Abstract
There are limited treatment options for carbapenem-resistant Gram-negative infections. Currently, there are suggestions in the literature that combination therapy should be used, which frequently includes antibiotics to which the causative pathogen demonstrates in vitro resistance. This case-control study evaluated risk factors associated with all-cause mortality rates for critically ill patients with carbapenem-resistant Gram-negative bacteremia. Adult patients who were admitted to an intensive care unit with sepsis and a blood culture positive for Gram-negative bacteria resistant to a carbapenem were included. Patients with polymicrobial, recurrent, or breakthrough infections were excluded. Included patients were classified as survivors (controls) or nonsurvivors (cases) at 30 days after the positive blood culture. Of 302 patients screened, 168 patients were included, of whom 90 patients died (53.6% [cases]) and 78 survived (46.4% [controls]) at 30 days. More survivors received appropriate antibiotics (antibiotics with in vitro activity) than did nonsurvivors (93.6% versus 53.3%; P < 0.01). Combination therapy, defined as multiple appropriate agents given for 48 h or more, was more common among survivors than nonsurvivors (32.1% versus 7.8%; P < 0.01); however, there was no difference in multiple-agent use when in vitro activity was not considered (including combinations with carbapenems) (87.2% versus 80%; P = 0.21). After adjustment for baseline factors with multivariable logistic regression, combination therapy was independently associated with decreased risk of death (odds ratio, 0.19 [95% confidence interval, 0.06 to 0.56]; P < 0.01). These data suggest that combination therapy with multiple agents with in vitro activity is associated with improved survival rates for critically ill patients with carbapenem-resistant Gram-negative bacteremia. However, that association is lost if in vitro activity is not considered.
INTRODUCTION
Carbapenem resistance among Gram-negative bacteria has been increasingly reported (1). Between 2004 and 2008, the proportion of Klebsiella pneumoniae isolates resistant to meropenem in the United States increased from 0.6% to 5.6% (2). In certain geographic areas, such as New York City, prevalence rates are substantially higher and carbapenem resistance is considered endemic (3). Additionally, carbapenem resistance is a significant problem for critically ill patients admitted to an intensive care unit (ICU), as an ICU stay has consistently been shown to be an independent predictor of the acquisition of a carbapenem-resistant Gram-negative infection (4, 5).
Furthermore, outcomes of carbapenem-resistant Gram-negative bacteremia are known to be poor. Infections with carbapenem-resistant Gram-negative organisms are associated with increased mortality rates, compared to carbapenem-susceptible organisms, and mortality rates are exceedingly high for these infections (4–8). In fact, attributable mortality rates for carbapenem-resistant Klebsiella pneumoniae bacteremia are as high as 50% (9). Additionally, outcomes such as hospital length of stay are worsened with carbapenem-resistant Gram-negative infections (4, 7, 8).
Unfortunately, there are limited treatment options available for carbapenem-resistant Gram-negative infections, because these organisms are typically resistant to all beta-lactams and other classes of antimicrobials in addition to carbapenems. Treatment options may include tigecycline, colistin, and/or aminoglycosides (10); however, the optimal treatment strategy for carbapenem-resistant Gram-negative infections has yet to be identified. Some recently published studies suggest that combination therapy is associated with improved survival rates, compared with monotherapy (11–13). Although the definitions of combination therapy varied in those studies, combination therapy regimens have commonly included the use of agents to which the causative pathogen demonstrates in vitro resistance. For example, in two studies of carbapenemase-producing Klebsiella pneumoniae bacteremia, a combination regimen including a carbapenem was associated with increased survival rates (11, 12). The use of a carbapenem for carbapenem-resistant Gram-negative infections is suggested based on in vitro synergistic activity and clinical experience with the drugs in combination with other antimicrobials (14); however, the data are inconsistent, and in vitro synergy is difficult to predict (15). Furthermore, the practice of using antimicrobials to which the causative organism displays in vitro resistance to treat an infection seems counterintuitive, even when the antimicrobials are used in combination with agents with in vitro activity.
Although there are suggestions in the literature that combination therapy is associated with improved survival rates, previous definitions of combination therapy did not consistently base the combination therapy on in vitro activity; therefore, it is still unknown whether combination therapy with two or more agents with in vitro activity is associated with decreased mortality rates. The purpose of this study is to evaluate the effect of combination therapy based on in vitro activity, compared with multiple antimicrobial use when in vitro activity is not considered, on 30-day all-cause mortality rates among patients admitted to an ICU with sepsis due to carbapenem-resistant Gram-negative bacteria.
MATERIALS AND METHODS
Study design.
This case-control study included adult patients at least 18 years of age who were admitted to an ICU at the time of or within 12 h after a positive blood culture for a carbapenem-resistant Gram-negative organism. Carbapenem resistance was defined based on Clinical and Laboratory Standards Institute (CLSI) guidelines for carbapenem resistance, i.e., MIC greater than 1 μg/ml for ertapenem or 2 μg/ml for doripenem, imipenem, or meropenem (16). A modified Hodge test was performed on all Enterobacteriaceae isolates, as described previously (17). For study inclusion, patients were also required to have sepsis, which was considered the presence of at least 2 of 4 possible systemic inflammatory response syndrome (SIRS) characteristics (18). Patients were excluded if they had a polymicrobial bloodstream infection, a recurrent infection with a carbapenem-resistant organism, or a breakthrough infection (3). A recurrent infection was considered any bloodstream infection with another carbapenem-resistant organism within the previous 6 months. A breakthrough infection was defined as having a positive blood culture despite receiving appropriate antimicrobial treatment (antibiotics with in vitro activity) for the organism of interest for more than 12 h. Breakthrough infections were excluded due to concerns regarding possible inadequate source control. Cases were identified as patients with carbapenem-resistant Gram-negative bacteremia who died from any cause at or before 30 days after the positive blood culture. Control patients were patients who survived 30 days. The study was performed at a large, tertiary-care, academic medical center and was approved by the Cleveland Clinic institutional review board (Cleveland, OH).
Data collection and outcomes.
Data, including demographic information, severity of illness, type of organism, source of infection, and antimicrobial therapy, were collected from the electronic or paper medical records and microbiological database. Baseline severity of illness was assessed through the acute physiology and chronic health evaluation II (APACHE II) score (19), Charlson comorbidity index (CCI) score (20), sequential organ failure assessment (SOFA) score (21), and baseline laboratory markers and vital signs. The source of infection was identified by review of the electronic records and microbiological database. The source of infection was further stratified into 3 categories based on mortality risk, i.e., high (>20%), moderate (10 to 20%), or low (<10%) (22). Data collected regarding antimicrobial therapy included antibiotic susceptibility, MIC, daily antibiotic use for the first 14 days after the positive blood culture, and time to appropriate antibiotic initiation after the positive blood culture.
Antibiotics evaluated for daily use included tigecycline, colistin, aminoglycosides, and beta-lactams. Loading doses were given for colistin and tigecycline. Subsequently, colistin was administered at 4.5 mg/kg of body weight/day, divided into doses administered every 8 h. Aminoglycosides were administered according to extended-interval dosing when applicable (5 to 7 mg/kg every 24 h for gentamicin and tobramycin and 15 to 18 mg/kg every 24 h for amikacin). Beta-lactams, including carbapenems, were administered by bolus infusion. Doses were adjusted based on creatinine clearance values if necessary.
The primary objective was to examine the prevalence of combination therapy, which was considered the concomitant use of ≥2 appropriate agents for ≥48 h within the first 14 days after the positive blood culture, for nonsurvivors (cases) and survivors (controls). Appropriate therapy was considered therapy with in vitro activity against the pathogen of interest and was defined according to published MIC breakpoints for carbapenem resistance, including those of the CLSI and the European Committee on Antimicrobial Susceptibility Testing (EUCAST), taking into account pharmacokinetic and pharmacodynamic parameters along with standard dosing (Table 1) (16, 23). When there was a discrepancy between published guidelines, the more conservative breakpoint was selected. Isolate identification and antimicrobial susceptibility testing were performed with the Vitek 2 automated system (bioMérieux, Durham, NC). A secondary objective of this study was to evaluate the impact of multiple-agent use, defined as the concomitant use of ≥2 agents with Gram-negative activity for ≥48 h within the first 14 days after the positive blood culture, when the appropriateness of therapy was not considered (11). Carbapenems were included as a component of multiple-agent use and were considered to be associated with resistance for the in vitro sensitivity analysis. An additional analysis was performed to evaluate multiple-agent use among patients who received a carbapenem. Other secondary objectives were analyses of the time to appropriate antibiotics and the type of first appropriate antibiotic. The time to appropriate antibiotics was calculated only for patients who received appropriate antibiotics.
TABLE 1.
MIC breakpoints according to antibiotic
| Antibiotic | MIC breakpoint (μg/ml) |
|---|---|
| Colistin | ≤2 |
| Tigecycline | ≤1 |
| Amikacin | ≤8 |
| Gentamicin | ≤2 |
| Tobramycin | ≤2 |
| Trimethoprim-sulfamethoxazole | ≤2/38 |
| Ciprofloxacin | ≤0.5 |
| Ampicillin-sulbactam | ≤8/4 |
| Aztreonam | ≤4 |
| Ceftazidime | ≤4 |
| Piperacillin-tazobactam | ≤16/4 |
Statistical analyses.
The primary outcome was analyzed with the chi-square test. Other nominal data were evaluated using the chi-square test or Fisher's exact test, as appropriate. Continuous data were assessed for normalcy using the Shapiro-Wilk test and visual examination of the histogram and normal quantile plots. Continuous nonnormally distributed data and ordinal data were evaluated using the Mann-Whitney U test.
Multivariable analysis with standard logistic regression was performed to identify independent risk factors for death. Variables were entered into the model if they had biological plausibility for affecting the primary outcome and met the statistical criterion, determined a priori, of P values of <0.1 for baseline univariate comparisons. After identification of variables that met these criteria, variables that were colinear were removed from the model. All analyses were two sided, and results were considered statistically significant with P values of <0.05. Statistics were computed using SPSS software, version 11.5 (SPSS Inc., Chicago, Illinois).
RESULTS
Between October 2007 and November 2012, 302 patients who were admitted to an ICU with a blood culture positive for carbapenem-resistant Gram-negative bacteria were identified. Of the patients who were initially screened, 134 patients were excluded. The primary reasons for exclusion were polymicrobial bloodstream infections (n = 58 [43%]) and breakthrough infections (n = 43 [32%]). After exclusions, 168 patients were included. The case group consisted of 90 nonsurvivors at day 30 after the blood culture (53.6%), and the control group included 78 patients who survived to day 30 (46.4%) (Fig. 1).
FIG 1.
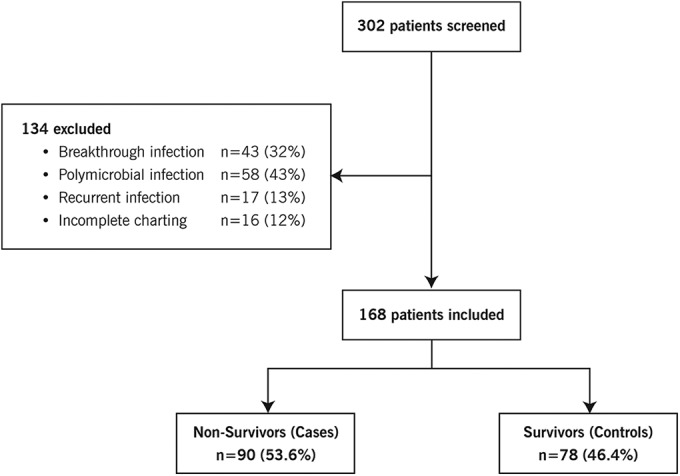
Study design and patient distribution.
Table 2 summarizes the baseline characteristics of survivors and nonsurvivors. In general, nonsurvivors had higher baseline levels of severity of illness than did survivors, based on both chronic and acute measures. The median age was significantly higher for nonsurvivors than survivors (62.1 versus 57.5 years; P < 0.01). CCI (score of 4 versus 3; P < 0.01), APACHE II (score of 26 versus 19; P < 0.01), and SOFA (score of 12 versus 7; P < 0.01) scores were all significantly higher for nonsurvivors than for survivors. Additionally, significantly more nonsurvivors were receiving vasopressors on day 1 of positive blood cultures than were survivors (n = 49 [54.4%] versus n = 21 [26.9%]; P < 0.01).
TABLE 2.
Univariate analysis of baseline factors associated with 30-day all-cause mortality rates for patients with carbapenem-resistant Gram-negative bacteremia
| Characteristica | Nonsurvivors (n = 90) | Survivors (n = 78) | Pb |
|---|---|---|---|
| Age (median [IQR]) (yr) | 62.1 (53–70.9) | 57.5 (45.3–65.9) | <0.01 |
| Male (no. [%]) | 47 (52.2) | 35 (44.9) | 0.34 |
| CCI score (median [IQR]) | 4 (3–7) | 3 (1.75–5.25) | <0.01 |
| Day 1 WBC count (median [IQR]) (103 cells/μl) | 12.6 (3.4–25) | 13 (8.6–19.8) | 0.44 |
| APACHE II score (median [IQR]) | 26 (22.75–33) | 19 (14–26) | <0.01 |
| SOFA score (median [IQR]) | 12 (9–17) | 7 (4.75–10) | <0.01 |
| Vasopressor use on day of positive culture (no. [%]) | 49 (54.4) | 21 (26.9) | <0.01 |
| Type of organism (no. [%]) | 0.1 | ||
| Acinetobacter baumannii | 27 (30) | 12 (15.4) | 0.03 |
| Klebsiella pneumoniae | 20 (22.2) | 16 (20.5) | 0.79 |
| Pseudomonas aeruginosa | 34 (37.8) | 37 (47.4) | 0.21 |
| Otherc | 9 (10) | 13 (16.7) | 0.2 |
| Carbapenem MICd (median [IQR]) (μg/ml) | |||
| Acinetobacter baumannii | 16 (16–16) | 16 (8–16) | 0.12 |
| Klebsiella pneumoniae | 16 (8–16) | 16 (8–16) | 0.5 |
| Pseudomonas aeruginosa | 16 (8–16) | 8 (5–14) | <0.01 |
| Source of bacteremia (no. [%]) | |||
| Abdominal | 19 (21.1) | 16 (20.5) | 0.92 |
| Bone | 1 (1.1) | 3 (3.8) | 0.34 |
| Catheter-related | 24 (26.7) | 22 (28.2) | 0.82 |
| Endovascular | 3 (3.3) | 5 (6.4) | 0.35 |
| Manipulation-related | 4 (4.4) | 5 (6.4) | 0.74 |
| Respiratory tract | 24 (26.7) | 16 (20.5) | 0.35 |
| Skin and soft tissue | 6 (6.7) | 5 (6.4) | 1.0 |
| Urinary tract | 4 (4.4) | 22 (28.2) | <0.01 |
| Unknown | 25 (27.8) | 5 (6.4) | <0.01 |
CCI, Charlson comorbidity index; WBC, white blood cell; APACHE II, acute physiology and chronic health evaluation II; SOFA, sequential organ failure assessment.
Comparisons of categorical values were performed using either Fisher's exact test or the chi-square test. The Mann-Whitney U test was used for continuous nonparametric and ordinal variables.
Stenotrophomonas maltophilia, Enterobacter sp., Morganella morganii, or Burkholderia cepacia.
Carbapenem MIC reported for imipenem or meropenem.
The most common causative organism was Pseudomonas aeruginosa (n = 71 [42.3% overall]), and the causative organisms did not vary between survivors and nonsurvivors overall (P = 0.1). There was a higher rate of Acinetobacter baumannii isolates for nonsurvivors than for survivors (n = 27 [30%] versus n = 12 [15.4%]; P = 0.03). Regarding the source of bacteremia, there were no differences between groups except that nonsurvivors were more likely to have an unknown cause of bacteremia (n = 22 [28.2%] versus n = 4 [4.4%]; P < 0.01) and were less likely to have urinary tract infection-related bacteremia (n = 5 [6.4%] versus n = 25 [27.8%]; P < 0.01). When sources were stratified based on mortality risk, infections with a low-risk source were associated with decreased mortality rates (P = 0.02).
More survivors received appropriate antibiotics (antibiotics with in vitro activity) than nonsurvivors (n = 73 [93.6%] versus n = 48 [53.3%]; P < 0.01) (Table 3). However, the median time to appropriate antibiotic treatment did not vary significantly between survivors (59 h [interquartile range [IQR], 27.5 to 102.2 h]) and nonsurvivors (66.7 h [IQR, 21.7 to 88.7 h]; P = 0.84). Overall, there was no difference in first appropriate antibiotic use between survivors and nonsurvivors (P = 0.42) (Table 4). Although the use of tigecycline, colistin, or aminoglycoside as the first appropriate antibiotic did not differ significantly between the groups, there were significantly higher rates of ciprofloxacin (n = 7 [9%] versus n = 1 [1.1%]; P = 0.02) and beta-lactam (n = 18 [23.1%] versus n = 5 [5.6%]; P < 0.01) use as the first appropriate antibiotic for survivors versus nonsurvivors.
TABLE 3.
Univariate analysis of antimicrobial factors associated with 30-day all-cause mortality rates for patients with carbapenem-resistant Gram-negative bacteremia
| Factor | Nonsurvivors (n = 90) | Survivors (n = 78) | Pa |
|---|---|---|---|
| Combination therapy (no. [%]) | 7 (7.8) | 25 (32.1) | <0.01 |
| Multiple-agent use (no. [%]) | 72 (80) | 68 (87.2) | 0.21 |
| Receipt of carbapenem (no. [%]) | 53 (58.9) | 41 (52.6) | 0.44 |
| Multiple-agent use for patients who received carbapenem (no. [%]) | 43 (59.7) | 34 (50) | 0.25 |
| Receipt of appropriate antibiotics (no. [%]) | 48 (53.3) | 73 (93.6) | <0.01 |
| No. of appropriate agents (median [IQR]) | 1 (0–1) | 1 (1–2) | <0.01 |
| Time to appropriate antibiotics (median [IQR]) (h) | 66.7 (21.7–88.7) | 59 (27.5–102.2) | 0.84 |
Comparisons of P values for categorical values were performed using either Fisher's exact test or the chi-square test. The Mann Whitney U test was used for continuous nonparametric variables.
TABLE 4.
Univariate analysis of the first appropriate antibiotic for patients with carbapenem-resistant Gram-negative bacteremia
| Antibiotic | No. (%) |
Pa | ||
|---|---|---|---|---|
| Overall (n = 168) | Nonsurvivors (n = 90) | Survivors (n = 78) | ||
| Colistin | 23 (13.7) | 12 (13.3) | 11 (14.1) | 0.89 |
| Tigecycline | 30 (17.9) | 14 (15.6) | 16 (20.5) | 0.4 |
| Aminoglycoside | 29 (17.3) | 13 (14.4) | 16 (20.5) | 0.3 |
| Amikacin | 14 (8.3) | 5 (5.6) | 9 (11.5) | |
| Gentamicin | 7 (4.2) | 4 (4.4) | 3 (3.8) | |
| Tobramycin | 8 (4.8) | 4 (4.4) | 4 (5.1) | |
| Trimethoprim-sulfamethoxazole | 8 (4.8) | 3 (3.3) | 5 (6.4) | NA |
| Ciprofloxacin | 8 (4.8) | 1 (1.1) | 7 (9) | 0.02 |
| Beta-lactam | 23 (13.7) | 5 (5.6) | 18 (23.1) | <0.01 |
| Ampicillin-sulbactam | 2 (1.2) | 1 (1.1) | 1 (1.3) | |
| Aztreonam | 1 (0.6) | 0 (0) | 1 (1.3) | |
| Ceftazidime | 7 (6.4) | 2 (2.2) | 5 (6.4) | |
| Piperacillin-tazobactam | 13 (7.7) | 2 (2.2) | 11 (14.1) | |
Comparisons of P values for categorical values were performed using either Fisher's exact test or the chi-square test. NA, not applicable.
Overall, the use of combination therapy was low (n = 32 [19%]). However, combination therapy was used significantly more often for survivors than for nonsurvivors (n = 25 [32.1%] versus n = 7 [7.8%]; P < 0.01). Similarly, there was a significantly higher median number of appropriate antimicrobial agents used for survivors than for nonsurvivors (1 agent [IQR, 1 to 2 agents] versus 1 agent [IQR, 0 to 1 agent]; P < 0.01). In contrast, the use of multiple agents was common (n = 140 [83.3% overall]), but there was no significant difference in multiple-agent use between survivors and nonsurvivors (n = 68 [87.2%] versus n = 72 [80%]; P = 0.21). Similarly, the receipt of carbapenems was common overall (n = 94 [56%]), with no difference between survivors and nonsurvivors (n = 41 [52.6%] versus n = 53 [58.9%]; P = 0.44). Among the group of patients who received a carbapenem, there was no difference in multiple-agent use between survivors and nonsurvivors (n = 34 [59.7%] versus n = 43 [50%]; P = 0.25).
Due to significant differences in baseline characteristics, logistic regression for the primary outcome was performed with adjustment for age, CCI score, SOFA score, vasopressor use on day 1 of the positive blood culture, and receipt of appropriate antibiotics (Table 5). In multivariable analysis, age, SOFA score, receipt of appropriate antibiotics, and combination therapy remained independent factors associated with 30-day mortality rates. Older age (odds ratio [OR], 1.05 [95% confidence interval [CI], 1.02 to 1.08]; P < 0.01) and higher SOFA score (OR, 1.15 [95% CI, 1.03 to 1.28]; P = 0.01) were associated with increased risk of death. Receipt of appropriate antibiotics was associated with decreased risk of death (OR, 0.14 [95% CI, 0.04 to 0.47]; P < 0.01). After adjustment, use of combination therapy was independently associated with decreased risk of death (OR, 0.19 [95% CI, 0.06 to 0.56]; P < 0.01).
TABLE 5.
Multivariable analysis, via standard logistic regression, of factors assessed and associated with 30-day all-cause mortality rates among patients with carbapenem-resistant Gram-negative bacteremia
| Independent risk factora | OR (95% CI) | P |
|---|---|---|
| Age | 1.05 (1.02–1.08) | <0.01 |
| SOFA score | 1.15 (1.03–1.28) | 0.01 |
| CCI score | 1.15 (0.98–1.34) | 0.08 |
| Vasopressor use on day of positive blood culture | 1.67 (0.62–4.49) | 0.31 |
| Receipt of appropriate antibiotics | 0.14 (0.04–0.47) | <0.01 |
| Combination therapy | 0.19 (0.06–0.56) | <0.01 |
SOFA, sequential organ failure assessment; CCI, Charlson comorbidity index.
DISCUSSION
Carbapenem resistance among Gram-negative isolates is increasing (1). More concerning, carbapenem resistance is associated with increased mortality rates, as seen with multiple Gram-negative pathogens, including Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa (4–8). Our study reports a 30-day mortality rate of 53.6%, which is similar to previous studies (8, 24). Unfortunately, there is very limited information to date regarding the optimal treatment for carbapenem-resistant Gram-negative bacteremia. There are suggestions in the literature that combination therapy with multiple agents may be effective (11–13); however, previous studies have differed in their definitions of combination therapy. For instance, the definition in one study considered combination therapy as administration of ≥2 agents with Gram-negative activity for 48 h, regardless of the appropriateness of therapy (11), while other studies defined combination therapy based on in vitro activity, often including sensitivity to carbapenems based on breakpoints that have been subsequently revised by the CLSI (12, 13). Other studies defined combination therapy based on specific drug regimens without consideration of the susceptibility of the individual pathogen (25, 26). No study to date has defined combination therapy based on multiple appropriate agents, and thus the results of this study will provide additional insights into the role of combination therapy for carbapenem-resistant Gram-negative bacteremia, particularly for critically ill patients.
The current study found that the use of combination therapy was more common for survivors than for nonsurvivors. Even after adjustment for baseline characteristics, combination therapy was independently associated with decreased risk of death (OR, 0.19 [95% CI, 0.06 to 0.56]; P < 0.01). Although this result has seemingly been reported previously, the use of combination therapy (19%) was much lower in the current study than in previous studies (33 to 63%) (11–13, 25). This is likely attributable to the definition of combination therapy, which was based on the in vitro activity of noncarbapenems in the current study. This definition of combination therapy was chosen because the appropriateness of therapy for Gram-negative infections has been shown consistently to be associated with decreased mortality rates, particularly for critically ill patients (27–29). Additionally, unlike in previous studies, all included patients had carbapenem MICs of >2 μg/ml; therefore, the use of a carbapenem was not considered a component of combination therapy. The rate of multiple-agent use, considered the administration of ≥2 agents with Gram-negative activity for more than 48 h, regardless of the appropriateness of therapy, was considerably higher (83%) than previously reported rates and likely was influenced by previous literature suggesting a benefit with multiple agents.
Although combination therapy was associated with decreased mortality rates, our study did not find a difference in mortality rates based on multiple-agent use, nor was there a difference in multiple-agent use in the subgroup of patients who received a carbapenem. This implies that the association of combination therapy and improved survival rates is lost if the appropriateness of therapy, or in vitro activity, is not considered. This finding is in opposition to previous studies, which found significant decreases in mortality rates with combination therapy defined similarly to our definition of multiple-agent use (11–13). These different results may be explained by the different populations studied, with the current study being performed specifically with critically ill patients with pathogens with very high carbapenem MICs. Although previous studies included critically ill patients, the proportions of critically ill patients were much smaller than in the current study (42 to 72% versus 100%) (11–13).
Previously used definitions of combination therapy, which were not based on in vitro activity, were supported by pharmacodynamic data from both humans and animals that demonstrated the possibility of in vivo target attainment despite in vitro resistance (14). The goals used in those pharmacodynamic studies were traditional target attainment parameters (e.g., 40% time above the MIC for carbapenems) based on pharmacokinetic parameters of the general population. However, it is well known that critically ill patients exhibit altered pharmacokinetics, such as increased volume of distribution, compared with the general population (30). Because all patients included in the current study had MICs of >2 μg/ml for a carbapenem and the median MICs for the most common organisms ranged from 8 to 16 μg/ml, it is highly unlikely the traditional target attainment parameters were met, due to the critically ill status of the patients and their elevated MICs. Additionally, there are suggestions in the literature that higher targets are needed for critically ill patients, such as 100% time above the MIC or goal concentrations of >4 times the MIC (31, 32). Thus, it is possible that the use of multiple agents including a carbapenem may be effective regardless of in vitro activity for non-critically ill patients but carbapenems are not beneficial as a component of combination therapy for critically ill patients because optimal pharmacodynamic targets are not attained.
Although the current study specifically evaluated the effects of combination therapy, the identified risk factors for death within 30 days have significant implications for the prevention and treatment of carbapenem-resistant Gram-negative bacteremia (8, 24, 33, 34). Additionally, because the current study included only ICU patients, the risk factors further inform the treatment of this patient population, for which ICU admission is already a well-known risk factor for death (4, 5). The current study found that, in addition to combination therapy, independent risk factors associated with 30-day mortality rates were older age, higher SOFA score, and receipt of appropriate antibiotics. These risk factors were similar to those seen in previous studies. Older age has been consistently identified as a risk factor for death among patients with carbapenem-resistant Gram-negative bacteremia (13, 33, 34). Although higher SOFA scores have not been identified in prior studies as a significant risk factor for death, other acute severity-of-illness markers, such as APACHE II and APACHE III scores, have been recognized (11, 13). Finally, receipt of appropriate antibiotics was shown in previous studies to be significantly associated with decreased risk of death with carbapenem-resistant Gram-negative bacteremia (4, 12, 13). Taking all of these factors into consideration, in areas with local susceptibility patterns that suggest high carbapenem resistance, it may be important to initiate therapy against carbapenem-resistant pathogens early for patients with risk factors such as ICU admission, older age, and higher SOFA scores.
There are several limitations to our study. First, this was a retrospective medical record review, and the study is subject to the inherent flaws of this design; we were reliant on the accuracy of medication administration records for our primary outcome measure, and incomplete medical records were reasons for exclusion for several patients. Second, this was a single-center study, and institutional practices may limit the generalizability. For instance, our institution does not utilize an extended-infusion dosing strategy for carbapenems, and thus the impact of multiple-agent use with extended-infusion carbapenem administration cannot be determined. Additionally, because the study spanned 5 years, institutional practice changes occurred during the period, with the most significant being the CLSI breakpoint changes for carbapenems. Because of this discrepancy, we chose to use the most conservative and most recent breakpoints (16). However, some of the isolates were reported by the microbiology laboratory as susceptible to carbapenem, based on the MIC criteria in place at the time, which could have influenced the clinician's decision to use a carbapenem. In fact, 56% of patients received a carbapenem despite in vitro resistance based on the current breakpoints; this may be attributable to the breakpoint changes. Finally, despite efforts to collect data on biologically plausible factors and to adjust for baseline differences, there may be additional unaccounted differences between groups that influenced the clinicians' decisions to administer monotherapy or combination therapy.
In conclusion, these data suggest that combination therapy with two or more agents with in vitro activity is associated with improved survival rates for critically ill patients with carbapenem-resistant Gram-negative bacteremia. However, the association with improved survival rates is lost if in vitro activity is not considered. These data are important as they are the first to define combination therapy based on in vitro activity without the use of a carbapenem in this population. Larger prospective studies are needed to further investigate the role of combination therapy for carbapenem-resistant Gram-negative bacteremia in ICU patients.
ACKNOWLEDGMENTS
We thank Emily Blum, Jason Mersek, and Ee Jye Poi for their assistance with data collection.
We have no conflicts of interest to declare.
REFERENCES
- 1.Gupta N, Limbago BM, Patel JB, Kallen AJ. 2011. Carbapenem-resistant Enterobacteriaceae: epidemiology and prevention. Clin Infect Dis 53:60–67. doi: 10.1093/cid/cir202. [DOI] [PubMed] [Google Scholar]
- 2.Rhomberg PR, Jones RN. 2009. Summary trends for the Meropenem Yearly Susceptibility Test Information Collection Program: a 10-year experience in the United States (1999–2008). Diagn Microbiol Infect Dis 65:414–426. doi: 10.1016/j.diagmicrobio.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen M, Eschenauer GA, Bryan M, O'Neil K, Furuya EY, Della-Latta P, Kubin CJ. 2010. Carbapenem-resistant Klebsiella pneumoniae bacteremia: factors correlated with clinical and microbiologic outcomes. Diagn Microbiol Infect Dis 67:180–184. doi: 10.1016/j.diagmicrobio.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP. 2008. Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29:1099–1106. doi: 10.1086/592412. [DOI] [PubMed] [Google Scholar]
- 5.Schwaber MJ, Klarfeld-Lidji S, Navon-Venezia S, Schwartz D, Leavitt A, Carmeli Y. 2008. Predictors of carbapenem-resistant Klebsiella pneumoniae acquisition among hospitalized adults and effect of acquisition on mortality. Antimicrob Agents Chemother 52:1028–1033. doi: 10.1128/AAC.01020-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sheng WH, Liao CH, Lauderdale TL, Ko WC, Chen YS, Liu JW, Lau YJ, Wang LH, Liu KS, Tsai TY, Lin SY, Hsu MS, Hsu LY, Chang SC. 2010. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis 14:e764–e769. doi: 10.1016/j.ijid.2010.02.2254. [DOI] [PubMed] [Google Scholar]
- 7.Lautenbach E, Synnestvedt M, Weiner MG, Bilker WB, Vo L, Schein J, Kim M. 2010. Imipenem resistance in Pseudomonas aeruginosa: emergence, epidemiology, and impact on clinical and economic outcomes. Infect Control Hosp Epidemiol 31:47–53. doi: 10.1086/649021. [DOI] [PubMed] [Google Scholar]
- 8.Ben-David D, Kordevani R, Keller N, Tal I, Marzel A, Gal-Mor O, Maor Y, Rahav G. 2012. Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 18:54–60. doi: 10.1111/j.1469-0691.2011.03478.x. [DOI] [PubMed] [Google Scholar]
- 9.Borer A, Saidel-Odes L, Riesenberg K, Eskira S, Peled N, Nativ R, Schlaeffer F, Sherf M. 2009. Attributable mortality rate for carbapenem-resistant Klebsiella pneumoniae bacteremia. Infect Control Hosp Epidemiol 30:972–976. doi: 10.1086/605922. [DOI] [PubMed] [Google Scholar]
- 10.Carmeli Y, Akova M, Cornaglia G, Daikos GL, Garau J, Harbarth S, Rossolini GM, Souli M, Giamarellou H. 2010. Controlling the spread of carbapenemase-producing Gram-negatives: therapeutic approach and infection control. Clin Microbiol Infect 16:102–111. doi: 10.1111/j.1469-0691.2009.03115.x. [DOI] [PubMed] [Google Scholar]
- 11.Qureshi ZA, Paterson DL, Potoski BA, Kilayko MC, Sandovsky G, Sordillo E, Polsky B, Adams-Haduch JM, Doi Y. 2012. Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 56:2108–2113. doi: 10.1128/AAC.06268-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, Marchese A, Spanu T, Ambretti S, Ginocchio F, Cristini F, Losito AR, Tedeschi S, Cauda R, Bassetti M. 2012. Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 55:943–950. doi: 10.1093/cid/cis588. [DOI] [PubMed] [Google Scholar]
- 13.Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, Ranellou K, Prekates A, Themeli-Digalaki K, Tsakris A. 2011. Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 17:1798–1803. doi: 10.1111/j.1469-0691.2011.03514.x. [DOI] [PubMed] [Google Scholar]
- 14.Daikos GL, Markogiannakis A. 2011. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 17:1135–1141. doi: 10.1111/j.1469-0691.2011.03553.x. [DOI] [PubMed] [Google Scholar]
- 15.van Belkum A, Halimi D, Bonetti EJ, Renzi G, Cherkaoui A, Sauvonnet V, Martelin R, Durand G, Chatellier S, Zambardi G, Engelhardt A, Karlsson A, Schrenzel J. 2015. Meropenem/colistin synergy testing for multidrug-resistant Acinetobacter baumannii strains by a two-dimensional gradient technique applicable in routine microbiology. J Antimicrob Chemother 70:167–172. doi: 10.1093/jac/dku342. [DOI] [PubMed] [Google Scholar]
- 16.Clinical and Laboratory Standards Institute. 2011. Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI document M100-S21 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 17.Lee K, Chong Y, Shin HB, Kim YA, Yong D, Yum JH. 2001. Modified Hodge and EDTA-disk synergy tests to screen metallo-β-lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect 7:88–91. doi: 10.1046/j.1469-0691.2001.00204.x. [DOI] [PubMed] [Google Scholar]
- 18.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, SCCM/ESICM/ACCP/ATS/SIS . 2003. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med 31:1250–1256. doi: 10.1097/01.CCM.0000050454.01978.3B. [DOI] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985. APACHE II: a severity of disease classification system. Crit Care Med 13:818–829. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira FL, Bota DP, Bross A, Melot C, Vincent JL. 2001. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 22.Moore CL, Osaki-Kiyan P, Haque NZ, Perri MB, Donabedian S, Zervos MJ. 2012. Daptomycin versus vancomycin for bloodstream infections due to methicillin-resistant Staphylococcus aureus with a high vancomycin minimum inhibitory concentration: a case-control study. Clin Infect Dis 54:51–58. doi: 10.1093/cid/cir764. [DOI] [PubMed] [Google Scholar]
- 23.European Committee on Antimicrobial Susceptibility Testing. 2011. Breakpoint tables for interpretation of MICs and zone diameters. European Committee on Antimicrobial Susceptibility Testing, Basel, Switzerland: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/EUCAST_breakpoints_v1.3_pdf.pdf. [Google Scholar]
- 24.Neuner EA, Yeh JY, Hall GS, Sekeres J, Endimiani A, Bonomo RA, Shrestha NK, Fraser TG, van Duin D. 2011. Treatment and outcomes in carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Diagn Microbiol Infect Dis 69:357–362. doi: 10.1016/j.diagmicrobio.2010.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopez-Cortes LE, Cisneros JM, Fernandez-Cuenca F, Bou G, Tomas M, Garnacho-Montero J, Pascual A, Martinez-Martinez L, Vila J, Pachon J, Rodriguez Bano J. 2014. Monotherapy versus combination therapy for sepsis due to multidrug-resistant Acinetobacter baumannii: analysis of a multicentre prospective cohort. J Antimicrob Chemother 69:3119–3126. doi: 10.1093/jac/dku233. [DOI] [PubMed] [Google Scholar]
- 26.Paul M, Carmeli Y, Durante-Mangoni E, Mouton JW, Tacconelli E, Theuretzbacher U, Mussini C, Leibovici L. 2014. Combination therapy for carbapenem-resistant Gram-negative bacteria. J Antimicrob Chemother 69:2305–2309. doi: 10.1093/jac/dku168. [DOI] [PubMed] [Google Scholar]
- 27.Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2005. Bloodstream infections caused by antibiotic-resistant Gram-negative bacilli: risk factors for mortality and impact of inappropriate initial antimicrobial therapy on outcome. Antimicrob Agents Chemother 49:760–766. doi: 10.1128/AAC.49.2.760-766.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micek ST, Lloyd AE, Ritchie DJ, Reichley RM, Fraser VJ, Kollef MH. 2005. Pseudomonas aeruginosa bloodstream infection: importance of appropriate initial antimicrobial treatment. Antimicrob Agents Chemother 49:1306–1311. doi: 10.1128/AAC.49.4.1306-1311.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zahar JR, Timsit JF, Garrouste-Orgeas M, Francais A, Vesin A, Descorps-Declere A, Dubois Y, Souweine B, Haouache H, Goldgran-Toledano D, Allaouchiche B, Azoulay E, Adrie C. 2011. Outcomes in severe sepsis and patients with septic shock: pathogen species and infection sites are not associated with mortality. Crit Care Med 39:1886–1895. doi: 10.1097/CCM.0b013e31821b827c. [DOI] [PubMed] [Google Scholar]
- 30.Roberts JA, Lipman J. 2009. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 37:840–851. doi: 10.1097/CCM.0b013e3181961bff. [DOI] [PubMed] [Google Scholar]
- 31.McKinnon PS, Paladino JA, Schentag JJ. 2008. Evaluation of area under the inhibitory curve (AUIC) and time above the minimum inhibitory concentration (T>MIC) as predictors of outcome for cefepime and ceftazidime in serious bacterial infections. Int J Antimicrob Agents 31:345–351. doi: 10.1016/j.ijantimicag.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 32.Tam VH, McKinnon PS, Akins RL, Rybak MJ, Drusano GL. 2002. Pharmacodynamics of cefepime in patients with Gram-negative infections. J Antimicrob Chemother 50:425–428. doi: 10.1093/jac/dkf130. [DOI] [PubMed] [Google Scholar]
- 33.Pena C, Suarez C, Gozalo M, Murillas J, Almirante B, Pomar V, Aguilar M, Granados A, Calbo E, Rodriguez-Bano J, Rodriguez F, Tubau F, Martinez-Martinez L, Oliver A. 2012. Prospective multicenter study of the impact of carbapenem resistance on mortality in Pseudomonas aeruginosa bloodstream infections. Antimicrob Agents Chemother 56:1265–1272. doi: 10.1128/AAC.05991-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SY, Jung JY, Kang YA, Lim JE, Kim EY, Lee SK, Park SC, Chung KS, Park BH, Kim YS, Kim SK, Chang J, Park MS. 2012. Risk factors for occurrence and 30-day mortality for carbapenem-resistant Acinetobacter baumannii bacteremia in an intensive care unit. J Korean Med Sci 27:939–947. doi: 10.3346/jkms.2012.27.8.939. [DOI] [PMC free article] [PubMed] [Google Scholar]


