Abstract
Enterococci that are nonsusceptible (NS; MIC > 4 μg/ml) to daptomycin are an emerging clinical concern. The synergistic combination of daptomycin plus beta-lactams has been shown to be effective against vancomycin-resistant Enterococcus (VRE) species in vitro. This study systematically evaluated by in vitro time-kill studies the effect of daptomycin in combination with ampicillin, cefazolin, ceftriaxone, ceftaroline, ertapenem, gentamicin, tigecycline, and rifampin, for a collection of 9 daptomycin-NS enterococci that exhibited a broad range of MICs and different resistance-conferring mutations. We found that ampicillin plus daptomycin yielded the most consistent synergy but did so only for isolates with mutations to the liaFSR system. Daptomycin binding was found to be enhanced by ampicillin in a representative isolate with such mutations but not for an isolate with mutation to the yycFGHIJ system. In contrast, ampicillin enhanced the killing of the LL-37 human antimicrobial peptide against daptomycin-NS E. faecium with either the liaFSR or yycFGHIJ mutation. Antagonism was noted only for rifampin and tigecycline and only for 2 or 3 isolates. These data add support to the growing body of evidence indicating that therapy combining daptomycin and ampicillin may be helpful in eradicating refractory VRE infections.
INTRODUCTION
Daptomycin is a cyclic lipopeptide antimicrobial agent with bactericidal activity against Gram-positive bacteria, including Enterococcus spp. Daptomycin is not approved by the U.S. Food and Drug Administration (FDA) for the treatment of infections caused by vancomycin-resistant Enterococcus (VRE) strains (e.g., vancomycin-resistant Enterococcus faecalis) or by vancomycin-susceptible or -resistant E. faecium strains. However, due to a limited number of available therapeutic options, daptomycin is frequently used in clinical practice for treatment of serious infections caused by these bacteria. No daptomycin resistance breakpoint has been established for the enterococci by the Clinical and Laboratory Standards Institute or the U.S. FDA. Isolates with MICs above the susceptible breakpoint of 4 μg/ml are therefore referred to as daptomycin-nonsusceptible (DNS) isolates (1). The prevalence of DNS Enterococcus strains in the United States remains low, ranging from 0.02% for E. faecalis to 0.18% for E. faecium (2). Nonetheless, we and others have isolated DNS Enterococcus strains from both patients treated with and patients naive to daptomycin therapy (3–8) and much higher rates of DNS E. faecium have been reported in Europe (9).
The recent description of daptomycin-susceptible Enterococcus strains for which daptomycin exhibits only bacteriostatic activity (10, 11) brings further into question the role of daptomycin for the treatment of enterococcal infections. These isolates have decreased susceptibility to daptomycin (DSD), with daptomycin MICs ranging from 3 to 4 μg/ml, which is higher than the wild-type modal daptomycin MICs of 0.5 μg/ml for E. faecalis and 2.0 μg/ml for E. faecium (12). These isolates harbor point mutations in the liaFSR genes, which encode a three-component regulatory system involved in cell membrane stress response (10, 11). Because liaFSR mutation is thought to be one of the first events in the stepwise accumulation of genomic mutations that lead to the DNS phenotype (11, 13, 14), it is possible that the use of daptomycin for the treatment of infections caused by DSD isolates increases the risk of the organism acquiring further chromosomal mutations and DNS MICs. This concern is more than theoretical, as a recent clinical treatment failure was documented for a patient with a bloodstream infection caused by a DSD E. faecium isolate (daptomycin MIC of 3 μg/ml) that harbored T120A and W73C substitutions in LiaS and LiaR, respectively. The patient was treated with high-dose daptomycin (8 mg/kg of body weight/day) plus gentamicin (3 mg/kg/day) but had recurrent bacteremia with an E. faecium strain that eventually became DNS. Daptomycin MICs for this isolate progressed from 16 μg/ml to 256 μg/ml over the course of several months, while the patient was on daptomycin therapy (15). Supporting in vitro data from a simulated endocardial vegetation pharmacokinetic/pharmacodynamic model demonstrated that exposure of enterococci to daptomycin concentrations equivalent to FDA-cleared doses (i.e., 4 to 6 mg/kg/day) results in the development of the DNS phenotype (16).
In order to mitigate the development of DNS, a minimum dose of 10 mg/kg/day, particularly in cases with high bacterial burden, such as cases of endocarditis, has been suggested for the enterococci (16). Alternatively, combination therapy, such as with a β-lactam plus daptomycin, has been suggested for successful treatment of DSD Enterococcus infections (13, 16). The combinations of daptomycin plus ampicillin and daptomycin plus ceftaroline have been shown to enhance the activity of daptomycin against the enterococci, by improving binding to the target cytoplasmic membrane, even in ampicillin-resistant isolates (17–19). However, such synergy is not observed for all DNS Enterococcus isolates and may occur only for isolates of E. faecium with a DSD phenotype associated with mutation to the LiaFSR pathway. A second pathway to the DSD phenotype has also been previously described and is associated with mutations to the YycFGHIJ system, a second regulatory system involved in cell wall homeostasis in Gram-positive bacteria (13). Two DSD E. faecium isolates associated with mutations to yycFGHIJ did not display in vitro synergy between daptomycin and ampicillin in a recent study (13).
The intent of the present study was to further explore the in vitro killing kinetics of daptomycin against a collection of 9 DNS Enterococcus isolates with a variety of daptomycin MICs and previously identified genetic mutations conferring DSD. The daptomycin concentrations evaluated ranged from 0.5× the daptomycin MIC to 180 μg/ml, the mean total serum concentration achievable with maximal daptomycin dosing (12 mg/kg/day). In addition, as daptomycin may be combined with broad-spectrum β-lactams in critically ill hospitalized patients with DNS enterococcal infections, the effect of the combination of daptomycin with five β-lactams and three other antimicrobial agents plus a host defense peptide, LL-37, was evaluated for these isolates and was correlated with resistance mechanisms.
MATERIALS AND METHODS
Bacterial isolates.
Seven clinical isolates of E. faecium and two of E. faecalis were included in this study, all with daptomycin MICs of >4 μg/ml (Table 1). Typing of the isolates was performed as described previously, by repetitive sequence-based PCR (rep-PCR) analysis (bioMérieux, Durham, NJ), to confirm that the isolates were not clonal (3–5). Daptomycin MICs were determined by Etest (bioMérieux, Durham, NJ) on Mueller-Hinton agar according to the manufacturer's instructions, and by broth microdilution (BMD), in cation-adjusted Mueller-Hinton broth (CA-MHB; BBL, Sparks, MD) supplemented with 50 mg/liter CaCl2, on panels prepared in-house (1). Ampicillin, cefazolin, ceftriaxone, ceftaroline, ertapenem, rifampin, and tigecycline MICs were also determined by BMD, following Clinical and Laboratory Standards Institute standards (1). As expected, all isolates had high ceftriaxone, cefazolin, and ertapenem MICs (≥32 μg/ml; data not shown). High-level gentamicin resistance was determined by BMD, by the ability to grow in 500 μg/ml gentamicin in brain heart infusion (BHI) medium (BBL) (1). The minimum bactericidal concentration (MBC) of daptomycin was determined for each isolate, as described elsewhere (20), in CA-MHB supplemented with 50 mg/liter CaCl2, as the concentration of daptomycin that resulted in a ≥3 log10 reduction in CFU compared to the inoculum, after 24 h of incubation. MIC and MBC testing was performed in triplicate for each isolate, on separate testing days, and modal MIC and MBC were reported. Use of clinical isolates for this study was approved by the institutional review board of the University of California, Los Angeles (UCLA).
TABLE 1.
DNS Enterococcus isolates included in this studya
| Strain | DAP Etest MIC (μg/ml) | DAP MIC (μg/ml) | DAP MBC (μg/ml) | MIC (μg/ml) |
Susceptibility to Gent (500 μg/ml) | Predicted amino acid change(s) associated with daptomycin resistance in indicated proteins |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VAN | AMP | CPT | TIG | RIF | LiaF | LiaS | LiaR | ClsA | ClsB | ||||||
| E. faecalis | |||||||||||||||
| Efc01 | >256 | 64 | >180 | 1 | ≤2 | 4 | ≤0.25 | 4 | S | 171Idel | |||||
| Efc04 | 24 | 32 | 128 | >32 | >64 | 1 | ≤0.25 | >4 | S | 171Idel | |||||
| E. faecium | |||||||||||||||
| Efm12 | 256 | 64 | 128 | >32 | >64 | >8 | ≤0.25 | 4 | R | N251E | G53R, R215K | G174V | |||
| Efm13 | >256 | 64 | 180 | >32 | >64 | >8 | ≤0.25 | >4 | S | T120A | W73C | N23T | |||
| Efm15 | >256 | 16 | 180 | >32 | >64 | >8 | ≤0.25 | >4 | R | V38L G174V S298T | |||||
| Efm16 | >256 | 64 | 128 | >32 | >64 | >8 | ≤0.25 | >4 | R | N251E | G174V | ||||
| Efm19 | 48 | 16 | 128 | >32 | >64 | >8 | ≤0.25 | >4 | R | V38L G174V | |||||
| Efm23 | >256 | 64 | 128 | >32 | >64 | >8 | ≤0.25 | >4 | R | N251E | V38L G174V S298T | ||||
| Efm25 | >256 | 64 | >180 | >32 | >64 | >8 | ≤0.25 | >4 | S | T120A | W73C | N23T | |||
MICs and MBCs were determined by BMD unless otherwise indicated. VAN, vancomycin; AMP, ampicillin; CPT, ceftaroline; TIG, tigecycline; RIF, rifampin; Gent, gentamicin; S, susceptible; R, resistant.
Time-kill assays and synergy testing.
The bactericidal activity of daptomycin was performed by time-kill assays with an initial inoculum of 6 × 106 CFU/ml in 10 ml of CA-MHB supplemented with 50 mg/liter CaCl2. Daptomycin concentrations tested against each isolate were 0.5×, 1×, and 2× the daptomycin MIC (Table 1). In addition, each isolate was tested in the presence of 180 μg/ml daptomycin (DAP180), as 183.7 μg/ml is the mean maximum concentration of drug in serum (Cmax) reported in the CUBICIN package insert for a cohort of subjects administered 12 mg/kg/day daptomycin. Bacterial colony counts were performed at 0, 6, and 24 h, in duplicate, by removal of two 100-μl aliquots of the culture, serial dilution in sterile saline solution, and plating of 25 μl on sheep blood agar plates (BD, Sparks, MD). Preliminary experiments were performed to ensure that this method did not result in antimicrobial carryover (not shown [21]). The limit of detection for the time-kill experiments was 100 CFU/ml, assuming maximal plating efficiency. Bactericidal activity was defined as a ≥3 log10 reduction in CFU/ml at 24 h in comparison to the CFU/ml at 0 h.
Synergy testing was performed for daptomycin, at the concentrations listed above, and for eight other antimicrobials at Cmax concentrations based on pharmacokinetic studies in adults. The following concentrations were tested for the respective drugs based on routine doses given intravenously: ampicillin at 90 μg/ml to 1 to 2 g every 6 h (22); cefazolin at 185 μg/ml to 1 g every 8 h (23); ceftriaxone at 200 μg/ml to 1 g every 24 h (24), ceftaroline at 21 μg/ml to 600 mg every 12 h (25); ertapenem at 115 μg/ml to 1 g every 24 h (26); gentamicin at 25 μg/ml to 6 mg/kg daily (27); rifampin at 10 μg/ml to 600 mg every 24 h (28); and tigecycline at a loading dose of 0.8 μg/ml to 100 mg followed by 50 mg every 12 h (29). Synergy was defined as a decrease of ≥2 log10 CFU/ml in bacterial counts at 6 or 24 h for the combination, compared to the counts for the most active agent alone at the respective time point, provided that the counts for the combination were ≥2 log10 CFU/ml below the starting inoculum. Bactericidal activity of the combination was defined as a ≥3 log10 CFU/ml reduction in bacterial counts at 24 h compared to the starting inoculum (30). Antagonism was defined as an increase of ≥2 log10 CFU/ml in bacterial counts at 6 or 24 h for the combination, compared to the counts for the most active agent alone.
Mutational analysis.
Mutations in genes previously associated with DNS were evaluated by Sanger sequencing of PCR products, as described by Werth and colleagues (16). The following genes were evaluated: liaFSR, encoding a three-component regulatory system that is part of the cell envelope response to stress, and cls, which encodes cardiolipin synthetase. Sequences were compared against the genomes of E. faecalis V583 and E. faecium DO, two daptomycin-susceptible enterococcal isolates whose genomes are sequenced and publicly available.
BDP-daptomycin assays.
Tested strains were grown overnight (14 to 16 h) to stationary phase in Luria broth (LB), diluted 1:100 in fresh antibiotic-free LB or LB containing ampicillin at 50 mg/liter, grown at 37°C with shaking at 200 rpm to an optical density at 600 nm (OD600) of 0.6 (approximately 5 to 6 h), and stained for 20 min with boron-dipyrromethene (BODIPY)-labeled daptomycin (BDP-daptomycin) at 32 mg/liter (supplied courtesy of Cubist Pharmaceuticals, Lexington, MA) and with 50 mg/liter CaCl2 as previously described (18). The concentration of labeled daptomycin was established by pilot studies as optimal for fluorescence microscopy (data not shown). Excess unincorporated label was removed by washing the cells three times in antimicrobial-free LB. The cells were counterstained with 2 mg/liter DAPI (4′,6-diamidino-2-phenylindole) in the final LB wash to visualize the nucleoid and then imaged using a Delta Vision Deconvolution microscope (Applied Precision, Inc., Issaquah, WA) as previously described (18).
Human cathelicidin LL-37 killing assays.
Human cathelicidin LL-37 (net charge, +6 at pH 7.5) was purchased from AnaSpec, Inc. (Fremont, CA), and killing assays were performed at 1× MIC (2 μM) as previously described (18). Bacteria were grown overnight (14 to 16 h) in LB in the absence or presence of ampicillin at 50 μg/ml, pelleted, washed in phosphate-buffered saline (PBS), and resuspended to an OD600 of 0.5 in PBS (approximately 108 CFU/ml). Bacteria were diluted to 103 CFU/ml in RPMI medium–5% LB containing 1× MIC of LL-37 and incubated at 37°C. Aliquots (10 μl) were plated on sheep blood agar after 2 h of incubation, and colonies were enumerated after 24 h to determine the percentages of surviving bacteria (± standard deviations [SD]). Results represent experiments performed in quadruplicate.
RESULTS
Effect of daptomycin and of other antimicrobials alone against 9 DNS Enterococcus isolates.
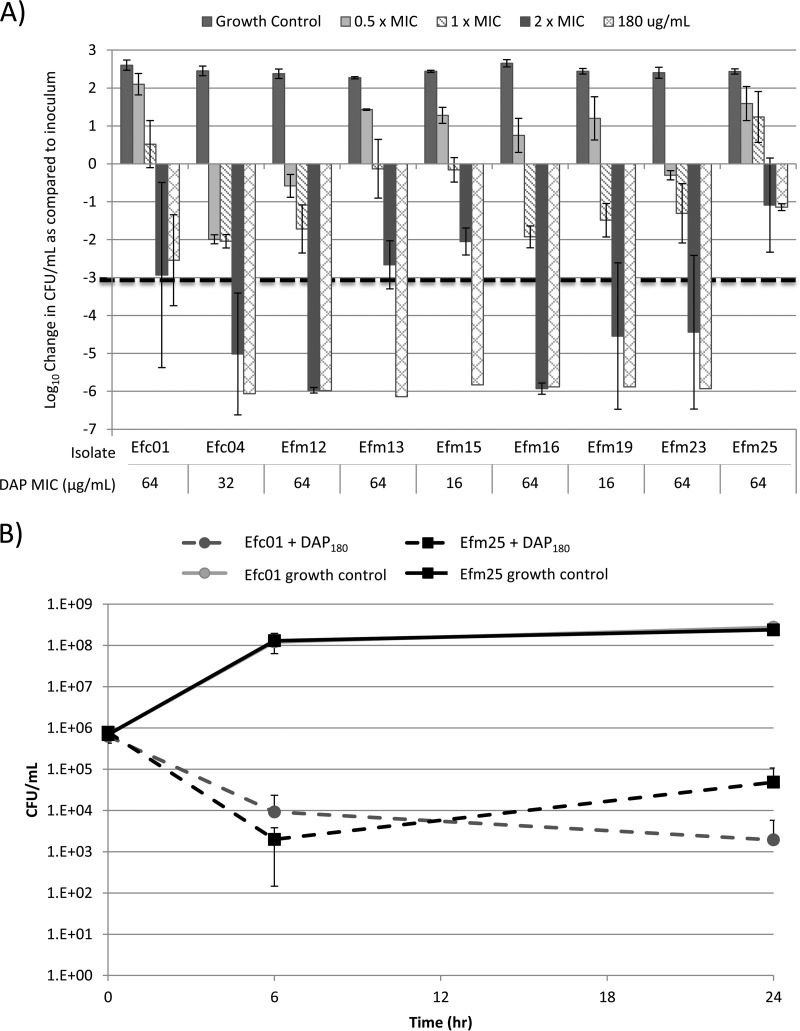
The accompanying mutations and daptomycin MICs and MBCs for the 9 isolates are shown in Table 1. Modal MICs ranged from 16 to 64 μg/ml by BMD and 24 to >256 μg/ml by Etest (Table 1), and MICs for each isolate were reproducible within a log2 dilution range on different testing days. Modal daptomycin MBCs ranged from 128 to >180 μg/ml (Table 1 and Fig. 1). Growth curves for the 9 isolates, in the absence of antimicrobials, were not appreciably different, with an average increase in CFU/ml of 2.45 log10 (±0.11) after 24 h of incubation (not shown). Daptomycin was bactericidal (i.e., ≥3 log reduction in CFU/ml versus the starting inoculum) at 2× the daptomycin MIC (DAP2xMIC) for 5 of 9 (55.6%) isolates (Fig. 1A) and was bactericidal at 180 μg/ml daptomycin (DAP180) for 7 of 9 (77.8%) isolates (Fig. 1A). Time-kill curves for the two isolates in this study for which DAP180 was not bactericidal are presented in Fig. 1B. Isolate E. faecalis c01 (Efc01) had a 2.51 (±1.01) log10 decrease in CFU/ml at 24 h, compared to the inoculum, in the presence of DAP180. In contrast, isolate Efm25, an E. faecium isolate, demonstrated 2.50 (±1.72) log10 killing at 6 h in the presence of DAP180, but by 24 h, the CFU/ml had increased 1.38 log10 (±0.09) from the 6-h reading, yielding an overall 1.2 log decrease in CFU/ml at 24 h compared to 0 h (Fig. 1B). Both of these isolates had daptomycin MICs of 64 μg/ml by BMD and >256 μg/ml by Etest. For the other 7 isolates, there was no growth from the 6-h or 24-h subcultures following incubation in the presence of DAP180 (i.e., <100 CFU/ml was present). No clear correlation between liaFSR or cls mutation and response to daptomycin in the time-kill studies was identified, and these differences may be attributed to other mutations in the genomes of these isolates.
FIG 1.
(A) Change in log10 CFU/ml after 24 h of antibiotic exposure to various concentrations of daptomycin in the kill curve. (B) Kill curves for isolates Efc01 and Efm25, the only two isolates that displayed growth after 24 h of incubation in 180 μg/ml daptomycin, in combination with test antimicrobials.
Ampicillin, cefazolin, ceftriaxone, ceftaroline, ertapenem, gentamicin, tigecycline, and rifampin did not display bactericidal activity at the concentrations used in this study (see Fig. S1 in the supplemental material), with one exception. Ertapenem was bactericidal against isolate Efc01 alone (see Fig. S1). Efc01 was susceptible to ampicillin (MIC ≤ 2 μg/ml), which predicts susceptibility to imipenem but not necessarily susceptibility to ertapenem (1).
liaFSR mutations associated with synergy between daptomycin and ampicillin.
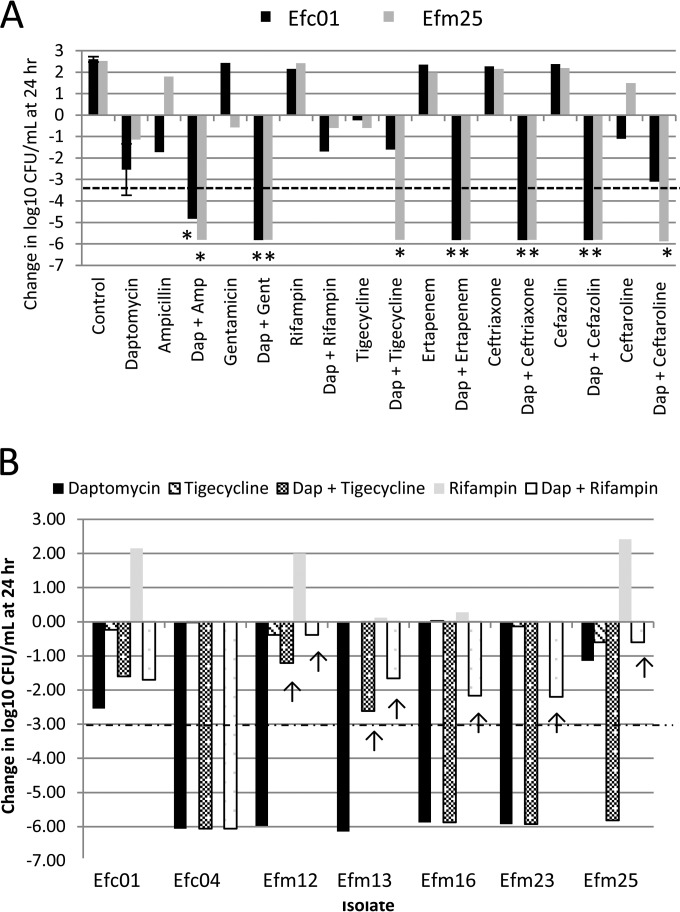
The changes in log10 CFU/ml after 24-h antimicrobial exposures, for experiments with ampicillin (90 μg/ml) in combination with 4 concentrations of daptomycin, are shown in Table 2. Ampicillin alone was not bactericidal for any of the DNS enterococci tested. At 0.5× the daptomycin MIC (DAP0.5xMIC), the addition of ampicillin yielded synergy in 7/9 isolates (77.8%), including both E. faecalis isolates, despite the fact that only one isolate, Efc01, was susceptible to ampicillin (Table 1). These 7 isolates all harbored mutation to the liaFSR system, whereas the 2 isolates for which the ampicillin-daptomycin combination was not synergistic with DAP0.5xMIC were the only isolates included in this study without liaFSR mutation. The combination of ampicillin and DAP0.5xMIC yielded bactericidal activity against only 3 of the isolates: Efc01, Efc04, (both E. faecalis), and Efm23 (an E. faecium isolate; Table 2). Ampicillin plus DAP1xMIC was both synergistic and bactericidal for 6/9 isolates (66.6%), all of which harbored mutations in liaFSR. Ampicillin, which was synergistic but not bactericidal, in combination with DAP0.5xMIC for isolate Efm13, no longer demonstrated synergy for this isolate when tested at this isolate's daptomycin MIC (DAP1xMIC). Daptomycin at twice the MIC (DAP2xMIC) was bactericidal for isolates Efm4, Efm12, Efm16, Efm19, and Efm23 (Fig. 1). For the 4 remaining isolates for which an effect of adding a second antimicrobial could be discerned, ampicillin yielded synergy for 3 (75.0%; Table 2). Daptomycin at 180 μg/ml (DAP180) was bactericidal against all but two isolates: Efc01 and Efm25 (Fig. 1A). Ampicillin acted synergistically with DAP180 for both isolates (Fig. 2A and Table 2).
TABLE 2.
Summary of 24-h time-kill testing for daptomycin at 4 concentrations in combination with 90 μg/ml ampicillin
| Isolate (daptomycin MIC) | Result for daptomycin at indicated tested concn or synergy resulta |
|||
|---|---|---|---|---|
| 0.5× MIC | 1× MIC | 2× MIC | 180 μg/ml | |
| Efc01 (64 μg/ml) | BC | BC | BC | — |
| Efc04 (32 μg/ml) | BC | BC | NA | NA |
| Efm12 (64 μg/ml) | — | BC | NA | NA |
| Efm13 (64 μg/ml) | — | BC | NA | |
| Efm15 (16 μg/ml) | NA | |||
| Efm16 (64 μg/ml) | — | BC | NA | NA |
| Efm19 (16 μg/ml) | NA | NA | ||
| Efm23 (64 μg/ml) | BC | BC | NA | NA |
| Efm25 (64 μg/ml) | — | BC | BC | — |
| Total no. (%) of isolates with synergyb | 7 (78) | 6 (67) | 3 (75) | 2 (100) |
Boldface data indicate synergy. —, daptomycin plus ampicillin yielded a colony count at 24 h that was ≥2 log10 CFU/ml lower than that seen with the most active antimicrobial alone. BC, bactericidal combination, where daptomycin plus ampicillin yielded a ≥3 log10 CFU/ml reduction in colony counts compared to the starting inoculum. NA, daptomycin at this concentration was bactericidal and synergy could not be assessed.
Data indicate the total numbers and percentages of isolates for which neither antimicrobial was bactericidal alone.
FIG 2.
Change in log10 CFU/ml after 24 h of antibiotic exposure to antimicrobials, alone or in combination, at 180 μg/ml daptomycin. (A) Results for isolates Efc01 and Efm25, the only two isolates that displayed growth after 24 h of incubation in 180 μg/ml daptomycin, in combination with test antimicrobials. Asterisks (*) represent synergistic interactions (e.g., ≥2 log change in CFU/ml compared to daptomycin and/or the test antimicrobial alone. (B) Results of tigecycline and rifampin in combination with 180 μg/ml daptomycin. Arrows (↑) indicate antagonistic interactions (e.g., ≥2 log higher CFU/ml compared to daptomycin at 180 μg/ml alone). In both panels, a dashed line indicates a bactericidal effect (e.g., ≥99.9% reduction in CFU/ml from time zero). Amp, ampicillin; Dap, daptomycin; Gent, gentamicin.
liaFSR mutations associated with synergy between daptomycin and other β-lactams.
The changes in log10 CFU/ml after 24-h antimicrobial exposures, for experiments with cefazolin, ceftriaxone, ceftaroline, and ertapenem in combination with 4 concentrations of daptomycin, are shown in Table 3. When combined with DAP0.5xMIC, cefazolin demonstrated synergy for only 2 isolates (22.2%; Table 3) and was bactericidal for 1 (11.1%). At DAP1xMIC, the addition of cefazolin yielded synergy for 5 isolates (55.5%), and for all 5, the combination was bactericidal (Table 3). At twice the daptomycin MIC, cefazolin yielded synergy for 2 of the 4 isolates (50.0%) for which DAP2xMIC alone was not bactericidal (Table 3). Cefazolin in combination with DAP180 was synergistic for both isolates that were not killed by 180 μg/ml daptomycin (Efc01 and Efm25).
TABLE 3.
Summary of 24-h time-kill testing for daptomycin at 4 concentrations in combination with the β-lactams cefazolin, ceftriaxone, ceftaroline, and ertapenem
| Isolate (daptomycin MIC) | Result for indicated concn of daptomycin or synergy resulta |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cefazolin (185 μg/ml) |
Ceftriaxone (200 μg/ml) |
Ceftaroline (21 μg/ml) |
Ertapenem (115 μg/ml) |
|||||||||||||
| 0.5× MIC | 1× MIC | 2× MIC | 180 μg/ml | 0.5× MIC | 1× MIC | 2× MIC | 180 μg/ml | 0.5× MIC | 1× MIC | 2× MIC | 180 μg/ml | 0.5× MIC | 1× MIC | 2× MIC | 180 μg/ml | |
| Efc01 (64 μg/ml) | BC | — | — | BC | — | — | — | NA | NA | NA | NA | |||||
| Efc04 (32 μg/ml) | BC | BC | NA | NA | BC | NA | NA | BC | BC | NA | NA | BC | BC | NA | NA | |
| Efm12 (64 μg/ml) | NA | NA | BC | NA | NA | NA | NA | NA | NA | |||||||
| Efm13 (64 μg/ml) | BC | NA | — | — | BC | NA | — | BC | NA | NA | ||||||
| Efm15 (16 μg/ml) | NA | NA | NA | NA | ||||||||||||
| Efm16 (64 μg/ml) | BC | NA | NA | BC | NA | NA | — | NA | NA | — | NA | NA | ||||
| Efm19 (16 μg/ml) | NA | NA | NA | NA | NA | NA | NA | NA | ||||||||
| Efm23 (64 μg/ml) | — | BC | NA | NA | — | BC | NA | NA | — | BC | NA | NA | BC | BC | NA | NA |
| Efm25 (64 μg/ml) | BC | — | — | — | BC | BC | — | — | BC | BC | — | — | BC | — | ||
| Total no. of isolates with synergy (%)b | 2 (22) | 5 (55) | 2 (50) | 2 (100) | 4 (44) | 7 (78) | 2 (50) | 2 (100) | 5 (55) | 4 (44) | 3 (75) | 0 (0) | 4 (50) | 3 (38) | 1 (33) | 1 (100) |
Boldface data indicate synergy. —, daptomycin plus β-lactam yielded a colony count at 24 h that was ≥2 log10 CFU/ml lower than that seen with the most active antimicrobial alone. BC, bactericidal combination, where daptomycin plus β-lactam yielded a ≥3 log10 CFU/ml reduction in colony counts compared to the starting inoculum. NA, daptomycin or the β-lactam alone at this concentration was bactericidal and synergy could not be assessed.
Data indicate the total numbers and percentages of isolates for which neither antimicrobial was bactericidal alone.
Ceftriaxone (200 μg/ml) demonstrated synergy for 4/9 (44.4%) isolates when combined with DAP0.5xMIC and for 7/9 (77.7%) when combined with DAP1xMIC (Table 3). The latter combination was bactericidal for 6 (66.6%) of the isolates, the same 6 isolates for which synergy was found for ampicillin with DAP1xMIC (Tables 2 and 3). In addition to these 6 isolates, isolate Efm13 yielded synergy with the ceftriaxone-plus-DAP1xMIC combination, but this combination was not bactericidal (Table 3). The combination of ceftriaxone plus DAP2xMIC was synergistic and bactericidal for 2/4 (50.0%) isolates not killed by DAP2xMIC, and the combination of ceftriaxone plus DAP180 was synergistic for the two isolates not killed by 180 μg/ml daptomycin alone (Table 3 and Fig. 2).
The addition of ceftaroline (21 μg/ml) to DAP0.5xMIC yielded synergy for 5/9 (55.5%) of the isolates. Synergy was observed for isolate Efc04 only with this combination at 6 h (not shown). Similarly, ceftaroline plus DAP0.5xMIC was bactericidal only against isolate Efc04. The ceftaroline MIC for isolate Efc04 was 1 μg/ml by BMD, but at the 21 μg/ml concentration used in the time-kill studies, ceftaroline was bacteriostatic for this isolate (see Fig. S1 in the supplemental material). When combined with DAP1xMIC, ceftaroline demonstrated synergy with 4 (44.4%) isolates and bactericidal activity against 3 (33.3%) isolates (Table 3). Ceftaroline plus DAP2xMIC was synergistic for 3/4 (75.0%) of the isolates not killed by DAP2xMIC and bactericidal for 2 (50.0%) of these isolates (Table 3). The combination of ceftaroline plus DAP180 was synergistic for the two isolates not killed by 180 μg/ml daptomycin (Table 3 and Fig. 2) and bactericidal against only Efm25.
Ertapenem was synergistic with DAP0.5xMIC for 4/8 isolates (50.0%, excluding Efc01, for which ertapenem alone was bactericidal) (see Fig. S1 in the supplemental material) and bactericidal in combination with DAP0.5xMIC for 2 isolates (25.0%) (isolates Efc4 and Efm23) (Table 3). When tested with DAP1xMIC, ertapenem yielded synergy with 3 isolates, and when tested with DAP, ertapenem was synergistic with DAP2xMIC for isolate Efm25 alone (Table 3). When combined with DAP180, ertapenem was synergistic for isolate Efm25.
Of note, none of the β-lactams when combined with any concentration of daptomycin demonstrated synergy against isolates Efm15 and Efm19, the two isolates evaluated without mutation to liaFSR genes.
Synergy between daptomycin and gentamicin, tigecycline, and rifampin.
Four of the Enterococcus isolates included in this study, Efc01, Efc04, Efm13, and Efm25, did not display high-level gentamicin resistance (Table 1). Gentamicin at 25 μg/ml was consistently synergistic in combination with daptomycin for 3 (75.0%) of these isolates, Efc01, Efc04, and Efm25, at 24 h (Table 4). Synergy was not observed for isolate Efm13 when gentamicin was combined with DAP0.5xMIC or DAP1xMIC, but gentamicin was synergistic with DAP2xMIC (Table 4). No synergy was observed at 6 h for any of these isolates. In addition, for isolate Efm16, there was synergy and bactericidal killing by the combination of DAP1xMIC plus gentamicin (Table 3), despite the fact that this isolate expressed high-level gentamicin resistance (Table 1).
TABLE 4.
Summary of 24-h time-kill testing for daptomycin at 4 concentrations in combination with gentamicin, rifampin, and tigecycline
| Isolate (daptomycin MIC) | Result for indicated concn of daptomycin or synergy or antagonism resulta |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gentamicin (25 μg/ml) |
Tigecycline (0.8 μg/ml) |
Rifampin (10 μg/ml) |
||||||||||
| 0.5× MIC | 1× MIC | 2× MIC | 180 μg/ml | 0.5× MIC | 1× MIC | 2× MIC | 180 μg/ml | 0.5× MIC | 1× MIC | 2× MIC | 180 μg/ml | |
| Efc01 (64 μg/ml) | — | — | BC | — | A | — | ||||||
| Efc04 (32 μg/ml) | BC | BC | NAc | NA | NA | NA | NA | NA | ||||
| Efm12 (64 μg/ml) | NA | NA | NA | A | A | A | ||||||
| Efm13 (64 μg/ml) | BC | NA | A | A | ||||||||
| Efm15 (16 μg/ml) | NA | BC | BC | BC | NA | BC | NA | |||||
| Efm16 (64 μg/ml) | BC | NA | NA | — | — | NA | NA | A | A | |||
| Efm19 (16 μg/ml) | NA | NA | NA | NA | NA | NA | ||||||
| Efm23 (64 μg/ml) | NA | NA | NA | NA | A | A | ||||||
| Efm25 (64 μg/ml) | BC | BC | BC | — | BC | BC | — | — | A | |||
| Total no. (%) of isolates with synergyb | 3 (33) | 4(44) | 3(75) | 2(100) | 2 (22) | 3 (33) | 2 (22) | 1 (50) | 0 (0) | 2 (22) | 1 (14) | 0(0) |
| Total no. (%) of isolates with antagonismb | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (11) | 2 (22) | 0(0) | 0(0) | 3 (33) | 5 (55) |
Boldface data indicate synergy. —, daptomycin plus antimicrobial yielded a colony count at 24 h that was ≥2 log10 CFU/ml lower than that seen with the most active antimicrobial alone. BC, bactericidal combination, where daptomycin plus antimicrobial yielded a ≥3 log10 CFU/ml reduction in colony counts compared to the starting inoculum. A, antagonism, where daptomycin plus antimicrobial yielded a ≥2 log10 CFU/ml increase in bacterial counts at 24 h compared to most active agent in combination. NA, daptomycin at this concentration was bactericidal and synergy could not be assessed.
Data indicate the total numbers and percentages of isolates for which neither antimicrobial was bactericidal alone.
Tigecycline was synergistic in combination with DAP0.5xMIC for 2 isolates and in combination with DAP1xMIC for 3 isolates (Table 3). Tigecycline was synergistic with DAP2xMIC for 2 isolates, Efm15 and Efm25, but was antagonistic for isolate Efc01. The addition of tigecycline to DAP180 was also antagonistic for 2 isolates, Efm12 (5 log10 less killing by the combination than by DAP180 alone) and Efm13 (3.2 log10 less killing by the combination than by DAP180 alone) (Table 3 and Fig. 2B).
The addition of rifampin to daptomycin yielded differing results. When combined with DAP0.5xMIC, rifampin was indifferent (i.e., exhibited no activity) for all the isolates tested (Table 3). Synergy was seen for two isolates (22.2%) with DAP1xMIC and for one isolate (11.1%) with DAP2xMIC. However, while rifampin showed either no activity or a bacteriostatic effect on its own, the addition of rifampin to DAP180 for isolates Efm12, Efm13, Efm16, Efm23, and Efm25 (55.5% of isolates tested) resulted in antagonism against the DAP180 activity (Fig. 2B and Table 4).
liaFSR mutations associated with enhanced ampicillin-induced BODIPY-daptomycin binding.
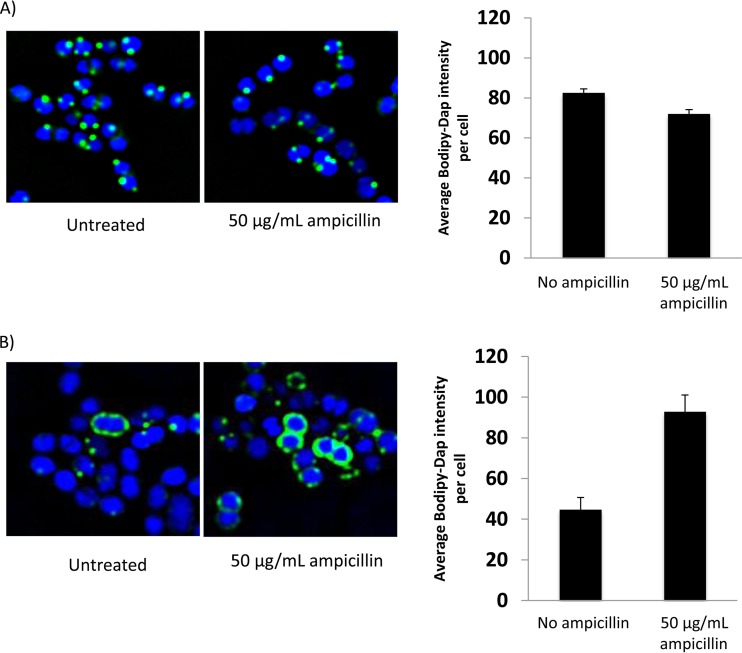
Two isolates, Efm19 and Efm25, were chosen to evaluate the interaction of daptomycin with the cytoplasmic membrane, in the absence or presence of ampicillin. The isolates were chosen based on the presence (Efm25) or absence (Efm19) of liaFSR mutations and of associated synergy between daptomycin and ampicillin (Table 2) and because they were both E. faecium isolates. These studies revealed that the addition of 50 μg/ml ampicillin yielded a significant increase in BODIPY-daptomycin binding for isolate Efm25 (Fig. 3B) (P = 0.01, t test) but no appreciable increase in binding for Efm19 (Fig. 3A), consistent with the results of the time-kill synergy testing. Interestingly, Efm19 and Efm25 demonstrated the same LL-37 MIC (2 μM) but liaFSR mutant Efm25 demonstrated a significant reduction in killing by LL-37 at 1× MIC compared to the Efm19 strain. Ampicillin significantly (P < 0.001, t test) increased the activity of human cathelicidin LL-37 for both Efm19 and Efm25, but the percent survival was significantly lower for the wild-type strain than for the liaFSR mutant strain (Fig. 4).
FIG 3.
BDP-daptomycin (32 μg/ml) binding (20 min) studies for VRE Efm19 (no liaFSR mutation) (A) and Efm25 (with liaFSR mutation) (B) grown in the presence or absence of ampicillin at 50 mg/liter.
FIG 4.
Effect of ampicillin on LL-37 killing of Efm19 (no liaFSR mutation) and Efm25 (with liaFSR mutation). Results are averages from 4 experiments, and error bars indicate standard deviations.
DISCUSSION
We have noted a significant increase in levels of DNS enterococci at our institution, including isolates with very high daptomycin MICs of >256 μg/ml, which is well above the daptomycin susceptibility breakpoint of 4 μg/ml. At such MICs, even 180 μg/ml daptomycin (the highest serum concentration achievable with 12 mg/kg of body weight/day dosing) can achieve only 1 to 2 log10 killing in vitro after 24 h (Fig. 1B). While such DNS enterococci remain uncommon, they are a major clinical concern (31). Several strategies have been suggested to prevent or overcome DNS or DSD phenotypes, including increasing daptomycin doses beyond those established in FDA labeling (16) and combination therapy with a second antimicrobial, such as a β-lactam (32). Data for staphylococci and enterococci suggest that the combination of daptomycin and a β-lactam agent, in particular, ampicillin or ceftaroline, is synergistic in vitro and is associated with therapeutic success against DNS isolates (17–19, 32). Ampicillin has been shown in several studies to enhance the activity of daptomycin against daptomycin-susceptible isolates (33–37). Furthermore, a retrospective clinical study demonstrated that the addition of β-lactam to daptomycin significantly improved treatment outcomes for vancomycin-resistant Enterococcus (VRE) bloodstream infections when the daptomycin MIC was 4 μg/ml (38), and several cases have had successful outcomes resulting from the addition of ampicillin to the treatment regimen for patients failing daptomycin therapy (8, 15, 17, 39).
Complicating this association are the recent findings of Diaz and colleagues, who demonstrated that ampicillin plus daptomycin was synergistic only against E. faecium with mutation to the liaFSR genes and not against an isolate that harbored wild-type alleles (13). Our present study confirmed this finding among a larger collection of isolates and further demonstrated that, for those isolates that harbor liaFSR mutations, synergy between ampicillin and daptomycin exists even for isolates with very high (i.e., >256 μg/ml) daptomycin MICs. In contrast, the isolates evaluated by Diaz and colleagues had daptomycin MICs of 3 to 16 μg/ml. It should be noted that, while we found synergistic activity between ampicillin and daptomycin for all 7 isolates with mutations to the LiaFSR pathway at DAP0.5xMIC, this combination was bactericidal for only 3 of the isolates. Similarly, at DAP1xMIC, the addition of ampicillin was synergistic for 6/7 (85.7%) of the isolates with mutations to liaFSR and bactericidal against all 6 of these (Table 2). Together, these findings suggest that, while mutation to liaFSR is more commonly associated with synergy between ampicillin and daptomycin, it is not predictive at all concentrations of daptomycin. To test the strains with high-level resistance, we chose the maximum total drug concentration achievable for a broad range of antibiotics in the study. However, it is notable that the free drug concentrations will likely differ depending on the degree of protein binding for each drug; it will be necessary to take this into consideration when extrapolating our results to the clinic. Given that a dose-dependent effect with daptomycin was observed to some extent for bactericidal activity and synergy with ampicillin against strains with liaFSR mutation, the maximally tolerated doses of daptomycin should be prescribed when daptomycin is used in combination with ampicillin. Regardless, no synergy was found between daptomycin and any β-lactam, at any concentration, for the two isolates with wild-type liaFSR alleles. As such, the absence of mutation to this region may rule out synergy. None of the other β-lactams evaluated here demonstrated consistent synergy with daptomycin.
The mechanism of synergy between ampicillin and daptomycin remains to be fully defined for enterococci. In a previous study, it was demonstrated that 50 μg/ml ampicillin caused a net decrease in the relative positive surface charge of an ampicillin-resistant, daptomycin-susceptible (daptomycin MIC, 1 μg/ml) E. faecium isolate, associated with increased binding of daptomycin to the enterococcal cell membrane (17). Daptomycin exhibited bacteriostatic activity against this isolate, so the presence of a liaFSR mutation, although not examined by those authors, is assumed. These data suggest a charge-based mechanism for daptomycin-ampicillin synergy, and a similar effect has been noted for ceftaroline for both daptomycin-susceptible and DNS E. faecium isolates (18). In addition, we previously demonstrated for isolate 5938 (which is the same strain as Efm16) that treatment with 50 μg/ml ampicillin caused an increase in cell wall thickness and increased LL-37 binding and activity (18). In the present study, treatment with 50 μg/ml ampicillin significantly increased the binding of BODIPY-daptomycin to the cell membrane of Efm25 but not to that of Efm19, which does not harbor mutation to liaFSR. Interestingly, while ampicillin enhanced the binding of daptomycin only to Efm25, it sensitized both Efm19 and Efm25 to LL-37, the human cathelicidin host defense peptide (Fig. 4). Further studies are required to more fully define these different mechanisms, but the data suggest that the concept of the charge-based mechanism for synergy may be overly simplistic. However, it is important that the relative levels of tolerance for LL-37 and, potentially, other cationic host defense peptides conferred by liaFSR mutations may be selected for by persistent endovascular infections, as has been shown in mprF for Staphylococcus aureus (40), perhaps even in the absence of daptomycin selective pressure.
The advantage conferred to the pathogen by its ability to resist killing by both the innate immune system and daptomycin appears, therefore, to result in an “Achilles' heel” whereby β-lactams such as ampicillin may be employed as adjunctive agents, and only in these settings would such a practice be beneficial. This hypothesis is supported circumstantially by the study by Moise et al. (38) which shows that the addition of β-lactams to daptomycin for VRE bloodstream infections is beneficial in improving outcome only in cases where the daptomycin MIC is 3 to 4 μg/liter (presumably in a liaFSR mutation-enriched subgroup, on the basis of prior data [10]) and not when the daptomycin MIC is ≤2 μg/liter (38).
Synergy between daptomycin and gentamicin, for E. faecium isolates with high-level susceptibility to gentamicin, has been noted previously (41), but little to no clinical data exist for this combination (42). Unlike the case with the glycopeptides (43), it would appear that high-level gentamicin resistance does not necessarily abolish in vitro synergy, as was seen for isolate Efm16; however, this interaction requires further evaluation, and no data exist to suggest that this may be the case in vivo.
Several reports have demonstrated effective use of daptomycin plus tigecycline for the treatment of endocarditis caused by E. faecium (44–46). In our hands, this combination was frequently antagonistic, but again, in vivo data are required to confirm this finding. Similarly, we found antagonism for a significant number of isolates with daptomycin plus rifampin, but this has not been demonstrated by an in vivo model.
In summary, we systematically evaluated by in vitro time-kill studies the effect of daptomycin in combination with other antimicrobials for a collection of 9 DNS enterococcal isolates that exhibited a broad range of MICs and different resistance-conferring mutations. We found that ampicillin plus daptomycin yielded the most consistent synergy but did so only for isolates with mutations to the liaFSR system. Daptomycin binding was found to be enhanced by the addition of ampicillin for such mutations but not for an isolate without mutations to this system. In contrast, ampicillin enhanced the killing of LL-37 against DNS E. faecium, regardless of the presence of liaFSR mutation. These data lend support to the growing body of evidence that combination therapy consisting of daptomycin plus ampicillin may be helpful in eradicating refractory VRE infections by counteracting the fitness advantages of reduced daptomycin susceptibility and resistance to killing by cathelicidin and other host defense peptides conferred by these mutations.
Supplementary Material
ACKNOWLEDGMENTS
Funding for this research was provided by Cubist Pharmaceuticals (R.H.) and by grant U54 HD071600-01 09/26/2011-06/30/2016 (Developmental and Translational Pharmacology of Pediatric Antimicrobial Therapy) from the National Institute of Child Health and Human Development (NICHD) (G.S. and V.N.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.05077-14.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2014. Performance standards for antimicrobial susceptibility testing; 24th informational supplement, vol M100 S24. CLSI, Wayne, PA. [Google Scholar]
- 2.Sader HS, Farrell DJ, Flamm RK, Jones RN. 2014. Daptomycin activity tested against 164457 bacterial isolates from hospitalised patients: summary of 8 years of a Worldwide Surveillance Programme (2005–2012). Int J Antimicrob Agents 43:465–469. doi: 10.1016/j.ijantimicag.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 3.Kelesidis T, Chow AL, Humphries R, Uslan DZ, Pegues D. 2012. Case-control study comparing de novo and daptomycin-exposed daptomycin-nonsusceptible Enterococcus infections. Antimicrob Agents Chemother 56:2150–2152. doi: 10.1128/AAC.05918-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelesidis T, Humphries R, Uslan DZ, Pegues D. 2012. De novo daptomycin-nonsusceptible enterococcal infections. Emerg Infect Dis 18:674–676. doi: 10.3201/eid1804.110932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelesidis T, Humphries R, Chow AL, Tsiodras S, Uslan DZ. 2013. Emergence of daptomycin-non-susceptible enterococci urinary tract isolates. J Med Microbiol 62:1103–1105. doi: 10.1099/jmm.0.056630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lewis JS II, Owens A, Cadena J, Sabol K, Patterson JE, Jorgensen JH. 2005. Emergence of daptomycin resistance in Enterococcus faecium during daptomycin therapy. Antimicrob Agents Chemother 49:1664–1665. doi: 10.1128/AAC.49.4.1664-1665.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munoz-Price LS, Lolans K, Quinn JP. 2005. Emergence of resistance to daptomycin during treatment of vancomycin-resistant Enterococcus faecalis infection. Clin Infect Dis 41:565–566. doi: 10.1086/432121. [DOI] [PubMed] [Google Scholar]
- 8.Arias CA, Torres HA, Singh KV, Panesso D, Moore J, Wanger A, Murray BE. 2007. Failure of daptomycin monotherapy for endocarditis caused by an Enterococcus faecium strain with vancomycin-resistant and vancomycin-susceptible subpopulations and evidence of in vivo loss of the vanA gene cluster. Clin Infect Dis 45:1343–1346. doi: 10.1086/522656. [DOI] [PubMed] [Google Scholar]
- 9.Fluit AC, Schmitz FJ, Verhoef J, Milatovic D. 2004. Daptomycin in vitro susceptibility in European Gram-positive clinical isolates. Int J Antimicrob Agents 24:59–66. doi: 10.1016/j.ijantimicag.2003.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Munita JM, Panesso D, Diaz L, Tran TT, Reyes J, Wanger A, Murray BE, Arias CA. 2012. Correlation between mutations in liaFSR of Enterococcus faecium and MIC of daptomycin: revisiting daptomycin breakpoints. Antimicrob Agents Chemother 56:4354–4359. doi: 10.1128/AAC.00509-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munita JM, Tran TT, Diaz L, Panesso D, Reyes J, Murray BE, Arias CA. 2013. A liaF codon deletion abolishes daptomycin bactericidal activity against vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother 57:2831–2833. doi: 10.1128/AAC.00021-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Streit JM, Jones RN, Sader HS. 2004. Daptomycin activity and spectrum: a worldwide sample of 6737 clinical Gram-positive organisms. J Antimicrob Chemother 53:669–674. doi: 10.1093/jac/dkh143. [DOI] [PubMed] [Google Scholar]
- 13.Diaz L, Tran TT, Munita JM, Miller WR, Rincon S, Carvajal LP, Wollam A, Reyes J, Panesso D, Rojas NL, Shamoo Y, Murray BE, Weinstock GM, Arias CA. 2014. Whole-genome analyses of Enterococcus faecium isolates with diverse daptomycin MICs. Antimicrob Agents Chemother 58:4527–4534. doi: 10.1128/AAC.02686-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller C, Kong J, Tran TT, Arias CA, Saxer G, Shamoo Y. 2013. Adaptation of Enterococcus faecalis to daptomycin reveals an ordered progression to resistance. Antimicrob Agents Chemother 57:5373–5383. doi: 10.1128/AAC.01473-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munita JM, Mishra NN, Alvarez D, Tran TT, Diaz L, Panesso D, Reyes J, Murray BE, Adachi JA, Bayer AS, Arias CA. 8 August 2014, posting date Failure of high-dose daptomycin for bacteremia caused by daptomycin-susceptible Enterococcus faecium harboring LiaSR substitutions. Clin Infect Dis doi: 10.1093/cid/ciu642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Werth BJ, Steed ME, Ireland CE, Tran TT, Nonejuie P, Murray BE, Rose WE, Sakoulas G, Pogliano J, Arias CA, Rybak MJ. 23 June 2014. Defining daptomycin resistance prevention exposures in vancomycin resistant Enterococcus (VRE) faecium and E. faecalis. Antimicrob Agents Chemother doi: 10.1128/AAC.00098-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sakoulas G, Bayer AS, Pogliano J, Tsuji BT, Yang SJ, Mishra NN, Nizet V, Yeaman MR, Moise PA. 2012. Ampicillin enhances daptomycin- and cationic host defense peptide-mediated killing of ampicillin- and vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 56:838–844. doi: 10.1128/AAC.05551-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sakoulas G, Rose W, Nonejuie P, Olson J, Pogliano J, Humphries R, Nizet V. 2014. Ceftaroline restores daptomycin activity against daptomycin-nonsusceptible vancomycin-resistant Enterococcus faecium. Antimicrob Agents Chemother 58:1494–1500. doi: 10.1128/AAC.02274-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sakoulas G, Nonejuie P, Nizet V, Pogliano J, Crum-Cianflone N, Haddad F. 2013. Treatment of high-level gentamicin-resistant Enterococcus faecalis endocarditis with daptomycin plus ceftaroline. Antimicrob Agents Chemother 57:4042–4045. doi: 10.1128/AAC.02481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moodey J, Knapp C. 2009. Minimum bactericidal concentration testing, p 5.10.1–5.10.14. In Hindler JF. (ed), Clinical microbiology procedures handbook, 3rd ed, vol 2 ASM, Washington, DC. [Google Scholar]
- 21.Pillai SK, Moellering RC, Eliopoulos GM. 2005. Antimicrobial combinations, p 365–405. In Lorian V. (ed), Antibiotics in laboratory medicine, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 22.Foulds G. 1986. Pharmacokinetics of sulbactam/ampicillin in humans: a review. Rev Infect Dis 8(Suppl 5):S503–S511. [DOI] [PubMed] [Google Scholar]
- 23.Sagent Pharmaceuticals. 2012. Cefazolin for injection, USP (package insert). Sagent Pharmaceuticals, Schaumburg, IL. [Google Scholar]
- 24.Goonetilleke AK, Dev D, Aziz I, Hughes C, Smith MJ, Basran GS. 1996. A comparative analysis of pharmacokinetics of ceftriaxone in serum and pleural fluid in humans: a study of once daily administration by intramuscular and intravenous routes. J Antimicrob Chemother 38:969–976. doi: 10.1093/jac/38.6.969. [DOI] [PubMed] [Google Scholar]
- 25.Forest Laboratories Inc. 2010. Teflaro (ceftaroline fosamil) injection for intravenous (IV) use. Forest Pharmaceuticals, Inc, St. Louis, MO. [Google Scholar]
- 26.Frasca D, Marchand S, Petitpas F, Dahyot-Fizelier C, Couet W, Mimoz O. 2010. Pharmacokinetics of ertapenem following intravenous and subcutaneous infusions in patients. Antimicrob Agents Chemother 54:924–926. doi: 10.1128/AAC.00836-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baxter Corporation. 2012. Gentamicin sulfate in 0.9% sodium chloride injection. Baxter Corporation, Mississauga, ON, Canada. [Google Scholar]
- 28.Peloquin CA, Namdar R, Singleton MD, Nix DE. 1999. Pharmacokinetics of rifampin under fasting conditions, with food, and with antacids. Chest 115:12–18. doi: 10.1378/chest.115.1.12. [DOI] [PubMed] [Google Scholar]
- 29.Sun HK, Ong CT, Umer A, Harper D, Troy S, Nightingale CH, Nicolau DP. 2005. Pharmacokinetic profile of tigecycline in serum and skin blister fluid of healthy subjects after multiple intravenous administrations. Antimicrob Agents Chemother 49:1629–1632. doi: 10.1128/AAC.49.4.1629-1632.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Society for Microbiology. 2014. In vitro susceptibility tests. In Instructions to authors (Antimicrobial Agents and Chemotherapy). American Society for Microbiology, Washington, DC. [Google Scholar]
- 31.Kelesidis T, Humphries R, Uslan DZ, Pegues DA. 2011. Daptomycin nonsusceptible enterococci: an emerging challenge for clinicians. Clin Infect Dis 52:228–234. doi: 10.1093/cid/ciq113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gould IM, Miro JM, Rybak MJ. 2013. Daptomycin: the role of high-dose and combination therapy for Gram-positive infections. Int J Antimicrob Agents 42:202–210. doi: 10.1016/j.ijantimicag.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 33.Cilli F, Aydemir S, Tunger A. 2006. In vitro activity of daptomycin alone and in combination with various antimicrobials against Gram-positive cocci. J Chemother 18:27–32. doi: 10.1179/joc.2006.18.1.27. [DOI] [PubMed] [Google Scholar]
- 34.Bingen E, Lambert-Zechovsky N, Leclercq R, Doit C, Mariani-Kurkdjian P. 1990. Bactericidal activity of vancomycin, daptomycin, ampicillin and aminoglycosides against vancomycin-resistant Enterococcus faecium. J Antimicrob Chemother 26:619–626. doi: 10.1093/jac/26.5.619. [DOI] [PubMed] [Google Scholar]
- 35.Duez JM, Péchinot A, Siébor E, Cordin X, Kazmierczak A. 1989. Bactericidal activity of daptomycin and vancomycin alone or in combination with tobramycin, netilmicin or ampicillin against enterococcus. Pathol Biol (Paris) 37:263–268. (In French.) [PubMed] [Google Scholar]
- 36.Rand KH, Houck H. 2004. Daptomycin synergy with rifampicin and ampicillin against vancomycin-resistant enterococci. J Antimicrob Chemother 53:530–532. doi: 10.1093/jac/dkh104. [DOI] [PubMed] [Google Scholar]
- 37.Mobarakai N, Quale JM, Landman D. 1994. Bactericidal activities of peptide antibiotics against multidrug-resistant Enterococcus faecium. Antimicrob Agents Chemother 38:385–387. doi: 10.1128/AAC.38.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moise P, Lamp K, DePestel D. 2012. Abstr 52nd Intersci Conf Antimicrob Agents Chemother, San Francisco, CA, p 53. [Google Scholar]
- 39.Sierra-Hoffman M, Iznaola O, Goodwin M, Mohr J. 2012. Combination therapy with ampicillin and daptomycin for treatment of Enterococcus faecalis endocarditis. Antimicrob Agents Chemother 56:6064. doi: 10.1128/AAC.01760-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mishra NN, Yang SJ, Chen L, Muller C, Saleh-Mghir A, Kuhn S, Peschel A, Yeaman MR, Nast CC, Kreiswirth BN, Cremieux AC, Bayer AS. 2013. Emergence of daptomycin resistance in daptomycin-naive rabbits with methicillin-resistant Staphylococcus aureus prosthetic joint infection is associated with resistance to host defense cationic peptides and mprF polymorphisms. PLoS One 8:e71151. doi: 10.1371/journal.pone.0071151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leclercq R, Bingen E, Su QH, Lambert-Zechovski N, Courvalin P, Duval J. 1991. Effects of combinations of beta-lactams, daptomycin, gentamicin, and glycopeptides against glycopeptide-resistant enterococci. Antimicrob Agents Chemother 35:92–98. doi: 10.1128/AAC.35.1.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Munita JM, Murray BE, Arias CA. 2 September 2014, posting date Daptomycin for the treatment of bacteraemia due to vancomycin-resistant enterococci. Int J Antimicrob Agents doi: 10.1016/j.ijantimicag.2014.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahm DF, Kissinger J, Gilmore MS, Murray PR, Mulder R, Solliday J, Clarke B. 1989. In vitro susceptibility studies of vancomycin-resistant Enterococcus faecalis. Antimicrob Agents Chemother 33:1588–1591. doi: 10.1128/AAC.33.9.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schutt AC, Bohm NM. 2009. Multidrug-resistant Enterococcus faecium endocarditis treated with combination tigecycline and high-dose daptomycin. Annals Pharmacother 43:2108–2112. doi: 10.1345/aph.1M324. [DOI] [PubMed] [Google Scholar]
- 45.Polidori M, Nuccorini A, Tascini C, Gemignani G, Iapoce R, Leonildi A, Tagliaferri E, Menichetti F. 2011. Vancomycin-resistant Enterococcus faecium (VRE) bacteremia in infective endocarditis successfully treated with combination daptomycin and tigecycline. J Chemother 23:240–241. doi: 10.1179/joc.2011.23.4.240. [DOI] [PubMed] [Google Scholar]
- 46.Jenkins I. 2007. Linezolid- and vancomycin-resistant Enterococcus faecium endocarditis: successful treatment with tigecycline and daptomycin. J Hosp Med 2:343–344. doi: 10.1002/jhm.236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.