatments B and C, a between-subject comparison based on a two-sample t statistic on the log scale was used.
RESULTS
Subject disposition/baseline demographics.
A total of 71 subjects signed informed consent forms consenting to participation in screening procedures; to allow for discontinuations, 36 subjects (9 per treatment sequence) were treated. Thirty-five subjects completed the study, with one subject in sequence group 2 (treatment sequence A-D-B) being discontinued from the study on day 23 for reasons of poor compliance/noncompliance. Baseline demographics were generally balanced between the sequence groups: the overall median body weight was 80 kg (range, 52 to 106 kg), the median age was 30 years (range, 18 to 49 years), 26 (72%) of the subjects were male, and 19 (53%) of the subjects were black/African-American.
Pharmacokinetics of BMS-626529.
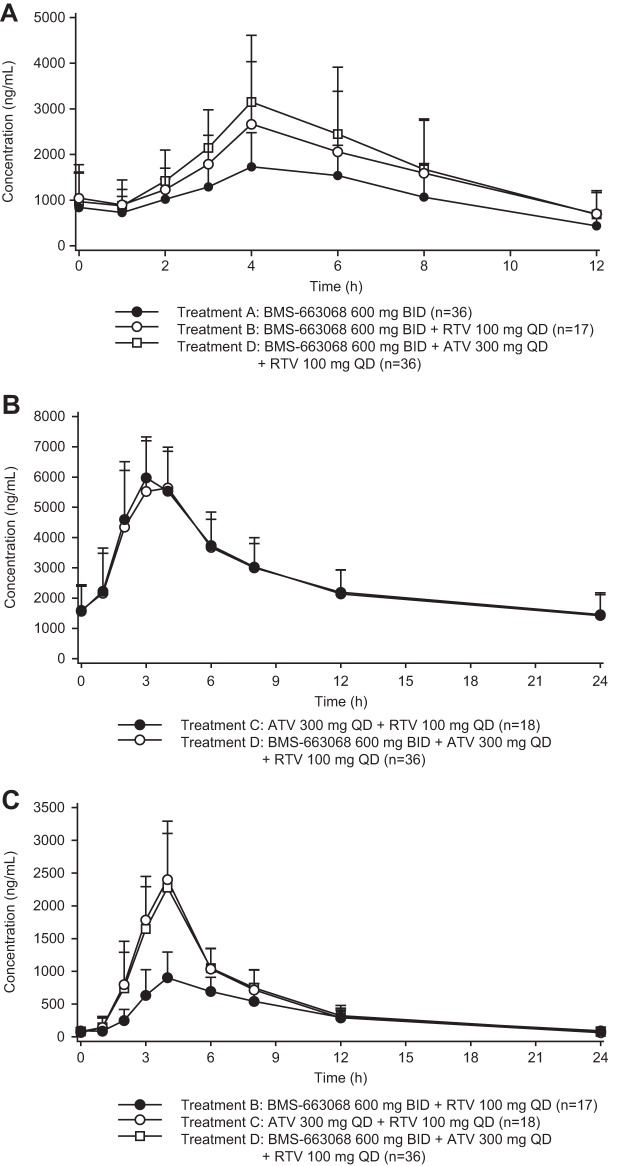
Mean BMS-626529 plasma concentration-time profiles for all subjects receiving treatment A (BMS-663068 at 600 mg BID, n = 36), treatment B (BMS-663068 at 600 mg BID plus RTV at 100 mg QD, n = 17), and treatment D (BMS-663068 at 600 mg BID plus ATV at 300 mg QD plus RTV at 100 mg QD, n = 36) are shown in Fig. 2A.
FIG 2.
(A) Mean ± SD plasma concentration-time profiles for BMS-626529 for all subjects receiving treatment A, treatment B, and treatment D. (B) Mean ± SD plasma concentration-time profiles for ATV for all subjects receiving treatment C and treatment D. (C) Mean ± SD plasma concentration-time profiles for RTV for all subjects receiving treatment B, treatment C, and treatment D.
(i) Effect of ATV/r when coadministered with BMS-663068.
To assess the effect of ATV/r on the PKs of BMS-626529, within-subject comparisons were used to compare BMS-626529 systemic exposures following administration of treatment D with those following administration of treatment A (Table 1). Compared with the results obtained with treatment with BMS-663068 alone, coadministration of BMS-663068 with ATV/r increased the adjusted geometric mean Cmax, AUCtau, and C12 of BMS-626529 by 68%, 54%, and 57%, respectively.
TABLE 1.
Summary of BMS-626529 PK parameters and treatment comparisonsa
| PK parameter | Geometric mean value (% CV) for the following treatments: |
Adjusted geometric mean ratio (90% CI) for the following treatment comparisons: |
||||
|---|---|---|---|---|---|---|
| A (n = 36) | B (n = 17) | D (n = 36) | D vs A | B vs Ab | D vs Bb | |
| Cmax | 1,878 ng/ml (42) | 2,688 ng/ml (52) | 3,150 ng/ml (47) | 1.68 (1.58, 1.79) | 1.53 (1.31, 1.79) | 1.15 (1.01, 1.30) |
| AUCtau | 12,040 ng · h/ml (38) | 16,615 ng · h/ml (44) | 18,559 ng · h/ml (47) | 1.54 (1.44, 1.65) | 1.45 (1.29, 1.61) | 1.08 (0.99, 1.17) |
| C12 | 358 ng/ml (65) | 522 ng/ml (73) | 560 ng/ml (70) | 1.57 (1.28, 1.91) | 1.44 (1.00, 2.08) | 0.991 (0.803, 1.22) |
Treatment A, BMS-663068 at 600 mg BID; treatment B, BMS-663068 at 600 mg BID plus RTV at 100 mg QD; treatment D, BMS-663068 at 600 mg BID plus ATV at 300 mg QD plus RTV at 100 mg QD; AUCtau, area under the plasma concentration-time curve in one dosing interval (12 h); CI, confidence interval; Cmax, maximum concentration in plasma; C12, plasma concentration at 12 h postdose; CV, coefficient of variation; PK, pharmacokinetic.
Only data for subjects randomized to treatment sequence A-B-D or A-D-B were included in the analysis.
(ii) Effect of RTV when coadministered with BMS-663068.
To assess the effect of RTV on the PKs of BMS-626529, within-subject comparisons were used to compare BMS-626529 systemic exposures following administration of treatment B with those following administration of treatment A (Table 1). Compared with the results obtained with treatment with BMS-663068 alone, coadministration of BMS-663068 with RTV increased the adjusted geometric mean Cmax, AUCtau, and C12 of BMS-626529 by 53%, 45%, and 44%, respectively.
(iii) Effect of ATV when coadministered with BMS-663068 plus RTV.
To assess the effect of ATV on the PKs of BMS-626529 when BMS-663068 was used in the presence of RTV, within-subject comparisons were used to compare BMS-626529 systemic exposures following administration of treatment D with those following administration of treatment B (Table 1). When comparing the coadministration of BMS-663068 plus RTV with the coadministration of BMS-663068 plus ATV plus RTV, the adjusted geometric mean AUCtau and C12 values for BMS-626529 were similar; however, Cmax increased by 15% in the presence of ATV (adjusted geometric mean; Table 1).
Pharmacokinetics of ATV.
The mean ATV plasma concentration-time profiles for all subjects receiving treatment C (ATV at 300 mg QD plus RTV at 100 mg QD, n = 18) and treatment D (n = 36) are shown in Fig. 2B.
(i) Effect of BMS-663068 when coadministered with ATV/r.
To assess the effect of BMS-663068 on the PKs of ATV, within-subject comparisons were used to compare ATV systemic exposures following administration of treatment D with those following administration of treatment C (Table 2). Compared with the results obtained with treatment with ATV/r alone, the adjusted geometric mean Cmax and AUCtau values for ATV remained similar following coadministration of BMS-663068 with ATV/r; however, C24 increased by 19%.
TABLE 2.
Summary of ATV PK parameters and treatment comparisonsa
| PK parameter | Geometric mean value (% CV) for the following treatments: |
Adjusted geometric mean ratio (90% CI) for treatment D vs Cb | |
|---|---|---|---|
| C (n = 18) | D (n = 36) | ||
| Cmax | 6,142 ng/ml (21) | 6,121 ng/ml (20) | 1.03 (0.963, 1.10) |
| AUCtau | 59,077 ng · h/ml (32) | 59,674 ng · h/ml (29) | 1.09 (1.03, 1.15) |
| C24 | 1,233 ng/ml (52) | 1,318 ng/ml (45) | 1.19 (1.10, 1.30) |
Treatment C, ATV at 300 mg QD plus RTV at 100 mg QD; treatment D, BMS-663068 at 600 mg BID plus ATV at 300 mg QD plus RTV at 100 mg QD; ATV, atazanavir; AUCtau, area under the plasma concentration-time curve in one dosing interval (24 h); CI, confidence interval; Cmax, maximum concentration in plasma; C24, plasma concentration at 24 h postdose; CV, coefficient of variation; PK, pharmacokinetic.
Only data for subjects randomized to treatment sequence A-C-D or A-D-C were included in the analysis.
Pharmacokinetics of RTV.
The mean RTV plasma concentration-time profiles for all subjects receiving treatment B (n = 17), treatment C (n = 18), and treatment D (n = 36) are shown in Fig. 2C.
(i) Effect of coadministration of BMS-663068 with ATV/r.
To assess the effect of BMS-663068 on the PKs of RTV, within-subject comparisons were used to compare RTV systemic exposures following administration of treatment C with those following administration of treatment D (Table 3). RTV systemic exposures (adjusted geometric mean Cmax and AUCtau) remained similar whether RTV was coadministered with ATV or ATV plus BMS-663068; however, the RTV C24 was slightly higher (22%) when RTV was coadministered with ATV and BMS-663068 than when it was administered with ATV alone.
TABLE 3.
Summary of RTV PK parameters and treatment comparisonsa
| PK parameter | Geometric mean value (% CV) for the following treatments: |
Adjusted geometric mean ratio (90% CI) for the following treatment comparisons: |
|||
|---|---|---|---|---|---|
| B (n = 17) | C (n = 18) | D (n = 36) | D vs Cb (90% CI) | B vs Cc (90% CI) | |
| Cmax | 933 ng/ml (37) | 2,327 ng/ml (36) | 2,188 ng/ml (36) | 1.02 (0.957, 1.09) | 0.401 (0.331, 0.484) |
| AUCtau | 7,373 ng · h/ml (35) | 11,994 ng · h/ml (28) | 12,138 ng · h/ml (27) | 1.07 (1.03, 1.10) | 0.615 (0.509, 0.742) |
| C24 | 78.2 ng/ml (65) | 54.5 ng/ml (52) | 67.9 ng/ml (51) | 1.22 (1.12, 1.32) | |
Treatment B, BMS-663068 at 600 mg BID plus RTV at 100 mg QD; treatment C, ATV at 300 mg QD plus RTV at 100 mg QD; treatment D, BMS-663068 at 600 mg BID plus ATV at 300 mg QD plus RTV at 100 mg QD; AUCtau, area under the plasma concentration-time curve in one dosing interval (24 h); CI, confidence interval; Cmax, maximum concentration in plasma; C24, plasma concentration at 24 h postdose; CV, coefficient of variation; PK, pharmacokinetic; RTV, ritonavir.
Only data for subjects randomized to treatment sequence A-C-D or A-D-C were included in the analysis.
This was a between-subject comparison; no subject received both treatment B and treatment C.
(ii) Effect of coadministration of BMS-663068 and RTV versus coadministration of ATV and RTV.
To assess the effect of BMS-663068 versus that of ATV on the PKs of RTV, between-subject comparisons were used to compare RTV systemic exposures following administration of treatment B with those following administration of treatment C (Table 3). Compared with the results obtained by coadministration of RTV with ATV, coadministration of RTV with BMS-663068 resulted in decreased RTV systemic exposures, with reductions in adjusted geometric mean Cmax and AUCtau values of 60% and 38%, respectively. As the RTV systemic exposure approximately doubles when RTV is coadministered with ATV (19), these results suggest that coadministration with BMS-663068 does not significantly affect RTV systemic exposure.
Safety and tolerability.
In total, AEs were reported in 24/36 (66.7%) treated subjects, with the majority of AEs being reported in subjects receiving BMS-663068, ATV, and RTV in combination (treatment D; 19 subjects, 52.8%) (Table 4). AEs deemed to be related to the study drugs were reported in 21/36 treated subjects (58.3%). The most frequently reported drug-related AEs were scleral icterus (13 subjects, 36.1%), abdominal pain (6 subjects, 16.7%), and headache (5 subjects, 13.9%). Drug-related scleral icterus occurred most frequently following treatment with BMS-663068, ATV, and RTV in combination (treatment D; 10 subjects, 27.8%) than following treatment with BMS-663068 plus RTV (treatment B; 1 subject, 5.6%) and treatment with ATV/r (treatment C; 2 subjects, 11.1%); no incidence of AEs was reported following treatment with BMS-663068 alone (treatment A). These observations are consistent with the known AE profile of ATV, which includes AEs of moderate to severe intensity, such as asymptomatic hyperbilirubinemia (including scleral icterus), abdominal pain, headache, nausea, and skin rash (13). Drug-related AEs (diarrhea and headache) occurred in 2/36 subjects (5.6%) following administration of BMS-663068 alone (treatment A). Elevations in total bilirubin levels considered to be marked laboratory abnormalities were observed during administration of ATV/r and occurred in 27 subjects (75%) on day 16 and in 13 subjects (36%) on day 27 (discharge). No deaths, SAEs, or AEs leading to discontinuation were reported during the study. There were no other clinically significant laboratory abnormalities, changes in vital signs, or ECG-related AEs reported.
TABLE 4.
Summary of adverse eventsa
| AE | No. (%) of subjects |
||||
|---|---|---|---|---|---|
| Treatment A (n = 36) | Treatment B (n = 18) | Treatment C (n = 18) | Treatment D (n = 36) | Any BMS-663068 treatment (n = 36) | |
| All events | 4 (11.1) | 5 (27.8) | 7 (38.9) | 19 (52.8) | 21 (58.3) |
| Scleral icterus | 0 | 1 (5.6) | 2 (11.1) | 10 (27.8) | 11 (30.6) |
| Abdominal pain | 0 | 1 (5.6) | 3 (16.7) | 4 (11.1) | 5 (13.9) |
| Headache | 2 (5.6) | 0 | 2 (11.1) | 5 (13.9) | 7 (19.4) |
| Dizziness | 0 | 0 | 2 (11.1) | 2 (5.6) | 2 (5.6) |
| Nausea | 0 | 1 (5.6) | 1 (5.6) | 3 (8.3) | 4 (11.1) |
| Musculoskeletal chest pain | 0 | 0 | 2 (11.1) | 1 (2.8) | 1 (2.8) |
Adverse events reported in >10% subjects in at least one treatment. Treatment A, BMS-663068 at 600 mg BID; treatment B, BMS-663068 at 600 mg BID plus RTV at 100 mg QD; treatment C, ATV at 300 mg QD plus RTV at 100 mg QD; treatment D, BMS-663068 at 600 mg BID plus ATV at 300 mg QD plus RTV at 100 mg QD.
DISCUSSION
BMS-663068 is a prodrug metabolized to the active moiety BMS-626529, a first-in-class attachment inhibitor that binds to HIV-1 gp120, blocking attachment of the virus to the host cell and preventing subsequent viral entry (5, 6). In an ongoing phase IIb study, BMS-663068 demonstrated efficacy comparable to that of ATV/r when both were combined with TDF and RAL (11). Treatment-experienced patients with extensive drug resistance require new therapies with novel modes of action to achieve virologic suppression, and it is important that new agents have few and/or manageable DDIs and are well tolerated. As BMS-663068 may be used in combination with ATV and/or RTV, this study assessed the potential DDIs following coadministration of BMS-663068, ATV, and RTV in healthy adults.
In this study, compared with the values obtained with BMS-663068 alone, coadministration of ATV/r with BMS-663068 led to increases in BMS-626529 Cmax, AUCtau, and C12 values of 68%, 54%, and 57%, respectively. Similarly, the coadministration of BMS-663068 with RTV at 100 mg QD resulted in a moderate increase in BMS-626529 systemic exposure, with approximate increases in Cmax, AUCtau, and C12 values being 53%, 45%, and 44%, respectively. The latter observations are in agreement with those from a previous absorption, distribution, metabolism, and excretion (ADME) study, where coadministration of 100 mg RTV with a single 300-mg oral dose of [14C]BMS-663068 resulted in 45% and 66% increases in the BMS-626529 Cmax and the BMS-626529 AUC from time zero to infinity, respectively, compared with the values obtained with administration of BMS-663068 alone (Bristol-Myers Squibb, unpublished). A moderate increase in steady-state BMS-626529 systemic exposure (Cmax, 45 to 51%; AUC from time zero to 24 h, 30 to 42%) was also seen in a proof-of-concept study following a comparison of multiple-dose PKs in HIV-1-infected subjects receiving BMS-663068 at 1,200 mg every 12 h (q12h) plus RTV at 100 mg q12h or every morning with those in subjects receiving BMS-663068 alone at 1,200 mg q12h (15).
The moderate increase in BMS-626529 systemic exposure observed in the presence of RTV is consistent with the metabolic profile of the compound, in that RTV inhibits the CYP3A-mediated metabolism of BMS-626529 but has a minimal effect on the esterase-mediated hydrolysis pathway. Notably, the increased systemic exposure of BMS-626529 following RTV coadministration is modest compared with the increased systemic exposures of PIs, such as darunavir (20) and lopinavir (21), whose plasma concentrations have been pharmacokinetically enhanced by low-dose RTV. It is also expected that in the presence of the potent CYP3A inhibitor RTV, the addition of ATV, which is also a potent inhibitor of CYP3A, would not further potentiate the DDI. This is demonstrated by the lack of a significant change in the BMS-626529 AUCtau and C12 and the minor 15% increase in the BMS-626529 Cmax observed following the coadministration of ATV/r with BMS-663068 compared with that observed following the coadministration of RTV plus BMS-663068 alone. Collectively, these results suggest that a moderate increase in BMS-626529 systemic exposure may be expected upon coadministration of BMS-663068 with a potent CYP3A inhibitor, such as RTV, or RTV-boosted PIs, such as ATV/r. However, addition of ATV to BMS-663068 administered in the presence of RTV does not result in any further changes in BMS-626529 systemic exposure.
The modest increases in BMS-626529 systemic exposure observed on BMS-663068 coadministration with RTV and/or ATV were not associated with any additional safety signals. In particular, no increases in the frequency or severity of BMS-663068-related AEs were observed (11). These findings suggest that no BMS-663068 dose adjustment is anticipated when coadministered with either ATV/r or RTV. Studies are ongoing to analyze potential DDIs between BMS-663068 and other antiretroviral agents.
As neither BMS-663068 nor the active moiety, BMS-626529, is an inhibitor or inducer of CYP3A, coadministration of BMS-663068 is not expected to affect the systemic exposures of CYP3A substrates. In line with this, compared with the results obtained by administration of ATV/r alone, coadministration of BMS-663068 at 600 mg BID with ATV/r had no effect on ATV or RTV systemic exposures (Cmax or AUCtau), although small increases in C24 were seen (19% and 22%, respectively). In addition, the effect of coadministration of RTV with BMS-663068 at 600 mg BID on RTV systemic exposures was determined. Following coadministration of RTV with BMS-663068, the RTV Cmax and AUCtau were approximately 60% and 38% lower, respectively, than those obtained with coadministration of RTV with ATV. Both ATV and RTV are potent CYP3A4 inhibitors, and it has been shown that coadministration of ATV with RTV roughly doubles RTV systemic exposures (19). Thus, the results from this study imply that BMS-663068 has only a minimal impact on RTV systemic exposures. It should, however, be noted that this was a between-subject comparison, and the precision of such a comparison is lower than that of a within-subject comparison. Thus, as BMS-663068 does not affect the systemic exposure of RTV or ATV/r, no dose adjustment for RTV or ATV/r is anticipated when these agents are used in any combination.
Throughout the study, BMS-663068 was generally well tolerated when administered as a 600-mg BID dose, either alone or in combination with RTV or ATV/r, in healthy subjects. No SAEs, discontinuations due to AEs, or deaths were reported. Drug-related AEs were observed in 58.3% of subjects, with the most frequently reported drug-related AEs being scleral icterus (36.1%), abdominal pain (16.7%), and headache (13.9%). These AEs are consistent with findings from previous clinical studies, and increases in bilirubin levels and rates of occurrence of scleral icterus were expected following administration of ATV/r (13). Scleral icterus occurred more frequently in subjects receiving BMS-663068 plus ATV/r (10/36, 27.8%) than in subjects receiving only ATV/r (2/18, 11%). Total bilirubin levels returned to normal in all subjects during the postdosing follow-up period. These findings are consistent with the unconjugated hyperbilirubinemia observed with administration of ATV/r secondary to competitive inhibition of the UGT1A1 enzyme (13).
Conclusions.
The results of this study show that in the presence of ATV/r or RTV, modest increases in BMS-626529 systemic exposure can be detected. As these moderate increases were not associated with any additional safety signals, dose adjustment of BMS-663068 is not required when coadministered with either ATV/r or RTV. Additionally, BMS-663068 did not significantly affect ATV (administered as ATV/r) or RTV systemic exposures, suggesting that dose adjustments of ATV or RTV are not required when used in combination with BMS-663068. BMS-663068 was generally well tolerated when administered alone or in combination with ATV/r and RTV. These findings contribute toward the design of a subsequent phase III clinical study of BMS-663068 in heavily treatment-experienced patients, for which the 600-mg BID dose has been selected for investigation.
ACKNOWLEDGMENTS
This study was funded by Bristol-Myers Squibb. Editorial assistance was provided by Sharmin Naaz of MediTech Media, funded by Bristol-Myers Squibb.
L.Z., M.H., C.H., V.S., G.J.H., R.B., and I.S.L. are employees of Bristol-Myers Squibb and obtain stock as partial compensation. M.F. was an employee of Bristol-Myers Squibb at the time that the work was conducted.
REFERENCES
- 1.Adamson C, Freed EO. 2010. Novel approaches to inhibiting HIV-1 replication. Antiviral Res 85:119–141. doi: 10.1016/j.antiviral.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhattacharya S, Osman H. 2009. Novel targets for anti-retroviral therapy. J Infect 59:377–386. doi: 10.1016/j.jinf.2009.09.014. [DOI] [PubMed] [Google Scholar]
- 3.Panel on Antiretroviral Guidelines for Adults and Adolescents. 2014. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. U.S. Department of Health and Human Services, Washington, DC: http://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf only retain. [Google Scholar]
- 4.European AIDS Clinical Society. 2014. European AIDS Clinical Society Guidelines for treatment of HIV-infected adults in Europe. European AIDS Clinical Society, Brussels, Belgium: http://eacsociety.org/Portals/0/140601_EACS%20EN7.02.pdf. [Google Scholar]
- 5.Brown J, Chien C, Timmins P, Dennis A, Doll W, Sandefer E, Page R, Nettles RE, Zhu L, Grasela D. 2013. Compartmental absorption modeling and site of absorption studies to determine feasibility of an extended-release formulation of an HIV-1 attachment inhibitor phosphate ester prodrug. J Pharm Sci 102:1742–1751. doi: 10.1002/jps.23476. [DOI] [PubMed] [Google Scholar]
- 6.Langley DR, Kimura SR, Sivaprakasam P, Zhou N, Dicker I, McAuliffe B, Wang T, Kadow JF, Meanwell NA, Krystal M. 2015. Homology models of the HIV-1 attachment inhibitor BMS-626529 bound to gp120 suggest a unique mechanism of action. Proteins 83:331–350. doi: 10.1002/prot.24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li Z, Zhou N, Sun Y, Ray N, Lataillade M, Hanna GJ, Krystal M. 2013. Activity of the HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068, against CD4-independent viruses and HIV-1 envelopes resistant to other entry inhibitors. Antimicrob Agents Chemother 57:4172–4180. doi: 10.1128/AAC.00513-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nowicka-Sans B, Gong YF, McAuliffe B, Dicker I, Ho HT, Zhou N, Eggers B, Lin PF, Ray N, Wind-Rotolo M, Zhu L, Majumdar A, Stock D, Lataillade M, Hanna GJ, Matiskella JD, Ueda Y, Wang T, Kadow JF, Meanwell NA, Krystal M. 2012. In vitro antiviral characteristics of HIV-1 attachment inhibitor BMS-626529, the active component of the prodrug BMS-663068. Antimicrob Agents Chemother 56:3498–3507. doi: 10.1128/AAC.00426-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ray N, Hwang C, Healy MD, Whitcomb J, Lataillade M, Wind-Rotolo M, Krystal M, Hanna GJ. 2013. Prediction of virological response and assessment of resistance emergence to the HIV-1 attachment inhibitor BMS-626529 during 8-day monotherapy with its prodrug BMS-663068. J Acquir Immune Defic Syndr 64:7–15. doi: 10.1097/QAI.0b013e31829726f3. [DOI] [PubMed] [Google Scholar]
- 10.Zhou N, Nowicka-Sans B, McAuliffe B, Ray N, Eggers B, Fang H, Fan L, Healy M, Langley DR, Hwang C, Lataillade M, Hanna GJ, Krystal M. 2014. Genotypic correlates of susceptibility to HIV-1 attachment inhibitor BMS-626529, the active agent of the prodrug BMS-663068. J Antimicrob Chemother 69:573–581. doi: 10.1093/jac/dkt412. [DOI] [PubMed] [Google Scholar]
- 11.Lalezari J, Latiff GH, Brinson C, Echevarría J, Treviño-Pérez S, Bogner JR, Stock D, Joshi SR, Hanna GJ, Lataillade M. 2014. Attachment inhibitor prodrug BMS-663068 in ARV-experienced subjects: week 24 analysis, abstr 86 Abstr 21st Annu Conf Retrovir Opportunist Infect, Boston, MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abbvie. 2013. Norvir prescribing information. Abbvie, North Chicago, IL. [Google Scholar]
- 13.Bristol-Myers Squibb. 2013. Reyataz prescribing information. Bristol-Myers Squibb, Princeton, NJ. [Google Scholar]
- 14.Nettles R, Chien C, Elefant E, Wang X, Chung E, Zhu L, Zhang D, Wu Y, Persson A, Grasela D. 2011. Single and multiple dose pharmacokinetics and safety in non-HIV-infected healthy subjects dosed with BMS-663068, an oral HIV attachment inhibitor, abstr O_04 Abstr 12th Int Workshop Clin Pharmacol HIV Ther, Miami, FL. [Google Scholar]
- 15.Nettles R, Schurmann D, Zhu L, Stonier M, Huang S-P, Chang I, Chien C, Krystal M, Wind-Rotolo M, Ray N, Hanna GJ, Bertz R, Grasela DM. 2012. Pharmacodynamics, safety, and pharmacokinetics of BMS-663068, an oral HIV-1 attachment inhibitor in HIV-1-infected subjects. J Infect Dis 206:1002–1011. doi: 10.1093/infdis/jis432. [DOI] [PubMed] [Google Scholar]
- 16.Zhu L, Chang I, Rubino C, Melhem M, Huang S-P, Stonier M, Furlong M, Krystal M, Ray N, Nettles R, Ahmad A, Bertz R. 2011. Exposure-response analyses of an oral HIV attachment inhibitor BMS-663068 following 8 days of monotherapy in HIV-infected patients, abstr O_08 Abstr 12th Int Workshop Clin Pharmacother HIV Ther, Miami, FL. [Google Scholar]
- 17.Zhu L, Hwang C, Shah V, Hruska M, Hu P, Vakkalagadda B, Furlong M, Xu X, Hanna GJ, Bertz R. 2013. Pharmacokinetic interactions between BMS-663068, the prodrug of the HIV-1 attachment inhibitor BMS-626529, and ritonavir or ritonavir-boosted atazanavir in healthy subjects, abstr 534. Abstr 20th Conf Retrovir Opportunist Infect, Atlanta, GA. [Google Scholar]
- 18.U.S. Department of Agriculture, Agricultural Research Service. 2008. USDA National nutrient database for standard reference, release 21. Nutrient Data Laboratory, U.S. Department of Agriculture, Agricultural Research Service, Washington, DC: http://www.ars.usda.gov/Services/docs.htm?docid=18880. [Google Scholar]
- 19.Molina JM, Andrade-Villanueva J, Echevarria J, Chetchotisakd P, Corral J, David N, Moyle G, Mancini M, Percival L, Yang R, Wirtz V, Lataillade M, Absalon J, McGrath D. 2010. Once-daily atazanavir/ritonavir compared with twice-daily lopinavir/ritonavir, each in combination with tenofovir and emtricitabine, for management of antiretroviral-naive HIV-1-infected patients: 96-week efficacy and safety results of the CASTLE study. J Acquir Immune Defic Syndr 53:323–332. doi: 10.1097/QAI.0b013e3181c990bf. [DOI] [PubMed] [Google Scholar]
- 20.Back D, Sekar V, Hoetelmans RM. 2008. Darunavir: pharmacokinetics and drug interactions. Antivir Ther 13:1–13. [PubMed] [Google Scholar]
- 21.Murphy RL, Brun S, Hicks C, Eron JJ, Gulick R, King M, White AC Jr, Benson C, Thompson M, Kessler HA, Hammer S, Bertz R, Hsu A, Japour A, Sun E. 2001. ABT-378/ritonavir plus stavudine and lamivudine for the treatment of antiretroviral-naive adults with HIV-1 infection: 48-week results. AIDS 15:F1–F9. doi: 10.1097/00002030-200101050-00002. [DOI] [PubMed] [Google Scholar]