Since their introduction in the 1960s, cochlear implants (CIs) have undergone several transformations, ultimately positioning themselves as the best-performing neural prosthesis available today. They have also been recognized as a unique tool for studying the potential protective effects of patterned electrical stimulation on the developing auditory system, with results from animal models often changing the manner in which CIs are used clinically to deliver auditory information to the brain (Moore and Shannon, 2009). From the development of the first successful commercial single-channel device, they have evolved into multi-channel devices that are part of the national health programmes of several countries. From the limited speech information provided by the early, rudimentary cochlear implants, these devices are now in a position to deliver intelligible speech information to the auditory system, largely due to advances in signal processing. Concerted efforts from several disciplines, including engineering, acoustics, neurobiology and otolaryngology have ensured that the continued development of CI technology has resulted in significant benefits to individuals with profound sensorineural hearing loss.
Could multisensory training increase cochlear implant candidacy? For normal-hearing listeners, interaural level differences (ILDs) are the most prominent localization cue for high-frequency sounds, whereas interaural time delays (ITDs) can be detected in the fine structure of low-frequency sounds (< 1.5 kHz) and in the envelopes of high-frequency, complex sounds (Gilkey and Anderson, 2014). Unilateral CI users are unable to exploit these cues, reducing their ability to localize sounds and to perceive acoustic signals in the presence of background noise (van Hoesel, 2004). Evidence suggests that most bilateral CI users, are sensitive to ILD cues, whereas ITDs are generally more difficult to hear. Current commercially-available stimulation strategies do not transmit fine-structure ITDs, due to the constant phase in the electrical pulse train. Thus, compared with unilateral CI, while bilateral CI can confer a significant binaural advantage to recipients, leading to improved sound localization ability and reduced hearing thresholds in noisy environments, we would not expect their performance in these tasks to necessarily reach that of listeners with normal hearing.
Previous work has shown that there exists a potential critical period for realizing the full benefits of patterned electrical stimulation on the developing auditory system (Sharma et al., 2002; Nicholas and Geers, 2006). These findings have been incorporated into technical reports and treatment guidelines established by government agencies throughout the world. Establishment of these guidelines thus excluded certain populations from receiving auditory rehabilitation using a CI, either unilateral or bilateral. Early bilateral cochlear implantation can potentially also exploit the sensitive period for spoken language development during the first few years of life (Holt and Svirsky, 2008). Bilateral CI may therefore be considered as the primary option for restoration of hearing in children and adults with profound sensorineural hearing loss, unless implantation is excluded medically or surgically. The concept of ‘saving the ear for future interventions’ does not, therefore, appear to be justified. The benefits reported by patients receiving the devices in both ears appear to confirm these findings. To date, no reliable data exists supporting the use of auditory training specifically to improve outcomes following bilateral CI in those individuals with early onset of profound hearing loss and presenting for hearing rehabilitation in adulthood.
Many investigators have highlighted the role of training in improving outcomes following unilateral CI. This training, provided either via a unisensory (Fu et al., 2005) or a multisensory approach (Strelnikov et al., 2009) appears to increasingly shift the trajectory of perceptual learning by CI recipients (Fu and Galvin, 2007). Thus it is conceivable that this training might also improve outcomes for those individuals who are normally ineligible for bilateral cochlear implantation, based on more traditional inclusion criteria.
Animal models of bilateral cochlear implantation: A few animal models of cochlear implantation have been described previously (Fallon et al., 2009; Eastwood et al., 2010). These span several species; many have been used to examine the effects of both acute and chronic intracochlear electrical stimulation on auditory processing using CIs. Nevertheless, an animal model to assess behavioural responses to bilateral CI use has not been described before. Such a model has many applications—especially in the setting of public health policies promulgating revisions in the indications for bilateral CI. Although many studies investigated beneficial effects of bilateral CI in humans, there are important questions that could be specifically answered using an animal model. These relate to the effects of (a) variable durations of deafness and age onset of hearing loss prior to implantation on binaural hearing outcomes, during development and in adulthood (b) synchronous versus asynchronous bilateral cochlear implantation on neurophysiological measures of binaural interactions in the brain, and (c) rehabilitative strategies, such as cross-modal training, that have been shown to be of benefit in other patterns of deafness (e.g., unilateral hearing loss).
Although data derived from animal models must be applied to humans with caution, these models are useful in the context of manipulation of sensory input to the developing auditory system, as the more compressed, yet predictable developmental timeframe in animals may be less affected by the variability inherent to a human clinical population. With these principles in mind, we developed a model of bilateral cochlear implantation in ferrets to study the potential protective effects of cochlear implantation on the developing binaural system (Hartley et al., 2010). This model could then be used to assess psychophysical and physiological aspects of hearing along with the structure of the auditory pathway in the same animals, a key aspect missing from prior animal models of CIs.
We studied the effects of age of onset of hearing loss and duration of deafness prior to implantation on free-field auditory localization accuracy (Isaiah et al., 2014). Ferrets deafened with ototoxic antibiotic injections were fitted with multi-channel electrode arrays, and subsequently passively stimulated with clinical speech processors that were programmed to provide optimal stimulation levels derived from electrophysiological thresholds. As in human CI recipients, we found that the best performance following CI occurred after late-onset hearing loss, compared with deafness early in life. Unilaterally-implanted animals performed no better than chance, which is also consistent with data from humans with a unilateral CI. Surprisingly, after the initial training task, animals did not improve any further in their sound localization abilities with auditory cues alone. Hence, the benefits of repeated perceptual training appeared to reach a plateau. In these circumstances, we considered the possibility that training using congruent auditory and visual stimuli (King, 2009) may facilitate the emergence of binaural hearing utilizing hitherto unknown connections between sensory cortices (Fuster et al., 2000).
Results from intermodal training support trials in human CI users: Ferrets grouped as a function of onset of hearing loss and whether they received unilateral or bilateral CIs were assessed for their sound localization performance in a free-field auditory task (Figure 1). In animals with early onset of hearing loss, sound localization was equally poor with unilateral or bilateral CIs. Subsequently, these animals were assigned a second task, where visual cues were randomly interleaved with regular auditory trials. Their auditory localization performance was then reassessed following removal of the visual trials. We found that ferrets with bilateral CIs showed an improvement in their auditory localization abilities, whereas this was not the case with unilateral CIs. Thus, the intermodal training paradigm that we developed can produce a clear and robust improvement in a task that relies on binaural hearing, and on which the ferrets with early-onset hearing loss and bilateral CIs otherwise performed poorly. The data presented here provides a unique argument against creation of candidacy criteria for bilateral CIs based solely upon critical or sensitive periods of auditory development, and suggest instead that intermodal training could warrant evaluation of candidates previously excluded by other criteria for bilateral CI.
Figure 1.
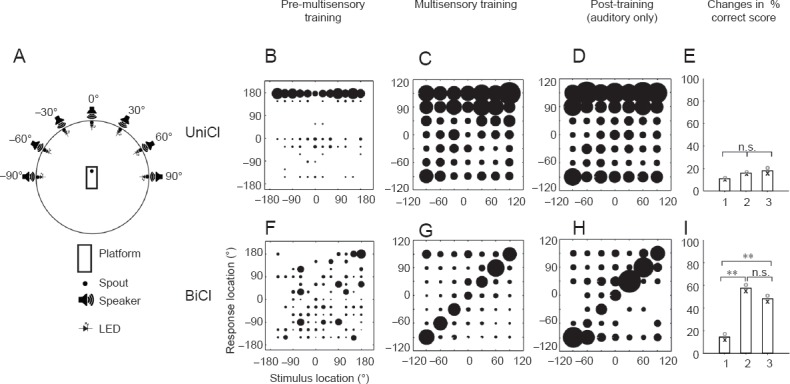
Effect of multisensory training on sound localization accuracy in ferrets with early-onset hearing loss.
(A) Testing chamber with 7 loudspeakers and light-emitting diodes arranged at 30° intervals in the frontal hemifield. Stimuli consisted of brief broadband noise bursts (1,000–2,000 ms) from one of the 7 speaker locations and were triggered by the animal licking the central spout. Each trial was concluded by a water reward if the animal approached the correct speaker location. The animals were first trained on a task with 12 speakers circumferentially arranged within the chamber. Auditory performance of the ferrets with a unilateral CI (UniCI, B–E) or bilateral CIs (BiCIs, F–I) are grouped by training experience. (B, F) Stimulus-response plots using all 12 loudspeakers covering the full 360° of azimuth (as in Figure 4A) prior to the start of multisensory training with the multisensory setup. The size of each solid circle in a given location represents the proportion of responses made at that location. At this stage, no difference was found between the performance of animals with a unilateral CI and those with bilateral CIs. (C, G) Stimulus-response plots for the final session of multisensory training. Subsequently, the visual stimuli were discontinued and animals were trained with auditory stimuli only for another 10 sessions. (D, H) Stimulus-response plots for the last of these sound-only sessions. (E, I) Mean percentage correct scores before, during and after multisensory training. No change in auditory localization performance (proportion of correct trials) was found in the ferrets with a single CI, whereas multisensory training resulted in a significant improvement in the bilaterally-implanted animals, which persisted after removal of the visual cues. n.s: Not significant; **P < 0.001.
Identifying a neural substrate for benefits of multisensory training: To determine the substrate for changes following behavioral training using the intermodal paradigm, we assessed binaural interactions in cortical neurons of animals previously fitted with bilateral CIs, after early or late onset of hearing loss. We observed that the primary auditory cortex (A1) is more responsive to binaural stimuli presented via bilateral CIs in ferrets implanted after shorter durations of hearing loss prior to CI. In addition, we also confirmed that neuronal responses from A1 are significantly reduced in ferrets with early-onset hearing loss, in agreement with previous studies that observed detrimental effects of sensory deprivation on the developing auditory pathway. Interestingly, following intermodal training ferrets had improved sound localization and binaural cue coding in A1, confirming a significant benefit for audiovisual training on binaural sensitivity of cortical neurons to auditory spatial cues. Using an information-theory-centric approach, we identified increases in the reliability of stimulus-evoked responses, as well as in the amount of information carried by A1 neurons. We obtained A1 responses over thousands of trials, increasing the validity of these findings. Indeed, since our recordings were limited to A1 based on previous work that described the detrimental effects on sound localization by inducing lesions within the auditory cortex, it may be interesting to examine whether similar changes can be induced in the subcortical circuitry, e.g., thalamocortical pathways. Similar questions may also be relevant to study changes within the auditory midbrain, e.g., the inferior colliculus.
Conclusions: There is strong evidence to conclude that early intervention in the form of cochlear implantation, and where possible bilateral, has the potential to provide sensory experience necessary for optimal development of the deafened auditory system. It may also be possible to extend CI candidacy to those individuals with early onset of hearing loss, if a suitable auditory-visual training paradigm could be designed to increase perceptual learning. The data presented here could argue for investigating the role of a suitable multisensory training paradigm in adults who lost their hearing early in childhood as potential candidates for bilateral, as opposed to unilateral CI. As this population is fairly sizeable, there is indeed a possibility for offering the significant benefits of binaural hearing to a previously excluded section of adults with profound hearing loss.
This work was funded in part by the Rhodes Trust, United Kingdom (AI) and the Wellcome Trust, United Kingdom (DEHH).
References
- Eastwood H, Pinder D, James D, Chang A, Galloway S, Richardson R, O’Leary S. Permanent and transient effects of locally delivered n-acetyl cysteine in a guinea pig model of cochlear implantation. Hear Res. 2010;259:24–30. doi: 10.1016/j.heares.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Fallon JB, Shepherd RK, Brown M, Irvine DR. Effects of neonatal partial deafness and chronic intracochlear electrical stimulation on auditory and electrical response characteristics in primary auditory cortex. Hear Res. 2009;257:93–105. doi: 10.1016/j.heares.2009.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Galvin JJ., 3rd Perceptual learning and auditory training in cochlear implant recipients. Trends Amplif. 2007;11:193–205. doi: 10.1177/1084713807301379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu QJ, Nogaki G, Galvin JJ., 3rd Auditory training with spectrally shifted speech: implications for cochlear implant patient auditory rehabilitation. J Assoc Res Otolaryngol. 2005;6:180–189. doi: 10.1007/s10162-005-5061-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuster JM, Bodner M, Kroger JK. Cross-modal and cross-temporal association in neurons of frontal cortex. Nature. 2000;405:347–351. doi: 10.1038/35012613. [DOI] [PubMed] [Google Scholar]
- Gilkey R, Anderson TR. Binaural and spatial hearing in real and virtual environments: Psychology Press. 2014 [Google Scholar]
- Hartley DE, Vongpaisal T, Xu J, Shepherd RK, King AJ, Isaiah A. Bilateral cochlear implantation in the ferret: a novel animal model for behavioral studies. J Neurosci Methods. 2010;190:214–228. doi: 10.1016/j.jneumeth.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt RF, Svirsky MA. An exploratory look at pediatric cochlear implantation: is earliest always best? Ear Hear. 2008;29:492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaiah A, Vongpaisal T, King AJ, Hartley DE. Multisensory training improves auditory spatial processing following bilateral cochlear implantation. J Neurosci. 2014;34:11119–11130. doi: 10.1523/JNEUROSCI.4767-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AJ. Visual influences on auditory spatial learning. Philos Trans R Soc Lond B Biol Sci. 2009;364:331–339. doi: 10.1098/rstb.2008.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Shannon RV. Beyond cochlear implants: awakening the deafened brain. Nat Neurosci. 2009;12:686–691. doi: 10.1038/nn.2326. [DOI] [PubMed] [Google Scholar]
- Nicholas JG, Geers AE. Effects of early auditory experience on the spoken language of deaf children at 3 years of age. Ear Hear. 2006;27:286–298. doi: 10.1097/01.aud.0000215973.76912.c6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A, Dorman MF, Spahr AJ. A sensitive period for the development of the central auditory system in children with cochlear implants: implications for age of implantation. Ear Hear. 2002;23:532–539. doi: 10.1097/00003446-200212000-00004. [DOI] [PubMed] [Google Scholar]
- Strelnikov K, Rouger J, Barone P, Deguine O. Role of speechreading in audiovisual interactions during the recovery of speech comprehension in deaf adults with cochlear implants. Scand J Psychol. 2009;50:437–444. doi: 10.1111/j.1467-9450.2009.00741.x. [DOI] [PubMed] [Google Scholar]
- van Hoesel RJ. Exploring the benefits of bilateral cochlear implants. Audiol Neurootol. 2004;9:234–246. doi: 10.1159/000078393. [DOI] [PubMed] [Google Scholar]


