Abstract
Campylobacter jejuni is a major cause of food-borne illness due to its ability to reside within the gastrointestinal tracts of chickens. Multiple studies have identified the flagella of C. jejuni as a major determinant of chicken colonization. An inhibitor screen of approximately 147,000 small molecules was performed to identify compounds that are able to inhibit flagellar expression in a reporter strain of C. jejuni. Several compounds that modestly inhibited motility of wild-type C. jejuni in standard assays were identified, as were a number of small molecules that robustly inhibited C. jejuni growth, in vitro. Examination of similar bacterial screens found that many of these small molecules inhibited only the growth of C. jejuni. Follow-up assays demonstrated inhibition of other strains of C. jejuni and Campylobacter coli but no inhibition of the closely related Helicobacter pylori. The compounds were determined to be bacteriostatic and nontoxic to eukaryotic cells. Preliminary results from a day-of-hatch chick model of colonization suggest that at least one of the compounds demonstrates promise for reducing Campylobacter colonization loads in vivo, although further medicinal chemistry may be required to enhance bioavailability.
INTRODUCTION
Campylobacter jejuni is a leading cause of food-borne illness in the United States, with a projected 1.3 million infections annually (1). This prevalence of infection with C. jejuni is primarily due to its ability to reside, asymptomatically, within the gastrointestinal tracts of agriculturally relevant animals, especially chickens, where it can reach high bacterial loads (∼109 CFU/g of cecal contents) (2). During harvest, C. jejuni is released from the gastrointestinal tract, contaminating meat products. Human infection often occurs following consumption of either undercooked meat or food that contacted a contaminated surface. As such, much work has focused on identifying factors of C. jejuni that enable the organism to colonize the chicken gastrointestinal tract.
Genetic screens have been used to identify C. jejuni factors involved in colonization of the chicken. These include signature-tagged mutagenesis and transposon insertion sequencing (Tn-Seq) approaches to identify mutants with decreased cecal abundance; these studies demonstrated that flagella are required for full colonization of the chicken cecum (2, 3). Additionally, the regulatory hierarchy of flagellar biosynthesis has been characterized (4).
With widespread antibiotic resistance and concern about dysbiosis resulting from treatment with broad-spectrum antibiotics, new approaches that target pathogenic microbes are needed (5). C. jejuni has recently been determined to be a “serious threat” for developing antibiotic resistance by the Centers for Disease Control and Prevention (6). This report specifically notes an observed increase in ciprofloxacin resistance among C. jejuni isolates from 1997 to 2011, an increase from 13% to 25%, respectively. Two approaches to combat pathogens are (i) to identify chemical inhibitors of specific virulence factors and (ii) to identify narrow-spectrum compounds that selectively inhibit growth of a target pathogen (7–10). An example of the former is the high-throughput screen for inhibitors of ToxT-dependent regulation in Vibrio cholerae, which identified a small molecule, termed virstatin, that inhibited expression of both cholera toxin (ctx) and the toxin coregulated pilus (11). Virstatin inhibits ToxT dimerization, affecting the ability of the regulator to bind the ctx promoter (12). Additionally, virstatin protected infant mice from intestinal colonization by V. cholerae (11). An example of the latter is the mycobacterium-specific antimicrobial bedaquiline, which inhibits the F1Fo-ATPase (10). While conserved across bacteria and mammals, variation in the mycobacterial F1Fo-ATPase confers selectivity of the compound for these species. Bedaquiline, the first antimicrobial identified using a high-throughput screen of a commercially available library, was approved by the Food and Drug Administration (FDA) for treatment of human multidrug-resistant tuberculosis (MDR-TB) infection (10).
For a precise anti-Campylobacter strategy, we developed a screen to identify small-molecule inhibitors of flagellum expression in C. jejuni. We used a reporter strain with a gene encoding chloramphenicol acetyltransferase expression (cat) under the control of the flgDE2 promoter. Given the nature of the regulatory hierarchy of flagellar biosynthesis in C. jejuni, any of several structural and regulatory gene products could be targets for inhibition with this strategy (4).
Following a screen of approximately 147,000 compounds against the C. jejuni reporter strain, we identified several potential flagellar inhibitors that ultimately exhibited modest effects on motility. Our approach, however, enabled simultaneous identification of compounds that specifically inhibited growth of our C. jejuni reporter strain but not of several other Gram-negative bacteria screened with the same library of compounds. Several lead compounds also inhibited growth of farm-isolated strains of C. jejuni and Campylobacter coli but did not inhibit growth of Helicobacter pylori. For in vivo efficacy, we determined that the Campylobacter growth inhibitors, which we term campynexins, are bacteriostatic and do not cause eukaryotic cell cytotoxicity. Finally, campynexins were administered to infected day-of-hatch chicks; one compound was effective, albeit inconsistently, toward C. jejuni in the gastrointestinal tract.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli strains used for subcloning were grown in LB medium at 37°C under aerobic conditions. C. jejuni strains and C. coli were grown at 37°C under microaerobic conditions (85% N2, 10% CO2, 5% O2) on either Mueller-Hinton (MH) agar containing 10% sheep's blood or in MH broth. H. pylori was grown on MH agar plates containing 10% sheep's blood under the same conditions as C. jejuni. In liquid culture, H. pylori was grown in 90% base medium (60% brucella broth and 40% calprotectin buffer [100 mM NaCl, 3 mM CaCl2, 20 mM Tris, pH 7.5]) and 10% heat-inactivated fetal bovine serum (FBS). For C. jejuni experiments, the following antibiotics were used at the indicated concentrations: chloramphenicol (Cam), 7.5 μg/ml; kanamycin (Kan), 150 μg/ml; and trimethoprim (Tmp), 10 μg/ml.
Animal protocols used in this study were approved by the University of Michigan's University Committee on Use and Care of Animals.
Construction of reporter strain.
A pUC19-based plasmid containing the flgDE2 operon (DRH351) was mutagenized with a mariner transposon that contains a promoterless cat gene linked to a constitutive kanamycin resistance gene (DRH164) (4). Transposon insertions into flgDE2 were identified by PCR and confirmed by sequencing. This plasmid was introduced into wild-type C. jejuni DRH212 (13) via electroporation, and transformants were selected for on MH blood plates containing kanamycin. Chromosomal integration was confirmed by PCR. To confirm that expression of cat was dependent on flgDE2 expression, the reporter construct was also introduced into C. jejuni DRH212 ΔflhA, ΔfliP, and ΔrpoN mutant chromosomes and growth in chloramphenicol-containing media was examined (4). Briefly, each strain was used to inoculate MH medium containing 7.5 μg/ml chloramphenicol at an optical density at 600 nm (OD600) of 0.025 in 96-well plates. Plates were incubated statically at 37°C under microaerobic conditions for 48 h before the cells were resuspended and the OD600 was recorded. Since several of these flagellar mutants exhibited decreased endpoint growth in medium lacking chloramphenicol, it was necessary to correct the results from chloramphenicol-containing medium in order to determine accurate statistical effect sizes (Z′ values). This was done by determining the extent of the growth defect for each mutant in medium lacking chloramphenicol and dividing mutant growth by wild-type growth (to determine % wild-type growth). The differences between wild-type growth (100%) and flagellar mutant growth (% wild-type growth as calculated above) were added to 1 and multiplied by those values observed for each mutant in chloramphenicol-containing medium.
Primary small-molecule screen.
A primary screen was performed using the C. jejuni flgDE2::′cat-kan reporter strain (the prime denotes that the cat gene is promoterless). Approximately 147,000 compounds from the small-molecule collection at the University of Michigan Center for Chemical Genomics (UM-CCG) were tested. Assay plates were prepared, with 20 μl MH broth in each well of 384-well microtiter plates (Corning product no. 3680). Compounds were added in 0.2 μl of 2 mM dimethyl sulfoxide (DMSO) stock solutions using a Beckman Biomek FX liquid handler with a high-density replication (HDR) “pintool” (Beckman Coulter, Brea, CA). Columns containing either DMSO alone (the vehicle for all compounds) or ciprofloxacin were used as negative and positive controls, respectively. An equal volume (20 μl) of bacterial suspension containing C. jejuni flgDE2::′cat-kan at an OD600 of 0.05 with chloramphenicol at 15 μg/ml was added to all wells, resulting in the following final concentrations: compound, 10 μM; C. jejuni flgDE2::′cat-kan,0.025 (OD600); and chloramphenicol, 7.5 μg/ml. Plates were incubated statically for 48 h at 37°C under microaerobic conditions before absorbances (OD600s) were recorded.
Triage and confirmation.
To identify inhibitors of flagellar biosynthesis, compounds that reduced the growth of C. jejuni flgDE2::′cat-kan by >40% in chloramphenicol-containing medium were selected for further confirmation. To eliminate leads that simply inhibited C. jejuni growth, compounds that inhibited by >40% were cherry-picked to triplicate wells in a set of 384-well plates by use of a TTP Labtech Mosquito X1 hit picking system (TTP Labtech, Inc., Cambridge, MA). Forty microliters of a suspension with an OD600 of 0.025 of C. jejuni flgDE2::′cat-kan without chloramphenicol was added to each well. Plates were grown exactly as described prior to recording the OD600. Small molecules that did not inhibit C. jejuni growth by >50% in medium lacking chloramphenicol were considered further as potential flagellar inhibitors. Of these compounds, all were similarly picked in triplicate, and C. jejuni flgDE2::′cat-kan in medium containing chloramphenicol was added. Following growth in the presence of chloramphenicol, those compounds that inhibited reporter strain growth by >3 standard deviations in all three replicate wells were considered further.
To identify inhibitors of C. jejuni growth, compounds described above that inhibited C. jejuni flgDE2::′cat-kan growth in medium lacking chloramphenicol by >90% were examined in other bacterial screens within the UM-CCG database, MScreen, including those using the Gram-negative bacteria E. coli ΔtolC, Shigella flexneri, and Vibrio cholerae (14). Compounds that inhibited C. jejuni growth by >90% but did not inhibit other Gram-negative bacteria by >10% were considered further as possible Campylobacter-specific growth inhibitors.
Dose-response analysis.
For both flagellar inhibitors and growth inhibitors, compounds from the small-molecule library were added to 384-well plates using the TTP Labtech Mosquito X1 system to achieve final concentrations between 1 and 100 μM. Forty microliters of 0.025 (OD600) suspensions of either C. jejuni flgDE2::′cat-kan or wild-type C. jejuni in MH broth, with or without chloramphenicol, respectively, were added to each well and grown for 48 h at 37°C under microaerobic conditions. Similarly, for the growth inhibitor screen, a dose-response curve for E. coli EC2880 ΔtolC (a generous gift from Michael Hubband, Pfizer Scientific) was generated in parallel, under the same conditions, but the mutant was grown for 24 h (15).
For both screens, dose-response curve analyses were repeated at least twice, in a similar manner, using newly purchased compounds (ChemDiv, Inc., San Diego, CA). Briefly, compounds were resuspended in deuterated-DMSO (d6-DMSO) to final concentrations between 10 and 20 mM and serially diluted (1:2 in MH broth) in 96-well plates to obtain 100 μl of medium containing compounds at concentrations between 3 and 200 μM. One hundred microliters of 0.05 (OD600) suspensions of either C. jejuni flgDE2::′cat-kan or wild-type C. jejuni DRH212 in MH broth was added to each well, resulting in final compound concentrations between 1.5 and 100 μM at a final OD600 of 0.025 in 200 μl of MH broth. These plates were incubated without shaking for 48 h at 37°C under microaerobic conditions. For dose-response curves of growth inhibitors, E. coli EC2880 ΔtolC was treated similarly, in parallel, but was again grown for 24 h. Data were analyzed using SigmaPlot software, and either 50% effective concentrations (EC50s), the effective molar concentrations of compound where 50% of bacterial growth was inhibited, or pEC50s, the effective molar concentrations expressed as −log10, were calculated (Systat Software, Inc., San Jose, CA).
Motility assays.
Compounds were added to a final concentration of 100 μM in 4 ml of MH motility agar (MH broth plus 0.4% agar), which was allowed to solidify in individual wells of a six-well tissue culture plate. Control wells were prepared similarly but contained only DMSO, the vehicle of all compounds. The center of each well was inoculated with an overnight culture of wild-type C. jejuni, except for a single DMSO control well that was inoculated with a nonmotile C. jejuni ΔrpoN mutant. Cultures were grown for 24 h at 37°C under microaerobic conditions before wells were imaged and motility diameters were measured.
Dose-response analysis of additional strains.
To examine for specificity of growth inhibition, dose-response curves for wild-type C. jejuni DRH212, C. jejuni MTVDSCj20 (16), C. coli MTVDSCc1 (M. Taveirne and V. J. DiRita, unpublished), and E. coli EC2880 ΔtolC were generated from 96-well plates, like those described above. For H. pylori J75 (17), the strain was grown under the same conditions but in medium containing brucella broth, calprotectin buffer, and FBS. After 24 h, OD600s were recorded and analyzed using SigmaPlot.
MIC/MBC assays.
Dose-response curves were generated for the wild type as described, but cultures were grown for 24 h at 37°C under microaerobic conditions. The concentration where endpoint growth was inhibited by approximately 90% (by OD600) was identified, and cells were removed from these wells, serially diluted, and plated onto plates containing MH agar plus Tmp. Colonies were enumerated after growing microaerobically at 37°C for 48 h. Control wells contained DMSO or the inhibitory compounds chloramphenicol and kanamycin, which are bacteriostatic and bactericidal, respectively.
Cell viability assays.
The effect of each compound on eukaryotic cell viability was determined using the CellTiter-Glo assay (Promega, Madison, WI). Briefly, 2-fold dilutions of each compound were made in Dulbecco's modified Eagle medium (reference no. 11965-092; Life Technologies, Carlsbad, CA) containing glucose and l-glutamine with 10% fetal bovine serum, 10 mM HEPES, and 1 mM sodium pyruvate (final concentrations). Fifty microliters of a 2 × 106/ml suspension (1 × 105) of Caco-2 cells was added to 50 μl of medium containing compound, resulting in final compound concentrations of 100, 50.0, 25.0, 12.5, 6.25. 3.12, and 1.56 μM. Additionally, Caco-2 cells were added to control wells containing either DMSO or 0.1% Triton X-100. Cultures were incubated for 24 h in 96-well Optilux plates (Becton Dickinson, Franklin Lakes, NJ) under standard tissue culture conditions. After incubation, 100 μl of CellTiter-Glo reagent was added to each well and ATP levels were determined according to the manufacturer's instructions.
Chicken colonization studies.
To determine whether lead compounds can reduce C. jejuni loads within the chicken gastrointestinal tract, day-of-hatch chickens were inoculated by oral gavage with approximately 104 CFU (100 μl of a 105-CFU/ml suspension) of wild-type C. jejuni DRH212 in phosphate-buffered saline (PBS) containing compound. Briefly, a common inoculum was split into aliquots that received either compound CCG-84443, CCG-187741, CCG-187751, CCG-187769, or CCG-198215 to 100 μM concentrations. A positive-control group was infected with the same inoculum but did not receive compound, only an equivalent amount of DMSO. A negative-control group was mock infected with sterile PBS containing an equivalent amount of DMSO. Birds within each group were dosed further with their respective compound at days 3 and 6 postinoculation via oral gavage of 100 μl of sterile PBS containing 100 μM each compound. Control groups were dosed with sterile PBS containing only DMSO. At day 7 postinoculation, cecal contents were isolated and plated for viable C. jejuni.
RESULTS
An flgDE2 reporter for screening compound libraries.
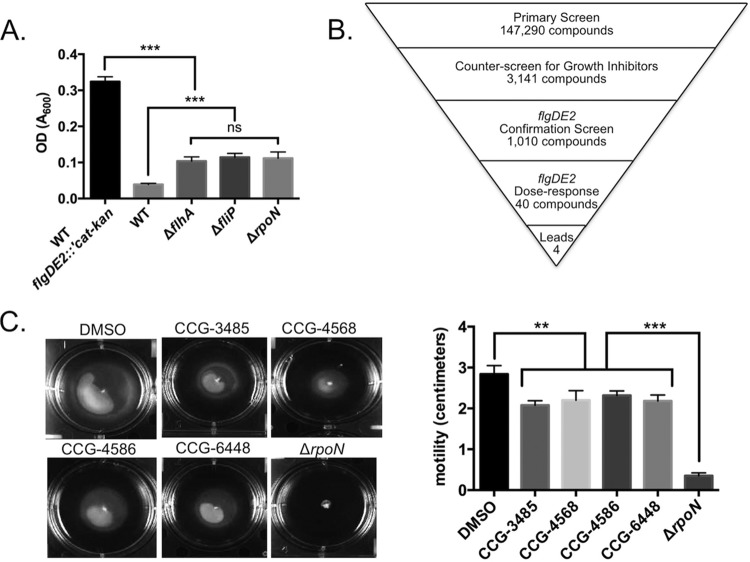
Growth in chloramphenicol-containing medium was dependent on a chromosomally encoded flgDE2::′cat-kan reporter; this strain grew to an average OD600 of 0.347 ± 0.037, whereas the wild-type strain grew to an average OD600 of 0.039 ± 0.004. The average statistical effect size of this difference was calculated with a Z′ of 0.783 (18). To demonstrate that flgDE2 reporter output was linked to flagellar expression, we moved the reporter into mutant strains (ΔflhA, ΔfliP, and ΔrpoN mutants). However, those strains exhibited a slight general growth defect even in medium lacking chloramphenicol. As this would magnify any differences in flgDE2 expression observed in chloramphenicol-containing medium, the growth defect in medium without chloramphenicol was used to correct values obtained with chloramphenicol-containing medium. The corrected OD600 values for flagellar mutants expressing flgDE2::′cat-kan in medium containing chloramphenicol were as follows: ΔflhA mutant, 0.104 ± 0.011; ΔfliP mutant, 0.115 ± 0.011; and ΔrpoN mutant, 0.111 ± 0.018, representing >3-fold decreases in endpoint growth of the mutants (Fig. 1A). The corrected growth of each strain, while not significantly different from one another, was greater than that observed for wild-type C. jejuni and significantly less than the growth of the flgDE2::′cat-kan reporter strain (P < 0.0001). Compared to the flgDE2::′cat-kan reporter strain, these reductions produced Z′ values of 0.661 for the ΔflhA mutant, 0.656 for the ΔfliP mutant, and 0.561 for the ΔrpoN mutant.
FIG 1.
Identification of flagellar inhibitors by high-throughput screening of a small-molecule library. (A) Effect of flagellar mutations on flgDE2 expression in a C. jejuni flgDE2::′cat-kan reporter strain; (B) schematic of triage process used to identify putative small-molecule inhibitors of flgDE2 expression; (C) effect of compounds on wild-type C. jejuni motility in swim-agar medium. Statistical analyses were performed using Student's t test (**, P < 0.001, ***, P < 0.0001). ns, not significant.
Identification of putative flagellar inhibitors.
After screening of the flgDE2::′cat-kan reporter strain against a library of 147,290 compounds, 3,141 compounds were found to inhibit growth of C. jejuni flgDE2::′cat-kan in medium containing chloramphenicol by >40% (Fig. 1B). Since a certain number of these hits likely represent nonspecific bacterial growth inhibitors, a counterscreen was performed by testing each compound in triplicate against C. jejuni flgDE2::′cat-kan in medium without chloramphenicol. This counterscreen identified 1,010 compounds that inhibited growth by <50%, indicating that these small molecules do not significantly inhibit bacterial growth and that the results observed in chloramphenicol are likely due to inhibition of flgDE2 expression (Fig. 1B). Reproducibility of growth inhibition by these 1,010 compounds—and the likely inhibition of flagellar expression—was confirmed in triplicate using the C. jejuni flgDE2::′cat-kan strain in medium containing chloramphenicol. Of these 1,010 compounds, 40 inhibited C. jejuni flgDE2::′cat-kan growth in chloramphenicol-containing medium by more than 3 standard deviations from the uninhibited mean (Fig. 1B).
Dose-response analysis of flagellar inhibitors identified few reproducibly potent compounds.
Of the 40 compounds that specifically inhibited C. jejuni flgDE2::′cat-kan growth in chloramphenicol-containing medium, four produced pEC50s greater than 5.0, indicating adequate potency of flgDE2 inhibition (Fig. 1B). These compounds—CCG-3485, CCG-4568, CCG-4586, and CCG-6448—are from a subset of UM-CCG's compound library (Chembridge 3028 and Chembridge 10000) and exhibited pEC50s of 5.61, 5.67, 5.57, and 5.78, respectively (data not shown). Subsequent dose-response analysis of these inhibitors using fresh compound determined that they reproducibly inhibited growth of the reporter strain under the conditions used in the screen.
Flagellar inhibitors modestly reduce motility.
After growth of wild-type C. jejuni in motility agar containing 100 μM CCG-3485, CCG-4568, CCG-4586, or CCG-6448, all compounds significantly reduced motility compared to an untreated control. Motility diameters were as follows: 2.84 ± 0.09 cm for the DMSO control, 2.08 ± 0.05 cm for CCG-3485 (P < 0.0001), 2.20 ± 0.11 cm for CCG-4568 (P = 0.002), 2.32 ± 0.05 cm for CCG-4586 (P = 0.001), and 2.18 ± 0.06 cm for CCG-6448 (P < 0.001) (Fig. 1C). While these represent reproducible reductions in motility, none of the compounds inhibited motility to the level of a ΔrpoN mutant (0.35 ± 0.05 cm, P < 0.0001).
Identification of campynexins, anti-Campylobacter compounds.
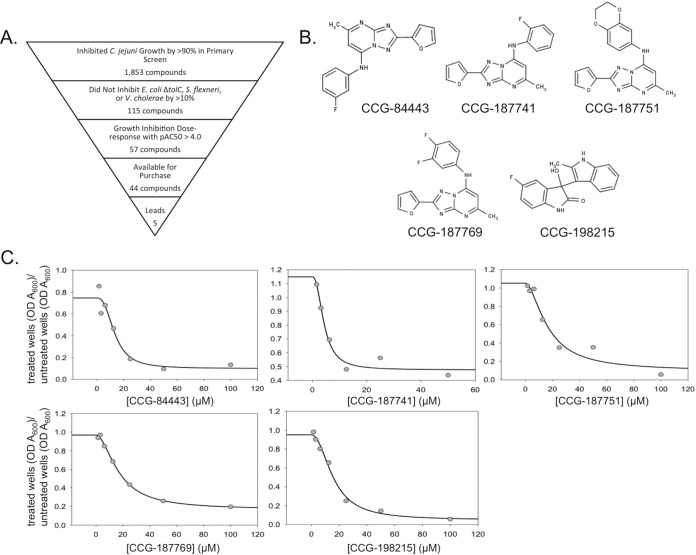
While separating inhibitors of flgDE2 expression from those that inhibited bacterial growth, we identified 1,853 compounds that inhibited C. jejuni growth by >90% in medium lacking chloramphenicol, indicating the presence of potent growth inhibitors (Fig. 2A). These were analyzed for general growth inhibition of Gram-negative bacteria by examining data from other screens performed at the UM-CCG using E. coli ΔtolC, S. flexneri, and V. cholerae. The E. coli ΔtolC mutant was of particular interest because its lack of the TolC efflux protein makes it hypersensitive to toxic compounds (19). From this analysis, 115 of the 1,853 C. jejuni inhibitors did not inhibit growth of the above-mentioned strains by >10% (Fig. 2A). Initial dose-response analysis determined that 57 of the 115 potential C. jejuni-specific growth inhibitors yielded pEC50s greater than 4.0, with 31 of those compounds producing pEC50s greater than 5.0 (Fig. 2A). Forty-four of these 57 compounds were available for purchase, and we performed dose-response analyses with these molecules. Following this analysis, the compounds selected for further study were CCG-84443, CCG-187741, CCG-187751, CCG-187769, and CCG-198215, which yielded average EC50s of 8.18, 8.96, 11.9, 10.4, and 37.4 μM, respectively (Fig. 2B and C); we term these compounds campynexins A to E, respectively. Cultures containing E. coli EC2880 ΔtolC did not exhibit a dose response to any of the campynexins, and therefore EC50s could not be calculated.
FIG 2.
Identification of C. jejuni-specific growth inhibitors. (A) Triage process used to identify growth inhibitors from the high-throughput screen for flagellar inhibitors; (B) structures of selected lead compounds; (C) dose-response curves of selected lead compounds.
Campynexins exhibit activity toward Campylobacter but not Helicobacter bacteria.
To rule out that growth inhibition by the campynexins was limited to our laboratory strain DRH212, and to examine how broadly the campynexins inhibited bacterial growth, dose-response assays against farm isolates C. jejuni MTVDSCj20 and C. coli MTVDCc1 were performed similarly to those described above. Compounds exhibited mean EC50s for these strains that were similar to those observed for C. jejuni DRH212 (Table 1). In contrast, no response at any dose was observed with H. pylori strain J75, similar to the results using E. coli EC2880 ΔtolC (Table 1).
TABLE 1.
Campynexin effectiveness on different bacterial species
| Strain | Compound EC50 (μM)a |
||||
|---|---|---|---|---|---|
| CCG-84443 | CCG-187741 | CCG-187751 | CCG-187769 | CCG-198215 | |
| C. jejuni DRH212 | 12.93 | 9.83 | 16.14 | 2.95 | 51.78 |
| C. jejuni MTVDSCj20 | 3.99 | 8.08 | 12.68 | 2.72 | 50.06 |
| C. coli MTVDSCc1 | 7.55 | 16.8 | 15.4 | 3.82 | 37.54 |
| E. coli EC2880 ΔtolC | >100 | >100 | >100 | >100 | >100 |
| H. pylori J75 | >100 | >100 | >100 | >100 | >100 |
The EC50 was calculated from a dose-response curve that was generated from the average result for triplicate cultures at the given concentrations.
Inhibitors of C. jejuni growth are bacteriostatic.
Prior to testing the compounds in infected chicks, we determined whether they act bacteriostatically or bactericidally. This was done for the five lead compounds, campynexins A to E, by identifying concentrations that inhibited endpoint growth by ≥90% and plating serial dilutions to determine the number of viable bacteria present. This level of inhibition occurred at concentrations of 50 μM for compounds CCG-187741 and CCG-187769 and at 100 μM for compounds CCG-84443, CCG-187751, and CCG-198215. Similarly, we also identified the lowest concentrations of chloramphenicol and kanamycin that were needed to inhibit growth by ≥90%. This level of inhibition occurred at concentrations of 2 μg/ml and 20 μg/ml for chloramphenicol and kanamycin, respectively. Following serial dilution, cultures treated with campnexins A to E had mean viable bacterial counts of 1.34 × 107, 9.65 × 106, 8.98 × 106, 2.73 × 107, and 3.38 × 106 CFU/ml, respectively (Fig. 3). These values were significantly different from those for control cultures treated with DMSO, with a mean of 1.42 × 109 CFU/ml (P < 0.0001), and those of the bactericidal control kanamycin, with a mean of 6.14 × 104 CFU/ml (P < 0.05). Viable cell counts from the bacteriostatic control, chloramphenicol, were not significantly different from those of the campynexins (P > 0.05), with a mean count of 1.41 × 107 CFU/ml.
FIG 3.
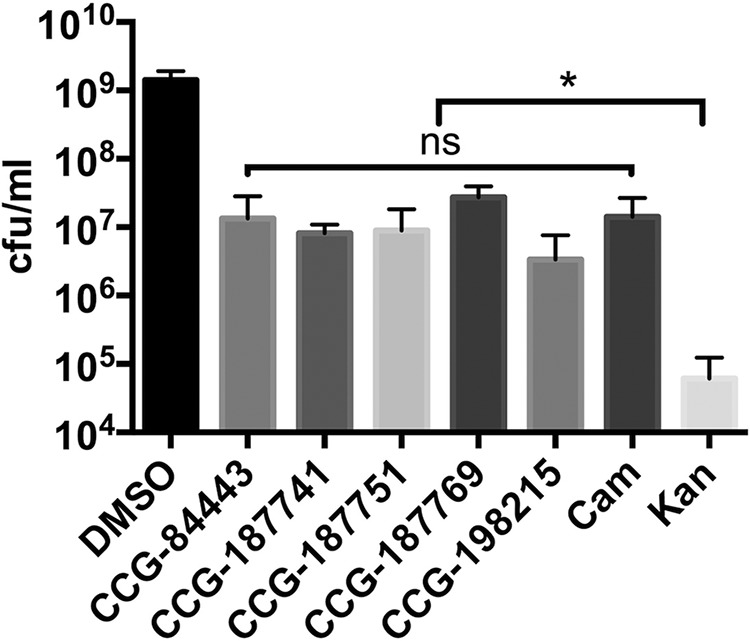
Determining the class of C. jejuni growth inhibitors (bacteriostatic versus bactericidal) by using selected compounds in an MIC/MBC assay. Statistical analysis was performed using Student's t test (*, P < 0.05).
Lead compounds do not affect eukaryotic cell viability.
Human intestinal epithelial cells (Caco-2) were grown in the presence of increasing amounts of compound (1.56 μM to 100 μM) for 24 h under tissue culture conditions. Cell viability was examined by determining ATP levels using CellTiter-Glo reagent. Percent cell viability was determined by dividing luminescence units for each compound by those recorded from wells where Caco-2 cells were untreated. Cell viability for DMSO-treated Caco-2 cells was found to exhibit luminescence at a mean (± standard deviation) of 106.95% ± 53.03%, while nonviable, 0.1% Triton-treated control wells produced luminescence at a mean of 0.06% ± 0.03%. For the five lead compounds, mean percent cell viabilities of 120.82% ± 20.41%, 125.55% ± 24.71%, 161.46% ± 27.90%, 187.21% ± 25.74%, and 74.84% ± 37.54% were recorded at 100 μM concentrations of CCG-84443, CCG-187741, CCG-187751, CCG-187769, and CCG-198215, respectively. Based on these results, Caco-2 cells do not exhibit a cytotoxic dose-response to these compounds, so 50% cytotoxic concentrations (CC50) could not be calculated. Only cells treated with 0.1% Triton X-100 exhibited a significant decrease in cell viability.
Campynexin A reduces C. jejuni carriage in the chicken gastrointestinal tract.
The ability of campynexins to reduce the load of C. jejuni was tested using day-of-hatch chickens, a standard colonization model in the natural host for C. jejuni. Following administration of the compounds at the time of inoculation and 3 and 6 days postinoculation, only campynexin A was capable of reducing C. jejuni loads, albeit inconsistently (Fig. 4). In more than half of the chickens (5 of 9) treated with campynexin A, the number of viable C. jejuni present was at or below the limit of detection (2 × 103 CFU/g cecal content) while approximately half of the chickens (4 of 9) exhibited full levels of cecal colonization (∼108 to 109 CFU/g cecal content). The mean cecal load of treated chickens was 3.63 × 108 CFU/g of cecal content, representing a nearly 1-log average decrease (P < 0.01) in colonization compared to the mean cecal load of chickens treated with only DMSO (2.28 × 109 CFU/g cecal content).
FIG 4.

Colonization levels of C. jeuni in the chicken cecum following treatment with lead compound CCG-84443. Statistical analysis was performed using a Mann-Whitney test (P < 0.01).
DISCUSSION
C. jejuni is a significant cause of food-borne infection in the United States, primarily due to its ability to reside within the gastrointestinal tracts of chickens. Infection of humans often occurs following ingestion of undercooked meat or by consuming uncooked food that has been cross-contaminated. As such, much emphasis has been placed on identifying and characterizing factors of C. jejuni with the goal of using those insights to inform the identification and/or development of strategies or compounds that limit C. jejuni loads in the food supply (2, 3). One such factor that has repeatedly been shown to be required for full colonization of the chicken gastrointestinal is the flagella of C. jejuni.
Other work has shown that a majority of the flagellar biosynthetic cascade terminates with expression of the flagellar hook-encoding operon, flgDE2, immediately upstream of flagellin production. We constructed an flgDE2 reporter strain that links the expression of flgDE2 to the expression of the gene that encodes C. coli chloramphenicol acetyltransferase, making growth in chloramphenicol-containing medium dependent on flagellar expression.
Following both the primary screen and the dose-response analysis of flagellar inhibitors, few compounds were found to reproducibly inhibit growth of the flgDE2 reporter strain and many of those that were identified did not exhibit sufficient potency for us to pursue further. Instead, we chose to focus on those that were the most reproducible and potent, reasoning that those compounds were the most likely to provide us with detectable inhibition of flagellar motility. Unfortunately, at compound concentrations that almost completely inhibited growth of the reporter strain in the dose-response analysis, we observed very little inhibition of flagellar motility in a standard assay. This likely reflects that either (i) the assay is somewhat insensitive at detecting flgDE2 expression, where even though we observe significant decreases in reporter strain growth, there is still productive flgDE2 expression and flagellar biosynthesis, or (ii) the compounds are simply not amenable for use in this standard assay. We are currently pursuing both of these possibilities in the hope of optimizing the primary screen and/or motility assays for future identification of antiflagellar compounds.
In our primary screen for flagellar inhibitors, we identified ones that inhibited growth of the reporter strain, but these likely represented three different classes of molecules in terms of their effects: (i) those that inhibited flgDE2 expression, which is the class we originally sought, (ii) those that inhibited the function of the chloramphenicol acetyltransferase enzyme, or (iii) those that inhibited growth of C. jejuni independently of flgDE2 reporter inhibition. This made the screen attractive not only from the standpoint of identifying inhibitors of flagellar biosynthesis but also for identifying compounds capable of inhibiting growth of C. jejuni in vitro. While the ability to inhibit growth of C. jejuni is interesting by itself, ideally one would want to identify compounds that specifically inhibit C. jejuni growth while not affecting the viability or growth of other bacteria within the chicken gastrointestinal tract. As the facility in which we carried out the primary screen has performed multiple primary screens on other Gram-negative bacteria, we mined the data from these screens to eliminate compounds that broadly inhibit Gram-negative bacterial growth.
When many of these compounds were purchased and reexamined, many either did not inhibit to the level we would have expected or exhibited an inhibitory effect toward our bacterial toxicity control, E. coli EC2880 ΔtolC. This is likely due to the gradual degradation of the small molecules in the library; the initial growth inhibition may have been due to a degradation product rather than the original progenitor molecule. Nevertheless, several compounds were confirmed to inhibit the growth of C. jejuni in vitro, and these, which we term campynexins, became the focus of additional study.
While a comparison of the results of our growth inhibitor screen to those using other Gram-negative bacteria indicated some measure of specificity, we also examined the campynexins for inhibition of farm-isolated strains of C. jejuni and C. coli, as well as the closely related epsilonproteobacterium H. pylori J75. Campynexins inhibited growth of members of the Campylobacter genus but not those of Helicobacter. This indicates differences in effectiveness within the order Campylobacterales but potentially uniform effectiveness within the Campylobacter genus. Such specificity would be attractive for infection control within an agricultural setting since one could potentially limit proliferation of all food-borne pathogens within the Campylobacter genus. We are currently expanding the above-described screen to determine whether this is a possibility by using Campylobacter strains from multiple sources in dose-response assays. Also of obvious further interest is the identification of targets of growth inhibition that make these compounds so specific to Campylobacter spp.
Initial tests to examine the feasibility of using these compounds to reduce C. jejuni colonization of the chicken gastrointestinal tract centered on determining whether compounds are bacteriostatic or bactericidal in nature and whether these compounds affect eukaryotic cell viability. The finding that these compounds are bacteriostatic indicates that they will likely need to be administered early and for an extended period of time to prevent outgrowth within the chicken gastrointestinal tract. Since we observed that eukaryotic cells tolerate high levels of these compounds in vitro, we attempted to reduce C. jejuni loads in the chicken by administering each compound individually at the time of inoculation and with doses at 3 and 6 days postinoculation. One of these compounds, campynexin A (CCG-84443), reduced C. jejuni loads on one occasion but was unable to significantly decrease C. jejuni carriage upon a subsequent attempt. The reasons for this are unclear and indicate that more information on the fates of these molecules in vivo is required. As such, pharmacokinetic analysis of these inhibitors is being carried out in order to determine their half-life within the chicken bloodstream and gastrointestinal tract. These data will inform the further development of these compounds for use in chickens. Additionally, studies are also under way to determine whether these compounds have any effect on other Campylobacter species in the chicken gastrointestinal tract, including C. coli. Long-term goals also include examining the efficacy of these compounds as feed or water additives in an agricultural setting.
ACKNOWLEDGMENTS
We thank Jennifer Gaddy (Vanderbilt University) for helping with H. pylori growth experiments and Michael Taveirne (University of Michigan) for providing C. jejuni and C. coli farm isolates.
This work was supported by grants from the National Institute of Allergy and Infectious Diseases to V.J.D. (R01 AI069383), and J.G.J. was a fellow of the Molecular Mechanisms of Microbial Pathogenesis Training Program (T32 AI007528). Further support for J.G.J. was from the U.S. Department of Agriculture's National Institute for Food and Agriculture (awards 2010-65201-20594 and 2013-67012-21136). Funding for the primary screen was provided by the University of Michigan Center for the Discovery of New Medicines.
REFERENCES
- 1.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, Jones JL, Griffin PM. 2011. Foodborne illness acquired in the United States—major pathogens. Emerg Infect Dis 17:7–15. doi: 10.3201/eid1701.091101p1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hendrixson DR, DiRita VJ. 2004. Identification of Campylobacter jejuni genes involved in commensal colonization of the chick gastrointestinal tract. Mol Microbiol 52:471–484. doi: 10.1111/j.1365-2958.2004.03988.x. [DOI] [PubMed] [Google Scholar]
- 3.Johnson JG, Livny J, Dirita VJ. 2014. High-throughput sequencing of Campylobacter jejuni insertion mutant libraries reveals mapA as a fitness factor for chicken colonization. J Bacteriol 196:1958–1967. doi: 10.1128/JB.01395-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hendrixson DR, DiRita VJ. 2003. Transcription of sigma54-dependent but not sigma28-dependent flagellar genes in Campylobacter jejuni is associated with formation of the flagellar secretory apparatus. Mol Microbiol 50:687–702. doi: 10.1046/j.1365-2958.2003.03731.x. [DOI] [PubMed] [Google Scholar]
- 5.Anthouard R, DiRita VJ. 2015. Chemical biology applied to the study of bacterial pathogens. Infect Immun 83:456–469. doi: 10.1128/IAI.02021-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hampton T. 2013. Report reveals scope of US antibiotic resistance threat. JAMA 310:1661–1663. doi: 10.1001/jama.2013.280695. [DOI] [PubMed] [Google Scholar]
- 7.Koppolu V, Osaka I, Skredenske JM, Kettle B, Hefty PS, Li J, Egan SM. 2013. Small-molecule inhibitor of the Shigella flexneri master virulence regulator VirF. Infect Immun 81:4220–4231. doi: 10.1128/IAI.00919-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun H, Xu Y, Sitkiewicz I, Ma Y, Wang X, Yestrepsky BD, Huang Y, Lapadatescu MC, Larsen MJ, Larsen SD, Musser JM, Ginsburg D. 2012. Inhibitor of streptokinase gene expression improves survival after group A streptococcus infection in mice. Proc Natl Acad Sci U S A 109:3469–3474. doi: 10.1073/pnas.1201031109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sully EK, Malachowa N, Elmore BO, Alexander SM, Femling JK, Gray BM, DeLeo FR, Otto M, Cheung AL, Edwards BS, Sklar LA, Horswill AR, Hall PR, Gresham HD. 2014. Selective chemical inhibition of agr quorum sensing in Staphylococcus aureus promotes host defense with minimal impact on resistance. PLoS Pathog 10:e1004174. doi: 10.1371/journal.ppat.1004174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis K. 2013. Platforms for antibiotic discovery. Nat Rev Drug Discov 12:371–387. doi: 10.1038/nrd3975. [DOI] [PubMed] [Google Scholar]
- 11.Hung DT, Shakhnovich EA, Pierson E, Mekalanos JJ. 2005. Small-molecule inhibitor of Vibrio cholerae virulence and intestinal colonization. Science 310:670–674. doi: 10.1126/science.1116739. [DOI] [PubMed] [Google Scholar]
- 12.Shakhnovich EA, Hung DT, Pierson E, Lee K, Mekalanos JJ. 2007. Virstatin inhibits dimerization of the transcriptional activator ToxT. Proc Natl Acad Sci U S A 104:2372–2377. doi: 10.1073/pnas.0611643104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendrixson DR, Akerley BJ, DiRita VJ. 2001. Transposon mutagenesis of Campylobacter jejuni identifies a bipartite energy taxis system required for motility. Mol Microbiol 40:214–224. doi: 10.1046/j.1365-2958.2001.02376.x. [DOI] [PubMed] [Google Scholar]
- 14.Jacob RT, Larsen MJ, Larsen SD, Kirchhoff PD, Sherman DH, Neubig RR. 2012. MScreen: an integrated compound management and high-throughput screening data storage and analysis system. J Biomol Screen 17:1080–1087. doi: 10.1177/1087057112450186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill SK, Garcia GA. 2011. Rifamycin inhibition of WT and Rif-resistant Mycobacterium tuberculosis and Escherichia coli RNA polymerases in vitro. Tuberculosis 91:361–369. doi: 10.1016/j.tube.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Taveirne ME, Dunham DT, Miller WG, Parker CT, Huynh S, DiRita VJ. 2014. Complete genome sequence and annotation of a Campylobacter jejuni strain, MTVDSCj20, isolated from a naturally colonized farm-raised chicken. Genome Announc 2(4):e00852-14. doi: 10.1128/genomeA.00852-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smoot DT, Wynn Z, Elliott TB, Allen CR, Mekasha G, Naab T, Ashtorab H. 1999. Effects of Helicobacter pylori on proliferation of gastric epithelial cells in vitro. Am J Gastroenterol 94:1508–1511. doi: 10.1111/j.1572-0241.1999.01134.x. [DOI] [PubMed] [Google Scholar]
- 18.Zhang JH, Chung TD, Oldenburg KR. 1999. A simple statistical parameter for use in evaluation and validation of high throughput screening assays. J Biomol Screen 4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
- 19.Blair JM, Richmond GE, Piddock LJ. 2014. Multidrug efflux pumps in Gram-negative bacteria and their role in antibiotic resistance. Future Microbiol 9:1165–1177. doi: 10.2217/fmb.14.66. [DOI] [PubMed] [Google Scholar]