Abstract
Valnemulin, a semisynthetic pleuromutilin antibiotic derivative, is greatly active against Mycoplasma. The objective of our study was to evaluate the effectiveness of valnemulin against Mycoplasma gallisepticum in a neutropenic intratracheal model in chickens using a pharmacokinetic/pharmacodynamic (PK-PD) method. The PK of valnemulin after intramuscular (i.m.) administration at doses of 1, 10, and 20 mg/kg of body weight in M. gallisepticum-infected neutropenic chickens was evaluated by liquid chromatography-tandem mass spectrometry (LC-MS/MS). Real-time PCR (RT-PCR) was used for quantitative detection of M. gallisepticum. The ratio of the 24-h area under the concentration-time curve divided by the MIC (AUC24/MIC) correlated well with the in vivo antibacterial effectiveness of valnemulin (R2 = 0.9669). The AUC24/MIC ratios for mycoplasmastasis (a reduction of 0 log10 color-changing unit [CCU] equivalents/ml), a reduction of 1 log10 CCU equivalents/ml, and a reduction of 2.5 log10 CCU equivalents/ml are 28,820, 38,030, and 56,256, respectively. In addition, we demonstrated that valnemulin at a dose of 6.5 mg/kg resulted in a reduction of 2.5 log10 CCU equivalents/ml. These investigations provide a solid foundation for the usage of valnemulin in poultry with M. gallisepticum infections.
INTRODUCTION
Mycoplasma gallisepticum is a primary pathogen in chickens, turkeys, pheasants, red-legged partridges, chukar, and members of the Fringillidae family (1). It is the etiologic agent of chronic respiratory disease (CRD), with clinical symptoms of rales, nasal discharge, air sacculitis, and depression (2). It has been deemed the most economically important of the four pathogenic Mycoplasma species, as it causes vast losses in the commercial poultry industry (3). M. gallisepticum infection causes not only direct losses, such as reduced hatchability, increased feed conversion ratio, lower quality of processed chicken carcasses, and increased mortality, but it also causes indirect losses, like costs of eradication procedures, monitoring programs, and control procedures (4). Moreover, it leads to a negative effect on foreign trade (2). M. gallisepticum can be transmitted vertically through eggs and horizontally by susceptible individuals through direct/indirect contact with contaminated surfaces, infected equipment, and airborne particles (2). Although both vaccination and antimicrobial drugs are utilized against M. gallisepticum, vaccination for M. gallisepticum control has been greatly discouraged, because of considerable organism strain variability and the uselessness of stopping horizontal spread (5). Given this, antimicrobial chemotherapy plays a significant role in treating infections of M. gallisepticum. Valnemulin, as a semisynthetic pleuromutilin antibiotic derivative, was developed solely for veterinary use due to its potent activity against Mycoplasma (6). This peculiarity indicates valnemulin to be another prospective therapeutic choice in M. gallisepticum infections.
A rational antimicrobial drug dosage to be used in veterinary clinical practice should be based on the relationship of pharmacokinetics (PK) and pharmacodynamics (PD) (7). Therefore, in the current study, we investigated the in vivo PK-PD profiles of valnemulin against M. gallisepticum. This in vivo PK-PD model avoids the limitations of ex vivo PK-PD. For instance, for ex vivo PK-PD, there is continuous exposure to a fixed concentration of agent for a defined time (24 h), which does not mimic the concentration of antibacterial decline in animals because of body clearance and drug metabolism. In addition, the concentration in the target site usually varies from that in serum. Therefore, an in vivo PK-PD model provides more actual approximate clinical data. Unfortunately, due to the difficulty of isolating and culturing M. gallisepticum from chickens, little is known about the in vivo PK-PD profiles of valnemulin against M. gallisepticum. Molecular methods, such as hybridization techniques, PCR, or combinations of both techniques to detect the presence of bacterium-specific DNA sequences, were used in both qualitative and quantitative detection of the organism (8, 9). Recently, real-time PCR (RT-PCR) methods that monitor the production of specific PCR products by fluorimetric detection of amplified products and use melting curve analysis or specific hybridization probes for identification have been used for qualitative and quantitative detection of various pathogens (10, 11). This method for the quantitative detection of M. gallisepticum was also reported in several studies (12, 13).
In the current study, an experimental M. gallisepticum-infected neutropenic chicken model was developed, and the PK of valnemulin at 1, 10, and 20 mg/kg of body weight was evaluated. The dose proportionality of valnemulin was evaluated in a range of 1 to 20 mg/kg. RT-PCR was used for quantitative detection of M. gallisepticum in vivo. An in vivo PK-PD study of valnemulin against M. gallisepticum was established to identify the PK-PD index associated with variable valnemulin effectiveness and to elucidate the magnitude of the PK-PD parameters most predictive of effectiveness. These studies were used with MICs to provide a rational approach to the design of a dosage regimen that optimizes effectiveness with respect to bacteriological and clinical outcomes.
MATERIALS AND METHODS
Organisms, chemicals, and susceptibility assay.
The M. gallisepticum standard strain S6 was purchased from the Chinese Veterinary Microorganism Culture Collection Center (Beijing, China). Valnemulin hydrochloride (>99%) was kindly supplied by Guangdong Dahuanong Animal Health Products Co., Ltd. (Guangdong, China). The structure of valnemulin is shown in Fig. 1. The MIC of valnemulin against the study strain M. gallisepticum S6 was determined by a broth dilution method, according to recommended protocols (14). Briefly, the M. gallisepticum strain was cultured to reach exponential phase and diluted to ∼2 × 105 color-changing units (CCU)/ml. A series of concentrations of valnemulin were prepared by doubling dilution (final concentration, 3.5 × 10−4 to 1.44 μg/ml) with M. gallisepticum medium. A 0.1-ml aliquot of the medium with different concentrations of valnemulin was added to a 96-well plate, and another 0.1-ml aliquot of prepared M. gallisepticum S6 was supplemented to each well, achieving final titers of ∼1 × 105 CCU/ml. The tests were conducted in triplicate and included growth controls (M. gallisepticum in the medium only), endpoint controls (blank medium adjusted to pH 6.8), and germfree controls (blank medium only). The plates were cultured at 37°C. Changes in color were monitored every 4 h until the color of the growth control was the same as that of the endpoint control. The MIC was determined as the minimal concentration of valnemulin that resulted in no change in color.
FIG 1.
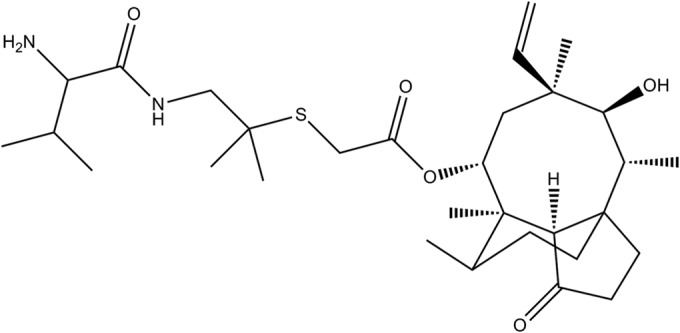
Chemical structure of valnemulin.
In vitro standard DNA preparation.
Overnight cultures of the M. gallisepticum strain were used to inoculate 20 ml of medium specifically sold as “M. gallisepticum medium,” purchased from Qingdao Hope Bio-Technology Co., Ltd. (Shandong, China), to a starting concentration of 105 CCU/ml, and these were allowed to grow for 36 h at 37°C. At 36 h of incubation, 20 ml of the sample was centrifuged for 10 min at 1,500 rpm, and the pellet was resuspended in 0.6 ml of M. gallisepticum medium. The sample was serially diluted for organism counting by culture. Meanwhile, DNA was isolated from serial 10-fold dilutions (100 to 10−6) prepared from the 0.6-ml sample with a bacterial DNA kit (Omega Bio-Tek, Inc., Norcross, GA). Isolated DNA was aliquoted in 30-μl volumes and stored at −80°C. The DNA copies of M. gallisepticum were determined by real-time PCR (RT-PCR) (12). The in vitro standard DNA curve was plotted by the number of M. gallisepticum organisms derived from the culture method and the cycle threshold (CT) values obtained using RT-PCR results, as previously described (12, 13).
Neutropenia model.
In order to study the effectiveness of valnemulin solely and to eliminate immunity caused by different variables among different chickens, a neutropenic model was used. The South China Agriculture University Animal ethics committee approved all in vivo experiments (March 2014). In addition, all husbandry practices and experimental operations were performed with full consideration of animal welfare. One-day-old chickens (weighing 35 to 45 g) were provided from the Guangdong Academy of Agricultural Sciences (Guangzhou, Guangdong, China). The chickens were M. gallisepticum free and fed with antibacterial-free food and water ad libitum. Two days postarrival, the chickens were rendered neutropenic with cyclophosphamide (Puboxin Biotechnology Co., Ltd., Beijing, China) administered intramuscularly (i.m.) at 60 mg/kg for 3 days (15). Blood was drawn from the neck vein, and leukocytes were counted with smears. The animals were severely granulocytopenic (absolute leukocyte count, <1,000/mm3) and remained so for 8 days after the last injection of cyclophosphamide.
M. gallisepticum intratracheal infection model.
M. gallisepticum is a major cause of CRD in chickens. Thus, a M. gallisepticum intratracheal infection model was utilized in this study. Briefly, neutropenic chickens were inoculated with a 0.2-ml aliquot solution containing approximately 108 CCU of the M. gallisepticum strain, a 95% infective dose (ID95) for the study strain, as established in pilot studies, via intratracheal injection for 3 days. Clinical symptoms and bacteriological examinations were performed to ensure infection. To quantify the pathogen load, tracheae, air sacs, and lungs were removed at the time of sacrifice, homogenized in 2 ml of phosphate-buffered saline (PBS), and centrifuged at 500 rpm for 5 min. A 0.5-ml aliquot of supernatant was used for DNA extraction with a bacterial DNA kit (Omega Bio-Tek, Inc., Norcross, GA). A DNA template was used for CT value determination through RT-PCR. To confirm the recovery rate of DNA extraction from chicken lung, trachea, and air sac tissue samples, the same amount of 10-fold dilutions (100 to 10−6) of an M. gallisepticum sample from an in vitro DNA standard curve was added to blank chicken lung, trachea, and air sac tissue samples, and the same procedure as described above for the samples was used.
Determination of DNA copies of M. gallisepticum using RT-PCR.
RT-PCR was used to identify DNA copies of M. gallisepticum using M. gallisepticum-specific primers Mg14F (5′-GAG CTA ATC TGT AAA GTT GGT C-3′; melting temperature [Tm], 57.80°C) and Mg13R (5′-GCT TCC TTG CGG TTA GCA AC-3′; Tm, 63.6°C), as described in a previous study (12). All RT-PCRs were performed on a Bio-Rad iQ5 (Bio-Rad Laboratories, Inc., USA) using the SYBR Ex Taq premix (TaKaRa, Shiga, Japan). The DNA template extracted from samples or elution buffer (negative control), the forward and reverse primers per reaction, SYBR premix, and water were mixed according to recommended procedures, at a final volume of 25 μl. The PCR mixture was incubated for 40 cycles, and fluorescence was monitored during each cycle at 72°C. After amplification, a melting curve analysis was performed starting at 65°C, using a temperature transition rate of 0.2°C/s to determine the Tm values of the PCR products. The CT value was defined as the cycle number yielding a maximum value of the second derivative of the amplification curve of the sample. Samples were regarded as positive when both a measurable CT and the expected Tm (± 0.5°C) were seen.
Valnemulin PK in the neutropenic intratracheal infection model.
Infected neutropenic chickens were treated with i.m. valnemulin at a single doses of 1, 10, or 20 mg/kg. Blood samples in 1.5-ml aliquots were collected from the neck vein at 10 min, 30 min, and 1, 2, 4, 6, 8, 12, and 24 h after drug administration. Ten chickens were sampled at each time point. Serum was obtained by centrifuging blood samples at 3,000 rpm for 10 min and freezing at −20°C immediately until analysis (within 2 weeks). The valnemulin concentrations in serum were determined via a high-performance liquid chromatography-tandem mass spectrometry (LC-MS/MS) method, as described previously (16–18). The limit of quantitation was confirmed to be 2.5 ng/ml (19). The coefficient of correlation (R2) was 0.9992 for the linear range of 2.5 to 500 ng/ml. To overcome the carryover effect, water was inserted in the detection queue for every 10 samples. The intra- and interday coefficients of variation were determined to be 5.2% and 8.7%, respectively.
Efficacy of valnemulin in neutropenic chicken intratracheal infection model.
To evaluate the effectiveness of valnemulin, infected neutropenic chickens were treated with either 0.85% NaCl (control) or i.m. valnemulin at 1, 2, 4, 6, 8, 10, 15, or 20 mg/kg once daily at 24 h postinfection for 3 days. At 24 h after the last drug administration, the animals were euthanized, and the tracheae, air sacs, and lungs were collected, homogenized in 2 ml of PBS, and centrifuged at 500 rpm for 5 min. A 0.5-ml aliquot of supernatant was used for DNA extraction with a bacterial DNA kit (Omega Bio-Tek, Inc., Norcross, GA), as described above. The number of DNA copies of M. gallisepticum in these samples was measured by RT-PCR, as described above. The amount of M. gallisepticum was expressed as color-changing unit equivalents per milliliter.
PK and PD analysis.
The PK profiles of valnemulin were analyzed by the noncompartmental model with uniform weighting using the WinNonlin software (version 6.1; Pharsight, CA, USA). The surrogate marker of antibacterial effectiveness, AUC24/MIC, was calculated using in vitro MIC values and PK parameters derived from three i.m. administrations of valnemulin. The bacterial load for each sample was calculated according to the in vitro standard DNA curve of the CT value and bacteria counting by the culture method, measured as the color-changing unit equivalents per milliliter. The effectiveness of valnemulin was expressed as the reduction in M. gallisepticum load after treatment compared to that before treatment. The in vivo PK-PD relationship of valnemulin was described using a sigmoid maximum effect (Emax) model in the WinNonlin software (version 6.1; Pharsight), with the following equation:
| (1) |
where E is the change in log10 color-changing unit (CCU) equivalents per milliliter for different dosage regimens, E0 is the change in log10 CCU equivalents per milliliter in the control sample (absence of valnemulin), Emax is the difference in effect between the greatest amount of growth (as seen for the growth control, E0) and the greatest amount of kill, Ce is the AUC24/MIC in the effect compartment, EC50 is the AUC24/MIC value producing a 50% reduction in bacterial counts, and N is the Hill coefficient that describes the steepness of the AUC24/MIC effect curve (20).
Dosage calculation.
In order to deduce a more rational regimen, the general formula adapted by Toutain, Bousquet-Mélou, and Martinez (21) was employed to estimate the dosages for different magnitudes of efficiency:
| (2) |
where dose is the optimal dose (in milligrams per kilogram of body weight per day), CL is body clearance (in liters per kilogram per hour), AUC24/MIC is the breakpoint marker for the desired effect, MIC90 is the MIC inhibiting 90% of strains (in milligrams per liter), F is the bioavailability, and fu is the free drug fraction.
RESULTS
Susceptibility testing.
The MIC of valnemulin against the study strain was 0.0014 μg/ml.
In vitro standard DNA curve and recovery rate.
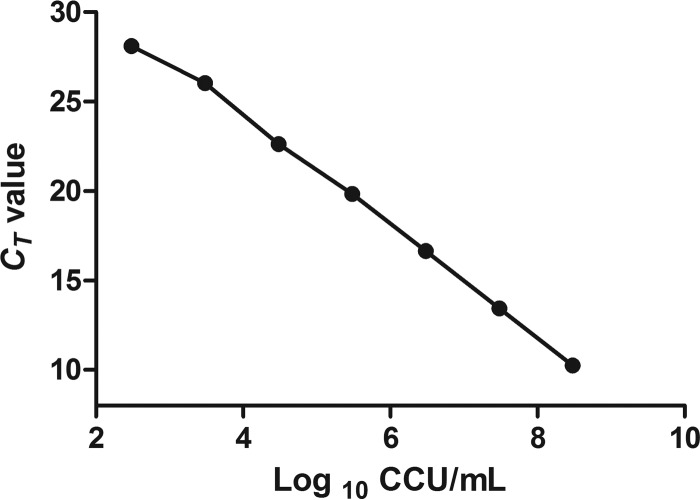
There was a significant correlation between the CT values and log10 color-changing units per milliliter (y = −3.028x + 36.155; R2 = 0.9976; Fig. 2), indicating that the correlation between the molecular and culturing methods is significant. The limit of detection was 3 × 102 CCU equivalents/ml. A small range (0.087 to 0.45) of standard deviations of the CT values was observed. The recovery rates at different dilutions were 56.5% ± 5.08%.
FIG 2.
In vitro DNA standard curve. Relationship of DNA standards between CT value and culture results (in log10 CCU per milliliter). The mean values from three different RT-PCR runs are shown.
Neutropenia model.
The animals were severely granulocytopenic (absolute leukocyte count, <1,000/mm3) and remained so for 8 days after the last injection of cyclophosphamide.
M. gallisepticum intratracheal infection model.
An evaluation of the M. gallisepticum infection model mainly depended on clinical symptoms and bacteriological assays. Depression, mouth breathing, and eye closures were observed in infected animals. In addition, air sacculitis, the cardinal sign of M. gallisepticum, infection was noticed in 95% of the infected chickens. The mean M. gallisepticum load was 0.3 × 106 CCU for all inoculated chickens. The morbidity and mortality rates were 95% and 17%, respectively, at 5 days after infection. Neither clinical signs nor air sacculitis were observed in noninfected control animals. The bacteriological assay was negative in the control chickens.
PK profiles of valnemulin in neutropenic intratracheal infection model.
The main PK parameters are presented in Table 1. The maximum concentration of drug in serum (Cmax) values were 6.34, 71.32, and 111.13 μg/ml for 1-, 10-, and 20-mg/kg doses, respectively, which were observed at 30 min after administration (Fig. 3). The half-life (t1/2β) was approximately 2.8 h for all three different doses. In addition, a concentration-dependent AUC24 was seen (13.88, 125.96, and 213.61 μg · h/ml for 1-, 10-, and 20-mg/kg doses, respectively). Importantly, a significant correlation between dose and AUC24 or Cmax was observed (R2 = 0.9899 and 0.972 for dose-to-AUC24 and dose-to-Cmax ratios, respectively; Fig. 4). As the AUC24 increased in a dose-dependent manner from 1 to 20 mg/kg, the AUC24 of 2, 4, 6, 8, and 15 mg/kg was calculated according to the linear relationship. These results indicate that the analytic chemistry method is a reasonable tool to derive meaningful PK profiles and may predict valnemulin efficacy.
TABLE 1.
Pharmacokinetic parameters of valnemulin in serum following single-dose intramuscular administration various doses in M. gallisepticum infected chickens (n = 10/group)
| Parametera | Results for valnemulin dose (mg/kg) of: |
||
|---|---|---|---|
| 1 | 10 | 20 | |
| Cmax (μg/ml) | 6.34 | 71.32 | 111.13 |
| Tmax (h) | 0.5 | 0.5 | 0.5 |
| t1/2β (h) | 2.78 | 2.81 | 2.71 |
| AUC24 (μg · h/ml) | 13.88 | 125.96 | 213.61 |
| CLβ/F (liters/h/kg) | 0.072 | 0.079 | 0.093 |
| Vz/F (liters/kg) | 0.29 | 0.32 | 0.36 |
Cmax, maximum serum concentration; Tmax, time of maximum serum concentration; t1/2β, elimination half-life; AUC24, 24-h area under serum concentration-time curve; F, bioavailability; CLβ/F, clearance divided by bioavailability; Vz/F, volume of distribution scaled by bioavailability.
FIG 3.
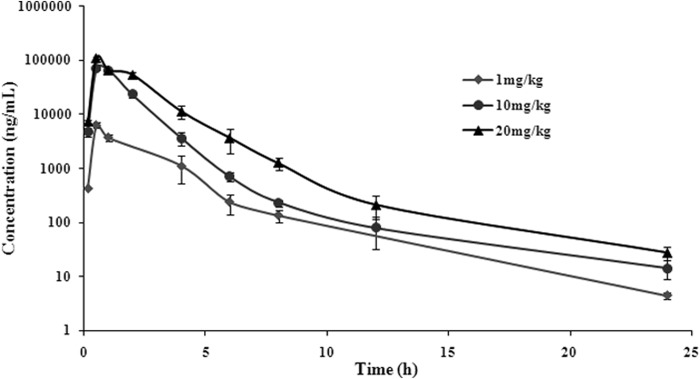
Serum concentrations of valnemulin following single-dose intramuscular administration at 1, 10, or 20 mg/kg in M. gallisepticum intratracheal infection model (n = 10/time point).
FIG 4.
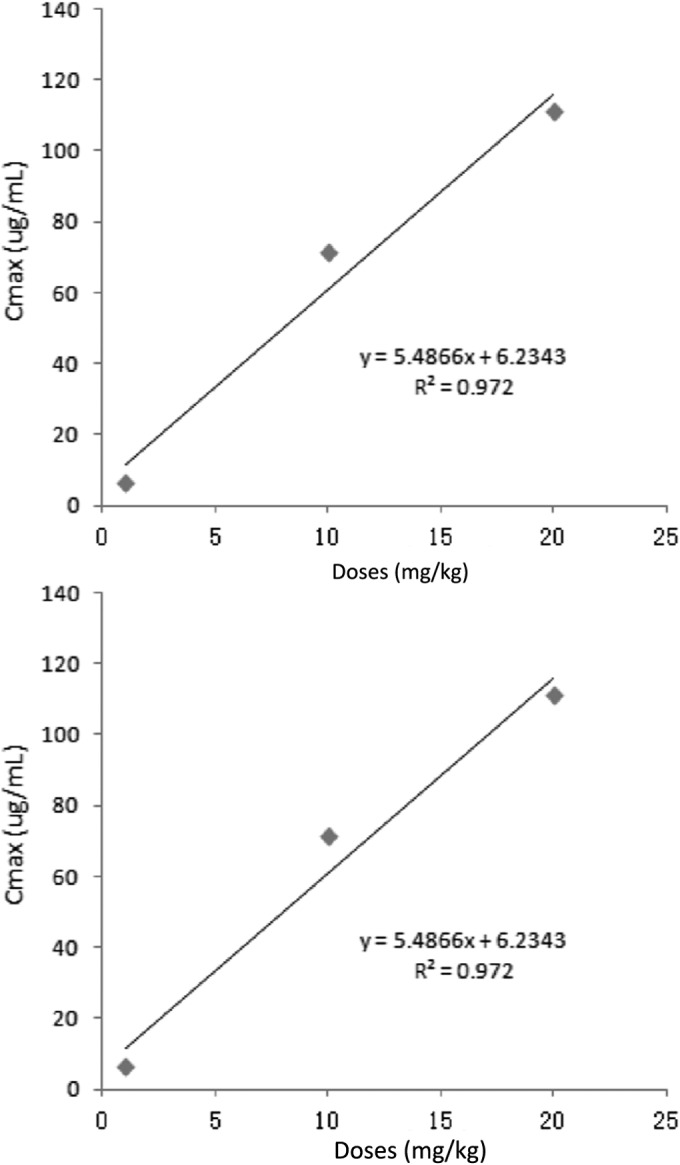
Linear regression plots between administered dose and Cmax values, and between administered dose and AUC24 values.
In vivo effectiveness of valnemulin in neutropenic chicken intratracheal infection model.
The CT values of respiratory tissues from chickens administered valnemulin doses of 0, 1, 2, 4, 6, 8, 10, 15, or 20 mg/kg increased with increasing dose, implying that the bacteria load decreased. The CT value increased sharply between the doses of 1 and 8 mg/kg and gently from 8 to 20 mg/kg. The calculated numbers in vivo of M. gallisepticum after drug treatment are shown in Fig. 5.
FIG 5.
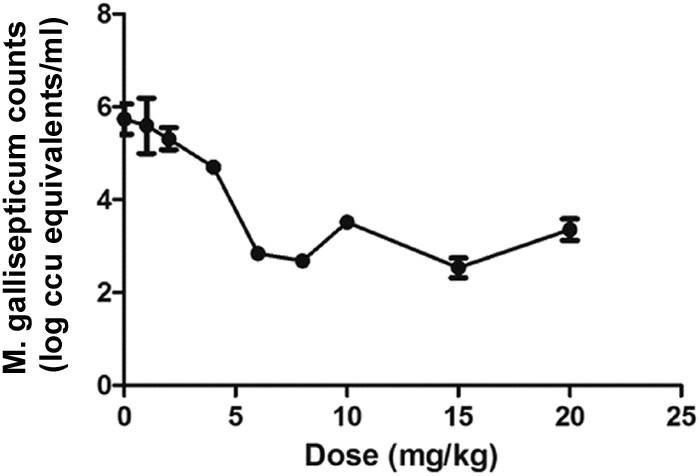
Calculated in vivo M. gallisepticum counts after valnemulin treatment (n = 4/dose).
Valnemulin PK-PD profiles.
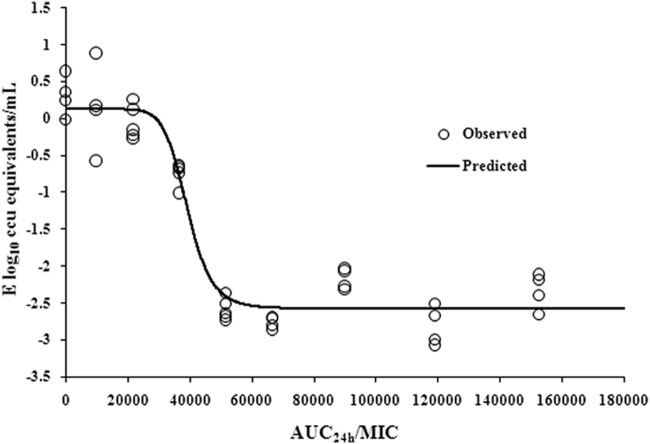
The AUC24/MIC values for doses of 0, 1, 2, 4, 6, 8, 10, 15, and 20 mg/kg were 0, 9,917, 21,778, 36,750, 51,714, 66,692, 89,971, 119,092, and 152,579, respectively. The parameter AUC24/MIC correlated significantly with effectiveness (R2 = 0.9669). The profile of sigmoid Emax model describing the relationship of antibiotic effectiveness and AUC24/MIC is presented in Fig. 6. The AUC24/MIC ratios for mycoplasmastasis (reduction of 0 log10 CCU/ml), a reduction of 1 log10 CCU/ml, and a reduction of 2.5 log10 CCU/ml were 28,820, 38,030, and 56,256, respectively (Table 2). The EC50 was 39,299, and the slope of the graph of the AUC24/MIC ratio versus effectiveness was 9.98.
FIG 6.
Sigmoid Emax relationships between antimycoplasmal effect (E, log10 CCU equivalents/ml) and in vivo AUC24/MIC ratio against M. gallisepticum in serum of chickens.
TABLE 2.
Pharmacodynamic analysis of valnemulin in M. gallisepticum intratracheal infection model
| Parametera | Value |
|---|---|
| Emax (log10 CCU equivalents/ml) | 2.69 |
| E0 (log10 CCU equivalents/ml) | 0.11 |
| EC50 | 39,299 |
| AUC/MIC for reduction of (log10 CCU equivalents/ml): | |
| 0 | 28,820 |
| 1 | 38,030 |
| 2.5 | 56,256 |
| Slope (N) | 9.98 |
E0, change in log10 CCU equivalents/ml in the control sample (absence of valnemulin); Emax, difference in effect between the greatest amount of growth (as seen for the growth control, E0) and the greatest amount of kill; EC50, AUC24/MIC ratio producing a 50% reduction in bacterial counts; N, Hill coefficient that describes the steepness of the AUC24/MIC effect curve.
Dosage calculation.
For dosing calculations, bioavailability had been taken into account, owing to the extravascular route of administration, and the free-drug fraction was not required for using PD data generated in vivo. The MIC of S6 was substituted for the MIC90, because not enough valnemulin MIC data were available to provide an estimate of the MIC90. A dosage of 6.5 mg/kg was calculated for a reduction of 2.5 log10 CCU/ml.
DISCUSSION
M. gallisepticum is a highly transmissible and persistent pathogen in chickens, turkeys, and some wild birds. Significant losses in all sectors of the poultry industry have occurred due to infection by this organism (22). Outbreaks have been seen even in vaccinated birds, demonstrating that further means of control are needed (23).
Valnemulin shows excellent effectiveness for treating mycoplasmal infection and rarely induces significant resistance (16). Its PK character is also favorable; however, its chemotherapeutic properties are sparse. The use of a PK-PD model to identify the PD activity by integrating the PK character, MIC, and pathogen load outcome has been proven helpful in the design of rational dosage regimens in humans and animals and in the determination of susceptibility breakpoints (24, 25).
In the present investigation, a number of interesting findings emerged. First, to the best of our knowledge, this is the first time the dose proportionality of valnemulin PK in the range of 1 mg/kg to 20 mg/kg has been described in chickens. The rate and extent of absorption were constant among tested doses (1, 10, and 20 mg/kg), with clearance and distribution volume values of 0.072, 0.079, and 0.093 h and 0.29, 0.32, 0.36 liters/kg, respectively. The significant correlation between dose and AUC24 (R2 = 0.9899) allows us to calculate the AUC24 for other dosages. This phenomenon was confirmed in other research, i.e., for marbofloxacin PK in pigs within doses ranging from 4 to 16 mg/kg (26); however, our estimated PK parameters were somewhat different from those published previously for chickens infected by M. gallisepticum plus Escherichia coli (17). After i.m. administration at 10 mg/kg, the drug was absorbed quickly, with a Cmax of 71.32 μg/ml achieved at 0.5 h in 9-day-old infected chickens, while the mean Cmax values of 27.94 μg/ml and time to Cmax (Tmax) of 1.57 h occurred in adult chickens infected with M. gallisepticum plus E. coli. In addition, the t1/2β was much shorter in our model, with a value of 2.81 h compared to the value of 6.50 h in the chickens infected with M. gallisepticum plus E. coli. This difference may be explained by age and pathological situation. In our study, M. gallisepticum caused only respiratory system injury in 9-day-old chickens, while the injury from M. gallisepticum plus E. coli affected the whole body (lungs, liver, kidneys, heart, and others) in the adult chicken (17). These results emphasize the influence of age and physiological status on pharmacokinetics (27, 28).
Our data show a significant correlation between the PK-PD index, AUC24/MIC, and the in vivo antibacterial effects of valnemulin against M. gallisepticum (R2 = 0.9669), which was in accordance with previous studies of valnemulin against Staphylococcus aureus (18, 29). The AUC24/MIC ratios for mycoplasmastasis (a reduction of 0 log10 CCU equivalents/ml), a reduction of 1 log10 CCU equivalents/ml, and a reduction of 2.5 log10 CCU equivalents/ml (28,820, 38,030, and 56,256, respectively) were much higher than those resulting ex vivo (634, 1,297, and 1,987 for reductions of 0, 3, and 4 log10 CCU/ml, respectively) (30). This may due to the following reasons. First, the antibacterial concentration is constant in ex vivo PK-PD studies, while the concentration of antibacterial drug declined in animals because of body clearance. Next, in ex vivo studies, the drug acts on M. gallisepticum directly, but in vivo, the concentration in serum was not the real one acting against M. gallisepticum, as the infection site is the air sac or respiratory system, and valnemulin concentrations in air sacs or lungs are usually different from that in serum. Finally, in vivo PK-PD takes all the infection factors into consideration, which may influence the ratio. The in vivo PK-PD model showed great advantage over in vitro or ex vivo PK-PD models, as it considered the complex environment of the infected animals. In addition, we recognize that the dose fractionation studies were not included in the current manuscript. Therefore, the PD index of valnemulin was not entirely demonstrated. Future studies will be designed to adjudicate the effects of these limitations.
We also demonstrated that in order to achieve a reduction of ≥2.5 log10 CCU equivalents/ml in bacterial counts, the recommended daily dose would be ≥6.5 mg/kg daily for 3 days when valnemulin was applied to treat M. gallisepticum-infected chickens, with an MIC of 0.0014 μg/ml, which is almost half of the recommended dose range for swine enzootic pneumonia (10 to 12 mg/kg) (31). This recommended daily dose has been proven to be effective but less expensive for farmers. Additionally, a much lower drug dosage can be more ecofriendly, cost-effective, and minimize the drug residue burden on public health via human food consumption.
The gold standard for evaluating the effectiveness of an antibacterial drug is culturing and counting bacteria; however, the results for M. gallisepticum were usually influenced by overgrowth of faster growing Mycoplasma species or were impeded by other organisms or no growth in subculture. In addition, the cultivation technique is expensive, laborious, and requires a lot of time (32). Real-time PCR methodology, which is reported to be capable of quantitative detection of M. gallisepticum in an in vivo model and from clinical samples has significantly overcome the problems with time, specificity, and sample size (12).
In this study, neutropenic chickens were used to remove host immunity factors that may play an important role in M. gallisepticum infections and in antimicrobial effectiveness. Previous studies suggest that the PK-PD index magnitude necessary for successful therapy is reduced in animal models with neutrophils present (33). Therefore, further studies on the elucidation of host immunity-pathogen interactions are essential. In addition, a population PK approach should be conducted to derive a population clearance of valnemulin for use in dose calculations. Moreover, MIC distributions should be evaluated to take variations in susceptibility of M. gallisepticum to valnemulin into account. This dose regimen should be validated in clinical practice by evaluating therapeutic outcomes and monitoring resistance development.
In conclusion, the present study characterized the in vivo effectiveness of valnemulin against M. gallisepticum in a neutropenic chicken model. The in vivo data suggest that animal dosage regimens should supply an AUC24/MIC of valnemulin of 56,256 for a reduction of 2.5 log10 CCU equivalents/ml of M. gallisepticum. These studies suggest that valnemulin, if used for the treatment of M. gallisepticum, with an MIC90 at 0.0014 μg/ml, would benefit from a valnemulin dose of 6.5 mg/kg once daily for 3 days, which is convenient for use in the clinical setting.
ACKNOWLEDGMENTS
This work was supported by the Program for Changjiang Scholars and Innovative Research Team of the University of Ministry of Education of China (grant IRT13063), the National Science Fund for Distinguished Young Scholars (grant 31125026), and the Science and Technology Planning Project of Guangdong Province, China (grant 2012A020800004).
REFERENCES
- 1.Benskin CM, Wilson K, Jones K, Hartley IR. 2009. Bacterial pathogens in wild birds: a review of the frequency and effects of infection. Biol Rev Camb Philos Soc 84:349–373. doi: 10.1111/j.1469-185X.2008.00076.x. [DOI] [PubMed] [Google Scholar]
- 2.Levisohn S, Kleven SH. 2000. Avian mycoplasmosis (Mycoplasma gallisepticum). Rev Sci Tech 19:425–442. [PubMed] [Google Scholar]
- 3.Mohammed HO, Carpenter TE, Yamamoto R. 1987. Economic impact of Mycoplasma gallisepticum and M. synoviae in commercial layer flocks. Avian Dis 31:477–482. doi: 10.2307/1590727. [DOI] [PubMed] [Google Scholar]
- 4.Maurer FP, Bruderer VL, Ritter C, Castelberg C, Bloemberg GV, Bottger EC. 2014. Lack of antimicrobial bactericidal activity in Mycobacterium abscessus. Antimicrob Agents Chemother 58:3828–3836. doi: 10.1128/AAC.02448-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Feberwee A, von Banniseht-Wysmuller T, Vernooij JC, Gielkens AL, Stegeman JA. 2006. The effect of vaccination with a bacterin on the horizontal transmission of Mycoplasma gallisepticum. Avian Pathol 35:35–37. doi: 10.1080/03079450500465700. [DOI] [PubMed] [Google Scholar]
- 6.Karlsson M, Gunnarsson A, Franklin A. 2001. Susceptibility to pleuromutilins in Brachyspira (Serpulina) hyodysenteriae. Anim Health Res Rev 2:59–65. doi: 10.1079/AHRR200118. [DOI] [PubMed] [Google Scholar]
- 7.Andes D, Craig WA. 2002. Animal model pharmacokinetics and pharmacodynamics: a critical review. Int J Antimicrob Agents 19:261–268. doi: 10.1016/S0924-8579(02)00022-5. [DOI] [PubMed] [Google Scholar]
- 8.Silveira RM, Fiorentin L, Marques EK. 1996. Polymerase chain reaction optimization for Mycoplasma gallisepticum and M. synoviae diagnosis. Avian Dis 40:218–222. doi: 10.2307/1592392. [DOI] [PubMed] [Google Scholar]
- 9.Marois C, Dufour-Gesbert F, Kempf I. 2002. Polymerase chain reaction for detection of Mycoplasma gallisepticum in environmental samples. Avian Pathol 31:163–168. doi: 10.1080/03079450120118658. [DOI] [PubMed] [Google Scholar]
- 10.Wittwer CT, Ririe KM, Andrew RV, David DA, Gundry RA, Balis UJ. 1997. The LightCycler: a microvolume multisample fluorimeter with rapid temperature control. Biotechniques 22:176–181. [DOI] [PubMed] [Google Scholar]
- 11.Carli KT, Eyigor A. 2003. Real-time polymerase chain reaction for Mycoplasma gallisepticum in chicken trachea. Avian Dis 47:712–717. doi: 10.1637/6041. [DOI] [PubMed] [Google Scholar]
- 12.Mekkes DR, Feberwee A. 2005. Real-time polymerase chain reaction for the qualitative and quantitative detection of Mycoplasma gallisepticum. Avian Pathol 34:348–354. doi: 10.1080/03079450500179954. [DOI] [PubMed] [Google Scholar]
- 13.Grodio JL, Dhondt KV, O'Connell PH, Schat KA. 2008. Detection and quantification of Mycoplasma gallisepticum genome load in conjunctival samples of experimentally infected house finches (Carpodacus mexicanus) using real-time polymerase chain reaction. Avian Pathol 37:385–391. doi: 10.1080/03079450802216629. [DOI] [PubMed] [Google Scholar]
- 14.Hannan PC. 2000. Guidelines and recommendations for antimicrobial minimum inhibitory concentration (MIC) testing against veterinary mycoplasma species. International Research Programme on Comparative Mycoplasmology. Vet Res 31:373–395. doi: 10.1051/vetres:2000100. [DOI] [PubMed] [Google Scholar]
- 15.Craig WA, Andes DR. 2008. In vivo pharmacodynamics of ceftobiprole against multiple bacterial pathogens in murine thigh and lung infection models. Antimicrob Agents Chemother 52:3492–3496. doi: 10.1128/AAC.01273-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang R, Yuan LG, He LM, Zhu LX, Luo XY, Zhang CY, Yu JJ, Fang BH, Liu YH. 2011. Pharmacokinetics and bioavailability of valnemulin in broiler chickens. J Vet Pharmacol Ther 34:247–251. doi: 10.1111/j.1365-2885.2010.01215.x. [DOI] [PubMed] [Google Scholar]
- 17.Xiao X, Zhao DH, Yang X, Shi W, Deng H, Ma J, Zhang S, Liu YH. 2014. Mycoplasma gallisepticum and Escherichia coli mixed infection model in broiler chickens for studying valnemulin pharmacokinetics. J Vet Pharmacol Ther 37:99–102. doi: 10.1111/jvp.12065. [DOI] [PubMed] [Google Scholar]
- 18.Zhao DH, Zhou YF, Yu Y, Shi W, Yang X, Xiao X, Deng H, Qiao GG, Fang BH, Liu YH. 2014. Integration of pharmacokinetic and pharmacodynamic indices of valnemulin in broiler chickens after a single intravenous and intramuscular administration. Vet J 201:109–115. doi: 10.1016/j.tvjl.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Patel N, Pai MP, Rodvold KA, Lomaestro B, Drusano GL, Lodise TP. 2011. Vancomycin: we can't get there from here. Clin Infect Dis 52:969–974. doi: 10.1093/cid/cir078. [DOI] [PubMed] [Google Scholar]
- 20.Burton ME, Shaw LM, Schentag JJ, Evans W. 2005. Applied pharmacokinetics & pharmacodynamics: principles of therapeutic drug monitoring, p 61–65. Lippincott Williams and Wilkins Press, Philadelphia, PA. [Google Scholar]
- 21.Toutain PL, Bousquet-Mélou A, Martinez M. 2007. AUC/MIC: a PK/PD index for antibiotics with a time dimension or simply a dimensionless scoring factor? J Antimicrob Chemother 60:1185–1188. doi: 10.1093/jac/dkm360. [DOI] [PubMed] [Google Scholar]
- 22.Mohammed HO, Carpenter TE, Yamamoto R, McMartin DA. 1986. Prevalence of Mycoplasma gallisepticum and M. synoviae in commercial layers in southern and central California. Avian Dis 30:519–526. doi: 10.2307/1590416. [DOI] [PubMed] [Google Scholar]
- 23.Evans JD, Leigh SA, Branton SL, Collier SD, Pharr GT, Bearson SMD. 2005. Mycoplasma gallisepticum: current and developing means to control the avian pathogen. J Appl Poult Res 14:757–763. doi: 10.1093/japr/14.4.757. [DOI] [Google Scholar]
- 24.Craig WA. 1998. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 26:1–10, quiz 11–12. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 25.Andes D, Craig WA. 1998. In vivo activities of amoxicillin and amoxicillin-clavulanate against Streptococcus pneumoniae: application to breakpoint determinations. Antimicrob Agents Chemother 42:2375–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schneider M, Paulin A, Dron F, Woehrle F. 2014. Pharmacokinetics of marbofloxacin in pigs after intravenous and intramuscular administration of a single dose of 8 mg/kg: dose proportionality, influence of the age of the animals and urinary elimination. J Vet Pharmacol Ther 37:523–530. doi: 10.1111/jvp.12125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blot SI, Pea F, Lipman J. 2014. The effect of pathophysiology on pharmacokinetics in the critically ill patient–concepts appraised by the example of antimicrobial agents. Adv Drug Deliv Rev 77:3–11. doi: 10.1016/j.addr.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Shi S, Klotz U. 2011. Age-related changes in pharmacokinetics. Curr Drug Metab 12:601–610. doi: 10.2174/138920011796504527. [DOI] [PubMed] [Google Scholar]
- 29.Novak R. 2011. Are pleuromutilin antibiotics finally fit for human use? Ann N Y Acad Sci 1241:71–81. doi: 10.1111/j.1749-6632.2011.06219.x. [DOI] [PubMed] [Google Scholar]
- 30.Xiao X, Sun J, Chen Y, Zou M, Zhao DH, Liu YH. 2015. Ex vivo pharmacokinetic and pharmacodynamic analysis of valnemulin against Mycoplasma gallisepticum S6 in Mycoplasma gallisepticum and Escherichia coli co-infected chickens, in press. Vet J doi: 10.1016/j.tvjl.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 31.European Medicines Agency. 2014. Econor product profile. European Medicines Agency, London, United Kingdom: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/veterinary/000042/WC500064379.pdf Accessed 9 July 2014. [Google Scholar]
- 32.Garcia M, Jackwood MW, Levisohn S, Kleven SH. 1995. Detection of Mycoplasma gallisepticum, M synoviae, and M. iowae by multi-species polymerase chain reaction and restriction fragment length polymorphism Avian Dis 39:606–616. [PubMed] [Google Scholar]
- 33.Pechère M, Letarte R, Pechère JC. 1987. Efficacy of different dosing schedules of tobramycin for treating a murine Klebsiella pneumoniae bronchopneumonia. J Antimicrob Chemother 19:487–491. doi: 10.1093/jac/19.4.487. [DOI] [PubMed] [Google Scholar]