Abstract
The presynaptic active zone is a dynamic structure that orchestrates regulated release of neurotransmitters. Developmental and aging processes, and changes in neuronal network activity can all modulate the number, size and composition of active zone and thereby synaptic efficacy. However, very little is known about the mechanism that controls the structural stability of active zone. By studying a model synapse, the Drosophila neuromuscular junction, our recent work shed light on how two scaffolding proteins at the active zone regulate active zone stability by promoting a localized dephosphorylation event at the nerve terminal. Here we discuss the major insights from our findings and their implications for future research.
Keywords: active zone stability, Drosophila, neuromuscular junction, dephosphorylation, Liprin-α, Syd-1, PP2A, GSK-3β
In the Drosophila neuromuscular junction (NMJ), a large synaptic connection mediates neurotransmission between the motor neuron and muscle. Each synaptic terminal contains 20–50 synaptic boutons. Within each bouton, there are about 10 active zones (AZs) – specialized membrane compartments at the axonal terminal – where orchestrated release of the neurotransmitter glutamate occurs. Opposing each AZ is a cluster of postsynaptic glutamate receptors that responds to the released transmitter (Collins and Di-Antonio, 2007). Neither synaptic terminals nor AZs are permanent structures. The size of synaptic terminals and the number, density and distribution of AZs can all change during synaptic development, activity-dependent structure plasticity, synaptic elimination and aging. All these changes have a profound impact on the function of the NMJs and their modulation by internal or external cues. Studies of synaptic terminal retraction at the Drosophila NMJ have identified several proteins required for synaptic terminal stability, including the dynactin protein complex (Eaton et al., 2002), the presynaptic spectrin skeleton (Pielage et al., 2005), a giant isoform of Ankyrin2 (Koch et al., 2008; Pielage et al., 2008), the actin-capping protein Adducin (Pielage et al., 2011), and a microtubule-binding protein Stathmin (Graf et al., 2011). However, much less is known about the presynaptic mechanisms that control the stability and localization of AZs at the presynaptic sites. Recent studies on presynaptic development at the Drosophila NMJs have shed some light on how AZs are stabilized at the motoneuron nerve terminal.
AZs are composed of an evolutionarily conserved protein complex containing scaffolding proteins such as ELKS/CAST/BRP, Munc13, RIM, Syd-1 and Liprin-α (Sudhof, 2012), cell adhesion molecules such as SYG-1/Neph1, SYG-2/Nephrin, Neurexin and Neuroligin, and actin cytoskeletal components such as the WVE-1/WAVE complex (Chia et al., 2014). The assembly of the AZ is a fascinating process in which hundreds of AZ proteins are generated in the cell body, transported along the axon by specific motors, and finally deposited on the nerve terminal to achieve site-specific AZ formation in a coordinated manner. The presynaptic multi-domain scaffolding proteins ELKS/CAST/BRP, RIM, Syd-1 and Liprin-α were identified through genetic and biochemical studies, and they play essential roles in organizing the AZ assembly and modulating synaptic function. BRP constitutes the T-bar structure of Drosophila AZs and actively recruits Voltage-gated Ca2+ channel; RIM is an integrated component of the presynaptic cytomatrix that interacts with many other AZ and synaptic vesicle proteins, including Munc-13 and Rab3, and plays critical roles in synaptic vesicle docking and priming, as well as Ca2+ channel clustering (Sudhof, 2012); Syd-1 and Liprin-α are identified as two master organizer proteins since imaging analysis indicates that both proteins accumulate very early at defined AZ sites during AZ assembly, probably serving as AZ nucleation activity (Owald et al., 2010). Despite recent progress on understanding the molecular actions of Syd-1 and Liprin-α at the AZs (Owald et al., 2012; Chia et al., 2013; Kittelmann et al., 2013), how exactly Syd-1 and Liprin-αcoordinate AZs development together is not entirely clear.
Through proteomic means, we identified the B’ regulatory subunit (called Wrd) of protein phosphatase 2A (PP2A) as a novel binding partner of Liprin-α at the active zone and demonstrated that the Liprin-α-Wrd physical interaction is necessary and sufficient to localize Wrd-containing PP2A to the active zones. Previous genetic analysis suggested that Syd-1 functions upstream of Liprin-α to regulate AZ formation (Dai et al., 2006; Patel et al., 2006). Our findings that Wrd may work downstream of Liprin-α led us to hypothesize that Syd-1/Liprin-α/Wrd work in a linear pathway to regulate presynaptic development. To test this hypothesis, we first screened for synaptogenic phenotype shared by loss-of-function of each of the three genes. Interestingly, we found ectopic accumulation of AZ components, including ELKS/CAST/BRP, Rim, and voltage-gated Ca2+ channels, at the distal side (toward the muscles), but not the proximal side (toward the motoneuron cell bodies), of the motoneuron axons in syd-1, liprin-α and wrd individual mutant NMJs. Some synaptic vesicles and dense core vesicles are also ectopically clustered at this axonal region near NMJs in these mutants. Through a series of genetic rescue experiments, we determined that Syd-1, Liprin-α and Wrd work in the same molecular pathway with a hierarchy from Syd-1 to Liprin-α, and from Liprin-α to Wrd to prevent ectopic localization of presynaptic materials (Li et al., 2014).
What is the nature of the unique synaptogenic phenotype caused by an impaired Syd-1/Liprin-α/Wrd pathway? It is clearly different from a typical axonal transport defect caused by mutations of transport motors or cargos, which normally lead to the entire axon being clogged with aggregated vesicles. It is also unlikely to be a secondary defect of abnormal presynaptic axonal retraction because the typical “footprint” phenotype, such as what is seen in stathmin loss-of-function, is absent in syd-1, liprin-α or wrd mutant NMJs. Our data support a notion that, when the syd-1/liprin-α/wrd pathway is impaired, a portion of AZs at the nerve terminal become destabilized and detached from the nerve terminal, and the floating AZ materials diffuse back to the adjacent axonal regions as ectopic docking sites for vesicles.
First, EM analysis detected floating, dense AZ-like structures inside the synaptic boutons and their adjacent axonal regions in syd-1 mutants, suggesting a defect in AZ stabilization and subsequent back-diffusion of detached AZ materials to distal axonal regions. Second, ectopically-accumulated vesicles do not participate in release or recycling, consistent with the notion that the vesicles do not dock on the axonal plasma membrane, but float with the AZ-like structures. Third, Syd-1, Liprin-α and Wrd show exclusive synaptic localization, consistent with a collaborative function of the three molecules at the presynaptic AZs, but not along the axon.
Wrd defines a specific form of PP2A. If AZ stability is regulated by a phosphatase pathway, then by default, a kinase pathway must exist to antagonize the action of such a phosphatase pathway. Among a number of serine-threonine kinases that share substrates with PP2A, we identified that GSK-3α shows genetic interaction with the syd-1/liprin-α/wrd pathway. Blocking GSK-3α (the fly homologue is called Shaggy, Sgg) suppresses the distal axon vesicle clustering defect in syd-1, liprin-α, or wrd mutants. These data confirm the linear relationship of the syd-1/liprin-α/wrd pathway and also suggest that dephosphorylation by Wrd-containing PP2A phosphatase and phosphorylation by GSK-3α converge to stabilize AZ at the synapse. Collectively, our data support a model that Syd-1 and Liprin-α control PP2A/Wrd-mediated dephosphorylation, which antagonizes GSK-3α-mediated phosphorylation through either common substrate(s) or downstream factors. Such a balanced action of a phasphotase pathway and a kinase pathway is required for the stabilization of AZs at the nerve terminal (Figure 1).
Figure 1.
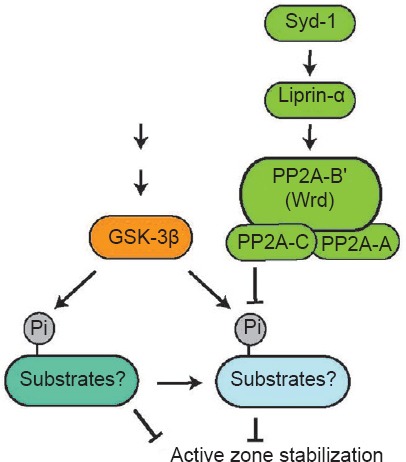
A Syd-1/Liprin-α/Wrd phosphatase pathway and a GSK-3β kinase pathway converge to regulate active zone stability.
The active zone (AZ) organizers Syd-1 and Liprin-α collaborate to localize a Wrd-containing protein phosphatase 2A (PP2A) phosphatase complex to the AZs, where it dephosphorylates specific substrates to promote AZ stability. A GSK-3β signaling pathway antagonizes the action of the Syd-1/Liprin-α/Wrd phosphatase pathway directly via phosphorylating the Wrd substrates, or indirectly via its downstream targets. GSK-3β: Glycogen synthase kinase-3beta.
What could be the downstream targets? Our data indicate that reducing BRP at the AZs is not sufficient to prevent AZ instability in syd-1, liprin-α and wrd mutants, suggesting that BRP may not be the target. Recent studies on how cell adhesion molecules and cytoskeleton-mediated mechanisms regulate AZ function and stability at the presynaptic sites revealed several hints. First, α-Catenin, as a multifunctional protein, binds to transcription factor TCF/LEF, neuronal cadherin (N-cadherin) and Axin. Phosphorylation/dephosphorylation of α-Catenin not only impacts its protein turn-over, but also actively regulates N-cadherin-mediated adhesion (Sadot et al., 2002; Lilien and Balsamo, 2005), which plays an important role in AZ function and stability (Salinas and Price, 2005). Second, studies in worms found that local assembly of the F-actin network at presynaptic sites regulates synaptogenesis. Such assembly requires interaction between a cell adhesion molecule SYG-1 and a key regulator of actin cytoskeleton, the WVE-1/WRC regulatory complex (Chia et al., 2014). In the loss of SYG-1 function, the localization of active zone components is no longer restricted to the normal synaptic region, suggesting a role of the F-actin network in regulating AZ stability (Patel et al., 2006). Third, a recent study showed that, in the presynaptic site of fly NMJs, the microtubule (MT) is closely associated with AZs, and that one of the components anchoring MT to AZs is the microtubule-associated protein Futsch (fly homologue of MAP1). Drosophila futsch mutants show reduced AZ number and density, suggesting that Futsch functions to stabilize AZs by locally reinforcing the linkage between the MT cytoskeleton and AZs (Lepicard et al., 2014). Intriguingly, both β-catenin and Futsch were shown to be substrates of both PP2A and GSK-3β in multiple cell types and signaling contexts. In future studies, it would be interesting to study the downstream targets of Wrd/PP2A and GSK-3β particularly, as well as how their phosphorylation status regulates AZ stability and presynaptic development.
References
- Chia PH, Patel MR, Wagner OI, Klopfenstein DR, Shen K. Intramolecular regulation of presynaptic scaffold protein SYD-2/liprin-alpha. Mol Cell Neurosci. 2013;56C:76–84. doi: 10.1016/j.mcn.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chia PH, Chen B, Li P, Rosen MK, Shen K. Local F-actin network links synapse formation and axon branching. Cell. 2014;156:208–220. doi: 10.1016/j.cell.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins CA, DiAntonio A. Synaptic development: insights from Drosophila. Curr Opin Neurobiol. 2007;17:35–42. doi: 10.1016/j.conb.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Dai Y, Taru H, Deken SL, Grill B, Ackley B, Nonet ML, Jin Y. SYD-2 Liprin-alpha organizes presynaptic active zone formation through ELKS. Nat Neurosci. 2006;9:1479–1487. doi: 10.1038/nn1808. [DOI] [PubMed] [Google Scholar]
- Eaton BA, Fetter RD, Davis GW. Dynactin is necessary for synapse stabilization. Neuron. 2002;34:729–741. doi: 10.1016/s0896-6273(02)00721-3. [DOI] [PubMed] [Google Scholar]
- Graf ER, Heerssen HM, Wright CM, Davis GW, DiAntonio A. Stathmin is required for stability of the Drosophila neuromuscular junction. J Neurosci. 2011;31:15026–15034. doi: 10.1523/JNEUROSCI.2024-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittelmann M, Hegermann J, Goncharov A, Taru H, Ellisman MH, Richmond JE, Jin Y, Eimer S. Liprin-alpha/SYD-2 determines the size of dense projections in presynaptic active zones in C. elegans. J Cell Biol. 2013;203:849–863. doi: 10.1083/jcb.201302022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch I, Schwarz H, Beuchle D, Goellner B, Langegger M, Aberle H. Drosophila ankyrin 2 is required for synaptic stability. Neuron. 2008;58:210–222. doi: 10.1016/j.neuron.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Lepicard S, Franco B, de Bock F, Parmentier ML. A presynaptic role of microtubule-associated protein 1/Futsch in Drosophila: regulation of active zone number and neurotransmitter release. J Neurosci. 2014;34:6759–6771. doi: 10.1523/JNEUROSCI.4282-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Tian X, Zhu M, Bulgari D, Bohme MA, Goettfert F, Wichmann C, Sigrist SJ, Levitan ES, Wu C. Drosophila Syd-1, liprin-alpha and protein phosphatase 2A B’ subunit Wrd function in a linear pathway to prevent ectopic accumulation of synaptic materials in distal axons. J Neurosci. 2014;34:8474–8487. doi: 10.1523/JNEUROSCI.0409-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilien J, Balsamo J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of beta-catenin. Curr Opin Cell Biol. 2005;17:459–465. doi: 10.1016/j.ceb.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Owald D, Fouquet W, Schmidt M, Wichmann C, Mertel S, Depner H, Christiansen F, Zube C, Quentin C, Korner J, Urlaub H, Mechtler K, Sigrist SJ. A Syd-1 homologue regulates pre- and postsynaptic maturation in Drosophila. J Cell Biol. 2010;188:565–579. doi: 10.1083/jcb.200908055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owald D, Khorramshahi O, Gupta VK, Banovic D, Depner H, Fouquet W, Wichmann C, Mertel S, Eimer S, Reynolds E, Holt M, Aberle H, Sigrist SJ. Cooperation of Syd-1 with Neurexin synchronizes pre- with postsynaptic assembly. Nat Neurosci. 2012;15:1219–1226. doi: 10.1038/nn.3183. [DOI] [PubMed] [Google Scholar]
- Patel MR, Lehrman EK, Poon VY, Crump JG, Zhen M, Bargmann CI, Shen K. Hierarchical assembly of presynaptic components in defined C. elegans synapses. Nat Neurosci. 2006;9:1488–1498. doi: 10.1038/nn1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J, Fetter RD, Davis GW. Presynaptic spectrin is essential for synapse stabilization. Curr Biol. 2005;15:918–928. doi: 10.1016/j.cub.2005.04.030. [DOI] [PubMed] [Google Scholar]
- Pielage J, Bulat V, Zuchero JB, Fetter RD, Davis GW. Hts/Adducin controls synaptic elaboration and elimination. Neuron. 2011;69:1114–1131. doi: 10.1016/j.neuron.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pielage J, Cheng L, Fetter RD, Carlton PM, Sedat JW, Davis GW. A presynaptic giant ankyrin stabilizes the NMJ through regulation of presynaptic microtubules and transsynaptic cell adhesion. Neuron. 2008;58:195–209. doi: 10.1016/j.neuron.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadot E, Conacci-Sorrell M, Zhurinsky J, Shnizer D, Lando Z, Zharhary D, Kam Z, Ben-Ze’ev A, Geiger B. Regulation of S33/S37 phosphorylated beta-catenin in normal and transformed cells. J Cell Sci. 2002;115:2771–2780. doi: 10.1242/jcs.115.13.2771. [DOI] [PubMed] [Google Scholar]
- Salinas PC, Price SR. Cadherins and catenins in synapse development. Curr Opin Neurobiol. 2005;15:73–80. doi: 10.1016/j.conb.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The presynaptic active zone. Neuron. 2012;75:11–25. doi: 10.1016/j.neuron.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]


