Abstract
Although amphotericin B-azole combination therapy has traditionally been questioned due to potential antagonistic interactions, it is often used successfully to treat refractory invasive aspergillosis. So far, pharmacodynamic (PD) interactions have been assessed with conventional in vitro tests, which do not mimic human serum concentrations and animal models using limited doses. We therefore simulated the human serum concentration profiles of amphotericin B and voriconazole in an in vitro dialysis/diffusion closed pharmacokinetic-pharmacodynamic (PK-PD) model and studied the pharmacodynamic interactions against an azole-resistant and an azole-susceptible Aspergillus fumigatus isolate, using Bliss independence and canonical mixture response surface analyses. Amphotericin B dosing regimens with the drug administered every 24 h (q24h) were combined with voriconazole q12h dosing regimens. In vitro PK-PD combination data were then combined with human PK data by using Monte Carlo analysis. The target attainment rate and the serum concentration/MIC ratio were calculated for isolates with different MICs. Synergy (20 to 31%) was observed at low amphotericin B-high voriconazole exposures, whereas antagonism (−6 to −16%) was found at high amphotericin B-low voriconazole exposures for both isolates. Combination therapy resulted in 17 to 48% higher target attainment rates than those of monotherapy regimens for isolates with voriconazole/amphotericin B MICs of 1 to 4 mg/liter. Optimal activity was found for combination regimens with a 1.1 total minimum concentration of drug in serum (tCmin)/MIC ratio for voriconazole and a 0.5 total maximum concentration of drug in serum (tCmax)/MIC ratio for amphotericin B, whereas the equally effective monotherapy regimens required a voriconazole tCmin/MIC ratio of 1.8 and an amphotericin B tCmax/MIC ratio of 2.8. Amphotericin B-voriconazole combination regimens were more effective than monotherapy regimens. Therapeutic drug monitoring can be employed to optimize antifungal combination therapy with low-dose (≤0.6 mg/kg) amphotericin B-based combination regimens against resistant isolates for minimal toxicity.
INTRODUCTION
Invasive aspergillosis is a serious life-threatening complication in immunocompromised patients. The main pathogen is Aspergillus fumigatus (1). The poor prognosis, the unsatisfactory response to first-line treatment, and the emergence of resistance among clinical isolates have raised interest in the use of combination therapy as an alternative therapeutic approach in an attempt to increase antifungal efficacy, particularly against difficult-to-treat infections (2). The combined use of antifungal compounds that belong to different pharmacological classes and possess different mechanisms of action is an attractive approach. Combination antifungal therapy may increase the extent and rate of pathogen killing even in difficult-to-treat anatomical sites of infections, lower the risk of acquired resistance, shorten the recovery time, reduce undesirable side effects by using smaller drug doses, overcome the problem of subtherapeutic drug levels, and expand the antifungal spectrum to cover mixed infections (3). However, there are risks which may outweigh the value of combination therapy and limit its use, such as a reduced efficacy due to antagonistic interactions, increasing toxicity with the use of both drugs, and the higher cost of treatment, especially when new compounds are combined (4).
The use of an amphotericin B (AMB)-azole combination has traditionally been questioned due to potential antagonistic interactions, as azoles inhibit the biosynthetic pathway of the main sterol of the cell membrane, ergosterol, which is involved in the action of amphotericin B (5). However, successful treatment was reported in several individual cases and in a retrospective case series study which concluded that a polyene-azole combination is not clinically antagonistic (6). Furthermore, preclinical studies showed various interactions, ranging from synergy to antagonism, depending on the technical and analytical methodology used for in vitro testing (7) and on the neutropenic status, mode of infection, doses, administration route, and time of dosing in animal models (8). Animal models show significant differences from humans in the pharmacokinetics (PK) of antifungal agents (distribution, protein binding, and concentration-time profiles) and the pathophysiology of fungal infections (9), while conventional in vitro combination testing using the microdilution checkerboard method does not simulate the changing serum concentration profiles of drugs in combination. In vitro pharmacokinetic-pharmacodynamic (PK-PD) models may help in the study of pharmacodynamic interactions of combination therapy regimens at clinically relevant drug exposures and allow researchers to infer useful conclusions about the benefit of combination therapy (10).
We recently developed an in vitro dialysis/diffusion closed PK-PD model that reliably simulated AMB and voriconazole (VOR) pharmacokinetics in human serum and correlated with in vivo outcomes observed in animal models and clinical trials (11, 12). We therefore applied this model to simulate the human serum pharmacokinetics of amphotericin B and voriconazole administered concomitantly, at the standard dosages of 1 mg/kg of body weight and 4 mg/kg of body weight, respectively, and to study the pharmacodynamic interactions against azole-susceptible and -resistant Aspergillus fumigatus. Target serum levels of combination therapy regimens were determined for isolates with different MICs, opening a new field of therapeutic drug monitoring and optimization of combination antifungal therapy.
MATERIALS AND METHODS
Test organism.
Two isolates with different susceptibilities to voriconazole and amphotericin B were tested: the azole-susceptible A. fumigatus strain NIH 4215 (ATCC no. MYA-3626), with amphotericin B/voriconazole CLSI MICs of 1/0.5 mg/liter (called strain AFM4215 in this study); and the azole-resistant A. fumigatus strain v5235, with amphotericin B/voriconazole CLSI MICs of 0.25/2 mg/liter (called strain AFM5235 in this study). The isolates were stored in normal saline with 10% glycerol at −70°C until the study was performed. Prior to testing, they were revived by subculturing twice onto Sabouraud dextrose agar (SDA; bioMérieux) at 30°C for 5 to 7 days. Each inoculum was prepared in sterile saline with 1% Tween 20, conidia were counted on a Neubauer hemacytometer in order to obtain a final concentration of 103 CFU/ml, and the count of the inoculum was affirmed each time by culturing serial dilutions on SDA plates.
Antifungal drugs and medium.
Laboratory-grade standard powders of amphotericin B (MP Biomedicals, LLC, Solon, OH) and voriconazole (Pfizer Inc., Groton, CT) were dissolved in sterile dimethyl sulfoxide (DMSO) (Carlo Erba Reactifs-SDS, Val de Reuil, France), and stock solutions were stored at −70°C until the day of the experiment. The medium used throughout was RPMI 1640 medium (with l-glutamine, without bicarbonate) (AppliChem, Darmstadt, Germany) buffered to pH 7.0 with 0.165 M MOPS (morpholinepropanesulfonic acid) (AppliChem, Darmstadt, Germany) and supplemented with 100 mg/liter chloramphenicol (AppliChem, Darmstadt, Germany).
In vitro PK-PD model.
A previously optimized two-compartment PK-PD dialysis/diffusion closed model was used (11, 13). The model consists of an external compartment (EC) comprised of a conical flask connected to a peristaltic pump and an internal compartment (IC) comprised of a 10-ml semipermeable cellulose dialysis tube (Spectra/Por Float-A-Lyzer G2; Spectrum Laboratories Inc., Breda, The Netherlands) inoculated with a conidial suspension. The in vitro system has been adapted to accommodate two drugs with different half-lives, thus enabling the study of drug combinations. Repeated samplings of 200 μl were made from the IC and stored at −70°C until the drug concentrations (100 μl) and galactomannan (GM) levels (100 μl) were determined.
In vitro pharmacokinetics.
Voriconazole and conventional amphotericin B serum concentration-time profiles were simulated in the in vitro PK-PD model, with a half-life of 6 h for voriconazole and half-lives of 2 h and 12 h for the alpha and beta elimination phases, respectively, of amphotericin B. Twenty-one different combination regimens, including monotherapies and a drug-free control, were investigated to simulate various maximum concentrations of the free, unbound fraction of drug in serum (fCmax) for voriconazole and amphotericin B (7.2, 3.4, 1.7, 0.8, and 0.4 mg/liter and 2.4, 0.6, 0.3, 0.1, 0.05, 0.025, and 0.012 mg/liter, respectively). For the azole-resistant AFM5235 strain, selected combinations were tested, with voriconazole fCmax values of 14, 7, and 3.4 mg/liter and amphotericin B fCmax values of 0.3, 0.1, and 0.05 mg/liter. The time-concentration profiles included the profiles observed in human serum with standard voriconazole and amphotericin B dosing regimens of 4 mg/kg and 1 mg/kg, respectively, and were expanded to cover high concentrations that will help to characterize the full exposure-effect relationship. After inoculation of the IC with Aspergillus conidia, voriconazole and amphotericin B were injected into both compartments of the model alone and in combination every 12 h and 24 h, respectively. The EC was covered with aluminum foil in order to minimize light exposure and placed on a heated magnetic stirrer (37°C) for 72 h. At the beginning of, during, and at the end of each experiment, the temperature and flow rate were measured to ensure that they were at the expected values. Drug levels were measured using microbiological agar diffusion assays as previously described (11, 12). A concentration-time curve was generated for each simulated dose and analyzed by nonlinear regression analysis, using one- and two-compartment models for voriconazole and amphotericin B, respectively, as described by the equations Ct = C0 × e−k × t and , where Ct (dependent variable) is the concentration of drug at a given time t (independent variable), C0 is the concentration of the drug at 0 h, Ca and Cb are the maximum concentrations for the alpha and beta phases, respectively, e is the physical constant 2.718, and k is the rate of drug removal. The half-life was calculated using the equation t1/2 = 0.693/k and compared with the respective values observed for human serum. Finally, the area under the dosing interval (τ) time-free drug concentration curve (fAUCτ) was calculated for each simulated dosage by applying the trapezoidal rule. For each simulated dose of amphotericin B and voriconazole, the fCmax/MIC and fAUC/MIC ratios, respectively, were calculated.
In vitro pharmacodynamics.
To estimate fungal growth and the antifungal effect of each monotherapy and combination dosing regimen, 100-μl aliquots from inoculated dialysis tubes were sampled at regular intervals for up to 72 h. The GM index levels were determined in the 100-μl samples after adding 200-μl saline to reach a final volume of 300 μl using a commercially available sandwich enzyme-linked immunoassay (Platelia Aspergillus EIA; Bio-Rad Laboratories), and a GM index-time profile was constructed. Moreover, the area under the GM index curve (AUCGI) was determined as a surrogate marker of fungal growth, as previously described (14). The percentage of growth inhibition at each dose was calculated as follows: 1 − AUCGI,DR/AUCGI,GC, where AUCGI,DR is the AUCGI at a certain dose for drug monotherapies and their combination, whereas AUCGI,GC is the AUCGI of the drug-free control.
Time-kill assays.
In order to verify the observed in vitro interactions, conidial suspensions (103 CFU/ml) of AFM4215 were incubated at 37°C for 72 h in 50 ml of RPMI medium containing voriconazole or amphotericin B alone or the respective combination regimens at the fCmax values used in the in vitro PK-PD model experiments. Because amphotericin B degrades over time, reaching a 30% loss within 24 h, amphotericin B was added to the test tubes every 24 h in order to compensate for this loss. Drug levels were measured over the 72-h period by using the above-described bioassays. At regular time points, the % viable conidia was calculated microscopically as the % germinated conidia. At high drug concentrations where no germinated conidia were present, the % viable conidia was calculated using CFU counts after subculture of 250 μl on SDA plates and incubation at 37°C for 48 h in duplicate. The lower limit of detection was 4 CFU/ml, whereas average fungal loads as low as 12 CFU/ml could be detected reproducibly, with an average coefficient of variation of 50% (35 to 70%).
Pharmacodynamic drug interaction analysis.
In order to assess the nature of in vitro interactions between voriconazole and amphotericin B, the data were analyzed using the Bliss independence model (15). Bliss independence is described by the equation EIND = EA × EB for a certain combination of two drugs, where EA and EB are the % fungal growth at x dose of drug A and y dose of drug B alone, respectively, and EIND is the expected % fungal growth of a noninteractive (independent) theoretical combination of the drugs. The difference (ΔE = EIND − EOBS) between the expected and the experimentally observed (EOBS) % growth values describes the interaction of each combination of concentrations of the two drugs. If ΔE was >0 (EIND > EOBS), then Bliss synergy was assigned, whereas if ΔE was <0 (EIND < EOBS), Bliss antagonism was assigned. In any other case, Bliss independence was claimed. For each combination, the ΔE value was calculated, its statistical significance was determined by Student's t test (P < 0.05), and the interaction was assessed as described above (16).
Response surface modeling.
The exposure response surface of all voriconazole-amphotericin B combinations was modeled using the previously described modified canonical mixture nonlinear global response surface Emax-based model (17), which is described by the following set of equations.
| (1) |
where E is the % growth corresponding to the total amount of units (U) of a given combination. U is the total number of units (UAMB + UVOR) for any combination, where UAMB and UVOR are the potency units, calculated as (fCmax/MIC)/EI50,AMB and (fAUC/MIC)/EI50,VOR, with EI50,AMB and EI50,VOR being the exposure indices fCmax/MIC and fAUC/MIC associated with 50% growth for amphotericin B and voriconazole, respectively. For each combination, the relative potency units x of amphotericin B and y of voriconazole, calculated as UAMB/U and UVOR/U, respectively, ranged from 0 to 1 and, by definition, x + y = 1. Emax is the maximum % growth in the absence of any drug, and B is the minimum % growth for infinite drug concentrations, given by the following equation:
| (2) |
U50 is the total amount of potency units producing 50% of Emax – B and is given by the following equation:
| (3) |
m is the slope and is given by the following equation:
| (4) |
Equations 2, 3, and 4 are full cubic canonical mixture polynomials which describe B, logU50, and m as functions of x and y. The coefficients a, b, and c are model parameters for B (aB1, aB2, bB12, and cB12), m (am1, am2, bm12, and cm12), and U50 (aD1, aD2, bD12, and cBD12) and were estimated from the data by regression analysis. In contrast to B, logU50, and m, Emax was not modeled as a function of x and y, because all drugs at very low concentrations resulted in 100% growth. Because the logarithms of the potency units were used, equation 1 was transformed to the following:
| (5) |
Equation 5 together with equations 2, 3, and 4 was used to fit the global model to the % fungal growth by using the nonlinear platform of JMP5.0.1 software and was weighted with the inverse of the standard deviation (SD) (SAS Institute, Cary, NC). The am1, am2, aD1, and aD2 values were set to the slopes and calculated from the individual exposure-effect relationships of amphotericin B and voriconazole alone, whereas the aB1 and aB2 values were set to 0, since there was no growth at high exposures of amphotericin B and voriconazole alone. The goodness of fit was checked with a variety of diagnostic parameters, such as R2 values, analysis of variance, a lack-of-fit test, residual and leverage plot analysis, a correlation matrix, and the standard errors of parameters. Parameters with coefficients not statistically significantly different from 0 were removed from the final model. Simpler models with fewer parameters were compared statistically to complex models with more parameters by using the F test. A P value of <0.05 indicates that the complex model had a significantly better fit than the simpler model.
Monte Carlo simulation.
In order to bridge the in vitro data with human PK data, Monte Carlo simulation analysis was performed using the normal random number generator function of Excel (MS Office 2007) for 10,000 patients infected with A. fumigatus isolates with MICs of voriconazole and amphotericin B ranging from 0.25 to 4 mg/liter and treated with the standard intravenous dosage of 4 mg/kg of voriconazole twice daily, 1 mg/kg of amphotericin B once daily, or both of them as a combination therapy regimen. For amphotericin B, this dosage resulted in a steady-state total maximum concentration in human serum (tCmax) of 2.83 ± 1.17 mg/liter (18), which corresponds to a free maximum concentration (fCmax) of 0.14 ± 0.06 mg/liter, based on the 95% protein binding rate of amphotericin B previously found at this concentration (19). Because of the concentration-dependent protein binding of amphotericin B, the protein binding rate at tCmax >5 mg/liter is >96% (19). For voriconazole, this dosage corresponded to a total tAUC0–12 of 50.40 ± 41.8 mg · h/liter (20), while the fAUC0–12 was calculated on the basis of the 0.42 unbound fraction of voriconazole in human serum and was 21.2 ± 17.6 mg · h/liter. We assumed that there was no pharmacokinetic interaction between polyenes and azoles, as previously found (16). In order to estimate the % fungal growth for each of the 10,000 simulated patients treated with either the monotherapy regimens or the combination therapy regimens, the above-described response surface model was used. The input parameters for each simulated patient were the fCmax/MIC ratio for amphotericin B and the fAUC/MIC ratio for voriconazole, and the output parameter was the % fungal growth for each particular patient. The % of patients with <50% estimated fungal growth was calculated for each MIC of amphotericin B and voriconazole alone and in combination. The PK-PD target corresponding to 50% growth was previously found to be associated with 6-week survival for voriconazole (11) and amphotericin B (12), using the same in vitro PK-PD model.
Therapeutic drug monitoring.
The required human serum levels necessary to attain the clinically relevant in vitro exposure index EI50 were calculated in relation to the MIC of the infecting isolate. For that purpose, the fCmax values for amphotericin B and the fCmin values for voriconazole in monotherapy and combination therapy regimens were determined for isolates with MICs ranging from 0.25 mg/liter to 4 mg/liter for both drugs by using equation 5, after determining the best-fit parameters that described the entire exposure-effect surface. Total drug concentrations (tCmax of amphotericin B and tCmin of voriconazole) were calculated on the basis of 95% and 58% protein binding rates, respectively (9, 19).
Statistical analysis.
All data were analyzed using the statistics software package GraphPad Prism, version 5.0, for Windows (GraphPad Software, San Diego, CA). All experiments were carried out in duplicate and were independently performed on two different days with individually prepared inocula.
RESULTS
Pharmacokinetic analysis.
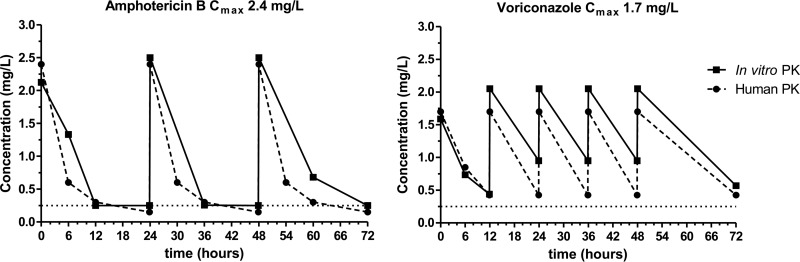
The in vitro model simulated steady-state human serum pharmacokinetics well. In particular, the initial fCmax values for both drugs in the IC were close to the target values (maximum deviation, 18%), with average half-lives of 5.5 to 6.7 h for voriconazole and 0.2 to 1 h and 6 to 8 h for the amphotericin B alpha and beta phases, respectively. Representative time-concentration profiles for both monotherapies are depicted in Fig. 1. Voriconazole dosages resulted in a mean fCmax/fCmin ratio of 4.1 (range, 3.3 to 5.2) and a mean fCmax/fAUC0–12 ratio of 6.1 (range, 5.4 to 6.6). Similarly, the mean amphotericin B fCmax/fCmin ratio was 8 (range, 5 to 11), and the mean fCmax/fAUC0–24 ratio was 5.3 (range, 4.8 to 6.2).
FIG 1.
Representative time-concentration profiles for simulated human once-daily dosing regimens of amphotericin B and twice-daily dosing regimens of voriconazole in an in vitro PK-PD model with target Cmax values of 2.4 and 1.7 mg/liter, respectively. Data represent drug levels in the internal compartment of the in vitro model (solid lines) and the respective target values observed in human serum (broken lines).
Pharmacodynamic analysis.
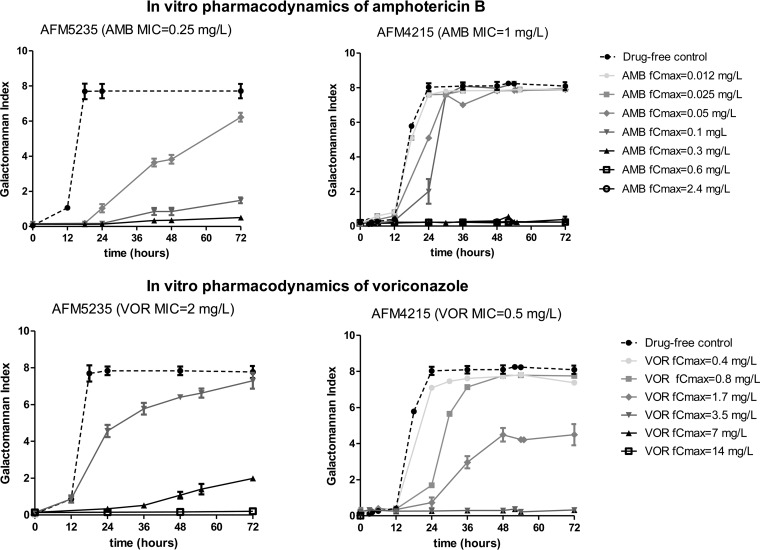
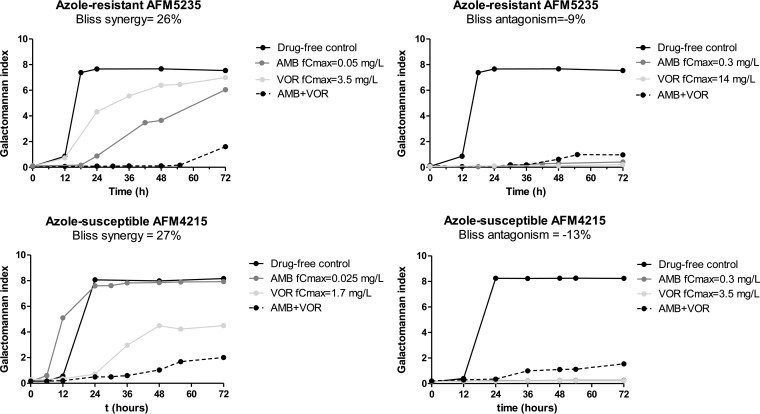
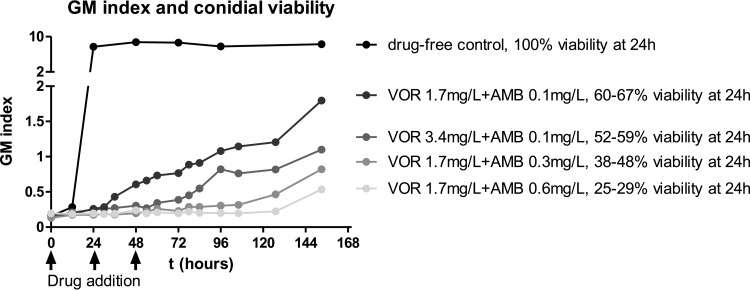
After 72 h of incubation, both drugs alone completely inhibited fungal growth of AFM4215 and AFM5234 at simulated doses with voriconazole fCmax values of ≥3.5 and 14 mg/liter, respectively, and amphotericin B fCmax values of ≥0.3 mg/liter (Fig. 2). The GM index-time curves for selected combination dosing regimens with inhibitory and noninhibitory doses of amphotericin B and voriconazole are shown in Fig. 3. For some combinations, the GM index was lower than those for both monotherapy regimens (Fig. 3, left graphs), whereas for others, the GM index was higher than that for the most effective monotherapy regimen (Fig. 3, right graphs) or similar to those for other monotherapy dosing regimens (not shown). Note that for some combinations, low GM indexes were observed (Fig. 3, right graphs). These GM index levels increased over time after the end of the experiment, when drugs were not added to the in vitro model, in a dose-dependent pattern (Fig. 4). This dose dependency was confirmed in time-kill studies. Combination regimens with higher GM index levels in the in vitro model had higher percentages of viable conidia than regimens with lower GM index levels (Fig. 4).
FIG 2.
In vitro pharmacodynamics of amphotericin B (top graphs) and voriconazole (bottom graphs) against the azole-susceptible A. fumigatus isolate AFM4215 (right graphs) and the azole-resistant A. fumigatus isolate AFM5235 (left graphs).
FIG 3.
Synergistic (left graphs) and antagonistic (right graphs) interactions of amphotericin B-voriconazole combinations against the azole-susceptible A. fumigatus strain AFM4215 (bottom graphs) and the azole-resistant A. fumigatus strain AFM5235 (top graphs). The fCmax and the Bliss interaction are shown for each combination regimen.
FIG 4.
Dose-dependent nature of Bliss antagonism based on galactomannan (GM) index-time curves for the in vitro PK-PD model and % viable conidia at 24 h in time-kill studies against the azole-susceptible A. fumigatus strain AFM4215. Monotherapy regimens suppressed galactomannan production and killed Aspergillus conidia more than combination regimens did.
Exposure-effect relationships.
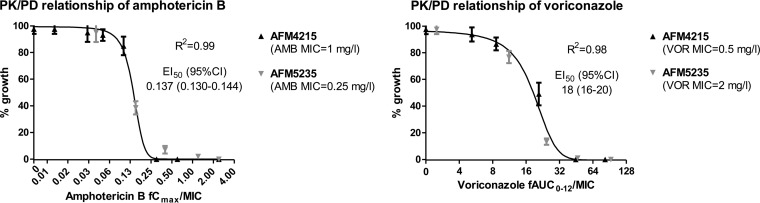
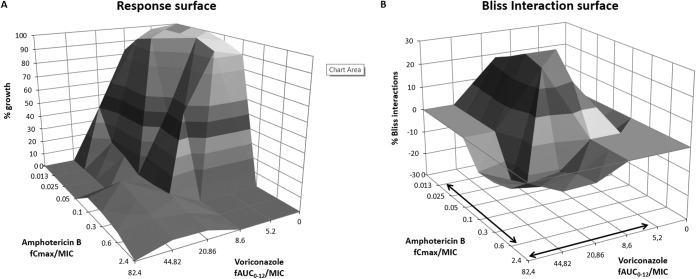
For amphotericin B, the fCmax/MIC relationship followed a sigmoid pattern (R2 = 0.99) with an EI50 (95% confidence interval [CI]) of 0.137 (0.130 to 0.144), while for voriconazole, the fAUC0–12/MIC relationship followed a sigmoid curve (R2 = 0.99) with an EI50 (95% CI) of 18 (16–20) for both the azole-susceptible and the azole-resistant isolate (Fig. 5). These values are similar to those previously determined using the same model with more isolates, indicating the excellent interexperimental reproducibility of the in vitro model (11, 12). The exposure-effect surface for all combination regimens is shown in Fig. 6A. The surface is concave at intermediate drug exposures, whereas it is convex at higher drug exposures. The final model that describes the entire exposure-effect surface is as follows: .
FIG 5.
In vitro exposure-effect relationships of amphotericin B (left graph) and voriconazole (right graph) in an in vitro PK-PD model simulating human serum concentrations. The lines for the exposure-effect relationship represent the regression lines obtained with the Emax model. Error bars represent SD.
FIG 6.
Response surface (A) and interaction surface based on Bliss independence analysis (B) for the combination of amphotericin B plus voriconazole against A. fumigatus AFM4215 in an in vitro PK-PD model simulating human serum concentration-time profiles. Arrows indicate clinically achievable drug exposures in serum for isolates with MICs of 0.25 to 4 mg/liter.
The reduced model with only five coefficients, namely, bB12 = 37 ± 3.7, cD12 = 2.12 ± 0.91, am1 = −6.8 ± 2.9, am2 = −2.7 ± 0.39, and bm12 = −18.9 ± 7.9 (data are means ± standard errors of the means [SEM]), described the entire response surface well (Fig. 6A) (R2 = 0.97). When the full model was compared with the reduced model, the P value of the F test was 0.88, indicating that the full model is not significantly better than the simple model. The coefficients of variations of most parameters were <20%, and most differences between predicted and observed values were <10%. The prediction of the model was very close to the growth observed for the azole-resistant isolate AFM5235 (r2 = 0.97), with <5% differences between predicted and observed values. For example, for the combinations of voriconazole/amphotericin B with fCmax values of 7/0.05 and 7/0.3 mg/liter, the model predicted 8.5% and 6.3% fungal growth, respectively, and it observed 10.9% and 2.5% fungal growth, respectively.
Pharmacodynamic interactions.
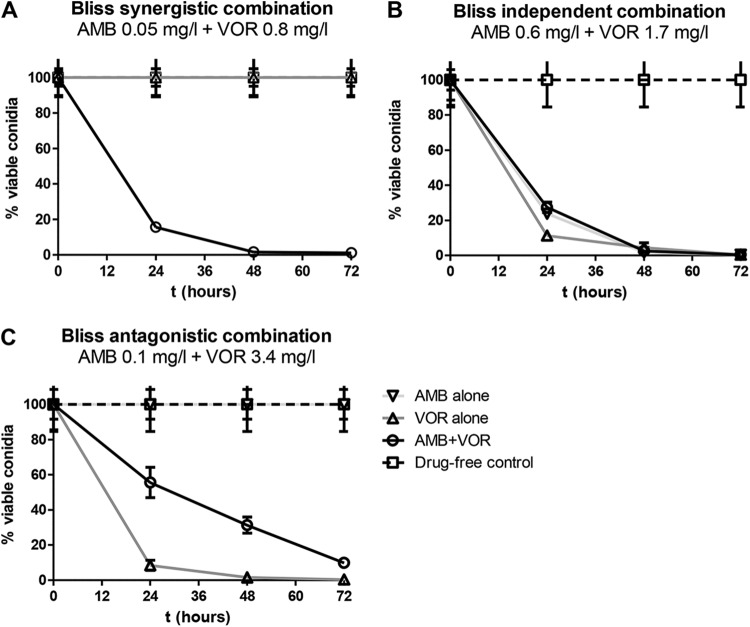
Most combination regimens exerted antagonistic effects (mean, −10%; range, −6 to −16%). These interactions were mainly observed at high drug exposures, with voriconazole fAUC0–12/MIC ratios of ≥20.86 and amphotericin B fCmax/MIC ratios of ≥0.1, as shown in Fig. 6B. Synergy (mean, 26%; range, 20 to 31%) was observed for a narrow range of drug exposures, corresponding to voriconazole fAUC0–12/MIC ratios of 8.6 to 20.86 and amphotericin B fCmax/MIC ratios of 0.05 to 0.1, whereas at lower drug exposures, with amphotericin B fCmax/MIC ratios of <0.05 and voriconazole fAUC0–12/MIC ratios of <8.6, antagonism (−12 to −15%) was found. Representative GM index-time curves for synergistic and antagonistic combinations are shown in Fig. 3. The antagonistic interactions were found for combinations where the monotherapy regimens were effective alone, with no galactomannan production, but where galactomannan production was observed when the drugs were combined (Fig. 3, right graphs). The synergistic interactions were observed for combinations where neither of the monotherapy regimens was able to fully suppress galactomannan production (Fig. 3, left graphs). Concentration-dependent interactions were confirmed with time-kill studies (Fig. 7). For the synergistic combination of AMB at 0.05 mg/liter and VOR at 0.8 mg/liter, the % viable conidia was lower than those for both monotherapy regimens at all time points (Fig. 7A). For the antagonistic combination of AMB at 0.1 mg/liter and VOR at 3.4 mg/liter, the % viable conidia of the combination regimen was higher than that of the most effective monotherapy regimen of voriconazole at all time points (Fig. 7C). For the independent combination of AMB at 0.6 mg/liter and VOR at 1.7 mg/liter, the % viable conidia was similar to those for the combination and monotherapy regimens (Fig. 7B).
FIG 7.
Time-kill curves for selected amphotericin B-voriconazole combinations which were Bliss synergistic, independent, and antagonistic against the azole-susceptible A. fumigatus AFM4215 isolate in the in vitro model. Error bars represent SD.
The concentration-dependent interactions shown in Fig. 6B were observed within the drug-exposure range achieved in human serum. In order to calculate the net effect of such complex interactions and to determine which patients will benefit from combination therapy with standard dosing regimens of amphotericin B and voriconazole for isolates commonly implicated in these infections, we performed a Monte Carlo analysis.
Monte Carlo analysis.
The results of Monte Carlo analysis of the monotherapy and combination regimens are shown in Table 1. More than 83% target attainment was observed for combination regimens for isolates with voriconazole MICs of ≤0.25 mg/liter and amphotericin B MICs of ≤0.5 mg/liter (Table 1, underlined data). Combination therapy against these isolates marginally (<10%) increased the % target attainment of monotherapy regimens. For isolates with voriconazole/amphotericin B MICs of 0.5 to 1/1 mg/liter and 0.5/2 to 4 mg/liter, combination therapy resulted in 75 to 87% target attainment rates, whereas the corresponding rates for monotherapy regimens were 41 to 72% (Table 1, data shown in bold). For isolates with voriconazole/amphotericin B MICs of ≥4/≥2 mg/liter, the % target attainment rate was very low (≤6%) for both monotherapy and combination therapy regimens (Table 1, shaded cells). For the remaining isolates, with voriconazole/amphotericin B MICs of 1 to 2/2 to 4 mg/liter and 2 to 4/1 mg/liter, although most combination regimens resulted in 17 to 48% higher target attainment rates than those for the corresponding monotherapy regimens, the % target attainment for these isolates was <70% (Table 1, nonhighlighted cells). This indicates that therapeutic drug monitoring will be required for these isolates, since only a subset of patients (31% to 69%) will benefit from combination therapy.
TABLE 1.
PK-PD target attainment rates associated with 6-week survival in 10,000 simulated patients infected with A. fumigatus isolates with various MICs of voriconazole and amphotericin B and treated with 1 mg/kg amphotericin B or 4 mg/kg voriconazole monotherapy or combination therapya
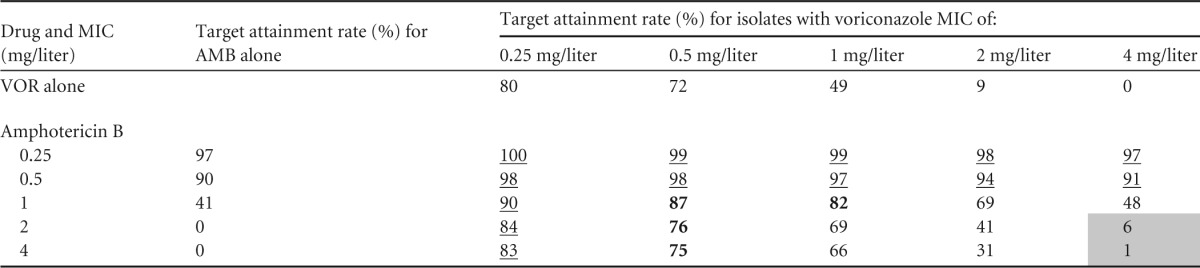
Underlining indicates isolates for which the % target attainment was ≥83% for combination therapy regimens and marginally (<10%) higher than those for monotherapy regimens. Bold values indicate isolates for which the % target attainment was 75 to 87% for combination therapy regimens and up to 4 to 33% higher than those for monotherapy regimens. Shading indicates isolates for which the % target attainment was ≤6% for combination therapy regimens.
Therapeutic drug monitoring.
The serum concentration/MIC ratios for amphotericin B and voriconazole required for combination therapy to attain the EI50 are shown in the isobologram in Fig. 8. A specific isolate could be treated efficiently with high concentrations of each drug alone (gray circles) or any set of serum levels of both drugs depicted by the line of the isobologram. The lowest concentrations that could be targeted without reducing efficacy were 1.1 tCmin/MIC for voriconazole and 0.5 tCmax/MIC for amphotericin B (Fig. 8, white circle). Low voriconazole serum concentration/MIC ratios should be avoided because of the potential antagonistic interactions with amphotericin B, in which case higher amphotericin B concentrations would be required to produce the same effect as that with monotherapy regimens (black circle). The target voriconazole and amphotericin B serum levels required to attain the EI50 are presented in Table 2 for isolates with different MICs.
FIG 8.
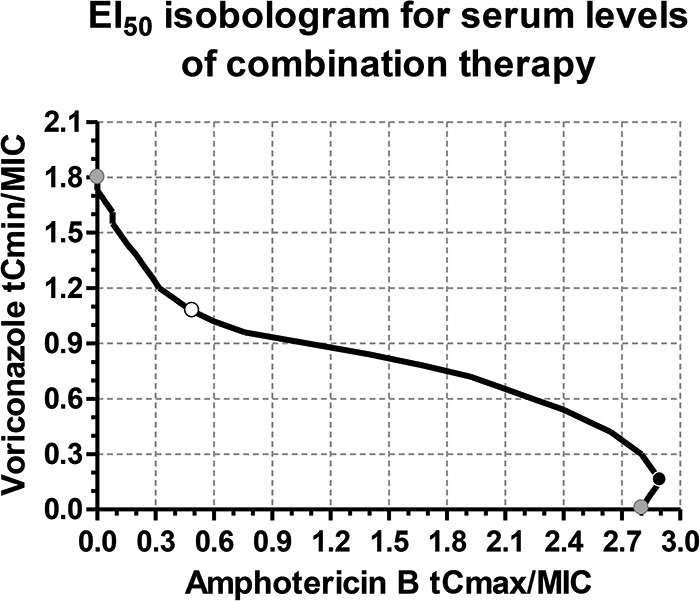
Isobologram for total serum level/MIC ratios for voriconazole and amphotericin B in combination that attain the PK/PD target EI50. Concave (white circle) and convex (black circle) parts of the curve represent synergistic and antagonistic interactions, respectively. However, any set of values are equally effective. The gray circles represent the serum level/MIC ratios for drugs alone, and the white circle represents the combination with the lowest drug concentrations, corresponding to a voriconazole tCmin/MIC ratio of 1.1 and an amphotericin B tCmax/MIC ratio of 0.5, considering 58% and 95% protein binding, respectively, or to a voriconazole fCmin/MIC ratio of 0.46 and an amphotericin B fCmax/MIC ratio of 0.025. For amphotericin B fCmax >0.1 mg/liter, tCmax should be calculated using protein binding rates >96% depending on the concentration of fCmax as previously described (19).
TABLE 2.
Target total serum levels for therapeutic drug monitoring of voriconazole-amphotericin B combination therapy in relation to the MIC of the infecting isolatec
| Drug and MIC (mg/liter) | Amphotericin B tCmaxa/voriconazole tCminb | Amphotericin B tCmaxa/voriconazole tCminb for isolates with a voriconazole MIC of: |
|||||
|---|---|---|---|---|---|---|---|
| 0.25 mg/liter | 0.5 mg/liter | 1 mg/liter | 2 mg/liter | 4 mg/liter | 8 mg/liter | ||
| Voriconazole | 0/0.45 | 0/0.9 | 0/1.8 | 0/3.6 | 0/7.2 | 0/14.4 | |
| Amphotericin B | |||||||
| 0.25 | 0.7/0 | 0.125/0.275 | 0.125/0.55 | 0.125/1.1 | 0.125/2.2 | 0.125/4.4 | 0.125/8.8 |
| 0.5 | 1.4/0 | 0.25/0.275 | 0.25/0.55 | 0.25/1.1 | 0.25/2.2 | 0.25/4.4 | 0.25/8.8 |
| 1 | 2.8/0 | 0.5/0.275 | 0.5/0.55 | 0.5/1.1 | 0.5/2.2 | 0.5/4.4 | 0.5/8.8 |
| 2 | 9.3/0 | 1/0.275 | 1/0.55 | 1/1.1 | 1/2.2 | 1/4.4 | 1/8.8 |
| 4 | >10/0 | 2/0.275 | 2/0.55 | 2/1.1 | 2/2.2 | 2/4.4 | 2/8.8 |
The tCmax value was calculated by dividing fCmax by 0.05, 0.03, and 0.01, the free fractions of amphotericin B at tCmax <5, 5 to 10, and >10 mg/liter concentrations, respectively (19). Amphotericin B trough levels can be calculated by dividing the tCmin by 8, since the tCmax/trough ratio found in the present and other clinical studies is approximately 8.
The tCmin value was calculated by dividing fCmin by 0.42, the free fraction of voriconazole.
Underlined numbers correspond to concentrations associated with toxicity. The first row and column correspond to target values of voriconazole and amphotericin B monotherapy regimens, respectively.
Voriconazole tCmin values of >5.5 mg/liter and amphotericin B tCmax values of >1 mg/liter should be avoided because of toxicity problems (Table 2, shaded numbers) (21, 22). Thus, combination therapy may reduce toxicity associated with monotherapy regimens employed for the treatment of isolates with amphotericin B MICs of ≥0.5 mg/liter and voriconazole MICs of ≥4 mg/liter. Targeting the concentrations presented in Table 2, the EI50 can be attained for isolates with amphotericin B and voriconazole MICs of up to 2 and 4 mg/liter, respectively, without significant toxicity, considering that there is no toxicological interaction between amphotericin B and voriconazole. Although there are no clinical trials addressing the toxicological interaction between polyenes and azoles, safety data from case reports do not indicate increased adverse events in patients treated with these combinations (23–25).
DISCUSSION
Concentration-dependent interactions were found for simulated serum concentration-time profiles of amphotericin B and voriconazole administered concomitantly. Synergy was observed at low amphotericin B-high voriconazole exposures, and antagonism was seen at high amphotericin B-low voriconazole exposures. By bridging in vitro PK-PD combination data with human pharmacokinetics, the combination of standard doses of voriconazole (4 mg/kg) and amphotericin B (1 mg/kg) resulted in higher target attainment rates than those for monotherapy regimens, particularly for isolates with voriconazole/amphotericin B MICs of 1 to 4 mg/liter. The pharmacodynamic target was attained for combination regimens with serum concentration/MIC ratios of 1.1 tCmin/MIC for voriconazole and 0.5 tCmax/MIC for amphotericin B, whereas the equally effective monotherapy regimens required a voriconazole tCmin/MIC ratio of 1.8 and an amphotericin B tCmax/MIC ratio of 2.8. These approximately 2- and 6-fold reductions in the PK-PD parameters of voriconazole and amphotericin B, respectively, when combined, allowed attainment of the EI50 for isolates with voriconazole/amphotericin B MICs of up to 4/2 mg/liter with lower concentrations than those required for monotherapy regimens to efficiently treat the same isolates, thus minimizing toxicity. Consequently, therapeutic drug monitoring of antifungal combination therapy can be employed in order to increase its efficacy, using Fig. 8 as a guidance chart. Because the MIC of the pathogen is usually not known, combination therapy can be optimized using local epidemiological MIC data, and predictions can be made using the MIC90, covering most A. fumigatus isolates.
The concentration-dependent nature of amphotericin B-azole interactions is supported by the mechanisms of action of the component drugs. Voriconazole inhibits ergosterol biosynthesis by blocking the C14-alpha-demethylation of lanosterol, resulting in depletion of ergosterol from the cell membrane (26). Amphotericin B interacts with ergosterol of the cell membrane and, at high concentrations (>0.1 mg/liter), forms transmembrane aqueous pores, causing an increase in the permeability of the membrane, rapid leakage of cytoplasmic components, and, eventually, cell death (27), whereas at lower concentrations (<0.1 mg/liter), amphotericin B interacts with phospholipids of the cell membrane without the direct involvement of sterol molecules strongly influencing the membrane environment (28, 29). The action of amphotericin B at low concentrations may provide greater access to azoles in the intracellular space, leading to increased inhibition of the enzymes of ergosterol biosynthesis (30). Thus, the polyene-azole synergistic interactions at low concentrations of amphotericin B can be interpreted as a consequence of increased inflow and/or ineffective outflow of the azole. Since ergosterol is not involved in this step, the inhibition of its biosynthesis by the azole does not antagonize the action of the polyene. In contrast, at high concentrations of amphotericin B, where hydrophilic pores are created, the presence of ergosterol is important. Thus, at these concentrations, the azole antagonizes the action of the polyene by depleting ergosterol from the fungal membrane. This dual mode of action was previously described for Leishmania spp. (31), whereas the concentration-dependent interaction of amphotericin B and an azole was previously found for Aspergillus fumigatus (32). Although in the latter study weak synergistic and strong antagonistic interactions were found using the standard microdilution checkerboard technique, with constant drug concentrations over time, in the present study the synergistic interactions were stronger than the antagonistic ones, indicating an enhancement of synergy and diminishment of antagonism at clinically relevant drug concentrations.
The in vivo interaction of amphotericin B-azole combination therapy in animal models of invasive aspergillosis showed mainly indifferent or antagonistic interactions (33). However, most experiments were carried out with various azoles and using a narrow range of (usually high) doses of amphotericin B which are associated with antagonism, as found in the present study. Regarding amphotericin B-voriconazole combination, in two models of invasive pulmonary aspergillosis, in guinea pigs and mice, no statistically significant difference was found in survival rates between monotherapy and combination therapy (34, 35). A possible explanation is that the high doses of amphotericin B resulted in increased drug levels, which, according to the results of the present in vitro study, are associated with independent or weakly antagonistic interactions. In a recent study, the combination of voriconazole with a suboptimal dose (0.3 mg/kg) of amphotericin B improved the efficacy (100% mouse survival) of the respective monotherapies in a murine model of Aspergillus fumigatus infection (36). The combination of voriconazole with a subtherapeutic dose (1 mg/kg), but not a higher dose (10 mg/kg), of liposomal amphotericin B resulted in increased effectiveness (100% survival and significant reductions of fungal burdens in the kidneys and brain) compared to monotherapy in a murine model of central nervous system aspergillosis (37). In both the latter studies, amphotericin B was used at suboptimal doses, resulting in low amphotericin B concentrations which are associated with synergistic interactions, as we found in the present report. Although sequential therapy with amphotericin B followed by voriconazole may resemble concomitant combination therapy with low doses of amphotericin B and standard doses of voriconazole because of the long half-life of amphotericin B, the investigation of these interactions requires another experimental design.
Given the absence of a controlled clinical trial evaluating the efficacy of amphotericin B-azole combination for the treatment of invasive aspergillosis, the only available clinical data are derived mainly from retrospective studies and case reports. In a retrospective study of patients treated with lipid formulations of amphotericin B alone or with itraconazole, the response to amphotericin B alone (10%) was not significantly higher than that to combination therapy (0%) (38). The combination of standard dosages of lipid formulations of amphotericin B and itraconazole may have resulted in high amphotericin B concentrations, which are associated with independent or weakly antagonistic interactions based on the present study, assuming that the pharmacodynamic interactions with lipid formulations of amphotericin B are the same as those with conventional amphotericin B. In contrast, in another retrospective study with patients treated with conventional amphotericin B alone or with itraconazole, 82% of patients who received combination therapy were cured or showed improvement, compared to 50% of those who received only amphotericin B (23). Since in the latter study most patients in the combination arm completed therapy with itraconazole alone, it is very likely that the low amphotericin B concentrations that persist in serum after discontinuation of amphotericin B therapy (39) interacted synergistically with the azole, as found in the present study. Interestingly, the Monte Carlo simulation analysis showed a similar increase in the target attainment rate (41% for amphotericin B monotherapy versus 87% for combination therapy) for frequently observed isolates with amphotericin B and voriconazole MICs of 1 and 0.5 mg/liter, respectively. Although individual cases do not provide information on the nature of an interaction, successful outcomes have been reported for patients treated with liposomal amphotericin B and voriconazole (24, 25, 40).
The only randomized clinical trial of amphotericin B-azole combination therapy was against candidemia, and it showed an improved treatment success with an amphotericin B-fluconazole combination using a lower-than-standard dose of conventional amphotericin B (0.6 mg/kg) (41). Monte Carlo analysis also showed an improvement of the target attainment rate for amphotericin B-voriconazole combination therapy against aspergillosis in the present study. The validity of this approach was recently shown for amphotericin B and voriconazole monotherapies against aspergillosis (11, 12). The target values of 1.8× MIC for trough levels of voriconazole and 2.8× MIC for amphotericin B peak concentrations for maximal activity observed in the present study were indeed found to be associated with maximal efficacy of monotherapy regimens in clinical and animal studies (42, 43). The latter target values are reduced 2- and 6-fold, respectively, in combination therapy, minimizing the potential for toxicity, particularly for amphotericin B, whose toxicity often results in discontinuation of therapy. Although the exposure-effect relationship of amphotericin B nephrotoxicity is not well defined, dosing regimens with a tCmax of 1.5 mg/liter were associated with significant increases of creatinemia (>18 mg/liter) and drug discontinuation (36%) compared to dosing regimens with a tCmax of 1 mg/liter, for which no discontinuation was observed in HIV-infected patients (22). Similar and higher rates of nephrotoxicity have been reported for doses of 0.6 to 1 mg/kg, which usually result in serum total Cmax values of >1 mg/liter (39, 44). Amphotericin B nephrotoxicity (>30%) was found to be associated with total doses of >0.5 g after a mean duration of therapy of 14 days, which corresponds to a daily dose of >0.6 mg/kg (45). Thus, a total Cmax of >1 mg/liter should be avoided because of the associated significant toxicity. Combination therapy can achieve this target for isolates with amphotericin B and voriconazole MICs of up to 2 and 4 mg/liter, respectively, as opposed to amphotericin B monotherapy, which can be used to treat isolates with MICs of up to 0.25 mg/liter with minimal toxicity (Table 2).
Patients treated with the standard doses of amphotericin B and voriconazole usually have low voriconazole exposures and high amphotericin B exposures. Based on the present findings, these exposures will result in independent and weakly antagonistic interactions. The latter may not be clinically significant because they are of a low magnitude (<10% Bliss antagonism) (Fig. 6) and will require a small increase of amphotericin B exposure, from 2.8 to 2.9 tCmax/MIC (Fig. 7), to compensate for the loss of antifungal efficacy due to antagonism. Thus, for combinations with low voriconazole and high amphotericin B exposures, the effect will be similar to the effect of amphotericin B monotherapy. The low-grade antagonism at the low voriconazole-high amphotericin B exposures that are usually observed in patients, together with the high-grade synergy at high voriconazole-low amphotericin B exposures that are seldom found in patients treated with standard doses of amphotericin B-voriconazole combination therapy, may result in a net improved treatment success, in agreement with the higher target attainment rates for combination therapy regimens than for monotherapy regimens found in the present study.
This balance between antagonism and synergy could be shifted toward synergy by therapeutic drug monitoring of amphotericin B and voriconazole to attain 0.5 tCmin/MIC and 1.1 tCmax/MIC ratios in serum, respectively. Considering that low-dose (≤0.3 mg/kg) amphotericin B regimens can reach tCmaxs of up to 0.6 mg/liter (46) and were safely and effectively used for prophylaxis of transplant patients (47, 48), these ratios can be obtained with low doses of amphotericin B in combination with the standard dose of voriconazole (4 mg/kg). The latter combination regimen will be associated with a reduced nephrotoxicity and can be used to treat isolates with amphotericin B/voriconazole MICs of up to 1/4 mg/liter, whereas for isolates with MICs of up to 2/4 mg/liter, a higher dose (0.6 mg/kg) should be combined with standard voriconazole doses, although this gives an increased risk for toxicity. A pilot clinical study with close monitoring of serum levels could test the low-dose amphotericin B-based combination regimens in order to show whether they are safe and effective and offer an alternative therapeutic approach against A. fumigatus isolates, including resistant isolates.
REFERENCES
- 1.Steinbach WJ, Marr KA, Anaissie EJ, Azie N, Quan SP, Meier-Kriesche HU, Apewokin S, Horn DL. 2012. Clinical epidemiology of 960 patients with invasive aspergillosis from the PATH Alliance registry. J Infect 65:453–464. doi: 10.1016/j.jinf.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Karthaus M, Buchheidt D. 2013. Invasive aspergillosis: new insights into disease, diagnostic and treatment. Curr Pharm Des 19:3569–3594. doi: 10.2174/13816128113199990330. [DOI] [PubMed] [Google Scholar]
- 3.Fohrer C, Fornecker L, Nivoix Y, Cornila C, Marinescu C, Herbrecht R. 2006. Antifungal combination treatment: a future perspective. Int J Antimicrob Agents 27(Suppl 1):S25–S30. doi: 10.1016/j.ijantimicag.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 4.Wirk B, Wingard JR. 2008. Combination antifungal therapy: from bench to bedside. Curr Infect Dis Rep 10:466–472. doi: 10.1007/s11908-008-0076-x. [DOI] [PubMed] [Google Scholar]
- 5.Marr K. 2004. Combination antifungal therapy: where are we now, and where are we going? Oncology (Williston Park) 18:24–29. [PubMed] [Google Scholar]
- 6.Johnson MD, MacDougall C, Ostrosky-Zeichner L, Perfect JR, Rex JH. 2004. Combination antifungal therapy. Antimicrob Agents Chemother 48:693–715. doi: 10.1128/AAC.48.3.693-715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meletiadis J, Mouton JW, Meis JF, Verweij PE. 2003. Methodological issues related to antifungal drug interaction modelling for filamentous fungi. Rev Med Microbiol 13:101–117. [Google Scholar]
- 8.Paulussen C, Boulet GA, Cos P, Delputte P, Maes LJ. 2014. Animal models of invasive aspergillosis for drug discovery. Drug Discov Today 19:1380–1386. doi: 10.1016/j.drudis.2014.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Roffey SJ, Cole S, Comby P, Gibson D, Jezequel SG, Nedderman AN, Smith DA, Walker DK, Wood N. 2003. The disposition of voriconazole in mouse, rat, rabbit, guinea pig, dog, and human. Drug Metab Dispos 31:731–741. doi: 10.1124/dmd.31.6.731. [DOI] [PubMed] [Google Scholar]
- 10.Blaser J. 1985. In-vitro model for simultaneous simulation of the serum kinetics of two drugs with different half-lives. J Antimicrob Chemother 15(Suppl A):125–130. doi: 10.1093/jac/15.suppl_A.125. [DOI] [PubMed] [Google Scholar]
- 11.Siopi M, Mavridou E, Mouton JW, Verweij PE, Zerva L, Meletiadis J. 2014. Susceptibility breakpoints and target values for therapeutic drug monitoring of voriconazole and Aspergillus fumigatus in an in vitro pharmacokinetic/pharmacodynamic model. J Antimicrob Chemother 69:1611–1619. doi: 10.1093/jac/dku023. [DOI] [PubMed] [Google Scholar]
- 12.Elefanti A, Mouton JW, Verweij PE, Zerva L, Meletiadis J. 2014. Susceptibility breakpoints for amphotericin B and Aspergillus species in an in vitro pharmacokinetic-pharmacodynamic model simulating free-drug concentrations in human serum. Antimicrob Agents Chemother 58:2356–2362. doi: 10.1128/AAC.02661-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meletiadis J, Al-Saigh R, Velegraki A, Walsh TJ, Roilides E, Zerva L. 2012. Pharmacodynamic effects of simulated standard doses of antifungal drugs against Aspergillus species in a new in vitro pharmacokinetic/pharmacodynamic model. Antimicrob Agents Chemother 56:403–410. doi: 10.1128/AAC.00662-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Saigh R, Elefanti A, Velegraki A, Zerva L, Meletiadis J. 2012. In vitro pharmacokinetic/pharmacodynamic modeling of voriconazole activity against Aspergillus species in a new in vitro dynamic model. Antimicrob Agents Chemother 56:5321–5327. doi: 10.1128/AAC.00549-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meletiadis J, Verweij PE, TeDorsthorst DT, Meis JF, Mouton JW. 2005. Assessing in vitro combinations of antifungal drugs against yeasts and filamentous fungi: comparison of different drug interaction models. Med Mycol 43:133–152. doi: 10.1080/13693780410001731547. [DOI] [PubMed] [Google Scholar]
- 16.Meletiadis J, Petraitis V, Petraitiene R, Lin P, Stergiopoulou T, Kelaher AM, Sein T, Schaufele RL, Bacher J, Walsh TJ. 2006. Triazole-polyene antagonism in experimental invasive pulmonary aspergillosis: in vitro and in vivo correlation. J Infect Dis 194:1008–1018. doi: 10.1086/506617. [DOI] [PubMed] [Google Scholar]
- 17.Meletiadis J, Stergiopoulou T, O'Shaughnessy EM, Peter J, Walsh TJ. 2007. Concentration-dependent synergy and antagonism within a triple antifungal drug combination against Aspergillus species: analysis by a new response surface model. Antimicrob Agents Chemother 51:2053–2064. doi: 10.1128/AAC.00873-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayestaran A, Lopez RM, Montoro JB, Estibalez A, Pou L, Julia A, Lopez A, Pascual B. 1996. Pharmacokinetics of conventional formulation versus fat emulsion formulation of amphotericin B in a group of patients with neutropenia. Antimicrob Agents Chemother 40:609–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob Agents Chemother 46:834–840. doi: 10.1128/AAC.46.3.834-840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfizer Inc. 2010. VFEND® (voriconazole) United States package insert. Pfizer Inc., New York, NY. [Google Scholar]
- 21.Pascual A, Calandra T, Bolay S, Buclin T, Bille J, Marchetti O. 2008. Voriconazole therapeutic drug monitoring in patients with invasive mycoses improves efficacy and safety outcomes. Clin Infect Dis 46:201–211. doi: 10.1086/524669. [DOI] [PubMed] [Google Scholar]
- 22.Chavanet P, Clement C, Duong M, Buisson M, D'Athis P, Dumas M, Bonnin A, Portier H. 1997. Toxicity and efficacy of conventional amphotericin B deoxycholate versus escalating doses of amphotericin B deoxycholate—fat emulsion in HIV-infected patients with oral candidosis. Clin Microbiol Infect 3:455–461. doi: 10.1111/j.1469-0691.1997.tb00282.x. [DOI] [PubMed] [Google Scholar]
- 23.Popp AI, White MH, Quadri T, Walshe L, Armstrong D. 1999. Amphotericin B with and without itraconazole for invasive aspergillosis: a three-year retrospective study. Int J Infect Dis 3:157–160. doi: 10.1016/S1201-9712(99)90038-3. [DOI] [PubMed] [Google Scholar]
- 24.Askari E, Jarque I, Nicolás Franco S, Cáceres Agra JJ. 2010. Case collection study of the safety of AmBisome in association with voriconazole in the treatment of patients with invasive fungal infection. Rev Esp Quimioter 23:210–212. [PubMed] [Google Scholar]
- 25.Vassiloyanakopoulos A, Falagas ME, Allamani M, Michalopoulos A. 2006. Aspergillus fumigatus tricuspid native valve endocarditis in a non-intravenous drug user. J Med Microbiol 55:635–638. doi: 10.1099/jmm.0.46398-0. [DOI] [PubMed] [Google Scholar]
- 26.Kathiravan MK, Salake AB, Chothe AS, Dudhe PB, Watode RP, Mukta MS, Gadhwe S. 2012. The biology and chemistry of antifungal agents: a review. Bioorg Med Chem 20:5678–5698. doi: 10.1016/j.bmc.2012.04.045. [DOI] [PubMed] [Google Scholar]
- 27.Baginski M, Czub J. 2009. Amphotericin B and its new derivatives—mode of action. Curr Drug Metab 10:459–469. doi: 10.2174/138920009788898019. [DOI] [PubMed] [Google Scholar]
- 28.Baginski M, Sternal K, Czub J, Borowski E. 2005. Molecular modelling of membrane activity of amphotericin B, a polyene macrolide antifungal antibiotic. Acta Biochim Pol 52:655–658. [PubMed] [Google Scholar]
- 29.Cohen BE. 1986. Concentration- and time-dependence of amphotericin-B induced permeability changes across ergosterol-containing liposomes. Biochim Biophys Acta 857:117–122. doi: 10.1016/0005-2736(86)90104-5. [DOI] [PubMed] [Google Scholar]
- 30.Mukherjee PK, Sheehan DJ, Hitchcock CA, Ghannoum MA. 2005. Combination treatment of invasive fungal infections. Clin Microbiol Rev 18:163–194. doi: 10.1128/CMR.18.1.163-194.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramos H, Valdivieso E, Gamargo M, Dagger F, Cohen BE. 1996. Amphotericin B kills unicellular leishmanias by forming aqueous pores permeable to small cations and anions. J Membr Biol 152:65–75. doi: 10.1007/s002329900086. [DOI] [PubMed] [Google Scholar]
- 32.Meletiadis J, te Dorsthorst DT, Verweij PE. 2006. The concentration-dependent nature of in vitro amphotericin B-itraconazole interaction against Aspergillus fumigatus: isobolographic and response surface analysis of complex pharmacodynamic interactions. Int J Antimicrob Agents 28:439–449. doi: 10.1016/j.ijantimicag.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Vazquez JA. 2008. Clinical practice: combination antifungal therapy for mold infections: much ado about nothing? Clin Infect Dis 46:1889–1901. doi: 10.1086/588475. [DOI] [PubMed] [Google Scholar]
- 34.Chandrasekar PH, Cutright JL, Manavathu EK. 2004. Efficacy of voriconazole plus amphotericin B or micafungin in a guinea-pig model of invasive pulmonary aspergillosis. Clin Microbiol Infect 10:925–928. doi: 10.1111/j.1469-0691.2004.00958.x. [DOI] [PubMed] [Google Scholar]
- 35.Clemons KV, Schwartz JA, Stevens DA. 2011. Therapeutic and toxicologic studies in a murine model of invasive pulmonary aspergillosis. Med Mycol 49:834–847. doi: 10.3109/13693786.2011.577822. [DOI] [PubMed] [Google Scholar]
- 36.Sandoval-Denis M, Pastor FJ, Capilla J, Guarro J. 2013. Efficacy of amphotericin B at suboptimal dose combined with voriconazole in a murine infection by Aspergillus fumigatus with poor in vivo response to the azole. Antimicrob Agents Chemother 57:4540–4542. doi: 10.1128/AAC.00563-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Clemons KV, Espiritu M, Parmar R, Stevens DA. 2005. Comparative efficacies of conventional amphotericin B, liposomal amphotericin B (AmBisome), caspofungin, micafungin, and voriconazole alone and in combination against experimental murine central nervous system aspergillosis. Antimicrob Agents Chemother 49:4867–4875. doi: 10.1128/AAC.49.12.4867-4875.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kontoyiannis DP, Boktour M, Hanna H, Torres HA, Hachem R, Raad II. 2005. Itraconazole added to a lipid formulation of amphotericin B does not improve outcome of primary treatment of invasive aspergillosis. Cancer 103:2334–2337. doi: 10.1002/cncr.21057. [DOI] [PubMed] [Google Scholar]
- 39.Bekersky I, Fielding RM, Dressler DE, Lee JW, Buell DN, Walsh TJ. 2002. Pharmacokinetics, excretion, and mass balance of liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate in humans. Antimicrob Agents Chemother 46:828–833. doi: 10.1128/AAC.46.3.828-833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdulaziz S, Al Jahdali H, Baharoon S. 2012. Combination antifungal therapy for invasive pulmonary aspergillosis. BMJ Case Rep 2012:bcr2012007824. doi: 10.1136/bcr-2012-007824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rex JH, Pappas PG, Karchmer AW, Sobel J, Edwards JE, Hadley S, Brass C, Vazquez JA, Chapman SW, Horowitz HW, Zervos M, McKinsey D, Lee J, Babinchak T, Bradsher RW, Cleary JD, Cohen DM, Danziger L, Goldman M, Goodman J, Hilton E, Hyslop NE, Kett DH, Lutz J, Rubin RH, Scheld WM, Schuster M, Simmons B, Stein DK, Washburn RG, Mautner L, Chu TC, Panzer H, Rosenstein RB, Booth J. 2003. A randomized and blinded multicenter trial of high-dose fluconazole plus placebo versus fluconazole plus amphotericin B as therapy for candidemia and its consequences in nonneutropenic subjects. Clin Infect Dis 36:1221–1228. doi: 10.1086/374850. [DOI] [PubMed] [Google Scholar]
- 42.Troke PF, Hockey HP, Hope WW. 2011. Observational study of the clinical efficacy of voriconazole and its relationship to plasma concentrations in patients. Antimicrob Agents Chemother 55:4782–4788. doi: 10.1128/AAC.01083-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wiederhold NP, Tam VH, Chi J, Prince RA, Kontoyiannis DP, Lewis RE. 2006. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob Agents Chemother 50:469–473. doi: 10.1128/AAC.50.2.469-473.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saliba F, Dupont B. 2008. Renal impairment and amphotericin B formulations in patients with invasive fungal infections. Med Mycol 46:97–112. doi: 10.1080/13693780701730469. [DOI] [PubMed] [Google Scholar]
- 45.Bates DW, Su L, Yu DT, Chertow GM, Seger DL, Gomes DR, Platt R. 2001. Correlates of acute renal failure in patients receiving parenteral amphotericin B. Kidney Int 60:1452–1459. doi: 10.1046/j.1523-1755.2001.00948.x. [DOI] [PubMed] [Google Scholar]
- 46.Undre NA, Stevenson P, Wilbraham D. 2014. Pharmacokinetic profile of micafungin when co-administered with amphotericin B in healthy male subjects. Int J Clin Pharmacol Ther 52:237–244. doi: 10.5414/CP202015. [DOI] [PubMed] [Google Scholar]
- 47.Koh LP, Kurup A, Goh YT, Fook-Chong SM, Tan PH. 2002. Randomized trial of fluconazole versus low-dose amphotericin B in prophylaxis against fungal infections in patients undergoing hematopoietic stem cell transplantation. Am J Hematol 71:260–267. doi: 10.1002/ajh.10234. [DOI] [PubMed] [Google Scholar]
- 48.Riley DK, Pavia AT, Beatty PG, Petersen FB, Spruance JL, Stokes R, Evans TG. 1994. The prophylactic use of low-dose amphotericin B in bone marrow transplant patients. Am J Med 97:509–514. doi: 10.1016/0002-9343(94)90345-X. [DOI] [PubMed] [Google Scholar]