Abstract
Ginsenoside Rg1 is the major pharmacologically active component of ginseng, and is reported to have various therapeutic actions. To determine whether it induces the differentiation of neural stem cells, and whether neural stem cell transplantation after induction has therapeutic effects on hypoxic-ischemic encephalopathy, we cultured neural stem cells in 10–80 μM ginsenoside Rg1. Immunohistochemistry revealed that of the concentrations tested, 20 mM ginsenoside Rg1 had the greatest differentiation-inducing effect and was the concentration used for subsequent experiments. Whole-cell patch clamp showed that neural stem cells induced by 20 μM ginsenoside Rg1 were more mature than non-induced cells. We then established neonatal rat models of hypoxic-ischemic encephalopathy using the suture method, and ginsenoside Rg1-induced neural stem cells were transplanted via intracerebroventricular injection. These tests confirmed that neural stem cells induced by ginsenoside had fewer pathological lesions and had a significantly better behavioral capacity than model rats that received saline. Transplanted neural stem cells expressed neuron-specific enolase, and were mainly distributed in the hippocampus and cerebral cortex. The present data suggest that ginsenoside Rg1-induced neural stem cells can promote the partial recovery of complicated brain functions in models of hypoxic-ischemic encephalopathy.
Keywords: nerve regeneration, hypoxic-ischemic brain damage, ginsenoside Rg1, neural stem cells, cell transplantation, cell differentiation, cognition, nerve reconstruction, neural regeneration
Introduction
Neonatal hypoxic-ischemic encephalopathy (HIE) is one of the main causes of permanent brain damage and often leads to cerebral palsy (Zheng et al., 2006; Ma et al., 2007; Pimentel-Coelho and Mendez-Otero, 2009; Lee et al., 2010; Pimentel-Coelho et al., 2012). Neural stem cell transplantation offers new hope for patients with the condition (Yang et al., 2014). Transplantation of exogenous neural stem cells (NSCs) corrects the balance of neurotransmitters released, establishes functional contact of neurons with surrounding cells, restores cognitive and neurological function, and stimulates the body to mobilize its own stem cells to contribute to nerve reconstruction (Nishino and Borlongan, 2000; Park, 2000; Li et al., 2002). It has recently been shown that ginsenoside Rg1, a steroid glycoside found in Panax (ginseng), slows cellular aging, reduces nerve cell damage, elevates protein synthesis in the brain, increases the number of synapses, improves memory, and promotes recovery of brain function after injury (Wang et al., 2008b; Zhou et al., 2011; Huang et al., 2012; Jiang et al., 2012; Wu et al., 2012). The purpose of the present study was to determine whether ginsenoside Rg1 induces the differentiation of NSCs, and whether the differentiated cells could be used therapeutically in a neonatal rat model of HIE (Thomaidou, 2014).
Materials and Methods
NSC separation and cultivation
Embryonic cells were derived from human embryos after miscarriage, with the informed consent of the patients. Human cerebral cortex was isolated and treated with 0.25% trypsin-ethylenediamine tetraacetic acid at 37°C for 30 minutes (Li et al, 2009). The cells were suspended in Dulbecco's modified Eagle's medium (DMEM) (Gibco, Grand Island, NY, USA) containing 20% fetal bovine serum (Biochrom, Berlin, Germany) and plated at a density of 800–1,000 cells/mm2 on glass coverslips in 35 mm dishes. After 24 hours, passage 3 NSCs were prepared for use. Cell medium was added to obtain a single-cell suspension of 1 × 106 cells/mL. Conventional laboratory cell culture and associated techniques were approved by the Ethics Committee of Chongqing Medical University in China.
Induction of NSC differentiation by ginsenoside Rg1
Passage 3 human NSCs were seeded in 24-well plates coated with poly-L-lysine, with glass covers (Wang et al., 2007; Li and Wang, 2009). The NSCs were divided into six groups: control, interleukin-1, and ginsenoside Rg1 at 10, 20, 40 and 80 µM. NSCs in the control group were cultured in DMEM/F12 (Hyclone Company, Grand Island, NY, USA) with 2% B27, epidermal growth factor (20 ng/mL), and basic fibroblast growth factor (20 ng/mL; all from Gibco). NSCs in the interleukin-1 group were cultured in DMEM/F12 containing 20 µM interleukin-1 (Gibco). NSCs in the 10, 20, 40 and 80 µM ginsenoside Rg1 groups were cultured in DMEM/F12 containing 10, 20, 40 or 80 µM ginsenoside Rg1 (Institute of Pharmaceutical and Biological Products, Beijing, China), respectively. Adherent cells were cultured for 3 days before growth-stimulating factors such as epidermal growth factor and basic fibroblast growth factor were removed and the induction factors, ginsenoside Rg1 and interleukin-1, were added. Cells were incubated in the compounds for 7 days.
Antigen retrieval was performed, and the sections were incubated with rabbit anti-neuron-specific enolase (neuronal marker), rabbit anti-glial fibrillary acidic protein (GFAP; astrocyte marker), and rabbit anti-Gal-c (oligodendrocyte marker) polyclonal antibodies (all 1:1,000; all from Abcam, Cambridge, UK) for 24 hours at 4°C. The sections were then incubated with goat anti-rabbit IgG/horseradish peroxidase antibody (Abcam) for 2 hours at room temperature. Cells were viewed at 400× magnification (Nikon, Japan, Tokyo) and five fields of view per coverslip were selected at random for quantification. Finally, the percentages of neurons expressing specific enolase, GFAP, and Gal-c were calculated to determine NSC differentiation in the presence of different concentrations of ginsenoside Rg1.
Whole-cell patch clamp recording
Membrane electrical properties and sodium channel functionality were analyzed by whole-cell patch clamp (Harvard Apparatus, Cambridge, USA) on neuron-like cells 7 days after differentiation. Before whole-cell patch clamp recording, glass microelectrodes were pulled using a micropipette puller (P-97, Sutter Instrument Company, Novato, CA, USA). All experiments were performed at 23°C, using pipette solution (140 mM KCl, 10 mM ethyleneglycol bis[2-aminoethyl ether]tetraacetic acid, 10 mM hydroxyethyl piperazine ethanesulfonic acid; pH adjusted to 7.2–7.4) and extracellular solution (140 mM NaCl, 4 mM MgCl2, 5 mM KCl, 10 mM hydroxyethyl piperazine ethanesulfonic acid, 10 mM glucose; pH adjusted to 7.2–7.4). The system was designed to keep the resistance within the range of 4–7 MΩ, filled with pipette solution. At the beginning of whole-cell recording, the input resistance, capacitance and resting membrane potential of the cell membrane were measured without injection of any current. Whole-cell currents were recorded at a holding potential of −80 mV, command pulses with 10 mV steps, ranging from −80 mV to +60 mV in calcium-free extracellular solution. The inward Na+ currents were recorded with a holding potential of −60 mV, command pulses with 10 mV steps, ranging from −60 mV to +60 mV in calcium-free extracellular solution with the K+ channel blockers tetraethylammonium (10 mM; Gibco) and 4-aminopyridine (1 mM; Gibco).
Construction of neonatal hypoxic-ischemic rat models
A total of 55 male Sprague-Dawley rat pups, aged 7 days and weighing 12–20 g, were obtained (with their dams) from the Experimental Animal Center of Chongqing Medical University in China (license No. SYXK (Yu) 2012-0001). The investigation conformed to the Guide for the Care and Use of Laboratory Animals (NIH publication No. 85-23, revised 1996) and the study was approved by the Animal Ethics Committee, Chongqing Medical University, China.
Forty rats were selected at random and divided into a model and a control group (n = 20 per group). Rats in the model group were anesthetized by isoflurane inhalation, and middle cerebral artery occlusion was performed. The rats were positioned on a clay mold (Yunna, Chongqing, China) and were continuously in contact with a gel heat pad to maintain thermoneutrality. The left common carotid artery was exposed via a 0.4–0.6 cm skin incision in the left side of the neck, adjacent to the trachea, and double ligation of the artery was performed 3 mm distal to its origin. Sterile techniques were observed throughout the process. After surgery, the animals were placed in jars submerged in a water bath to recover at 34°C for 2–4 hours, then exposed to 8% oxygen and 92% nitrogen for 2.5 hours, with the water bath temperature held at 34°C. When movement was observed upon waking, the rats were placed back in their home cages with their dams (Feng et al., 2013).
2,3,5-Triphenyl tetrazolium chloride (TTC) staining
Twelve hours after modeling, the rats were anesthetized intraperitoneally (i.p.) with sodium pentobarbital (30 mg/kg), perfused transcardially with saline, and the brains were removed and frozen at −20°C for about 20 minutes. Coronal sections, 2 mm thick, were cut, starting at the bifurcation point behind the sagittal suture and resulting in four sections per brain (herein identified as sections 1, 2, 3, and 4). The sections were placed in 2% TTC (Sigma, St. Louis, MO, USA), preheated to 37°C and stained for 20 minutes before being soaked in 4% paraformaldehyde for 16–20 hours. Image-Pro Plus 7.0 (Media Cybernetics, Houston, USA) was used to acquire images and analyze the injured area. The degree of injury (%) was calculated using the following formula: = (DA1 + DA2 + DA3 + DA4) / (CHA1 + CHA2 + CHA3 + CHA4) × 100%, where DA1, DA2, etc. represents the area of damage in brain sections 1, 2, etc., and CHA1, CHA2, etc. represents the area of the contralateral hemisphere in those sections (Hei et al., 2003).
NSC transplantation after induction with ginsenoside Rg1
The remaining 15 rats were divided into three groups: (1) control (n = 5), which did not undergo any procedures; (2) HIE + vehicle (n = 5), in which models of HIE were established and received an intracerebroventricular injection of normal saline; (3) HIE + NSCs (n = 5), in which models of HIE were established and received an intracerebroventricular injection of NSCs. Seven days after modeling, rats in the HIE + NSCs group were anesthetized and fixed on a stereotaxic frame (Beijing Channel Scientific Instrument Co., Beijing, China) and the skin on the head was prepared and disinfected. An incision was made in the middle of the scalp, and 5 µL single-cell suspension of human NSCs induced by ginsenoside Rg1 (20 µM) was injected into the left (ipsilateral) ventricle using a microsyringe (Beijing Channel Scientific Instrument Co.) at a concentration of 5.0 × 1010 cells/L. Rats in the HIE+ vehicle group received saline instead of NSCs. After adjusting for the smaller body mass of young rats, the injection sites were as follows: −1.0–0 mm posterior to the anterior fontanel; −1.0–1.5 mm lateral to the sagittal suture; −3.0–4.0 mm rostrocaudal. The injection proceeded at 1 µL/min and the needle was kept in place for 15 minutes after injection. The needle was then withdrawn at 1 mm/min. The incisions were closed and the animal was allowed to recover. Penicillin was injected (10,000 units intraperitoneal) for 7 consecutive days.
Morris water maze testing
One month after NSC transplantation, a Morris water maze (Beijing Channel Scientific Instrument Co.) was used to assess the functional recovery of the rats. The Morris water maze was divided into four quadrants for navigation and spatial probe testing. Navigation training and testing took place over 5 consecutive days, with four sessions per rat per day. During training, the rats were released at one of four sites separated by 90° around the periphery of the maze. Each rat was trained to navigate to the platform in three consecutive sessions with four trials per session, and was placed in an opaque drying cage between trials. The time required for each rat to find the hidden platform and climb onto it was recorded as the latency, and the length of path travelled to find the platform was recorded as the distance. After the rats found the platform, they were allowed to stand on it for 10 seconds. If the rats stayed in the water for 120 seconds without finding the platform, they were guided to it and allowed to remain on it for 10 seconds, before the next training session was started. The spatial probe test was used to measure spatial memory retention in rats that had learned to find the platform. In this part of the experiment, the platform was removed and the rats were placed into the water at the same points as previously. They were allowed to swim for 120 seconds and the time spent in the correct quadrant (where the platform had been) was measured.
Somatosensory evoked potential testing
One month after NSC transplantation, the somatosensory evoked potential was used to assess the functional recovery of rats. Rats were anesthetized with sodium pentobarbital (30 mg/kg intraperitoneal) and fixed on a stereotaxic frame, and the skull was fully exposed. In the posterior margin of the left coronal suture, a dental drill was used to penetrate the skull. The meninges were opened with a needle and cut with iris scissors. Near the right sciatic nerve, two needles were inserted as stimulating electrodes, 1 cm apart, and connected to the stimulation output of the computer system. Electrodes were placed at the hindlimb cortex (1–2 mm posterior to the anterior fontanel; 2–3 mm lateral to the midline at both sides) and connected to the signal input terminal. A reference electrode was placed on the skin. A biological signal collecting system (RM6240; Chengdu Instrument Factory, Chengdu, Sichuan Province, China) was used to perform cortical evoked potential testing with the following parameters: time constant, 0.001 seconds; filtering, 1 kHz; scanning speed, 5 ms/div; voltage, 100 µV; pulse width, 0.05 ms; stimulus intensity, 3 V. Upon single pulse stimulation, ipsilateral limb shaking could be seen. Cortical somatosensory evoked potential was recorded and 100 waveforms were superimposed.
Nissl staining
One month after NSC transplantation, rats were placed under deep anesthesia and sacrificed by transcardial perfusion with cold physiological saline solution followed by 4% paraformaldehyde (pH 7.4). The brains were dehydrated and embedded in paraffin. Coronal sections 4 µm thick were cut using a microtome. The paraffin-embedded brain sections were dewaxed using xylene, rehydrated through an ethanol series, and then washed with water. The sections were stained with 0.1% cresyl violet and examined under a light microscope (Canon, Japan, Tokyo) for histological assessment of neuronal cell damage.
Immunohistochemical staining
Paraffin-embedded sections were prepared (Li et al., 2009), dewaxed, and antigen retrieval was performed using citrate buffer solution (0.01 M, pH 6.0). The sections were incubated with rabbit anti-human neuron-specific enolase monoclonal antibody (1:1,000; Abcam) overnight at 4°C. They were rinsed with 0.01 M phosphate buffer solution, incubated with biotinylated goat anti-rabbit IgG (1:1,000; Abcam) at room temperature for 30 minutes, and incubated with peroxidase-labeled streptavidin for 15 minutes before mounting. The slides were viewed under a light microscope and stained cells were counted using Image-Pro Plus 6.0 software.
Statistical analysis
Measurement data are expressed as the mean ± SD. One-way analysis of variance and paired t-tests were used to identify differences between groups. P < 0.05 was regarded as statistically significant.
Results
Ginsenoside Rg1 induced NSC differentiation
Immunohistochemical staining showed that NSCs of the control group were the least differentiated of the three groups. When ginsenoside Rg1 was added to the culture medium at a concentration of 10 µM, the number of differentiated cells began to increase. A dose of 20 µM ginsenoside Rg1 produced the largest number of differentiated neurons, stromal cells, and oligodendrocytes. However, when the concentration of ginsenoside Rg1 was higher than 20 µM, fewer differentiated cells were observed. The number of differentiated cells after induction with 20 µM ginsenoside Rg1 was not significantly different from that after induction with 20 mM interleukin-1 (positive control) (P > 0.05; Figure 1).
Figure 1.
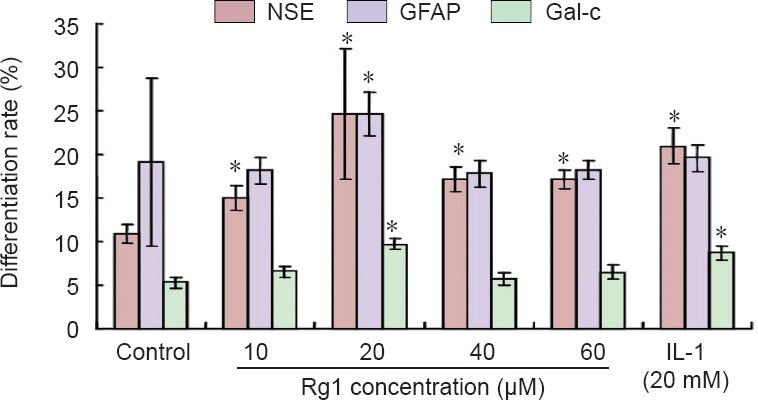
Effect of ginsenoside Rg1 on the differentiation of neural stem cells.
The percentage of cells expressing neuron-specific enolase (NSE), glial fibrillary acidic protein (GFAP), or Gal-c (differentiated neurons) was determined by immunocytochemistry. Data are expressed as the mean ± SD (n = 5). One-way analysis of variance and paired t-tests were used to identify differences between groups. *P < 0.05, vs. control group.
Effects of ginsenoside Rg1 on membrane properties of induced NSCs
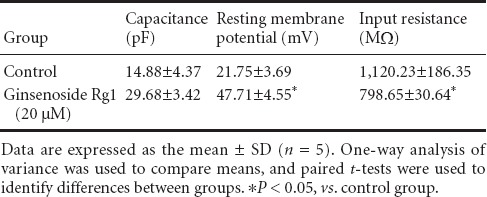
Whole-cell patch clamp recording at 7 days showed that the resting membrane potential and capacitance in NSCs in the 20 µM ginsenoside Rg1 group were significantly greater than that in the control group (P < 0.05), whereas input resistance was significantly lower (P < 0.05). These membrane properties showed that neurons induced by ginsenoside Rg1 were more mature than control neurons (Table 1).
Table 1.
Effects of ginsenoside Rg1 on the membrane properties of neural stem cells determined by whole-cell patch clamp recording
The amplitude of inward current in the group exposed to 20 µM ginsenoside Rg1 was elevated at 7 days, whereas inward current was often undetectable in the control group (Figure 2).
Figure 2.
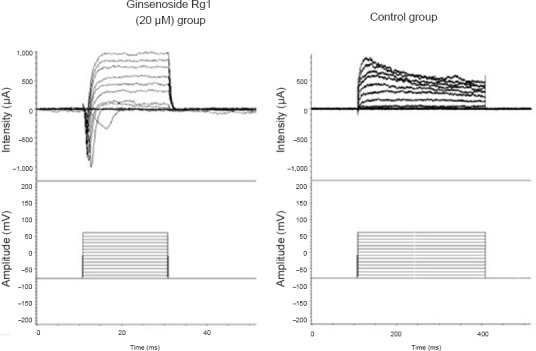
Effect of ginsenoside Rg1 on membrane function of induced neural stem cells at 7 days after differentiation (whole-cell patch clamp recording).
Cell activation was measured using the whole-cell patch clamp technique. Amplitudes in the 20 μM ginsenoside Rg1 group were notably greater than those in the control group.
The K+ and Ca2+ currents of cells were blocked by the K+ channel blockers 4-aminopyridine and tetraethylammonium, and by free Ca2+. In the control group, inward Na+ current was detected in 28% of cells, and the mean amplitude was 267.24 ± 71.15 pA. However, in the ginsenoside Rg1 group, the inward Na+ current was detected in 50% of cells and had a higher amplitude than in the control group (711.48 ± 158.03 pA; P < 0.05).
Validation of hypoxic-ischemic brain damage models
TTC staining revealed evident injury to rat brain tissue after modeling. The degree of injury was 9.33 ± 3.46% (Figure 3).
Figure 3.
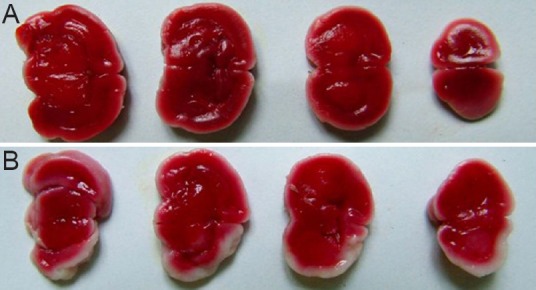
Coronal brain sections in rat models of hypoxic-ischemic encephalopathy (TTC staining).
(A) Control group; (B) model group. Red: Normal tissue; white: ischemic tissue.
Transplanted ginsenoside Rg1-induced NSCs improved learning and memory behavior in rat models of HIE
In the water maze experiments, rats in the HIE + vehicle group had longer latencies and swimming distances than control rats (P < 0.05). Furthermore, in the spatial probe test, rats in the HIE + vehicle group spent noticeably less time in the platform quadrant than the controls (P < 0.05). However, rats in the HIE + NSCs group showed shorter latencies and swimming distances with the platform present, and in the spatial probe test, the rats spent more time in the correct quadrant than HIE + vehicle rats (P < 0.05; Figure 4).
Figure 4.
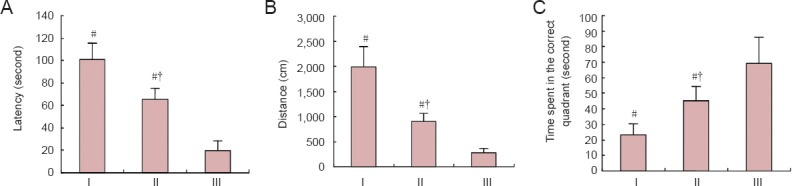
Effects of NSC transplantation on rat behavior.
Latency (A), distance (B) and (C) target quadrant time in the Morris water maze spatial probe test. Data are expressed as the mean ± SD (n = 5 rats per group). One-way analysis of variance and paired t-test were used to identify differences between groups. #P < 0.05, vs. III; †P < 0.05, vs. I. HIE: Hypoxic-ischemic encephalopathy; NSCs: neural stem cells. I: HIE + vehicle group; II: HIE + NSCs group; III: control group.
Effects of NSC transplantation on somatosensory evoked potential in rat models of HIE
One month after NSC transplantation, the latency of somatosensory evoked potentials was significantly longer in the HIE + NSCs group than in the HIE + vehicle group (P < 0.05), and the amplitude was smaller (P < 0.05). Transplantation of ginsenoside Rg1-induced NSCs resulted in significantly better properties than the control group (P < 0.05), but levels did not return to those of the control group (Figure 5).
Figure 5.
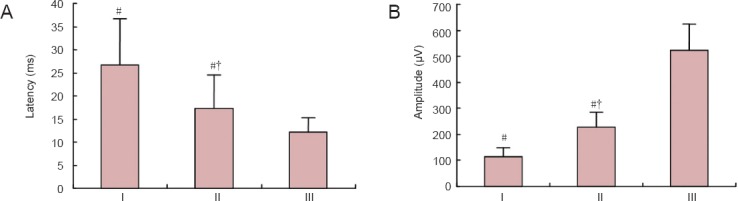
Effects of NSC transplantation on somatosensory evoked potential in HIE rats.
(A) Latency of somatosensory evoked potential. (B) Amplitude of somatosensory evoked potential. Data are expressed as the mean ± SD (n = 5). One-way analysis of variance and paired t-tests were used to identify differences between groups. #P < 0.05, vs. III; †P < 0.05, vs. I. HIE: Hypoxic-ischemic encephalopathy; NSCs: neural stem cells. I: HIE + vehicle group; II: HIE + NSCs group; III: control group.
Effects of NSC transplantation on morphological changes in neurons of rat models of HIE
One month after NSC transplantation, Nissl staining showed that the neurons in control rat brains were well demarcated and compactly arranged. The cellular structure was intact and the cytoplasm was lightly stained, and Nissl bodies were abundant. In the HIE + vehicle group, there were fewer neurons, and shrinkage of degenerated neurons could be observed. In the HIE + NSC group, fewer Nissl bodies were seen, and a number of well-preserved neurons remained (Figure 6).
Figure 6.
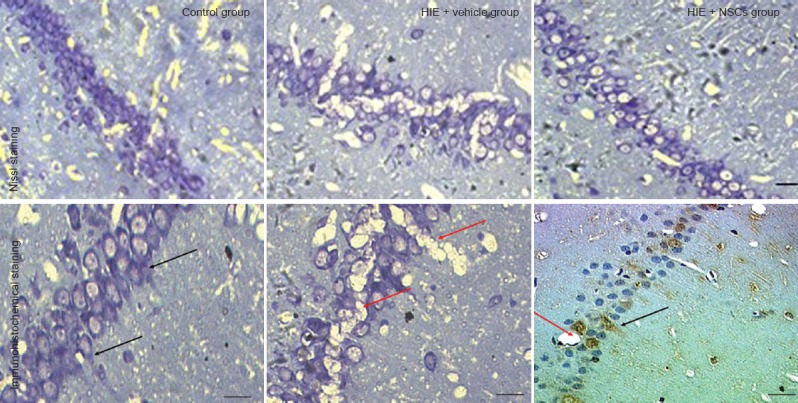
Changes in neuronal morphology in the brain of HIE rats 1 month after NSC transplantation.
NSC transplantation increases the number of neurons and reduces tissue damage in HIE rats. The red arrows show vacuoles, and the black arrows show neuron-specific enolase-positive neurons. Scale bars: 20 μm (upper panel); and 10 μm (lower panel). HIE: Hypoxic-ischemic encephalopathy; NSCs: neural stem cells.
Brain sections of rats in the HIE + NSCs rats showed that transplanted NSCs had differentiated into neurons. Neuron-specific enolase expression indicated that the transplanted neurons had become scattered through the cortex. Expression levels were greatest in the hippocampus, indicating a large number of neurons in that region. Because ischemia occurred after modeling, vacuoles caused by brain damage were present in the tissue sections, and transplanted neurons were growing around these regions (Figure 6).
Discussion
Neonatal HIE can cause asphyxia, maternal injuries, and hyperbilirubinemia, which may lead to permanent brain damage. The aim of the present study was to evaluate the therapeutic efficacy of transplantation of Rg1-induced NSCs into neonatal rats with HIE.
We have shown here that 20 µM ginsenoside Rg1 is the optimal concentration for producing the largest number of differentiated neurons, stromal cells, and oligodendrocytes from NSCs. We also previously found the optimal time of transplantation (7 days) and placement of the transplanted cells (−1.0–0 mm posterior to the anterior fontanel; −1.0–1.5 mm lateral to the sagittal suture; −3.0–4.0 mm rostrocaudal) (Luan et al., 2006; Wang et al., 2008a). The tests of membrane properties and Na+ currents reported here revealed that a high proportion of neurons induced by ginsenoside Rg1 reached maturity. As a result, the latency of the somatosensory evoked potential was increased, the amplitude was reduced, and brain function was noticeably impaired in the control and HIE + vehicle groups. However, rats in the HIE + NSCs group scored significantly better than those in the HIE + vehicle group, although they did not recover to the level of the control group. This directly reflects the complete process of nerve conduction, from the receptors to the somatosensory pathway, to the sensory area of the cerebral cortex. This is very similar to the clinical picture. Evoked potentials produced by visual, auditory, and somatosensory stimulation are measured in the clinic (Dive and Giffroy, 2004; Abend and Licht, 2008; Carrai et al., 2010), and changes in somatosensory evoked potential of the median nerve correlate with the degree of local cortical neuronal injury (Lai et al., 2011). Xiong et al. (2010) used somatosensory evoked potentials to detect HIE induced by cardiac arrest. Therefore, somatosensory evoked potential parameters may serve as independent predictors of outcome (Tzvetanov et al., 2005).
Nissl bodies were abundant in the neurons of the hippocampus in the HIE + NSCs group. Immunohistochemical analysis revealed that the transplanted NSCs differentiated into neurons and became scattered through the cortex, but were concentrated around the hippocampus.
When damaged, NSCs migrate from the ventricles along the external capsule of the corpus callosum to reach the damaged area, and exogenous NSCs migrate under the guidance of endogenous NSCs. Transplanting NSCs that can express specific neurotrophic growth factors, such as brain-derived neurotrophic factor and nerve growth factor, in hypoxic-ischemic brain regions can improve the results of NSC transplantation (Andsberg et al., 1998), as transplanted neurons can grow around the ischemic region. Our results suggest that transplanted cells may not only differentiate, but could also function and establish communication between cells. We plan to explore this further in future experiments.
Compared with the HIE + vehicle group, more neurons and fewer vacuoles were observed in the cortex and hippocampus of the HIE + NSCs group. NSCs can repair damage in many ways; one of the most important is that the differentiated cells can secrete a variety of cell growth factors, such as nerve growth factor, which can improve the local microenvironment of the spinal cord, induce axonal regeneration, and then produce a variety of extracellular matrices. At the same time, it can fill the cavity after HIE, and provide support for axonal regeneration. Our study shows that ginsenoside Rg1 stimulates the differentiation of NSCs, and indicates that the differentiated cells have the potential to be used in the treatment of brain damage in newborn hypoxic-ischemia. We therefore propose that transplanted Rg1-induced NSCs will play a role in many aspects of neural repair.
In summary, ginsenoside-induced NSC transplantation is a promising new treatment for brain injury; future studies will determine how the method can be applied in the clinic.
Footnotes
Funding: This study was supported by the Natural Science Foundation of Chongqing in China, No. CSTC2011jjA0013.
Conflicts of interest: None declared.
Copyedited by Slone-Murphy J, Robens J, Yu J, Qiu Y, Li CH, Song LP, Zhao M
References
- Abend NS, Licht DJ. Predicting outcome in children with hypoxic ischemic encephalopathy. Pediatr Crit Care Med. 2008;9:32–39. doi: 10.1097/01.PCC.0000288714.61037.56. [DOI] [PubMed] [Google Scholar]
- Andsberg G, Björklund ZKa, Lindvall O, Martínez-Serrano A. Amelioration of ischaemia-induced neuronal death in the rat striatum by NGF-secreting neural stem cells. Eur J Neurosci. 1998;10:2026–2036. doi: 10.1046/j.1460-9568.1998.00214.x. [DOI] [PubMed] [Google Scholar]
- Carrai R, Grippo A, Lori S, Pinto F, Amantini A. Prognostic value of somatosensory evoked potentials in comatose children: a systematic literature review. Intensive Care Med. 2010;36:1112–1126. doi: 10.1007/s00134-010-1884-7. [DOI] [PubMed] [Google Scholar]
- Dive D, Giffroy X. Somatosensory evoked potentials: clinical applications in peripheral neuropathies. Rev Med Liege. 2004;59(Suppl 1):157–169. [PubMed] [Google Scholar]
- Feng Z, Liu J, Ju R. Hyperbaric oxygen treatment promotes neural stem cell proliferation in the subventricular zone of neonatal rats with hypoxic-ischemic brain damage. Neural Regen Res. 2013;8:1220–1227. doi: 10.3969/j.issn.1673-5374.2013.13.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hei MY, Kuang SJ, Yin P. Difference of two HIE animal models using neonatal SD rat and C57 mouse assessed by TTC staining. Zhongguo Xiandai Yixue Zazhi. 2003;13:22–25. [Google Scholar]
- Huang T, Fang F, Chen L, Zhu Y, Zhang J, Chen X, Yan SS. Ginsenoside Rg1 attenuates oligomeric Aβ(1-42)-induced mitochondrial dysfunction. Curr Alzheimer Res. 2012;9:388–395. doi: 10.2174/156720512800107636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B, Xiong Z, Yang J, Wang W, Wang Y, Hu ZL, Wang F, Chen JG. Antidepressant-like effects of ginsenoside Rg1 are due to activation of the BDNF signalling pathway and neurogenesis in the hippocampus. Br J Pharmacol. 2012;166:1872–1887. doi: 10.1111/j.1476-5381.2012.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai PC, Huang YT, Wu CC, Lai CJ, Wang PJ, Chiu TH. Ceftriaxone attenuates hypoxic-ischemic brain injury in neonatal rats. J Biomed Sci. 2011;18:69. doi: 10.1186/1423-0127-18-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JA, Kim BI, Jo CH, Choi CW, Kim EK, Kim HS, Yoon KS, Choi JH. Mesenchymal stem-cell transplantation for hypoxic-ischemic brain injury in neonatal rat model. Pediatr Res. 2010;67:42–46. doi: 10.1203/PDR.0b013e3181bf594b. [DOI] [PubMed] [Google Scholar]
- Li Y, Chen J, Chopp M. Cell proliferation and differentiation from ependymal, subependymal and choroid plexus cells in response to stroke in rats. J Neurol Sci. 2002;193:137–146. doi: 10.1016/s0022-510x(01)00657-8. [DOI] [PubMed] [Google Scholar]
- Li YB, Wang SL. Total saponins of Panax ginseng effects on proliferation and differentiation of human embryonic neural stem cells and in a Parkinson's disease mouse model. Neural Regen Res. 2009;4:186–193. [Google Scholar]
- Luan Z, Qu SQ, Yin GC, Yan FQ, Hu XH, Wu HN. Hypoxic ischemic brain injury in different ways transplantation of human neural stem cells in experimental study. Zhonghua Xiaoer Waike Zazhi. 2006;27:497–500. [Google Scholar]
- Ma J, Wang Y, Yang J, Yang M, Chang KA, Zhang L, Jiang F, Li Y, Zhang Z, Heo C, Suh YH. Treatment of hypoxic-ischemic encephalopathy in mouse by transplantation of embryonic stem cell-derived cells. Neurochem Int. 2007;51:57–65. doi: 10.1016/j.neuint.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Nishino H, Borlongan CV. Restoration of function by neural transplantation in the ischemic brain. Prog Brain Res. 2000;127:461–476. doi: 10.1016/s0079-6123(00)27022-2. [DOI] [PubMed] [Google Scholar]
- Park KI. Transplantation of neural stem cells: cellular & gene therapy for hypoxic-ischemic brain injury. Yonsei Med J. 2000;41:825–835. doi: 10.3349/ymj.2000.41.6.825. [DOI] [PubMed] [Google Scholar]
- Pimentel-Coelho PM, Mendez-Otero R. Cell therapy for neonatal hypoxic-ischemic encephalopathy. Stem Cells Dev. 2009;19:299–310. doi: 10.1089/scd.2009.0403. [DOI] [PubMed] [Google Scholar]
- Pimentel-Coelho PM, Rosado-de-Castro PH, Barbosa da Fonseca LM, Mendez-Otero R. Umbilical cord blood mononuclear cell transplantation for neonatal hypoxic-ischemic encephalopathy. Pediatr Res. 2012;71:464–473. doi: 10.1038/pr.2011.59. [DOI] [PubMed] [Google Scholar]
- Thomaidou D. Neural stem cell transplantation in an animal model of traumatic brain injury. Methods Mol Biol. 2014;1210:9–21. doi: 10.1007/978-1-4939-1435-7_2. [DOI] [PubMed] [Google Scholar]
- Tzvetanov P, Rousseff RT, Atanassova P. Prognostic value of median and tibial somatosensory evoked potentials in acute stroke. Neurosci Lett. 2005;380:99–104. doi: 10.1016/j.neulet.2005.01.023. [DOI] [PubMed] [Google Scholar]
- Wang GF, Luan Z, Gao BQ, Yin GC, Bai JB, Qu SQ. Survival and distribution of human neural stem cells transplanted into cerebral ventricles of neonatal rats with hypoxic-ischemic brain damage. Zhongguo Zuzhi Gongcheng Yanjiu yu Linchuang Kangfu. 2008a;12:2201–2205. [Google Scholar]
- Wang SL, Li YB, Wang YP, Feng M. Effect of TSPG on proliferation and differentiation of human embryonic neural stem cell into dopaminergic neuron. Zhongguo Zhong Yao Za Zhi. 2007;32:1310–1313. [PubMed] [Google Scholar]
- Wang YZ, Wang YS, Chu SF, Chen J, Chen NH, Zhang JT. Nootropic signal transduction of ginsenoside, Rg1. Zhongguo Yaoli Xue Tongbao. 2008b;24:740–743. [Google Scholar]
- Wu J, Pan Z, Wang Z, Zhu W, Shen Y, Cui R, Lin J, Yu H, Wang Q, Qian J, Yu Y, Zhu D, Lou Y. Ginsenoside Rg1 protection against β-amyloid peptide-induced neuronal apoptosis via estrogen receptor α and glucocorticoid receptor-dependent anti-protein nitration pathway. Neuropharmacology. 2012;63:349–361. doi: 10.1016/j.neuropharm.2012.04.005. [DOI] [PubMed] [Google Scholar]
- Xiong W, Koenig MA, Madhok J, Jia X, Puttgen HA, Thakor NV, Geocadin RG. Evolution of somatosensory evoked potentials after cardiac arrest induced hypoxic-ischemic injury. Resuscitation. 2010;81:893–897. doi: 10.1016/j.resuscitation.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T, Liu H, Wang ZG, Gao JF, Xiao DJ, Wang YS. Neural stem cell transplantation: its actuality and future used for treatment of hypoxic-ischemic encephalopathy. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:143–148. [Google Scholar]
- Zheng T, Rossignol C, Leibovici A, Anderson KJ, Steindler DA, Weiss MD. Transplantation of multipotent astrocytic stem cells into a rat model of neonatal hypoxic-ischemic encephalopathy. Brain Res. 2006;1112:99–105. doi: 10.1016/j.brainres.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Jiang R, Yang B, Yao X, Wang P, Liu D, Wang Y. Changes of telomere and telomerase in effect of ginsenoside Rg1 to delay hematopoietic stem cell senescence. Zhongguo Zhong Yao Za Zhi. 2011;36:3172–3175. [PubMed] [Google Scholar]


