Human hearing relies on 15,000 hair cells (HCs) or mechanoreceptors sited in a 34 mm long helical shaped epithelial ridge. There may be several explanations for their vulnerability in man and regrettably, they do not regenerate. Approximately 35,000 bipolar neurons have their soma situated in the modiolus of the cochlea. Human afferents consist of two separate systems; one is represented by the large type I cells innervating the inner HCs (IHCs, n = 3,400) and the other one by the small type II cells innervating the outer HCs (OHCs, n = 12,000). Type I spiral ganglion neurons (SGNs) constitute 96% of the afferent nerve population. Unlike in other mammals, their somas together with the pre- and post-somatic segments are unmyelinated. Type II nerve cell soma and fibers are unmyelinated.
Comparative studies of the mammalian cochlea expose some significant structural dissimilarites that may be explained by evolutionary factors. The dissimilarites could explain the distinctive degenerative pattern of the human auditory nerve following loss of sensory mechanoreceptors. Loss of HCs and supporting cells are an instigation of a retrograde progressive defeat of dendrites (peripheral axons), presumably due to the loss of attractive forces from neurotrophic substances and receptor signaling. Supporting cells may provide trophic support through neuregulin (NRG)-erbB2/erbB3 receptor signaling (Stankovic, 2004). A reciprocal interaction between neurons and supporting cells, mediated by NRG and neurotrophins (brain-derived neurotrophic factor, neurotrophin-3) may therefore be critical for the survival of type I SGNs in the adult ear.
Degeneration pattern of the human type I SGNs – myelination and gap junctions: The human type I neurons show spectacular resistance to undergo retrograde degeneration after HC loss; a blessing for the deaf that can undergo CI surgery even after several years of deafness (Linthicum and Fayad, 2009) and for the continous function of CI. The etiology of hearing loss seems to be a more significant determinant of SGN survival. Deterioration is slow, incomplete and generally constrained to the peripheral dendrites. Neurons persist as “amputated” cells with unbroken connections to the brain stem (Figure 1). Histopathology and clinical experience imply that human SGNs can persist electrically excitable without dendrites. The biological background to this phenomenon remains elusive, since a retrograde degeneration generally proceeds centrally in animals. (We have personally experienced patients who receive open set word discrimination with CI after 40 years of single-sided complete deafness). Nerve degeneration seems to be arrested at the cell soma or progresses at a very slow rate. Why is this? Here, we attempted to portray some of the intrinsic mechanisms that may be responsible for these properties in man.
Figure 1.
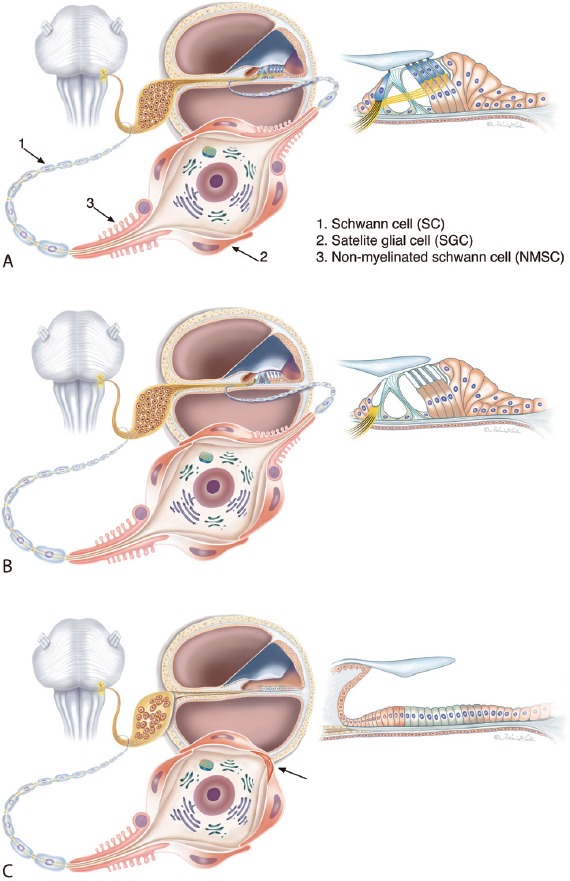
Illustration of different cell anatomies in patients with sensorineural deafness.
(A) Normal condition. The spiral ganglion neurons (SGNs) are surrounded by satellite glial cells (SGCs) while the pre- and post-somatic axonal segments are bordered by non-myelinated Schwann cells (NMSC). Axons are enwrapped by regular Schwann cells. (B) Deafness associated with loss of hair cells. Preservation of supporting cells maintains the integrity of the peripheral dendrite. (C) Atrophy of the sensory epithelium results in dendrite degeneration. The bordering cells (SGCs and NMSCs) consolidate neurons as mono-polar or “amputated” cells (arrow) with unbroken connections to the brain stem. Theoretically, these neurons could be re-sprouted. This condition could be prevalent in patients with long deafness duration (Modified from Liu et al., Neuroscience. 2015;284:470-482). Graphic Karin Lodin.
Firstly, the type I cell soma is not surrounded by a compact myelin sheath, which is otherwise typical in most mammals. 3.65% of the type I neurons are surrounded by a layer containing myelin basic protein (MBP)-positive). The absence of myelin persists (5–50 microns) along the distal and proximal segments of the axon. Secondly, a typical feature of the human type I SGNs is the cluster formation. The cell bodies appear in groups, some even devoid of separating satellite glial cells (SGCs) where inter-neural membrane specializations may exist. Thirdly, nerve cell bodies are surrounded by satellite cells that are intercellular coupled with gap junctions (GJs) expressing connexin 43 (Cx43) (Liu et al., 2014). GJs are intercellular channels for ions and second messengers (e.g., Ca2+, inositol triphosphate, cAMP, cGMP), metabolites (e.g., glucose, amino acids, glutathione, ATP) and neuroprotectants (e.g., adenosine). They are found between SGCs and between SGCs of neighboring type I nerve cell bodies (Figures 1, 2). Cx43 is recognized between SGCs cells in the trigeminal ganglion, dorsal root ganglia and autonomic ganglia. The essential role played by surrounding SGCs for the protection of neurons in various sensory ganglia was described by Hanani et al. (2002). At damage, SGCs are known to undergo structural changes and increase the expression of glial fibrillary acidic protein (Hanani et al., 2002). Effects of axotomy on mouse trigeminal ganglion include increased neuronal excitability, increased ectopic firing and coupling between SGCs, especially between neighboring cells. These effects are believed to play a major role in the induction of neuropathic pain. Similar changes can be detected after sensory deprivation, degeneration and inflammation (Hanani et al., 2002). Up-regulations of Cx43 and laminin-β2 genes were described after induced peripheral lesions in the non-neuronal dorsal root (DRG) and trigeminal ganglion cells (Beau et al., 1995). Likewise, RNA interference reducing Cx43 expression in glial cell GJs after nerve injury increased nociceptive behavior such as neuropathic pain (Ohara et al., 2008), a remarkable analogue to cochlear tinnitus. From the following we postulate that Cx43-mediated GJ signalling between SGCs might play an essential role for the preservation of auditory neurons following HC loss (Liu et al., 2013).
Figure 2.
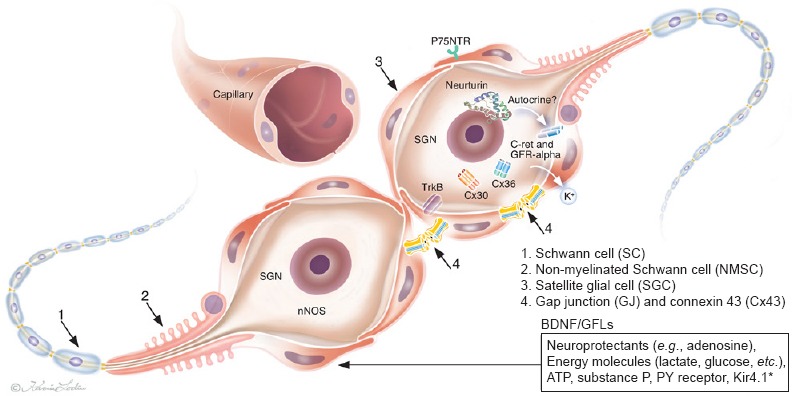
Molecular expression and illustration of human spiral ganglion neurons (SGNs) in the deaf lacking peripheral dendrites.
Surrounding SGCs (red) are intercellular coupled through gap junctions (GJs).
TrkB: Tyrosine kinase B receptor; BDNF: brain-derived neurotrophic factor; nNOS: nitric-oxide synthase; GFL: GDNF family ligand; C-ret: GFL receptor; GFRalpha: GDNF family receptor alpha; NTRN: neurturin; hSGNs: human spiral ganglion cells; SGCs: satellite glial cells; GDNF: glial-cell-line-derived neurotrophic factor; Cx30: connexin 30; Cx36: connexin 36; P75NTR: P75 neurotrophin receptor. (Modified from Liu et al., Cell Tissue Res 2014;355:267-278).
Fluorescent and confocal immunohistochemistry combined with transmission electron microscopy of well-fixed human cochleae, demonstrated a fairly distinct type of perineural cells. It surrounds the pre- and post-somatic initial segments of the human type I SGNs. Unlike the SGCs, they show a rich intracellular content of laminin-β2 and collagen IV but lack myelin and MBP expression. The cells were named non-myelinated Schwann cells (NMSCs, Liu et al., 2015) to separate them from the SGCs (Figure 1). The cell coat shows villous protrusions bordered by a folded basement membrane (BM). The phenotypic manifestations suggest that NMSCs may represent a cell with unique functional properties. Analyses of the pre- and post-somatic segments in pathological ears demonstrated the potential role of NMSCs to complete the glial ring around the SGN cell bodies following dendrite atrophy (“preservation”).
Thus, it is alleged that human type I SGNs are demarcated by three cell categories (Figure 1):
Over 340 million people suffer from sensorineural hearing loss globally. The reason is mostly deficient mechanoreceptors but also a decline in auditory nerve function. Many patients, particularly children, can now be treated successfully with medical devices that electrically and directly stimulate the auditory nerve fibers; by-passing the ailing sensory hair cells (HCs). This medical breakthrough (cochlear implants (CI)) occurred more than fifty years ago but its acceptance and progress escalated during the last 25 years. Thus, hearing is the only sense that can be restored in man. The treatment gives large benefit to patients producing open set speech comprehension but generally little tonal perception and fine hearing. Today, prizes are awarded to those responsible for this ingenious invention in medicine. The Lasker-DeBakey jury selected this topic for its Clinical Medicine Research Award, delivered on September 10th, 2013. Four countries, France, USA, Austria and Australia have played key roles in this progress (http://recorlsa.online.fr/implantcochleaire/historique.html). The tremendous achievement has brought many deaf people into the world of hearing. The devices depend on a patent auditory nerve whose condition varies among the deaf. A current research and therapeutic strategy is to regenerate the deficient auditory nerve to optimize electric stimulation? Even more superior would be to restore the sensory epithelium to combat hearing loss, since newly generated HCs could attract new synaptic contacts from pre-existing sprouting neurons. This review presents some recent findings on the fine structure and molecular expression of the human auditory nerve. Some results may explain the unique degeneration pattern following HC loss and can be relevant when considering future attempts to reconstruct the auditory nerve.
(1) myelinated Schwann cells, (2) NMSCs and (3) satellite glial cells (SGCs).
Neurotrophic factors and their receptors: Neurotrophins BDNF and NT3 play a crucial role for the normal development of the auditory nerve in mammals. Their roles in the adult human ear seem more uncertain. Rare studies aimed to investigate neurotrophin BDNF, BDNF/NT-3 growth factors receptors (TrkB/C or NTRK2 and NTRK3) as well as GDNF family ligands (GFLs) and their receptors in the human cochleae from surgical specimens. Such data should help to determine the target structures for neuron preservation and regeneration. The expression of GDNF, neurturin (NTRN, a member of GFLs) as well as cRet, GFRa-1 and GFRa-2 receptors were analyzed. TrkB receptor protein was found to be expressed in soma and processes of human spiral ganglion neurons (SGNs). In the organ of Corti, TrkB immunoreactivity was mainly present in nerve fibers underneath outer hair cells. BDNF expression was found neither in the organ of Corti nor in the spiral ganglion of human cochlea (Liu et al., 2011). Results suggest that alternate mechanisms fundamental for auditory nerve preservation may exist. cRet receptor immunoreactivity was seen in the SGNs, mainly inside the cell bodies but rarely in the nerve fibers and not in the organ of Corti. Immunolabeling for GFRa-1 and GFRa-2 receptors were identified mainly in the cell bodies of the SGNs. In the organ of Corti, GFRa-1 immunostaining could be demonstrated. However, GDNF immunoreactivity was not revealed in the human cochlea but instead NTRN; neurturin; a ligand that binds to GFRa-2 receptors related to Glial cell line-derived neurotrophic factor. Positive immunostaining was found in the supporting cells of organ of Corti, including Deiters’ cells, Hensen cells as well as Claudius’ cells. In the spiral ganglia, NTRN immunostaining was seen in both the cell bodies and the nerve fibers of neurons. Surprisingly, both Cx30 and Cx36 were identified in the SGN cell soma. Results are summarized in Figure 2.
Possible therapeutic strategies: It is apparent that attempts to re-sprout injured human SGNs is an appealing strategy. Drug-induced re-sprouting of dendrites could improve the functional outcome with CI. Outgrowth of perikaryon projections from SGNs has indicated that satellite cells play a key role. Axon regeneration following acute nerve section reveals that the BM can form “regenerating units” or buds with projecting growth cones. Laminin is a potent stimulator of neurite outgrowth in vitro and BM influences differentiation of regenerating nerve terminals at synaptic sites. BM scaffolds of Schwann cells can serve as conduits for regenerating axons (Ide, 1983). Electron microscopy studies have shown that regenerating axons can grow out even without Schwann cells with BM facing neural plasmalemma when treated with fibroblast growth factor (FGF). In addition, laminin-sulfatide binding initiates BM assembly and enables receptor signaling in Schwann cells and fibroblasts (Li et al., 2005). At present, several research groups endeavor advance nerve/electrode coupling in the deaf (such as the EU-based Consortium “NanoCI”). Hopefully results from such studies help to further improve outcome with CI.
Conclusions: An important clinical implication from these biological studies seems to be that patients should not be excluded from CI treatment solely based on long deafness duration (Lundin et al., 2014). Pertinent CI results can be achieved even in patients after long time deafness where the peripheral dendrites can be assumed to be non-existent. How and where spike generation occurs in the human auditory nerve is currently not known. Even in patients with good CI outcome it was histological verified that many neurons existed as “monopolar” cells. These “dormant” neurons are apparently excitable after many years of inactivity, indicating that the molecular and physiological requisites are maintained. Thus, divergent pattern of nerve decline may subsist in hearing impaired individuals with comparable auditory profiles. It raises questions as to how and where these “amputated” SGNs generate action potentials, which types of voltage-gated ion channels are involved and the potential for regenerative therapy.
Our research is part of the European Community 7th Framework Programme on Research, Technological Development and Demonstration. Project acronym: NANOCI. Grant agreement No. 281056. This study was supported by ALF grants from Uppsala University Hospital and Uppsala University and by the Foundation of “Tysta Skolan”, Swedish Deafness Foundation (HRF) and kind private funds from Börje Runögård, Sweden. Karin Lodin is acknowledged for skilful graphical art works.
References
- Beau JM, Liuzzi FJ, Depto AS, Vinik AI. Up-regulation of Laminin B2 gene expression in dorsal root ganglion neurons and non-neuronal cells during sciatic nerve regeneration. Exp Neurol. 1995;134:150–155. doi: 10.1006/exnr.1995.1045. [DOI] [PubMed] [Google Scholar]
- Hanani M, Huang TY, Cherkas PS, Ledda M, Pannese E. Glial cell plasticity in sensory ganglia induced by nerve damage. Neuroscience. 2002;114:279–283. doi: 10.1016/s0306-4522(02)00279-8. [DOI] [PubMed] [Google Scholar]
- Ide C. Nerve regeneration and Schwann cell basal lamina: observations of the long-term regeneration. Arch Histol Jpn. 1983;46:243–257. doi: 10.1679/aohc.46.243. [DOI] [PubMed] [Google Scholar]
- Li S, Liquari P, McKee KK, Harrison D, Patel R, Lee S, Yurchenco PD. Laminin-sulfatide binding initiates basement membrane assembly and enables receptor signaling in Schwann cells and fibroblasts. J Cell Biol. 2005;169:179–189. doi: 10.1083/jcb.200501098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthicum FH, Jr, Fayad JN. Spiral ganglion cell loss is unrelated to segmental cochlear sensory system degeneration in humans. Otol Neurotol. 2009;30:418–422. doi: 10.1097/mao.0b013e31819a8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Glueckert R, Linthicum FH, Rieger G, Blumer M, Bitsche M, Pechriggl E, Rask-Andersen H, Schrott-Fischer A. Possible role of gap junction intercellular channels and connexin 43 in satellite glial cells (SGCs) for preservation of human spiral ganglion neurons: A comparative study with clinical implications. Cell Tissue Res. 2014;355:267–278. doi: 10.1007/s00441-013-1735-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Edin F, Atturo F, Rieger G, Löwenheim H, Senn P, Blumer M, Schrott-Fischer A, Rask-Andersen H, Glueckert R. The pre- and post-somatic segments of the human type I spiral ganglion neurons - structural and functional considerations related to cochlear implantation. Neuroscience. 2015;284:470–482. doi: 10.1016/j.neuroscience.2014.09.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundin K, Stillesjö F, Rask-Andersen H. Experiences and results from cochlear implantation in patients with long duration of deafness. Audiol Neurotol Extra. 2014;4:46–55. [Google Scholar]
- Ohara PT, Vit JP, Bhargava A, Jasmin L. Evidence for a role of connexin 43 in trigeminal pain using RNA interference in vivo. J Neurophysiol. 2008;100:3064–3073. doi: 10.1152/jn.90722.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankovic K, Rio C, Xia A, Sugawara M, Adams JC, Liberman MC, Corfas G. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24:8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]


