Abstract
The pharmacokinetic profile of ceftaroline has not been well characterized in obese adults. The purpose of this study was to evaluate the pharmacokinetics of ceftaroline in 32 healthy adult volunteers aged 18 to 50 years in the normal, overweight, and obese body size ranges. Subjects were evenly assigned to 1 of 4 groups based on their body mass index (BMI) and total body weight (TBW) (ranges, 22.1 to 63.5 kg/m2 and 50.1 to 179.5 kg, respectively). Subjects in the lower-TBW groups were matched by age, sex, race/ethnicity, and serum creatinine to the upper-BMI groups. Serial plasma and urine samples were collected over 12 h after the start of the infusion, and the concentrations of ceftaroline fosamil (prodrug), ceftaroline, and ceftaroline M-1 (inactive metabolite) were assayed. Noncompartmental and population pharmacokinetic analyses were used to evaluate the data. The mean plasma ceftaroline maximum concentration and area under the curve were ca. 30% lower in subjects with a BMI of ≥40 kg/m2 compared to those <30 kg/m2. A five-compartment pharmacokinetic model with zero-order infusion and first-order elimination optimally described the plasma concentration-time profiles of the prodrug and ceftaroline. Estimated creatinine clearance (eCLCR) and TBW best explained ceftaroline clearance and volume of distribution, respectively. Although lower ceftaroline plasma concentrations were observed in obese subjects, Monte Carlo simulations suggest the probability of target attainment is ≥90% when the MIC is ≤1 μg/ml irrespective of TBW or eCLCR. No dosage adjustment for ceftaroline appears to be necessary based on TBW alone in adults with comparable eCLCR. Confirmation of these findings in infected obese patients is necessary to validate these findings in healthy volunteers. (This study has been registered at ClinicalTrials.gov under registration no. NCT01648127.)
INTRODUCTION
Ceftaroline fosamil is a novel intravenously administered antimicrobial agent approved for use in the United States as a treatment of acute bacterial skin and skin structure infections (ABSSSIs) and community-acquired bacterial pneumonia (1, 2). Unlike other regulatory approved cephalosporins, ceftaroline is uniquely active in vitro against methicillin-resistant Staphylococcus aureus (MRSA) (1–3). The current dosage regimen of ceftaroline fosamil is a fixed-dose of 600 mg every 12 h irrespective of body size in adult patients. Upon administration, the prodrug ceftaroline fosamil is converted by phosphatases to the active form ceftaroline that is principally (60%) eliminated unchanged in urine and to a small (6%) extent as an inactive metabolite (ceftaroline-M1). The dose should be reduced in patients with renal impairment when the estimated creatinine clearance (eCLCR) is ≤50 ml/min.
Obesity is defined as a body mass index (BMI) of ≥30 kg/m2 and has been independently associated with an increased risk of certain infections such as ABSSSIs (4–6). Some evidence suggests obese patients may be at risk of worse clinical outcomes attributed in part to inadequate antimicrobial exposure with standard dosage regimens (7, 8). A dedicated pharmacokinetic study comparing the concentration-time profile of ceftaroline with a standard dosage regimen across the spectrum of adult body size has not been previously investigated (2).
Pharmacokinetic studies of antimicrobials in obese subjects have often compared drug disposition between two groups of subjects: normal weight and obese class III (BMI ≥ 40 kg/m2) (9–11). Although this study design is helpful for identifying pharmacokinetic differences between these two groups, it limits identification of the optimal scalar of pharmacokinetic parameters across body size if differences are observed. This limitation is especially problematic when appraising the relationship of eCLCR to antimicrobial clearance (CL). CLCR is often estimated using the Cockcroft-Gault equation that includes age, sex, serum creatinine, and total body weight (TBW) (12). Discerning the independent relationship of TBW and ceftaroline pharmacokinetic parameters requires an adequate distribution of subjects across the entire spectrum of TBW while matching other characteristics of healthy subjects (e.g., age, sex, serum creatinine, race, and ethnicity).
We sought to evaluate the plasma and urine concentration-time profiles of ceftaroline fosamil, ceftaroline, and ceftaroline-M1 following a single dose of 600 mg in four groups of healthy adult subjects. This study was specifically designed to include a larger sample size (n = 32) of subjects across all BMI classification groups (normal weight to overweight, obese class I, obese class II, and obese class III) while matching other subject characteristics (11). The impact of measured creatinine clearance (mCLCR) and eCLCR on the primary pharmacokinetic parameters of ceftaroline was also evaluated.
MATERIALS AND METHODS
Study design.
The study protocol was reviewed and approved by the University of Illinois at Chicago Institutional Review Board and written informed consent was obtained from each subject prior to the study. All study procedures were performed at the Clinical Research Center of the University of Illinois at Chicago Center for Clinical and Translational Science, Chicago, IL. The study was registered with ClinicalTrials.gov (NCT01648127).
This was a prospective, phase I, open-label pharmacokinetic study in 32 healthy adult subjects. Each subject received a single dose of ceftaroline fosamil (CPTF) at 600 mg as a 1-h intravenous (i.v.) infusion through a peripheral i.v. catheter using a programmed infusion pump. Ceftaroline fosamil was supplied by Cerexa, Inc., Oakland, CA (a wholly owned subsidiary of Forest Laboratories, Inc., New York, NY). Vials of ceftaroline fosamil 600 mg were reconstituted with the appropriate amount of sterile water for injection and added to a normal saline solution to obtain a final infusion volume of 250 ml for each administration.
Study population.
Healthy men and women between 18 and 50 years of age (inclusive) were eligible to participate in the study. Screening for enrollment was performed after written informed consent was obtained and included a complete medical history, physical examination, assessment of vital signs (blood pressure, temperature, heart rate, and respiratory rate), and evaluation of clinical laboratory data (clinical chemistry, liver function panel, complete blood cell count with differential, and serum pregnancy test for female subjects of child-bearing potential). Subjects with clinically significant abnormalities identified on screening were ineligible for study inclusion. Females of childbearing potential were required to have a negative serum pregnancy test at screening and expected to be utilizing contraception if nonabstinent. A repeat negative serum pregnancy test was required for these female subjects within 24 h of study drug infusion. Subjects must have been nonsmokers, defined as abstinence from cigarette smoking for the previous 12 months before study enrollment.
Participants were evenly assigned to one of four groups based on BMI and TBW: normal to overweight (BMI, 18.5 to 29.9 kg/m2; TBW, 50 to 100 kg), obese class I (BMI, 30 to 34.9 kg/m2; TBW, 90 to 115 kg), obese class II (BMI, 35 to 39.9 kg/m2; TBW 105 to 130 kg), and obese class III (BMI, ≥40 kg/m2; TBW, ≥120 kg). Both BMI and TBW measurements for each subject were required to be within the range of one of the body size groups described. Subjects in the normal to overweight and obese class I groups were matched to subjects in the obese class II and III groups by age (±8 years), sex, race/ethnicity, and serum creatinine (SCr; ±0.2 mg/dl).
Subjects were excluded from participation if any of the following criteria were met: (i) history of significant hypersensitivity reaction or intolerance to ceftaroline or β-lactam agents; (ii) history of significant cardiac, neurological, thyroid, muscular, or immune disorders; (iii) positive serum pregnancy test or current breast-feeding; (iv) history of alcohol or substance abuse or dependence within 12 months of study enrollment; (v) history of prescription or nonprescription drug use within 7 days (or 14 days for potential enzyme inducers) or five half-lives (whichever was longer) prior to the first dose of study drug (except birth control pills or hormone replacement in females); (vi) participated in a clinical trial within the last 30 days; (vii) donated blood to the extent where participation would result in >500 ml of blood donated within a 56-day period; (viii) risk of noncompliance with study procedures; (ix) aspartate aminotransferase (AST) or alanine aminotransferase (ALT) levels of >3 times the upper limit of normal; (x) SCr ≥ 1.5 mg/dl or an eCLCR of <60 ml/min, as determined by the Cockcroft-Gault equation using the TBW (12).
Sample collection.
Blood samples were collected from a peripheral i.v. catheter in the arm contralateral to the i.v. catheter used for ceftaroline fosamil administration. Samples were collected prior to (predose) and at 0.5, 0.95, 1.05, 1.25, 1.5, 2, 3, 4, 6, 8, 10, and 12 h after the start of the ceftaroline fosamil infusion. Blood samples were collected using a prechilled 6-ml gray-top BD Vacutainer with 15 mg of sodium fluoride and 12 mg of potassium oxalate (Becton Dickinson, Franklin Lakes, NJ) to prevent ex vivo conversion of ceftaroline fosamil to ceftaroline. Samples were centrifuged immediately under refrigeration (4°C) at 1,500 × g for 10 min to obtain separated plasma. Plasma samples were immediately flash frozen in a liquid nitrogen bath and stored at −80°C until analysis of the study drug concentrations.
Urine samples were collected on wet ice prior to (predose) and at intervals of 0 to 2 h, 2 to 4 h, 4 to 8 h, and 8 to 12 h after the start of the CPTF infusion. At the end of each urine collection interval, urine aliquots (2 ml each) were flash frozen in a liquid nitrogen bath and then stored at −80°C until analysis. The remaining urine (excluding predose urine) was combined and stored under refrigeration (4°C) until analysis of 12-h urine creatinine (UCr).
Analytical procedures.
The concentrations of ceftaroline fosamil, ceftaroline, and ceftaroline-M1 were determined in plasma and urine using liquid chromatography coupled with tandem mass spectrometry at Keystone Bioanalytical, Inc. (North Wales, PA).
Deuterated forms of all three analytes served as internal standards for the assay methods. Plasma samples were deproteinated, and the supernatant was further diluted with a solution of ammonium formate in water. The entire extraction was performed rapidly (<90 min) in an ice bath or at 5°C to minimize degradation or conversion of the analytes. A 50-μl plasma sample aliquot was used for sample preparation and analysis. The validation showed the plasma assay was quadratic over the concentration range of 50 to 50,000 ng/ml for ceftaroline (R2 = 0.9978) and 50 to 10,000 ng/ml for ceftaroline fosamil and ceftaroline-M1 (R2 = 0.9960 and 0.9991, respectively). The lower limit of quantification (LLOQ) was 50 ng/ml for all analytes. The precision (i.e., the percent coefficient of variation) and accuracy (i.e., the percent bias) of the ceftaroline plasma standards were 1.2 to 4.2% and −7.2 to 5.4%, respectively. The precision and accuracy of the ceftaroline fosamil plasma standards were 1.6 to 8.5% and −3.9 to 2.3%, respectively. The precision and accuracy of the ceftaroline-M1 plasma standards were 0.3 to 3.5% and −2.6 to 3.0%, respectively.
For the urine assays, samples were extracted using dilution with a solution of ammonium formate-water-methanol-isopropanol (100:780:80:40 [vol/vol/vol/vol]). The entire extraction was also performed rapidly (<90 min) in an ice bath or at 10°C (centrifuged for 5 min) to minimize degradation or conversion of the analytes. A 50-μl urine sample aliquot was used for sample preparation and analysis. The validation showed the urine assay was linear over the concentration range of 0.5 to 200 μg/ml for ceftaroline (R2 = 0.9976) and 0.5 to 20 μg/ml for ceftaroline fosamil and ceftaroline-M1 (R2 = 0.9970 and 0.9985, respectively). The LLOQ was 0.5 μg/ml for all analytes. The precision and accuracy of the ceftaroline plasma standards were 0.4 to 2.5% and −7.5 to 5.1%, respectively. The precision and accuracy of the ceftaroline fosamil plasma standards were 0.3 to 3.2% and −4.8 to 5.7%, respectively. The precision and accuracy of the ceftaroline-M1 plasma standards were 0.9 to 2.7% and −4.0 to 3.0%, respectively.
Noncompartmental pharmacokinetic analysis.
Noncompartmental analysis of ceftaroline fosamil, ceftaroline, and ceftaroline-M1 plasma concentrations was performed using Phoenix WinNonlin version 6.3 (Pharsight Corp., St. Louis, MO). For all estimates of the area under the concentration-time curve (AUC), the linear trapezoidal rule and the logarithmic trapezoidal rule were used when concentration data were increasing and decreasing, respectively. Ceftaroline fosamil plasma concentrations decline rapidly due to an almost immediate conversion to ceftaroline in vivo. Consequently, the first measured plasma concentration of the ceftaroline fosamil below the LLOQ after infusion was fixed to 25 ng/ml (half the LLOQ), and subsequent ceftaroline fosamil concentrations below the LLOQ were treated as zero. Actual infusion and sampling times were used in the calculation of pharmacokinetic parameters. The amounts of ceftaroline fosamil, ceftaroline, and ceftaroline-M1 recovered in the urine were quantified as the summation of the measured concentration by urine volume (for each interval) over the 12-h urine collection period and used to compute the renal CL (CLR).
Population pharmacokinetic analysis.
Parametric population pharmacokinetic (POP-PK) analysis was performed using ADAPT 5 (13). Concentration-time data were modeled using the maximum-likelihood solution via the expectation-maximization algorithm. Candidate structural POP-PK models were evaluated sequentially for both ceftaroline fosamil and ceftaroline. The initial approach modeled plasma concentration data of each analyte separately as a two-compartment model with a zero-order infusion, linear elimination, transfer between the compartments, and an additive plus proportional residual error model. Models of higher and lower complexity were evaluated, including one- or three-compartments, nonlinear (Michaelis-Menten) elimination or parallel linear/nonlinear elimination, and various residual error models (i.e., additive, proportional, and exponential residual error models). Ceftaroline fosamil and ceftaroline plasma concentration-time data were then comodeled in a similar, sequential fashion. Ceftaroline urine concentration data were also evaluated for incorporation into the structural POP-PK model. Concentrations below the LLOQ for ceftaroline fosamil were treated as missing.
Standard goodness of fit criteria where used to discriminate between candidate POP-PK models, including the Akaike information criterion (AIC) (14), parameter estimates, between-subject variability, and diagnostic plots. For equivalent models, the rule of parsimony was used and the simpler model was chosen. Once the optimal structural POP-PK model was developed, post hoc pharmacokinetic parameter estimates from the base model were used to evaluate the influence of potential covariates via linear and nonlinear regression. Significant covariates were then incorporated into the structural POP-PK model using ADAPT 5. Covariates were retained in the final POP-PK model if their inclusion significantly improved the model fit (i.e., lowered the AIC).
Final POP-PK model validation was performed through evaluation of diagnostic plots, including observed versus predicted concentration plots (population and individual), residual versus time plots, and residual versus predicted concentration plots. Concentration-time data and area under the concentration-time curve integrated to 12 h (AUC0–12) and infinity (AUC0–∞) were simulated for 9,999 subjects using the final POP-PK model. Simulated concentration-time data were compared to observed concentration-time data using visual predictive checks (15). Simulated estimates of AUC were compared to individual estimates of AUC calculated via noncompartmental pharmacokinetic analysis.
Statistical analysis.
All statistical analysis was performed using Stata/SE version 12.1 (StataCorp LP, College Station, TX). Categorical variables were evaluated between body size groups using χ2 tests or the Fisher exact test, as appropriate. Continuous variables were compared between body size groups using a one-way analysis of variance (ANOVA) or Kruskal-Wallis one-way ANOVA if the data failed normality tests (both with the Bonferroni correction for multiple comparisons). Statistical significance was based on a two-sided P value of <0.05.
Scatter plots and graphs were created to visually assess covariate relationships with individual pharmacokinetic parameters. Univariate linear regression models, including log-log and polynomial regressions, were also performed to estimate the relationship between potential covariates and individual pharmacokinetic parameters. All relevant potential covariates were then evaluated in multiple linear regression models using forward and backward selection (α set to 0.01 and 0.001, respectively).
Potential covariates evaluated for inclusion in the structural POP-PK model were age, sex, race/ethnicity, height (HT), and various descriptors for renal function (mCLCR and eCLCR) (12) and body size (TBW, ideal body weight [IBW] [16], adjusted body weight [ABW] [17], lean body weight [LBW] [18], BMI [19], and BSA [20]), where (i) mCLCR = (12-h UCr) × (12-h urine volume)/SCr × 720 min, (ii) eCLCR = (140 − age) × TBW/72 × SCr, if female multiply the eCLCR by 0.85; (iii) IBW = 45.5 + [0.89 × (HT − 152.4)], if male add 4.5 to the IBW; (iv) ABW = IBW + 0.4 × (TBW − IBW); (v) LBW (males) = (9,270 × TBW)/[6,680 + (216 × BMI)] and LBW (females) = (9,270 × TBW)/[8,780 + (244 × BMI)]; (vi) BMI = TBW/[HT2 (in m)]; and (vii) BSA = [(TBW × HT)/3,600]0.5. In all calcuations, UCr and SCr are expressed in mg/dl, urine volume is expressed in ml, age is expressed in years, weight is expressed in kg, and height is expressed in cm (unless otherwise noted).
Probability of target attainment.
A Monte Carlo simulation of 9,999 subjects was performed to evaluate probability of target attainment following six 600-mg doses of ceftaroline fosamil administered i.v. every 12 h (steady state). The target attainment values chosen for analysis were 30, 40, and 50% fT>MIC, representing the range of static through 2-log bacterial killing for Staphylococcus aureus, Streptococcus pneumoniae, Escherichia coli, and Klebsiella spp. (21, 22). The simulated times based on total drug concentrations (% T>MIC) were transformed to those based on free drug concentrations (% fT>MIC) by factoring in the plasma protein binding of ceftaroline of 20% (23). The probability of target attainment (PTA) for the population was determined for MICs between 0.0625 and 32 μg/ml. Finally, MIC distribution data from respiratory isolates collected through the AWARE program from 2009 to 2011 were used to calculate the cumulative fraction of response (CFR) for a variety of microorganisms (Ronald Jones and Robert Flamm, unpublished data). CFRs represent the expected population PTA for a specific drug dose and specific population of microorganisms. In this analysis, CFRs for S. aureus, S. pneumoniae, E. coli, and Klebsiella spp. were calculated using the following formula:
where i is the MIC category ranked from lowest to highest MIC value of a population of microorganisms, PTAi is the PTA for the ith MIC category, and Fi is the fraction of the population of microorganisms for the ith MIC category (24). CFRs of ≥90% were considered acceptable since the majority of patients would be expected to achieve target attainment with the given dosage regimen over the expected population of microorganisms.
Laboratory and safety assessment.
Safety assessments, including physical examination findings, vital signs values, clinical laboratory measurements, and adverse events (AEs), were performed throughout the study. All AEs were evaluated by the investigators for severity and relationship to the study drug.
RESULTS
Subjects.
A total of 49 subjects were enrolled in the study. Seventeen subjects (37.8%) did not meet the study inclusion criteria. The baseline characteristics for the 32 enrolled subjects that met all study criteria and completed all study procedures are described in Table 1 by body size group. As expected, the lower TBW groups (normal to obese class I) and upper TBW groups were well matched by age, sex, race/ethnicity, baseline SCr, and height. Subjects in the obese class III group had a mean TBW and BMI that was ∼2-fold higher than the normal to overweight group. The mean mCLCR was 39.8% higher in the obese class III group compared to the normal to overweight group. The mean eCLCR was ∼2-fold higher in the obese class III group than the normal to overweight group, which corresponded with the TBW difference between groups.
TABLE 1.
Characteristics of study subjects receiving i.v. ceftaroline fosamila
| Characteristicb | Normal to overweight (n = 8) | Obese class I (n = 8) | Obese class II (n = 8) | Obese class III (n = 8) |
|---|---|---|---|---|
| General study subject characteristics | ||||
| Age, yr | 34.6 ± 11.6 | 35.8 ± 8.7 | 36.3 ± 11.3 | 34.8 ± 11.0 |
| Male, no. (%) | 7/8 (87.5) | 4/8 (50) | 5/8 (62.5) | 6/8 (75) |
| Race/ethnicity, no. (%) | ||||
| Black | 2/8 (25) | 5/8 (62.5) | 5/8 (62.5) | 2/8 (25) |
| Hispanic | 4/8 (50) | 1/8 (12.5) | 1/8 (12.5) | 4/8 (50) |
| White | 2/8 (25) | 2/8 (25) | 2/8 (25) | 2/8 (25) |
| Baseline SCr, mg/dl | 0.88 ± 0.24 | 1.01 ± 0.19 | 0.93 ± 0.17 | 0.88 ± 0.31 |
| Ht, cm | 173.2 ± 13.0 | 173.0 ± 7.2 | 174.6 ± 6.2 | 174.2 ± 9.0 |
| TBW, kg (range) | 74.5 ± 13.8 (50.1–91.1) | 101.4 ± 8.4 (91–111.2) | 113.8 ± 7.1 (105.4–128.9) | 145.4 ± 21.5 (125.2–179.5) |
| Calculated body size descriptors | ||||
| BMI, kg/m2 (range) | 24.6 ± 1.8 (22.1–27.7) | 33.9 ± 1.2 (31.2–34.9) | 37.4 ± 1.7 (35.5–39.9) | 48.1 ± 7.9 (40.5–63.5) |
| IBW, kg | 67.8 ± 12.8 | 65.9 ± 8.8 | 67.9 ± 7.3 | 68.2 ± 9.8 |
| ABW, kg | 70.5 ± 13.0 | 80.1 ± 8.4 | 86.3 ± 6.9 | 99.1 ± 11.0 |
| LBW, kg | 56.4 ± 11.6 | 61.6 ± 11.3 | 67.1 ± 9.9 | 75.8 ± 10.8 |
| BSA, m2 | 1.89 ± 0.24 | 2.21 ± 0.13 | 2.35 ± 0.17 | 2.65 ± 0.22 |
| Renal function descriptors | ||||
| mCLCR, ml/min | 130.1 ± 28.0 | 140.3 ± 20.2 | 164.0 ± 32.4 | 181.9 ± 40.4 |
| eCLCR, ml/min | 108.5 ± 17.1 | 120.7 ± 19.7 | 150.5 ± 33.5 | 222.4 ± 72.2 |
Values are reported as means ± the standard deviations, unless otherwise indicated.
SCr, serum creatinine; Ht, height; TBW, total body weight; BMI, body mass index; IBW, ideal body weight; ABW, adjusted body weight; LBW, lean body weight; BSA, body surface area; mCLCR, measured creatinine clearance; eCLCR, estimated creatinine clearance.
Noncompartmental pharmacokinetic analysis.
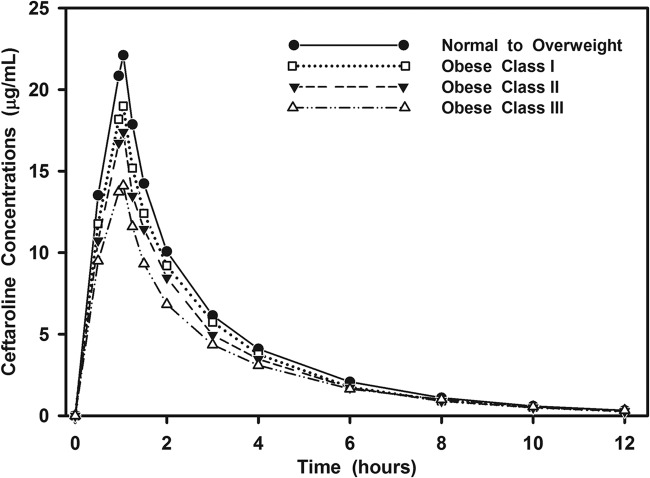
The plasma concentration-time profile of ceftaroline fosamil declined rapidly during infusion (due to rapid in vivo conversion to ceftaroline) and was below the LLOQ after the 2-h time point in all subjects. Approximately half of the ceftaroline fosamil dose was recovered in urine as ceftaroline (49.7% ± 6.3%) over the 12-h collection. There were no measurable concentrations of ceftaroline fosamil in urine at any time point, suggesting complete in vivo conversion to ceftaroline. The percentage of the ceftaroline fosamil dose recovered in urine as ceftaroline-M1 was 3.9% ± 0.8%. Plasma concentrations of ceftaroline-M1 reached a maximum value at a median of 4 h and were <1 μg/ml for the entire sampling period. Given these observations, Fig. 1 is limited to the mean plasma concentration-time profile of ceftaroline by body size group. As shown, the concentration-time profile declined in a mono-exponential manner over the 12-h period. Table 2 summarizes the key exposure and pharmacokinetic parameters derived by noncompartmental analysis of the ceftaroline concentration-time profile. As shown, statistically significant differences were primarily observed between the obese class III and normal to overweight group. Subjects in the obese class III group exhibited a 35.9% lower mean maximum serum concentration (Cmax) and 29.1% lower AUC0–12 value compared to normal weight to overweight group. These significant differences in exposure corresponded with a higher mean volume of distribution during the terminal phase (Vz), total clearance (CLT), and CLR values in the obese class III group. However, the mean minimum serum concentration (Cmin) and half-life (t1/2) were not different between the groups.
FIG 1.
Mean concentration-time profile of ceftaroline in plasma by body size group.
TABLE 2.
Noncompartmental pharmacokinetic parameters of ceftaroline
| Parameter | Mean ± SD |
||||
|---|---|---|---|---|---|
| All subjects (n = 32) | Normal to overweight (n = 8) | Obese class I (n = 8) | Obese class II (n = 8) | Obese class III (n = 8)a | |
| Cmax (μg/ml) | 18.3 ± 4.6 | 22.3 ± 5.9 | 19.2 ± 3.8 | 17.5 ± 2.4 | 14.3 ± 1.4* |
| Cmin (μg/ml) | 0.30 ± 0.15 | 0.32 ± 0.24 | 0.28 ± 0.07 | 0.27 ± 0.10 | 0.33 ± 0.17 |
| AUC0–12 (μg·h/ml) | 44.2 ± 9.8 | 51.9 ± 11.8 | 45.9 ± 8.1 | 42.3 ± 6.9 | 36.8 ± 6.2* |
| AUC0–∞ (μg·h/ml) | 45.3 ± 10.1 | 53.0 ± 12.3 | 46.8 ± 8.2 | 43.1 ± 7.2 | 38.1 ± 6.9* |
| t1/2 (h) | 2.3 ± 0.3 | 2.1 ± 0.3 | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.5 ± 0.3 |
| Vz (liters) | 45.4 ± 10.9 | 36.4 ± 9.7 | 42.9 ± 9.4 | 45.3 ± 6.7 | 56.9 ± 7.0† |
| Vz (liters/kg [TBW]) | 0.43 ± 0.07 | 0.49 ± 0.09 | 0.42 ± 0.07 | 0.40 ± 0.06 | 0.39 ± 0.04 |
| CLT (liters/h) | 13.9 ± 3.0 | 12.0 ± 3.3 | 13.2 ± 2.3 | 14.2 ± 2.3 | 16.2 ± 2.9* |
| CLR (liters/h) | 7.1 ± 1.9 | 5.8 ± 1.1 | 6.5 ± 1.1 | 7.5 ± 1.9 | 8.6 ± 2.3* |
*, P < 0.026 (obese class III compared with normal to overweight); †, P < 0.012 (obese class III compared with normal to overweight and obese class I).
Population pharmacokinetic analysis.
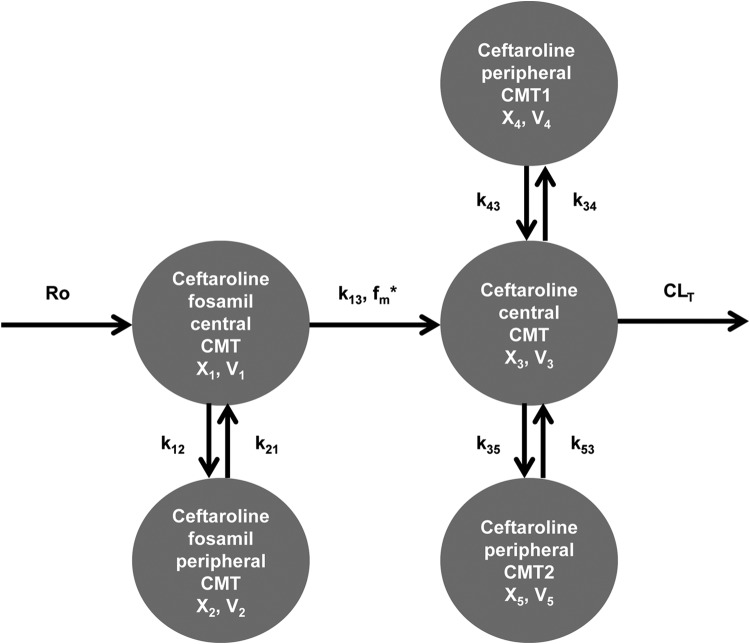
Population pharmacokinetic analysis was performed using 416 plasma samples from 32 study subjects. A five-compartment model with zero-order infusion and linear elimination fit the data best. This structural model with relevant parameters is illustrated in Fig. 2. This structural POP-PK model included both ceftaroline fosamil and ceftaroline plasma data which were best described as two- and three-compartment models, respectively. Ceftaroline-M1 was not comodeled with these data since it represented a low fraction of the systemic profile and the pharmacokinetics were not different between groups by noncompartmental analysis. Ceftaroline concentrations were transformed within the model into ceftaroline fosamil equivalents based on the ratio of molecular weights between the two analytes (a ceftaroline fosamil/ceftaroline ratio of 1.1323). Table 3 presents parameter estimates for the final POP-PK model. The model included a rate constant (k13) describing the first-order conversion of ceftaroline fosamil into ceftaroline. The large estimated mean value of k13 is in agreement with the high rate at which ceftaroline fosamil concentrations disappear. The fraction of drug metabolized from ceftaroline fosamil into ceftaroline by plasma phosphatases (fm) was assumed to be 1 based on previous pharmacokinetic information from the drug sponsor (Todd Riccobene, Forest Laboratories, unpublished data). This assumption is reasonable given that no ceftaroline fosamil concentrations were detectable in urine. Combination additive and proportional residual error models were used for both ceftaroline fosamil and ceftaroline.
FIG 2.
Final, five-compartment population pharmacokinetic model describing ceftaroline fosamil and ceftaroline pharmacokinetics in plasma. R0 represents the zero-order i.v. infusion. “X” represents the amount of drug in the respective compartment. “V” represents the volume of the respective compartment. k13, k12, k21, k34, k43, k35, and k53 represent microtransfer rate constants. CLT represents total body clearance. CMT, compartment. *fm represents the fraction of ceftaroline fosamil converted to ceftaroline and was assumed to be 1.
TABLE 3.
Final population pharmacokinetic model for ceftaroline fosamil and ceftaroline: parameter estimates and standard errorsa
| Parameterb | Mean | %RSE | SD | %CV |
|---|---|---|---|---|
| V1 (liters) | 17.9 | 47.1 | 9.49 | 52.9 |
| k13 (h−1) | 15.4 | 24.6 | 5.49 | 35.7 |
| k12 (h−1) | 1.02 | 47.5 | 0.425 | 41.5 |
| k21 (h−1) | 1.58 | 82.6 | 0.219 | 13.9 |
| V3 (liters)* | 17.2 | |||
| TBW power | 0.552 | 8.08 | ||
| CLT (liters/h)† | 16.4 | |||
| eCLCR intercept | 7.51 | 33.8 | ||
| eCLCR slope | 3.82 | 68.5 | ||
| k34 (h−1) | 0.401 | 38.3 | 0.0506 | 12.6 |
| k43 (h−1) | 0.581 | 11.7 | 0.0623 | 10.7 |
| k35 (h−1) | 1.72 | 58.5 | 0.266 | 15.5 |
| k53 (h−1) | 2.81 | 37.0 | 0.380 | 13.5 |
| σ2Add-CPTF | −0.0134 | 20.9 | ||
| σ2Prop-CPTF | 0.259 | 21.4 | ||
| σ2Add-CPT | 0.000930 | 909.0 | ||
| σ2Prop-CPT | 0.0551 | 8.97 |
%RSE, relative standard error; %CV, coefficient of variation.
V1, volume of distribution for ceftaroline fosamil (CPTF) central compartment; k13, rate constant from CPTF central compartment to ceftaroline (CPT) central compartment; k12, microtransfer rate constant from CPTF central to CPTF peripheral compartment; k21, microtransfer rate constant from CPTF peripheral to CPTF central compartment; V3, volume of distribution for CPT central compartment; TBW, total body weight; CLT, total body clearance of CPT; eCLCR, estimated creatinine clearance; k34, microtransfer rate constant from CPT central to first CPT peripheral compartment; k43, microtransfer rate constant from first CPT peripheral to CPT central compartment; k35, microtransfer rate constant from CPT central to second CPT peripheral compartment; k53, microtransfer rate constant from second CPT peripheral to CPT central compartment; σ2Add-CPTF, additive error for ceftaroline fosamil; σ2Prop-CPTF, proportional error for ceftaroline fosamil; σ2Add-CPT, additive error for ceftaroline; σ2Prop-CPT, proportional error for ceftaroline. *, Population mean V3 = TBW0.552; †, population mean CLT = 7.51 + 3.82 × (eCLCR/126).
Covariate analysis was performed on the primary descriptors of ceftaroline plasma pharmacokinetics, CLT and central compartment volume (V3). Following multiple linear regression, the sole significant covariates were renal function for CLT and body size for V3. Although both mCLCR and eCLCR significantly explained interindividual variability in CLT, the relationship with eCLCR was pursued for POP-PK model evaluation because of its clinical application and similar significance as mCLCR. The body size descriptors TBW, ABW, and BSA explained interindividual variability in V3. Each body size descriptor was evaluated separately in the POP-PK model. The POP-PK model CLT with eCLCR (linear function) and V3 with TBW (power function) as covariates were retained in the final POP-PK model.
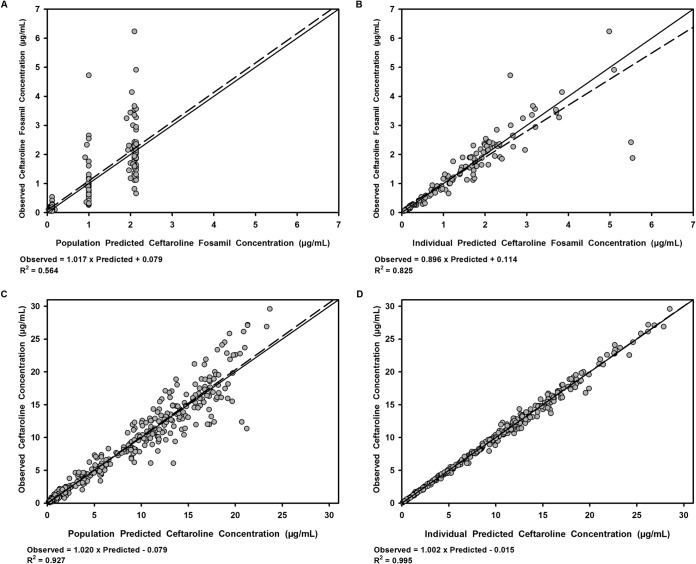
Table 3 details the pharmacokinetic parameter estimates for the final POP-PK model. Goodness-of-fit plots for ceftaroline fosamil and ceftaroline plasma concentrations fitted to the five-compartment model are displayed in Fig. 3. The observed versus population model-predicted and observed versus individual-predicted concentrations demonstrated a fair fit to ceftaroline fosamil data (R2 = 0.564 and 0.825, respectively) and more importantly an excellent fit to the active ceftaroline data (R2 = 0.927 and 0.995, respectively).
FIG 3.
Scatter and linear fit plots of observed versus population predicted (A) and observed versus individual predicted (B) concentrations of ceftaroline fosamil in plasma and of observed versus population predicted (C) and observed versus individual predicted (D) concentrations of ceftaroline in plasma. Bold dashed lines are linear fits of the data. Solid lines are lines of identity.
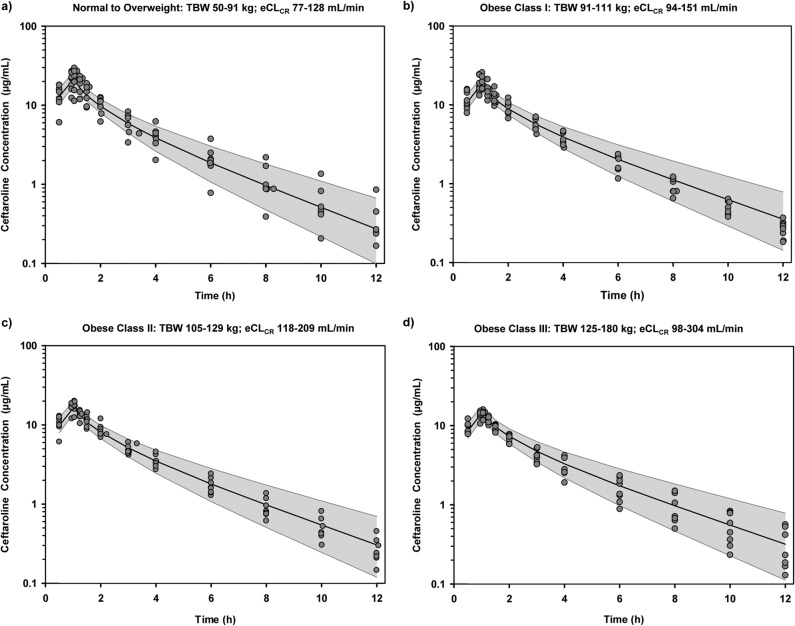
Visual predictive checks for ceftaroline in plasma stratified by each body size group of the original study population confirm that the final model represented the data well (Fig. 4). Each visual predictive check was based on a separate 9,999-subject simulation with uniform distributions of TBW and eCLCR within the specified ranges per group. The overall predicted versus observed ceftaroline concentrations (means ± the standard deviations [SD]) surrounding the end of the 1-h infusion were 16.8 ± 3.4 μg/ml at 0.95 h and 17.3 ± 3.3 μg/ml at 1.05 h. The predicted versus observed Cmin of ceftaroline were 0.39 ± 0.32 μg/ml at 12 h. The AUC estimates for the final POP-PK model were based on the 9,999 subject simulation with covariate distributions mirroring those of the original 32 study subjects. For ceftaroline, the final model based simulation predicted AUC0–12 and AUC0–inf were 44.3 ± 8.9 μg·h/ml and 45.9 ± 10.2 μg·h/ml, respectively, that were equivalent to those estimated via noncompartmental analysis (Table 2).
FIG 4.
Visual predictive checks of ceftaroline concentrations (log scale) in plasma stratified by body size group: normal to overweight (A), obese class I (B), obese class II (C), and obese class III (D). Shaded region indicates 90% prediction interval of ceftaroline concentration over time. Solid black line indicates median predicted ceftaroline concentration over time. Solid gray circles indicate observed ceftaroline concentrations over time.
Probability of target attainment.
A Monte Carlo simulation of 9,999 subjects was performed to estimate the % fT>MIC for ceftaroline in each simulated subject at steady state following six 600-mg doses of CPTF given i.v. every 12 h (in 1-h infusions). Covariate values were uniformly distributed across a prespecified range (TBW, 50 to 180 kg; eCLCR, 20 to 200 ml/min). These ranges were based on those of the original study population, with the exception of eCLCR, whose range extended to 20 ml/min (below the minimum eCLCR of 77.3 ml/min). These additional values of eCLCR were included to fully explore the effect of renal function on ceftaroline exposure. Only data for an eCLCR of >50 ml/min are presented here since this is the recommended lower limit of renal function for the full U.S. Food and Drug Administration (FDA)-approved dosage of 600 mg i.v. every 12 h (Forest Pharmaceuticals, Inc.). As a consequence, this represents a subset of the 9,999 simulated subjects (n = 8,344) with a TBW of 50 to 180 kg and an eCLCR of 50 to 200 ml/min.
The simulations predicted a ≥90% probability of achieving 30, 40, and 50% fT>MIC when isolates have an MICs of 2, 1, and 0.5 μg/ml, respectively. For the MIC distributions from clinical respiratory isolates collected from 2009 to 2011, the cumulative fractions of response for select microorganisms at various exposure targets are summarized in Table 4. The CFRs were well above 90% with all exposure targets for MSSA, S. pneumoniae, and non-ESBL-producing E. coli and Klebsiella spp. For MRSA, the cumulative fractions of response were 95.7 and 93.8% for the 30 and 40% fT>MIC targets, respectively. An 87.5% cumulative fraction of response was predicted for the 50% fT>MIC target against the evaluated MRSA MIC distribution.
TABLE 4.
Cumulative fractions of response for ceftaroline against select microorganisms from respiratory isolates collected from 2009 to 2011
| Microorganisma (no. of isolates) | CFR (%)b |
||
|---|---|---|---|
| 30% fT>MIC | 40% fT>MIC | 50% fT>MIC | |
| S. aureus (1,087) | 99.9 | 99.0 | 95.6 |
| MSSA (555) | 100.0 | 100.0 | 99.8 |
| MRSA (532) | 95.7 | 93.8 | 87.5 |
| S. pneumoniae (3,360) | 100.0 | 100.0 | 100.0 |
| Non-ESBL E. coli (178) | 96.8 | 94.9 | 93.6 |
| Non-ESBL Klebsiella spp. (479) | 99.3 | 98.8 | 97.8 |
MSSA, methicillin-susceptible S. aureus; MRSA, methicillin-resistant S. aureus; ESBL, extended-spectrum β-lactamase.
CFR, cumulative fraction of response; 30, 40, and 50% fT>MIC, percentage(s) of time that free drug concentrations exceeded the MIC. Estimate in boldface is <90%.
Safety.
Ceftaroline fosamil was well tolerated, and no serious events were reported. Six subjects experienced a total of 8 adverse events. The majority of AEs were mild (n = 7) with the most common being rash in the antecubital region where adhesive tape for the peripheral i.v. was placed (n = 3). Other mild AEs included headache, nausea, one episode of loose stool, and ecchymosis in the antecubital region (n = 1 per AE). One subject in obese class III experienced a moderate AE reported as an elevation in the AST/ALT from the baseline. Peak measurements were approximately 8 and 4 times the baseline values, respectively, at 4 weeks after study drug administration. The subject was clinically stable with no other significant signs or symptoms upon medical evaluation. Elevated hepatic enzymes returned to baseline following discontinuation of the concomitant medication, an oral hormonal combination drug (cyproterone/ethinyl estradiol). All AEs were classified as possibly related to the study drug, with the exception of nausea, which was probably related, and headache, which was not related due to a strong temporal relationship with significant caffeine consumption that occurred >12 h after the study drug administration.
DISCUSSION
Use of fixed antimicrobial doses irrespective of body size may increase the risk for inadequate antimicrobial exposure and decreased efficacy among obese patients (9–11). Body size is one of several covariates that may explain interindividual variability of antimicrobial pharmacokinetics, but the ability to discern the independent relationship of this covariate is not straightforward. As an example, pharmacokinetic analyses commonly identify estimated renal function as a parameter predictive of antimicrobial clearance (CL), especially when a drug such as ceftaroline is eliminated unchanged in urine (25). The evaluation of renal function is often based on eCLCR, which is a composite parameter of age, sex, SCr, and body size. As expected, the ability to discern the independent relationship between body size and drug CL requires that age, sex, and SCr be narrowly distributed relative to the body size distribution. The present study was uniquely designed to evaluate the pharmacokinetics of ceftaroline in 32 healthy adult volunteers across a wide distribution of body size while matching the covariates of age, sex, race/ethnicity, and SCr of these subjects.
Our study demonstrates that ceftaroline exposure in our obese subjects appeared lower than previously published estimates in healthy subjects who were nonobese (e.g., mainly normal to overweight) (25, 26). A previous report indicates that administration of a single 600-mg dose of ceftaroline fosamil in healthy subjects with a TBW (mean ± the SD) of 70.6 ± 10.2 kg and a BMI of 26.1 ± 2.7 kg/m2 resulted in a ceftaroline Cmax of 27.9 ± 4.3 μg/ml and AUC0–∞ of 62.2 ± 8.5 μg·h/ml by noncompartmental analysis (26). These observed values were higher than the Cmax of 18.3 ± 4.6 μg/ml and AUC0–∞ 45.3 ± 10.1 μg·h/ml observed in our study group with increased TBW and BMI (n = 32, 109 ± 29.1 kg). The respective mean Cmax and AUC0–∞ values were 36 and 28% lower in obese class III subjects than in normal to overweight subjects. The decrease in Cmax with larger body size groups was consistent with the expectation of a larger volume of distribution with body size. Similarly, the observed lower AUC values with increasing body size groups was a function of a higher CLT that could be explained, in part, by a higher CLR. The mean CLR was ca. 40% higher in obese class III subjects compared to normal to overweight subjects. The observed differences among the CLR values corresponded with a similar mean difference in the mCLCR.
Approximately 50% of ceftaroline and 4% of ceftaroline-M1 was recovered in the urine in the present study compared to previously reported recovery amounts of 60 and 6%, respectively (25, 26). Our slightly lower rates of recovery are consistent with the shorter 12-h urine collection compared to the 48-h collection that occurred in that previous study. Noting the general effects of increasing body size on estimated pharmacokinetic parameters with noncompartmental analysis, a more robust description of pharmacokinetics for ceftaroline was then defined by a POP-PK model.
A five-compartment model with linear elimination best described the data for ceftaroline fosamil and ceftaroline. This final POP-PK model was relatively similar to a previously published POP-PK model developed with ceftaroline pharmacokinetic data from clinical trials, albeit with some notable differences (27–30). The previous model utilized three and two compartments for ceftaroline fosamil and ceftaroline, respectively; parallel linear and Michaelis-Menten elimination pathways for ceftaroline; and additional covariates (age, gender, and phase 2/3 patient status). These structural model differences are due to the significantly larger, more heterogeneous data set used for model development, which included hundreds of subjects (both healthy volunteers and patients), ceftaroline fosamil doses ranging from 50 to 2,000 mg, and both i.v. and intramuscular drug administration.
In the previous model, the major determinant of variability in ceftaroline pharmacokinetics and the only covariate requiring dosage adjustment was eCLCR (27). Although the present POP-PK model utilized a linear relationship, the initial log-log regression of eCLCR on CLT estimated a β-exponent of 0.398 and the model from Van Wart et al. estimated β-exponents of 0.441 and 0.343 for intrinsic and linear CL, respectively. These comparable estimates suggest the underlying relationships between eCLCR and ceftaroline CL in each data set are similar.
The present model included the additional significant covariate of TBW on V3. Similar power relationships between body size descriptors and the volume of distribution of ceftaroline (TBW with β = 0.46; BSA with β = 1.35) have been reported in previous POP-PK analyses based on earlier clinical trial data sets (27, 31). The inclusion of this covariate in the present model is likely due to the wide, stratified distribution of body size descriptors in our study. Such a design has been suggested to increase the power to detect true covariate relationships (32).
Monte Carlo simulations with the present POP-PK model suggest increasing body size and/or renal function has minimal effect on target attainment at most MICs. Although lower single-dose exposures were observed in obese class III subjects, no dosage adjustment of ceftaroline fosamil is predicted based on multiple-doses in this study population. The CFRs estimated here, as well as those previously published, suggest that the current FDA-approved regimen of ceftaroline fosamil administered at 600 mg i.v. every 12 h as a 1-h infusion should provide adequate target exposure for the majority of clinical isolates of MSSA, S. pneumoniae, and non-ESBL-producing E. coli or Klebsiella spp. encountered in the United States (28–30). This regimen also appears to be relatively safe with no serious AEs reported in this study. This regimen is likely reliable for mild to moderate infections secondary to MRSA (CFR 95.7% for the stasis target of 30% fT>MIC). Consideration of a higher target of 50% fT>MIC for MRSA may support more aggressive dosing (e.g., every 8 h) and/or combination therapy for serious, deep-seated infections, but this requires further study.
A major strength of the present study is its underlying design. Stratification ensured even distribution of subject enrollment across a wide range of covariates of interest. This allowed for detailed covariate analysis and likely increased study power to identify true parameter-covariate relationships. In addition, matching of other known covariates (e.g., age, sex, serum creatinine, race, and ethnicity) helped minimize confounding, although matched covariates were still considered for inclusion in the model. Ample sampling of plasma ceftaroline concentrations also allowed for a relatively good fit of the structural POP-PK model even before the inclusion of covariates (Fig. 3).
Although our data represent a reasonable first step in exploring the complex relationships between body size, renal function, and ceftaroline CLT, some limitations should be considered. First, the study here enrolled healthy subjects who typically exhibit decreased pharmacokinetic variability and lower CLT levels compared to infected patients. Previous POP-PK models suggest that subjects in ceftaroline phase 2/3 studies had a 35% higher mean CLT than those in phase 1 studies (27–30). Given this background, obese class III adults who are acutely infected may theoretically have a higher CLT than that predicted by our model. As a consequence, our findings require validation in an obese patient population, including those with renal insufficiency who were not included here. Also, we assumed plasma protein binding to be 20%. It is unclear how altered protein binding in patients may affect the pharmacokinetics of ceftaroline. Additional considerations include a limited ability to evaluate the effect of matched covariates, single dose administration requiring extrapolation of concentration data at steady state, 12-h data collection, and reliance on 12-h mCLCR instead of a 24-h urine collection.
In conclusion, the pharmacokinetics of ceftaroline fosamil and ceftaroline in subjects who ranged from normal weight to obese were best described by a five-compartment, linear population pharmacokinetic model, including eCLCR and TBW as covariates. Despite significantly decreased Cmax and AUC0–∞ and increased CLT and V3 with increasing body size, target attainment data suggest that no dosage adjustment for ceftaroline appears necessary based on body weight alone in healthy subjects with comparable renal function. Our findings suggest that ceftaroline plasma exposures are lower in obese class III compared to normal to overweight adults after a single dose. Model based predictions of multiple doses indicate adequate achievement of target attainment for this antimicrobial with time-dependent pharmacodynamics. No signal of worse outcomes in obese patients compared to nonobese patients treated with standard doses of ceftaroline fosamil for ABSSSI has been reported to date. Despite this, confirmation of these pharmacokinetic findings for ceftaroline in obese patients will permit appropriate translation of our model-based predictions.
ACKNOWLEDGMENTS
We thank Ronald Jones and Robert Flamm for the generous provision of MIC distribution data, Alan Forrest and Scott Van Wart for guidance and expertise with population modeling and simulation, and the staff of the Clinical Interface Core at the Clinical Research Center within the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science whose contributions were instrumental in the conduct and completion of this study.
This project was an investigator-initiated study supported in part by a grant from Forest Laboratories, Inc. The project utilized services and facilities of the UIC Center for Clinical and Translational Science which is supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through grant UL1TR000050.
The content is solely the responsibility of the author and does not necessarily represent the official views of the National Institutes of Health.
M.M.S. is currently an employee of Actavis. L.H.D. and K.A.R. serve on advisory boards and speaker bureau of Forest Laboratories, Inc.
REFERENCES
- 1.Rodvold KA, McConeghy KW. 2014. Methicillin-resistant Staphylococcus aureus therapy: past, present, and future. Clin Infect Dis 58(Suppl 1):S20–S27. doi: 10.1093/cid/cit614. [DOI] [PubMed] [Google Scholar]
- 2.Merker A, Danziger LH, Rodvold KA, Glowacki RC. 2014. Pharmacokinetic and pharmacodynamics evaluation of ceftaroline fosamil. Expert Opin Drug Metab Toxicol 10:1741–1750. doi: 10.1517/17425255.2014.972932. [DOI] [PubMed] [Google Scholar]
- 3.Zhanel GG, Sniezek G, Schweizer F, Zelenitsky S, Lagace-Wiens PR, Rubinstein E, Gin AS, Oban DJ, Karlowsky JA. 2009. Ceftaroline: a novel broad-spectrum cephalosporin with activity against methicillin-resistant Staphylococcus aureus. Drugs 69:809–831. doi: 10.2165/00003495-200969070-00003. [DOI] [PubMed] [Google Scholar]
- 4.Ogden CL, Carroll MD, Kit BK, Flegal KM. 2014. Prevalence of childhood and adult obesity in the United States, 2011-2012. JAMA 311;806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamgbade OA, Rutter TW, Nafiu OO, Dorje P. 2007. Postoperative complications in obese and nonobese patients. World J Surg 31:556–560. doi: 10.1007/s00268-006-0305-0. [DOI] [PubMed] [Google Scholar]
- 6.Bochicchio GV, Buchicchio JM, Nehman S, Tracy JK, Scalea TM. 2006. Impact of obesity in the critically ill trauma patient: a prospective study. J Am Coll Surg 203:533–538. doi: 10.1016/j.jamcollsurg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Falagas ME, Athanasoulia AP, Peppas G, Karageorgopoulos DE. 2009. Effect of body mass index on the outcome of infections: a systematic review. Obes Rev 10:280–289. doi: 10.1111/j.1467-789X.2008.00546.x. [DOI] [PubMed] [Google Scholar]
- 8.Longo C, Bartlett G, Macgibbon B, Mayo N, Rosenberg E, Nadeau L, Daskalopoulou SS. 2013. The effect of obesity on antibiotic treatment failure: a historical cohort study. Pharmacoepidemiol Drug Saf 22:970–976. doi: 10.1002/pds.3461. [DOI] [PubMed] [Google Scholar]
- 9.Wurtz R, Itokazu G, Rodvold K. 1997. Antimicrobial dosing in obese patients. Clin Infect Dis 25:112–118. doi: 10.1086/514505. [DOI] [PubMed] [Google Scholar]
- 10.Pai MP, Bearden DT. 2007. Antimicrobial dosing considerations in obese adult patients: insights from the Society of Infectious Diseases Pharmacists. Pharmacotherapy 27:1081–1091. doi: 10.1592/phco.27.8.1081. [DOI] [PubMed] [Google Scholar]
- 11.Jain R, Chung SM, Jain L, Khurana M, Lau SWJ, Lee JE, Vaidyanathan J, Zadenzensky I, Choe S, Sahajwalla CG. 2011. Implications of obesity for drug therapy: limitations and challenges. Clin Pharmacol Ther 90:77–89. doi: 10.1038/clpt.2011.104. [DOI] [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.D'Argenio DZ, Schumitzky A, Wang X. 2009. ADAPT 5 user's guide: pharmacokinetic/pharmacodynamic systems analysis software. Biomedical Simulations Resource, Los Angeles, CA. [Google Scholar]
- 14.Akaike H. 1979. Bayesian extension of the minimum AIC procedure of autoregressive model fitting. Biometrika 66:237–242. doi: 10.1093/biomet/66.2.237. [DOI] [Google Scholar]
- 15.Bergstrand M, Hook AC, Wallin JE, Karlsson MO. 2011. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13:143–151. doi: 10.1208/s12248-011-9255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devine BJ. 1974. Gentamicin therapy. Drug Intell Clin Pharm 8:650–655. [Google Scholar]
- 17.Bauer LA, Edwards WA, Dellinger EP, Simonowitz DA. 1983. Influence of weight on aminoglycoside pharmacokinetics in normal weight and morbidly obese patients. Eur J Clin Pharmacol 24:643–647. doi: 10.1007/BF00542215. [DOI] [PubMed] [Google Scholar]
- 18.Janmahasatian S, Duffull SB, Ash S, Ward LC, Byrne NM, Green B. 2005. Quantification of lean body weight. Clin Pharmacokinet 44:1051–1065. doi: 10.2165/00003088-200544100-00004. [DOI] [PubMed] [Google Scholar]
- 19.NHLBI Obesity Education Initiative Expert Panel on the Identification Evaluation and Treatment of Obesity in Adults (US). 1998. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults: the evidence report. National Heat, Lung, and Blood Institute, Bethesda, MD: http://www.ncbi.nlm.nih.gov/books/NBK2003/. [Google Scholar]
- 20.Mosteller RD. 1987. Simplified calculation of body-surface area. N Engl J Med 317:1098. [DOI] [PubMed] [Google Scholar]
- 21.Andes D, Craig WA. 2006. Pharmacodynamics of a new cephalosporin, PPI-0903 (TAK-599), active against methicillin-resistant Staphylococcus aureus in murine thigh and lung infection models: identification of an in vivo pharmacokinetic-pharmacodynamic target. Antimicrob Agents Chemother 50:1376–1383. doi: 10.1128/AAC.50.4.1376-1383.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacGowan AP, Noel AR, Tomaselli S, Bowker KE. 2013. Pharmacodynamics of ceftaroline against Staphylococcus aureus studied in an in vitro pharmacokinetic model of infection. Antimicrob Agents Chemother 57:2451–2456. doi: 10.1128/AAC.01386-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forest Pharmaceuticals, Inc. 2013. Teflaro (ceftaroline fosamil) injection for intravenous use: prescribing information. Forest Pharmaceuticals, Inc., St. Louis, MO. [Google Scholar]
- 24.Mouton JW, Dudley MN, Cars O, Derendorf H, Drusano GL. 2005. Standardization of pharmacokinetic/pharmacodynamics (PK/PD) terminology for anti-infective drugs: an update. J Antimicrob Chemother 55:601–607. doi: 10.1093/jac/dki079. [DOI] [PubMed] [Google Scholar]
- 25.Riccobene T, Jakate A, Rank D. 2014. A series of pharmacokinetic studies of ceftaroline fosamil in select populations: normal subjects, healthy elderly subjects, and subjects with renal impairment or end-stage renal disease requiring hemodialysis. J Clin Pharmacol 54:742–752. doi: 10.1002/jcph.265. [DOI] [PubMed] [Google Scholar]
- 26.Riccobene TA, Su SF, Rank D. 2013. Single- and multiple-dose study to determine the safety, tolerability, and pharmacokinetics of ceftaroline fosamil in combination with avibactam in healthy subjects. Anitmicrob Agents Chemother 57:1496–1504. doi: 10.1128/AAC.02134-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van Wart SA, Forrest A, Khariton T, Rubino CM, Bhavnani SM, Reynolds DK, Riccobene T, Ambrose PG. 2013. Population pharmacokinetics of ceftaroline in patients with acute bacterial skin and skin structure infections or community-acquired bacterial pneumonia. J Clin Pharmacol 53:1155–1167. doi: 10.1002/jcph.153. [DOI] [PubMed] [Google Scholar]
- 28.Bhavnani SM, Hammel JP, Van Wart SA, Rubino CM, Reynolds DK, Forest A, Khariton T, Friedland HD, Riccobene TA, Ambrose PG. 2013. Pharmacokinetic-pharmacodynamic analysis for efficacy of ceftaroline fosamil in patients with community-acquired bacterial pneumonia. Antimicrob Agents Chemother 57:6348–6350. doi: 10.1128/AAC.01748-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Wart SA, Ambrose PG, Rubino CM, Khariton T, Riccobene TA, Friedland HD, Critchley IA, Bhavnani SM. 2014. Pharmacokinetic-pharmacodynamic target attainment analysis to evaluate in vitro susceptibility test interpretive criteria for ceftaroline against Staphylococcus aureus and Streptococcus pneumoniae. Antimicrob Agents Chemother 58:885–891. doi: 10.1128/AAC.01680-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhavnani SM, Hammel JP, Van Wart SA, Rubino CM, Reynolds DK, Forest A, Drusano GL, Khariton T, Friedland HD, Riccobene TA, Ambrose PG. 2015. Pharmacokinetic-pharmacodynamic analysis for efficacy of ceftaroline fosamil in patients with acute bacterial skin and skin structure infections. Antimicrob Agents Chemother 59:372–380. doi: 10.1128/AAC.02531-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ge Y, Liao S, Talbot GH. 2007. Population pharmacokinetics (PK) analysis of ceftaroline (CPT) in volunteers and patients with complicated skin and skin structure infections (cSSSI). Abstr 47th Intersci Conf Antimicrob Agents Chemother, abstr A-34. [Google Scholar]
- 32.Han PY, Kirkpatrick CJ, Green B. 2009. Informative study designs to identify true parameter-covariate relationships. J Pharmacokinet Pharmacodyn 36:147–163. doi: 10.1007/s10928-009-9115-y. [DOI] [PubMed] [Google Scholar]