Abstract
As the number of antibacterial medicines in the pipeline remains low, we anonymously surveyed pharmaceutical industry professionals on challenges and solutions for clinical development of these agents. Challenges were reported primarily as financial and regulatory. For multidrug-resistant organisms, there are needs for rapid diagnostic tests, new regulatory guidance, and adaptation of endpoints/trial designs. Regulators and public/private initiatives are addressing these challenges to help ensure that proposed solutions have the support of all involved stakeholders.
COMMENTARY
While resistance to the available arsenal of antibacterial agents is rising at an alarming rate (1), the number of new antibacterials in research and development (R&D) remains low (2, 3). The identification and a clear understanding of challenges hampering the clinical development of new antibacterial medicines are essential to develop and propose targeted solutions that will be implemented by pharmaceutical companies. Limited published information regarding the opinions of industry professionals on the topic is available (4–10). To fill this information gap, we surveyed professionals in the private sector working on the clinical development of new antibacterial agents. For methods and additional survey results, see the supplemental material.
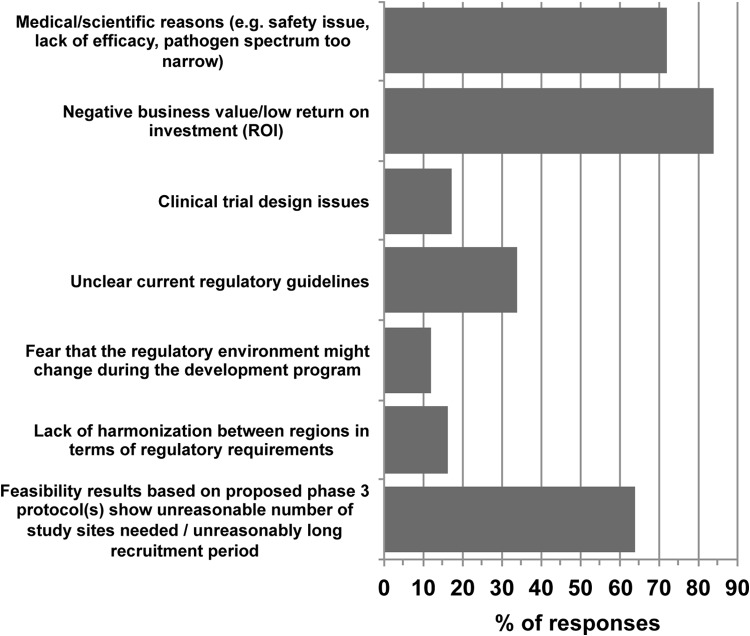
Sixty respondents (Table 1) entered the survey, and 82% (n = 49) completed it. Overall, financial issues came forward as the main challenge facing pharmaceutical companies developing antibacterial agents. The most frequent reason for stopping clinical development (Fig. 1) was reported to be due to low return on investment (ROI) (84%). This is no surprise, as the economics of antibacterial R&D is not favorable today and is one of the main reasons for the desertion of this therapeutic area by companies since the 1990s (11). Interestingly, the UK Review on Antimicrobial Resistance formed in 2014 recently published a survey of the pharmaceutical industry that focused mostly on financial barriers to antibacterial R&D (http://amr-review.org/sites/default/files/Surveyofactors.pdf), which also showed financial issues as being the main barrier to investment. To decrease financial challenges, the 2012 GAIN act in the United States defined incentives in the form of a 5-year additional market exclusivity, priority review, and fast track approval for new antibacterials that have obtained Qualified Infectious Disease Product (QIDP) designation. This designation can be obtained by new antibacterials targeting a defined list of pathogens that are considered a serious threat to public health and therefore would fulfill high unmet medical needs (12). Although there is discussion around whether this measure really fosters true innovation, 46 QIDP designations have been awarded to 32 compounds since 2012 (13). Interestingly, respondents supported clinical trial design options that would not result in the lowest clinical development costs. These options include prospectively gathering external control data for pathogen-based trials (versus the use of already-available historical control data) or gathering postmarketing surveillance data subsequent to acquisition of regulatory approval based on limited clinical data.
TABLE 1.
Survey respondents' main area of expertise and years of experience in antibacterial R&D
| Category of respondents | % (no.) of respondents |
|---|---|
| Main area of expertise | |
| Clinical development/medical affairs | 47 (28) |
| Preclinical development | 17 (10) |
| Research | 13 (8) |
| Regulatory affairs | 8 (5) |
| Clinical operations/clinical trial management | 5 (3) |
| Biostatistics | 5 (3) |
| Pharmacometrics | 2 (1) |
| Human pharmacology | 2 (1) |
| Business development | 2 (1) |
| No. of yrs of experience in antibacterial R&D | |
| ≤1 | 2 (1) |
| 2 to 5 | 27 (16) |
| 6 to 10 | 8 (5) |
| >10 | 63 (38) |
| Respondents experience with regulatory environments | |
| FDA | 95 (57) |
| EMA | 93 (56) |
| Othera | 25 (15) |
Pharmaceuticals and Medical Devices Agency (PMDA), Japan, n = 8; China Food and Drug Administration (CFDA), n = 6; Therapeutic Goods Administration (TGA), Australia, n = 5; Health Canada, n = 5; Health Sciences Authority, Singapore, n = 2; Africa, n = 2; South America, n = 1; Asia, n = 1; European Union member state regulatory agencies, n = 1.
FIG 1.
Response to the question “what are the three most frequent reasons for stopping the clinical development of new antibacterial agents?” n = 58.
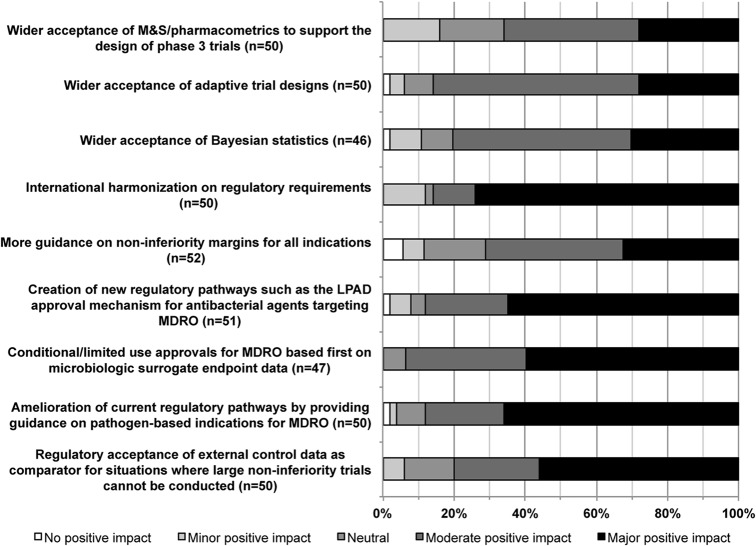
To adapt to the high unmet medical need caused by infections due to multidrug-resistant organisms (MDRO), the regulations for antibacterial R&D have been changing at a fast pace in the last few years; the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) updated their guidance for development of antibacterial medicines in case of high unmet medical need (14–16). Nevertheless, based on our survey, which was conducted after the release of the FDA guideline, the current regulations are still perceived as a hurdle, and more regulatory changes are needed. When asked about changes in regulatory pathways that would have a positive impact in simplifying the path to approval (Fig. 2), 74% of respondents indicated that harmonizing international regulatory requirements would have a major positive impact. Over 50% of respondents also rated as high impact the four following changes: creation of new regulatory pathways, provision of regulatory guidance on pathogen-based indications for MDRO, conditional/limited-use approvals for MDRO based first on microbiologic surrogate endpoint data, and regulatory acceptance of external control data. According to this survey, new approval pathways based on limited clinical data, such as the limited-population antibacterial drug (LPAD) pathway (17), would be well received by pharmaceutical companies, which also suggests that some pharmaceutical companies may envision stepping away from the current antibacterial-medicine business model based on sales volume.
FIG 2.
Opinions on the positive impact that changes in regulatory pathways and guidance might have on the successful development of new antibacterial agents. MDRO, multidrug-resistant organism; LPAD, limited-population antibacterial drug; M&S, modeling and simulation.
Following financial and regulatory challenges, the design and implementation of more-effective clinical development programs, including pathogen-based trials, were identified as challenging. As pathogen-based trials differ from the traditional large indication-based phase 3 randomized controlled trials, new trial designs (i.e., new clinical endpoints, new control group definitions) will have to be agreed upon between regulators and pharmaceutical companies. Regarding the design of such trials, the most favorable opinions went to single-arm trials supported by external control data gathered prospectively (78%), while the least-favored design option was represented by superiority trials (Fig. 3). The updated regulatory guidance mentions testing for superiority as an option to assess. However, situations in which such trials would be ethical and clinically important are still a matter of debate (14, 17). Two alternatives that have been discussed were met with a more favorable response: 53% of respondents agreed or strongly agreed with the concept of noninferiority trials with alternative statistical criteria (e.g., with relaxation of the type I error rate) as a feasible pathway to registration, and 61% agreed with the concept of indication-based trials where patients infected with MDRO are a subset. In terms of the required sample size as well as endpoints and hypothesis tested, there seems to be a need for flexibility, which is currently reflected in the guidance. Equally important is the general assumption that in noninferiority trials, the new drug will be tested in line with International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) guidelines against an approved standard of care, regardless of whether the control group is an arm of that same trial or is composed of external data gathered retrospectively or prospectively. Nevertheless, at the end of the day, new trial designs need to be operationally feasible and cost-effective while providing sufficient and quality data for regulators to make informed decisions. Innovative approaches, such as Bayesian statistics-based adaptive designs, pharmacokinetic/pharmacodynamic modeling, and the wider use of pharmacometrics, have limited support from the survey respondents since they seem to still be perceived as having a higher risk of delivering unsuccessful trials than traditional frequentist approaches.
FIG 3.
Opinions on study design for registration trials assessing safety and efficacy of new antibacterial agents for infections caused by MDRO.
Finally, regarding operational aspects, the lack of rapid diagnostic tests is a crucial R&D gap both for sensitive and MDRO infections. Indeed, even with streamlined development programs for MDRO, the absence of rapid diagnostic tests will still induce the recruitment of a high number of patients, only a small fraction of whom will be infected with the MDRO of interest. Development of rapid diagnostic tests for Gram-negative MDRO remains challenging (18), but two recent initiatives promote innovation in this field: the 2014 United Kingdom Longitude prize (http://www.longitudeprize.org/) and a new prize announced by the U.S. President in September 2014 (19).
Regarding our survey's limitations, the overall response rate was not assessable, as we had to rely on primary contacts within companies to distribute the survey. Second, we cannot guarantee that the opinions of this limited sample of respondents are representative of the whole pharmaceutical industry or of groups with different areas of expertise. Finally, our survey was conducted on the heels of the draft FDA guidance (15) release but shortly before the final EMA addendum was made public (16). This timing might have influenced respondents' answers.
Refueling the antibacterial pipeline will imply innovative R&D, regulatory pathways, and economic models (20) and is the aim of several ongoing initiatives. In 2013, the Biomedical Advanced Research and Development Authority (BARDA) (http://www.phe.gov/about/barda/Pages/default.aspx) initiated programs to provide public funds for R&D of agents against MDRO, and the Innovative Medicines Initiative (IMI) launched the New Drugs for Bad Bugs (ND4BB) program in the European Union (http://www.imi.europa.eu/content/nd4bb). ND4BB leverages the synergism of a public-private partnership to boost efforts in all R&D areas for antibacterial agents (i.e., basic research to new economic models) (21). Simplifying the design of antibacterial clinical trials is the focus of several initiatives, including the Clinical Trials Transformation Initiative (CTTI) (http://www.ctti-clinicaltrials.org/what-we-do/ctti-project-categories/ab-drug-development), the Foundation for the National Institute of Health (FNIH) (22), and the Combating Bacterial Resistance in Europe (COMBACTE) consortium, within the scope of which the present survey was run (https://www.combacte.com/).
In summary, the main challenges for the successful clinical development of new antibacterial medicines are rooted in financial and regulatory hurdles. Innovative trial designs were deemed important in the arsenal of tools to promote R&D of antibacterial agents, but their implementation within clinical development programs is still eclipsed by ROI concerns and an uncertain regulatory environment. For trials with antibacterial medicines active against MDRO, there is a need for improved rapid diagnostic tests, global regulatory guidance, surrogate endpoints, and alternative study designs which encompass external control data. Initiatives such as IMI's ND4BB program and others aimed at refueling the antibacterial-medicine pipeline should focus objectives on the requisite solutions to overcome the financial and regulatory challenges. Results from our survey as well as from all ongoing initiatives and public-private collaborations should help balance industry needs with regulatory requirements and ensure that proposed solutions have the support of both private and public stakeholders.
Supplementary Material
ACKNOWLEDGMENTS
The research leading to these results received support from the Innovative Medicines Initiative Joint Undertaking under grant agreement 115523 (COMBACTE), resources of which are composed of financial contribution from the European Union's 7th Framework Programme (FP7/2007–2013) and European Federation of Pharmaceutical Industries and Associations (EFPIA) companies' in-kind contributions.
We thank Scott White and Seamus O'Brien for helpful comments during development of the questions, as well as the test users and the respondents for having taken the time to complete the survey. We also thank David Wilson and Aaron Dane for critically reviewing this commentary.
S.H. has received peer-reviewed research grants funded by Pfizer and B. Braun and is a member of the advisory boards of Destiny Pharma, bioMérieux, Novartis, and DaVolterra. N.S. is a full-time employee of AstraZeneca, and J.D.W. is a full-time employee of GlaxoSmithKline.
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00638-15.
REFERENCES
- 1.WHO. 2014. Antimicrobial resistance: global report on surveillance. WHO, Geneva, Switzerland. [Google Scholar]
- 2.Boucher HW, Talbot GH, Benjamin DK Jr, Bradley J, Guidos RJ, Jones RN, Murray BE, Bonomo RA, Gilbert D. 2013. 10 × ′20 progress—development of new drugs active against gram-negative bacilli: an update from the Infectious Diseases Society of America. Clin Infect Dis 56:1685–1694. doi: 10.1093/cid/cit152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kinch MS, Patridge E, Plummer M, Hoyer D. 2014. An analysis of FDA-approved drugs for infectious disease: antibacterial agents. Drug Discov Today 19:1283–1287. doi: 10.1016/j.drudis.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Echols RM. 2012. A long and winding road; evolution of antimicrobial drug development—crisis management. Expert Rev Anti Infect Ther 10:1311–1319. doi: 10.1586/eri.12.131. [DOI] [PubMed] [Google Scholar]
- 5.Gwynn MN, Portnoy A, Rittenhouse SF, Payne DJ. 2010. Challenges of antibacterial discovery revisited. Ann N Y Acad Sci 1213:5–19. doi: 10.1111/j.1749-6632.2010.05828.x. [DOI] [PubMed] [Google Scholar]
- 6.Rex JH, Eisenstein BI, Alder J, Goldberger M, Meyer R, Dane A, Friedland I, Knirsch C, Sanhai WR, Tomayko J, Lancaster C, Jackson J. 2013. A comprehensive regulatory framework to address the unmet need for new antibacterial treatments. Lancet Infect Dis 13:269–275. doi: 10.1016/S1473-3099(12)70293-1. [DOI] [PubMed] [Google Scholar]
- 7.Rex JH, Goldberger M, Eisenstein BI, Harney C. 2014. The evolution of the regulatory framework for antibacterial agents. Ann N Y Acad Sci 1323:11–21. doi: 10.1111/nyas.12441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spellberg B, Sharma P, Rex JH. 2012. The critical impact of time discounting on economic incentives to overcome the antibiotic market failure. Nat Rev Drug Discov 11:168. doi: 10.1038/nrd3560-c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tomayko JF, Rex JH, Tenero DM, Goldberger M, Eisenstein BI. 2014. The challenge of antimicrobial resistance: new regulatory tools to support product development. Clin Pharmacol Ther 96:166–168. doi: 10.1038/clpt.2014.107. [DOI] [PubMed] [Google Scholar]
- 10.Bax R, Green S. 2015. Antibiotics: the changing regulatory and pharmaceutical industry paradigm. J Antimicrob Chemother 70:1281–1284. doi: 10.1093/jac/dku572. [DOI] [PubMed] [Google Scholar]
- 11.Projan SJ. 2003. Why is big Pharma getting out of antibacterial drug discovery? Curr Opin Microbiol 6:427–430. doi: 10.1016/j.mib.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Electronic Code of Federal Regulations. 2014. Title 21. Food and drugs. Chapter 1. Food and Drug Administration. Subchapter D. Drugs for human use. Part 317. List of qualifying pathogens that have the potential to pose a serious threat to public health. 21 CFR 317.2 http://www.ecfr.gov/cgi-bin/retrieveECFR?gp=1&SID=8508abd4d5a913bee24de949bb1920d2&ty=HTML&h=L&r=PARTvn=pt21.5.317 Accessed 19 May 2015. [Google Scholar]
- 13.Keener AB. 2014. First QIDP drug approved, but designation may fail urgent needs. Nat Med 20:690–691. doi: 10.1038/nm0714-690. [DOI] [PubMed] [Google Scholar]
- 14.Nambiar S, Laessig K, Toerner J, Farley J, Cox E. 2014. Antibacterial drug development: challenges, recent developments, and future considerations. Clin Pharmacol Ther 96:147–149. doi: 10.1038/clpt.2014.116. [DOI] [PubMed] [Google Scholar]
- 15.Center for Drug Evaluation and Research (CDER). 2013. Draft guidance for industry: antibacterial therapies for patients with unmet medical need for the treatment of serious bacterial diseases. UCM359184 US Department of Health and Human Services, Food and Drug Administration, Silver Spring, MD. [Google Scholar]
- 16.CHMP. 2013. Addendum to the guideline on the evaluation of medicinal products indicated for treatment of bacterial infections. EMA/CHMP/351889/2013. European Medicines Agency, London, United Kingdom. [Google Scholar]
- 17.Infectious Diseases Society of America. 2012. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis 55:1031–1046. doi: 10.1093/cid/cis688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Afshari A, Schrenzel J, Ieven M, Harbarth S. 2012. Bench-to-bedside review: rapid molecular diagnostics for bloodstream infection—a new frontier? Crit Care 16:222. doi: 10.1186/cc11202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.The White House. 2015. National action plan for combating antibiotic-resistant bacteria. The White House, Washington DC, USA. [Google Scholar]
- 20.Spellberg B, Bartlett J, Wunderink R, Gilbert DN. 2015. Novel approaches are needed to develop tomorrow's antibacterial therapies. Am J Respir Crit Care Med 191:135–140. doi: 10.1164/rccm.201410-1894OE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rex JH. 2014. ND4BB: addressing the antimicrobial resistance crisis. Nat Rev Microbiol 12:231–232. doi: 10.1038/nrmicro3245. [DOI] [Google Scholar]
- 22.Talbot GH, Powers JH, Fleming TR, Siuciak JA, Bradley J, Boucher H, CABP-ABSSSI Project Team . 2012. Progress on developing endpoints for registrational clinical trials of community-acquired bacterial pneumonia and acute bacterial skin and skin structure infections: update from the Biomarkers Consortium of the Foundation for the National Institutes of Health. Clin Infect Dis 55:1114–1121. doi: 10.1093/cid/cis566. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.