Abstract
Apelin-13 inhibits neuronal apoptosis caused by hydrogen peroxide, yet apoptosis following cerebral ischemia-reperfusion injury has rarely been studied. In this study, Apelin-13 (0.1 μg/g) was injected into the lateral ventricle of middle cerebral artery occlusion model rats. TTC, TUNEL, and immunohistochemical staining showed that compared with the cerebral ischemia/reperfusion group, infarct volume and apoptotic cell number at the ischemic penumbra region were decreased in the Apelin-13 treatment group. Additionally, Apelin-13 treatment increased Bcl-2 immunoreactivity and decreased caspase-3 immunoreactivity. Our findings suggest that Apelin-13 is neuroprotective against cerebral ischemia/reperfusion injury through inhibition of neuronal apoptosis.
Keywords: nerve regeneration, brain injury, neuroprotection, cerebral ischemia/reperfusion injury, lateral intracerebroventricular injection, Apelin-13, nerve apoptosis, Bcl-2, caspase-3, NSFC grants, neural regeneration
Introduction
Ischemic stroke is harmful to human health, and accounts for a large proportion of clinical cerebrovascular disease. Its main pathophysiological process is cerebral ischemia/reperfusion (I/R) injury, with apoptosis to be a key disease pathology (Hauk et al., 2002; Abas et al., 2010; Wang et al., 2014). Apelin is the endogenous ligand for the G protein coupled receptor, APJ. The Apelin gene encodes a group of functionally active endogenous peptides with different molecular structures, including Apelin-36, Apelin-31, Apelin-17, and Apelin-13 (Kleinz et al., 2005; Masri et al., 2005). By combining with the APJ receptor, Apelin regulates a number of physiological states including cardiovascular function (Barnes et al., 2010), endocrine function (Wei et al., 2005), nervous system function (Cheng et al., 2012), and control of feeding and drinking behavior (Falcao-Pires et al., 2009; Gu et al., 2013). Recent studies have shown that the Apelin/APJ system is expressed in neurons from many brain regions. Moreover, in vitro experiments show that hypoxia regulates neuronal Apelin expression (Zhang et al., 2011), and Apelin not only promotes survival of primary cultured neurons (O’Donnell et al., 2007; Cook et al., 2011), but also inhibits neuronal apoptosis induced by hydrogen peroxide (Zeng et al., 2010; Kasai et al., 2011). Current studies on Apelin have mainly focused on Apelin-13 and Apelin-36. Apelin-13 more readily combines with APJ and has stronger biological activity than Apelin-36.
Here, we performed lateral intracerebroventricular injection of Apelin-13 to observe the effect on apoptosis during cerebral I/R injury. We measured infarct volume, neuronal apoptosis and related factors (e.g., anti-apoptotic factor Bcl-2 and pro-apoptotic factor caspase-3) in rat brain to determine the neuroprotective effect of Apelin-13.
Materials and Methods
Animals
Thirty six healthy adult male Wistar rats aged 6–7 weeks, weighing 200 g, of specific-pathogen-free level, were provided by Shandong Lukang Pharmaceutical Co., Ltd. (Shandong Province, China; animal license No. SCXK (Lu) 20130001). Experimental animal management followed the National Experimental Animal Breeding Guide and Regulations of Jining Medical University. All experimental animals were maintained at 25 ± 3°C in a 12-hour light/dark cycle, with free access to food and water.
Animal grouping
The 36 rats were randomly divided into three groups: sham, cerebral I/R, and Apelin-13 treatment (n = 12 for each group). In the sham group, the right carotid artery was isolated with no further processing. In the cerebral I/R group, the right middle cerebral artery occlusion model was performed. While in the Apelin-13 treatment group, after reperfusion, Apelin-13 (0.1 μg/g; Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA) diluted in 10 μL physiological saline was injected into the lateral ventricle using a brain stereotaxic instrument (Stoelting Co., Wood Dale, IL, USA).
Middle cerebral artery occlusion (MCAO) model
The rat right middle cerebral artery occlusion model was performed using the suture-occluded method (Longa et al., 1989). In brief, rats were fasted for 12 hours before surgery, and anesthetized by intraperitoneal injection of 10% chloral hydrate (0.3 mL/100 g). Anesthetized rats were fixed on the operating table, and hair removed and disinfected. An incision was made along the cervical midline to isolate the carotid, external carotid, and internal carotid arteries, and then the carotid and external carotid arteries were ligated. The distal end of the internal carotid artery was occluded using clips, the carotid artery cut via an incision using ophthalmic scissors, and the thread line inserted to cut the arterioles. Line insertion was terminated at a depth of 18 mm and the skin wounds sutured. Lines were removed after 2 hours of ischemia and 24 hours of reperfusion.
Verification of model establishment
Two hours after the operation, rats were scored using the five stage evaluation method of Zea Longa (Longa et al., 1989). Specifically, 0 point: rats have no neurological symptoms; 1 point: rats cannot fully extend the contralateral forepaw; 2 points: rats circle towards the contralateral side; 3 points: rats fall towards the contralateral side; 4 points: rats cannot walk spontaneously or show loss of consciousness; 5 points: death. Rats with 1–3 points and no subarachnoid hemorrhaging qualified as established models.
2, 3, 5-Triphenyl-2H-tetrazolium chloride (TTC) staining
After model establishment, rat brains were harvested 24 hours after injury in the sham group, and 24 hours after reperfusion in the other two groups. Three brains were randomly selected from each group and kept at −20°C. Brains were cut into 2-mm-thick coronal sections placed in 1% TTC solution (Sigma, St. Louis, MO, USA) at 37°C, and stained for 20 minutes in the dark. Slices were then removed for imaging. Infarcted areas appeared white. Infarct areas were determined using Image-Pro Plus v 6.0 software (Media Cybernetics Inc., Bethesda, MD, USA) and the percentage of cerebral infarct volume to total brain volume calculated.
Slice preparation
Nine rats were randomly selected from each group at 24 hours after injury. Rats underwent a thoracotomy under intraperitoneal anesthesia, and then cardiac perfusion with normal saline until the liquid became clear. Rat brains were fixed in 200 mL of 4% paraformaldehyde solution for internal fixation. Next, brains were removed and immersed in 4% paraformaldehyde solution for 24 hours for external fixation, and then sunk in sucrose solution gradients of 10%, 20% and 30%. Brain slices were cut into 30-μm-thick coronal slices using a microtome (Thermo Scientific, Inc., New York, NY, USA).
TdT-mediated dUTP nick-end labeling (TUNEL) staining
Slices were immersed in 0.85% sodium chloride for 5 minutes, washed in PBS for 5 minutes, immersed in 4% paraformaldehyde solution for 15 minutes, and then washed in PBS twice for 5 minutes each. Subsequently, slices were incubated in protease K for 15 minutes, washed in PBS for 5 minutes, fixed in 4% paraformaldehyde for 5 minutes, and again washed in PBS for 5 minutes (TUNEL Kit; Promega Co., Madison, WI, USA). Slices were incubated in equilibration buffer for 10 minutes at room temperature. For labeling, slices were incubated in terminal deoxynucleotidyl transferase (TdT) reaction mix for 60 minutes at 37°C, and immersed in 2 × SSC for 15 minutes to terminate the reaction. Next, slices were washed in PBS three times for 5 minutes each, and incubated in streptavidin horseradish peroxidase for 30 minutes followed by three PBS washes. Slices were developed using DAB, which was incubated for 10 minutes, and then repeatedly washed in deionized water and sealed. TdT was used instead of deionized water for negative controls. Brown stained apoptotic cells were observed using an inverted fluorescence microscope (Olympus, Tokyo, Japan). Optical density of apoptotic cells was calculated from five randomly selected views at the ischemic penumbra using Image-Pro Plus v 6.0 software.
Immunohistochemistry
After washing in PBS, slices were incubated in 3% hydrogen peroxide solution for 10 minutes followed by B cell lymphoma/leukemia 2 (Bcl-2) and cysteinyl aspartate-specific proteinase-3 (caspase-3) rabbit anti-rat polyclonal antibodies (Bioworld Technology, Inc., Louis Park, MN, USA) at 4°C overnight. Next, slices were rinsed with PBS three times for 2 minutes each, and incubated in biotin labeled goat anti-rabbit secondary antibody (Beijing Sequoia Jinqiao Biological Technology Co., Ltd., Beijing, China) for 15 minutes at 37°C, followed by washing in PBS three times for 2 minutes each. Finally, slices were developed using DAB (Beijing Sequoia Jinqiao Biological Technology Co., Ltd.) and then sealed. Negative control antibody was treated with PBS. Brown stained positive cells were observed using an inverted fluorescence microscope (Olympus). Optical density of Bcl-2-and caspase-3-immunoreactive cells was calculated from five randomly selected views at the ischemic penumbra using Image-Pro Plus v 6.0 software.
Statistical analysis
Data were analyzed using GraphPad Prism software (GraphPad Software, Inc., La Jolla, CA, USA) and are expressed as the mean ± SD. Groups differences were analyzed using one-way analysis of variance and if a significant difference was detected, followed by the least significance difference test. P < 0.05 was considered significantly different.
Results
Apelin-13 reduced infarct volume in MCAO rats
In the Apelin-13 treatment group, infarct volume in brain tissue was reduced compared with the cerebral I/R group (Figure 1), demonstrating that Apelin-13 attenuates ischemic injury (P < 0.05; Figure 1B) and has a neuroprotective effect against cerebral I/R injury.
Figure 1.
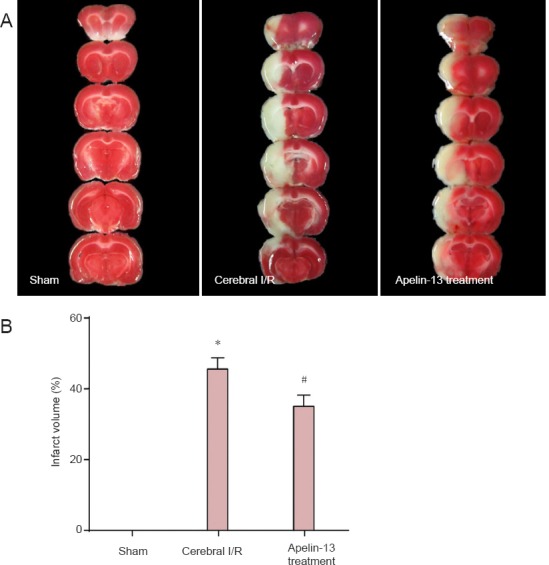
Apelin-13 reduced infarct volume in a middle cerebral artery occlusion rat model after 2 hours of ischemia and 24 hours of reperfusion, as detected by 2,3,5-triphenyl-2H-tetrazolium chloride (TTC) staining.
(A) Representative coronal brain sections of sham, cerebral I/R, and Apelin- 13 treatment groups. Brain tissue in the sham group showed uniform red color with no infarction. The infarct volume in the Apelin-13 treatment group was reduced compared with the cerebral I/R group. (B) Percentage of cerebral infarct volume to total brain volume. *P < 0.01, vs. sham group; #P < 0.05, vs. cerebral I/R group (one-way analysis of variance followed by the least significance difference test). Data are expressed as the mean ± SD (n = 3 for each group). I/R: Ischemia/reperfusion.
Apelin-13 reduced apoptosis in MCAO rats
There were few apoptotic cells in the sham group, which increased significantly in the cerebral I/R group (P < 0.01). The number of apoptotic cells decreased in the Apelin-13 treatment group compared with the cerebral I/R group (P < 0.05; Figure 2), showing that Apelin-13 treatment reduces apoptotic cell death after cerebral I/R injury.
Figure 2.
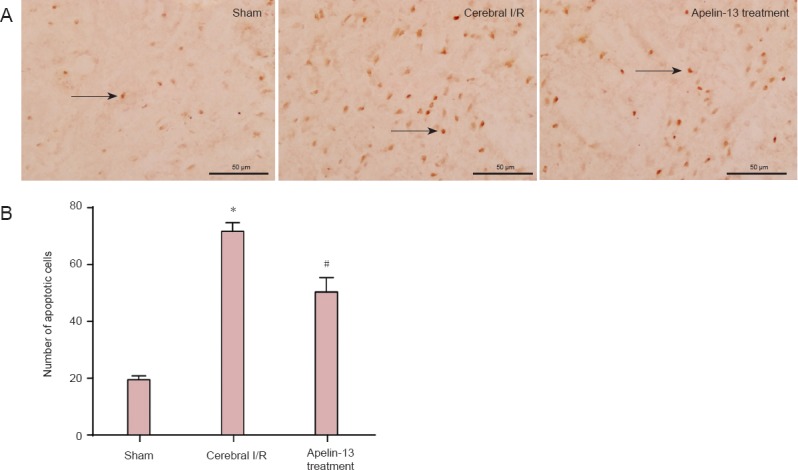
Apelin-13 reduced apoptosis in the ischemic penumbra region in a middle cerebral artery occlusion rat model after 2 hours of ischemia and 24 hours of reperfusion, as detected by TdT-mediated dUTP nick-end labeling (TUNEL) staining.
(A) Representative photomicrographs of apoptotic cells with TUNEL in sham, cerebral I/R, and Apelin-13 treatment groups (fluorescence microscope, × 400, scale bars: 50 μm). Arrows indicate TUNEL positive cells. (B) Optical density values were used to calculate apoptotic cell number. *P < 0.01, vs. sham group; #P < 0.05, vs. cerebral I/R group (one-way analysis of variance followed by the least significance difference test). Data are expressed as the mean ± SD (n = 3 for each group). I/R: Ischemia/reperfusion.
Apelin-13 increased Bcl-2 immunoreactivity at the ischemic penumbra in MCAO rats
Bcl-2 immunoreactivity was minimal in the sham group, but increased in the cerebral I/R group compared with controls (P < 0.01). In addition, Bcl-2 immunoreactivity increased in the Apelin-13 treatment group compared with the cerebral I/R group (P < 0.05; Figure 3), showing that Apelin-13 has protective effects against cerebral I/R injury by increasing immunoreactivity of anti-apoptotic factors.
Figure 3.
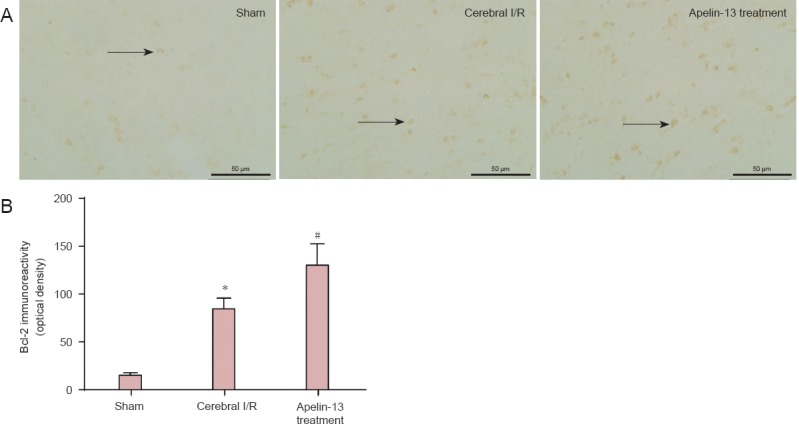
Bcl-2 immunoreactivity at the ischemic penumbra region in a middle cerebral artery occlusion rat model after Apelin-13 treatment, as detected by immunohistochemical staining.
(A) Representative photomicrographs of Bcl-2-immunoreactive cells in sham, cerebral I/R, and Apelin-13 treatment groups (fluorescence microscope, × 400, scale bars: 50 μm). Arrows indicate Bcl-2 positive cells. (B) Optical density values were used to calculate Bcl-2 immunoreactivity. *P < 0.01, vs. sham group; #P < 0.05, vs. cerebral I/R group (one-way analysis of variance followed by the least significance difference test). Data are expressed as the mean ± SD (n = 3 for each group). I/R: Ischemia/reperfusion.
Apelin-13 decreased caspase-3 immunoreactivity at the ischemic penumbra in MCAO rats
Caspase-3 immunoreactivity was weak in the sham group, but relatively high in the cerebral I/R group (P < 0.01). Caspase-3 immunoreactivity was decreased in the Apelin-13 treatment group compared with the cerebral I/R group (P < 0.05; Figure 4), indicating that Apelin-13 has protective effects on cerebral I/R injury by inhibiting immunoreactivity of pro-apoptotic factors.
Figure 4.
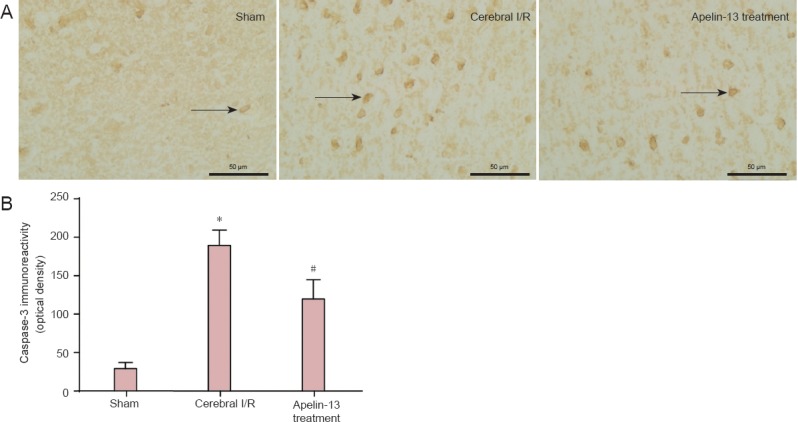
Caspase-3 immunoreactivity in the ischemic penumbra region in a middle cerebral artery occlusion rat model after Aplin-13 treatment, as detected by immunohistochemical staining.
(A) Representative photomicrog raphs of caspase-3-immunoreactive cells in sham, cerebral I/R, and Apelin-13 treatment groups (fluorescence microscope, × 400, scale bars: 50 μm). Arrows indicate caspase-3 immunoreactive cells. (B) Optical density values were used to calculate caspase-3 immunoreactivity. *P < 0.01, vs. sham group; #P < 0.05, vs. cerebral I/R group (one-way analysis of variance followed by the least significance difference test). Data are expressed as the mean ± SD (n = 3 for each group). I/R: Ischemia/reperfusion.
Discussion
In ischemic stroke, the cerebral artery is obstructed absolutely or relatively by thrombosis, resulting in injury to the corresponding brain tissue by ischemia and hypoxia (Sun et al., 2014; Zheng et al., 2014). Rapid restoration of blood flow to the brain is the most effective means of relieving ischemic hypoxic injury, but inevitably causes reperfusion injury (Pan et al., 2013; Shen et al., 2013). With a stroke, the infarct caused by cerebral I/R injury is divided into two regions. At the center of the infarct region, nerve cells die rapidly and the infarction injury is irreversible. However, there is an ischemic penumbra around the infarct area (Hughes et al., 2010; Du et al., 2014), where neuronal injury is mainly apoptotic and neurons are in a state of electrical failure. Nevertheless, ischemic neuronal apoptosis can be delayed and is reversible, which provides an opportunity for treatment of ischemic stroke using drugs that can reduce neuronal damage and cerebral infarct volume. Thus, effectively preventing neuronal apoptosis within the ischemic penumbra region may relieve brain injury and improve a patients’ quality of life.
In the central nervous system, apoptosis-related genes include anti-apoptotic and pro-apoptotic genes, with Bcl-2 and caspase-3 the main factors associated with cell apoptosis (Liu et al., 2014). Bcl-2 was first discovered at a chromosomal translocation, t(14, 18) that associated with B cell lymphoma. In 1988, Vaux (1988) found that Bcl-2 inhibits apoptosis and prolongs cell survival. The Bcl-2 gene is regulated by programmed cell death to extend cell lifespan, and is therefore directly related to cell apoptosis. Bcl-2 has a direct inhibitory effect on cell apoptosis and operates at all stages of the cell cycle. Numerous studies have shown that Bcl-2 upregulation reduces cerebral ischemia in animal models (Okazaki et al., 2008; Xing et al., 2008), indicating that Bcl-2 acts as a neuroprotective factor, and can inhibit neuronal damage caused by ischemia and hypoxia (Zhang et al., 2006). We found increased Bcl-2 immunoreactivity in the I/R group compared with the sham group, suggesting that Bcl-2 expression is increased by a self-protective mechanism during the process of cerebral I/R injury. Additionally, Bcl-2 immunoreactivity was significantly increased in the Apelin-13 treatment group compared with the cerebral I/R group, indicating that Apelin-13 increases expression of this anti-apoptotic protein and thus inhibits cell apoptosis. The caspase family is a group of proteases with cysteine restriction enzyme sites. Cascade activation of caspase proteases is the main operational mode of cellular apoptosis. Previous studies have demonstrated that caspase family members are mostly involved in neuronal apoptosis (Yuan et al., 1993; Nicholson et al., 1997). In addition, caspase-3 is the key protease and operator of cell apoptosis (Salvesen, 2002; Shi, 2004), which plays an important regulatory role in biological processes such as cell differentiation, adhesion, and neural development (Nakamoto et al., 2005; Puga et al, 2008; D’ Amelio et al., 2010). Caspase-3 is known as the death protein and most closely associated with cell apoptosis (Prabhakar et al., 2003; Meloni et al., 2011). Moreover, caspase-3 induces apoptosis after ischemic brain injury (Ayyash et al., 2012), and caspase inhibitors reduce cerebral I/R injury in animal models (Fink et al., 1998). We found increased number of caspase-3 positive cells in the cerebral I/R group compared with the sham group, with expression mainly located in the ischemic infarct region, suggesting that caspase-3 is activated during apoptosis caused by cerebral I/R injury and also promotes apoptosis. Caspase-3 immunoreactivity in the Apelin-13 treatment group was reduced compared with the cerebral I/R group, indicating that Apelin-13 inhibits caspase-3 immunoreactivity during cerebral I/R injury and plays an important anti-apoptotic role.
Our findings show that intracerebroventricular injection of Apelin-13 effectively reduces cerebral I/R injury in rats and inhibits apoptosis of neuronal cells, thereby exerting a neuroprotective effect. The protective mechanism of intracerebroventricular injection of Apelin-13 inhibits immunoreactivity of pro-apoptotic factors and promotes immunoreactivity of anti-apoptotic factors, thereby reducing apoptosis. Our research adds more theoretical basis to development of new drug treatments for Apelin and its receptor, and may provide greater drug choice for patients with ischemic stroke.
Acknowledgments
We would like to thank Lin Wang and Yan-you Pan from Neurobiology Institute of Jining Medical University in China for providing technical assistance.
Footnotes
Funding: This study was financially supported by the National Natural Science Foundation of China, No. 30971081, 31271243, 81070961 and 81241052; and the Natural Science Foundation of Shandong Province of China, No. ZR2011CM027 and 2012GGA08100.
Conflicts of Interest: None declared.
Copyedited by James R, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- Abas F, Alkan T, Goren B, Taskapilioglu O, Sarandol E, Tolunay S. Neuroprotective effects of postconditioning on lipid peroxid-ation and apoptosis after focal cerebral ischemia/reperfusion injury in rats. Turk Neurosurg. 2010;20:1–8. [PubMed] [Google Scholar]
- Ayyash M, Tamimi H, Ashhab Y. Developing a powerful in silico tool for the discovery of novel caspase-3 substrates: a preliminary screening of the human proteome. BMC Bioinformatics. 2012;13:14. doi: 10.1186/1471-2105-13-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes G, Japp AG, Newby DE. Translational promise of the apelin-APJ system. Heart. 2010;96:1011–1016. doi: 10.1136/hrt.2009.191122. [DOI] [PubMed] [Google Scholar]
- Cheng B, Chen J, Bai B, Xin Q. Neuroprotection of apelin and its signaling pathway. Peptides. 2012;37:171–173. doi: 10.1016/j.peptides.2012.07.012. [DOI] [PubMed] [Google Scholar]
- Cook DR, Gleichman AJ, Cross SA, Doshi S, Ho W, Jordan-Sciutto KL, Lynch DR, Kolson DL. NMDA receptor modulation by the neuropeptide apelin:implications for excitotoxic injury. J Neurochem. 2011;118:1113–1123. doi: 10.1111/j.1471-4159.2011.07383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelio M, Cavallucci V, Cecconi F. Neuronal caspase-3 signaling: not only cell death. Cell Death Differ. 2010;17:1104–1114. doi: 10.1038/cdd.2009.180. [DOI] [PubMed] [Google Scholar]
- Du GJ, Dang MR, Tan ZM, Su RQ, Shen Q, Fang J. Limb ischemic postconditioning promotes the proliferation of endogenous neural stem cells in rats with cerebral ischemia injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:1597–1602. [Google Scholar]
- Falcao-Pires I, Goncalves N, Henriques-Coelho T, Moreira-Goncalves D, Roncon-Albuquerque R, Jr, Leite-Moreira AF. Apelin decreases myocardial injury and improves right ventricular function in monocrotaline-induced pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2009;296:H2007–2014. doi: 10.1152/ajpheart.00089.2009. [DOI] [PubMed] [Google Scholar]
- Fink K, Zhu J, Namura S, Shimizu-Sasamata M, Endres M, Ma J, Dalkara T, Yuan J, Moskowitz MA. Prolonged therapeutic window for ischemic brain damage caused by delayed caspase activation. J Cereb Blood Flow Metab. 1998;18:1071–1076. doi: 10.1097/00004647-199810000-00003. [DOI] [PubMed] [Google Scholar]
- Gu Q, Zhai L, Feng X, Chen J, Miao Z, Ren L, Qian X, Yu J, Li Y, Xu X, Liu CF. Apelin-36, a potent peptide, protects against ischemic brain injury by activating the P13K/Akt pathway. Neurochem Int. 2013;63:535–540. doi: 10.1016/j.neuint.2013.09.017. [DOI] [PubMed] [Google Scholar]
- Hauck L, Hansmann G, Dietz R, von Harsdorf R. Inhibition of hypoxia-induced apoptosis by modulation of retinoblastoma protein-dependent signaling in cardiomyocytes. Circ Res. 2002;91:782–789. doi: 10.1161/01.res.0000041030.98642.41. [DOI] [PubMed] [Google Scholar]
- Hughes JL, Beech JS, Jones PS, Wang D, Menon DK, Baron JC. Mapping selective neuronal loss and microglial activation in the salvaged neocortical penumbra in the rat. Neuroimage. 2010;49:19–31. doi: 10.1016/j.neuroimage.2009.08.047. [DOI] [PubMed] [Google Scholar]
- Kasai A, Kinjo T, Ishihara R, Sakai I, Ishimaru Y, Yoshioka Y, Yamamuro A, Ishige K, Ito Y, Maeda S. Apelin deficiency accelerates the progression of amyotrophic lateral sclerosis. PLoS One. 2011;6:e23968. doi: 10.1371/journal.pone.0023968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107:198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- Liu DS, Xiao SL, Wang B, Zhou C. Expressions of Bcl-2 and Bax and apoptotic rate of bone marrow mesenchymal stem cells transfected with thymosin beta4 in a hypoxic environment. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:6573–6577. [Google Scholar]
- Longa EZ, Weinsten PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rat. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Masri B, Knibiehler B, Audigier Y. Apelin signalling a promising pathway from cloning to phamacology. Cell Signal. 2005;17:415–426. doi: 10.1016/j.cellsig.2004.09.018. [DOI] [PubMed] [Google Scholar]
- Meloni BP, Meade AJ, Kitikomolsuk D, Knuckey NW. Characterisation of neuronal cell death in acute and delayed in vitro ischemia (oxygen-glucose deprivation) models. Neurosci Methods. 2011;195:67–74. doi: 10.1016/j.jneumeth.2010.11.023. [DOI] [PubMed] [Google Scholar]
- Nakamoto K, Kuratsu J, Ozawa M. Beta-catenin cleavage in non-apoptotic cells with reduced cell adhesion activity. Int J Mol Med. 2005;15:973–979. [PubMed] [Google Scholar]
- Nicholson DW, Thornberry NA. Caspase: killer proteases. Trends Biochem Sci. 1997;22:299–306. doi: 10.1016/s0968-0004(97)01085-2. [DOI] [PubMed] [Google Scholar]
- O’Donnell LA, Agrawal A, Sabnekar P, Dichter MA, Lynch DR, Kolson DL. Apelin, an endogenous neuronal peptide, protects hippocampal neurons against excitotoxic injury. J Neurochem. 2007;102:1905–1917. doi: 10.1111/j.1471-4159.2007.04645.x. [DOI] [PubMed] [Google Scholar]
- Okazaki T, Magaki T, Takeda M, Kajiwara Y, Hanaya R, Sugiyama K, Arita K, Nishimura M, Kato Y, Kurisu K. Intravenous adminiatration of bone marrow stromal cells increases survivin and Bcl-2 protein expression and improves sensorimotor function following ischemia in rats. Neurosci Lett. 2008;430:109–114. doi: 10.1016/j.neulet.2007.10.046. [DOI] [PubMed] [Google Scholar]
- Pan Q, Wang Z, Gao Y, Yu D, Wang Y, Jing H, Wang SY, Wang J, Guan HQ. Eye acupuncture therapy affects intercellular adhesion molecule 1 expression in rat hippocampus of acute cerebral ischemia-reperfusion injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:6636–6640. [Google Scholar]
- Prabhakar G, Vona-Davis L, Murray D, Lakhani P, Murray G. Phosphocreatine restores high-energy phosphates in ischemic myocardium: implication for off-pump cardiac revascularization. J Am Coll Surg. 2003;197:786–791. doi: 10.1016/j.jamcollsurg.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Puga I, Rao A, Macian F. Targeted cleavage of signaling proteins by caspase 3 inhibits T cell receptor signaling in anergic T cells. Immunity. 2008;29:193–204. doi: 10.1016/j.immuni.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen GS. Caspase and apoptosis. Essays Biochem. 2002;38:9–19. doi: 10.1042/bse0380009. [DOI] [PubMed] [Google Scholar]
- Shen LP, Wang SS, Dong LG, Shen X, Hua F, Ye XC, Cui GY. Intranasal administration of the conditioned medium of human umbilical cord-derived mesenchymal stem cells for treatment of cerebral ischemia-reperfusion injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2013;17:7891–7897. [Google Scholar]
- Shi Y. Caspase activation, inhibition, and reactivation: a mechanistic view. Protein Sci. 2004;13:1979–1987. doi: 10.1110/ps.04789804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun NX, Gao WJ, Yi ZL. Improved suture occlusion method to prepare focal cerebral ischemia reperfusion models in Sprague-Dawley rats. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:225–230. [Google Scholar]
- Vaux DL, Cory S, Adanms JM. Bcl-2 gene promotes haemopoietic cell survival and cooperates with cmyc to immortalize pre-B cell. Nature. 1988;335:440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Wang YR, Mao HF, Chen JQ. Effects of huwentoxin on tumor necrosis factor apoptotic pathway in the hippocampus of a rat model of cerebral ischemia. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:5813–5818. [Google Scholar]
- Wei L, Hou X, Tatemoto K. Regulation of apelin mRNA expression by insulin and glucocorticoids in mouse 3T3-L1 adipocytes. Regul Pept. 2005;132:27–32. doi: 10.1016/j.regpep.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Xing B, Chen H, Zhang M, Zhao D, Jiang R, Liu X, Zhang S. Ischemic postconditioning inhibits apoptosis after focal cerebral ischemia/reperfusion injury in the rat. Stroke. 2008;39:2362–2369. doi: 10.1161/STROKEAHA.107.507939. [DOI] [PubMed] [Google Scholar]
- Yuan J, Shaham S, Ledoux S, Ellis HM, Horvitz HR. The C. elegans cell gene ced-3 encodes a protein similar to mammalian inter lenkin-1 beta-converting enzyme. Cell. 1993;75:641–652. doi: 10.1016/0092-8674(93)90485-9. [DOI] [PubMed] [Google Scholar]
- Zeng XJ, Yu SP, Zhang L, Wei L. Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp Cell Res. 2010;316:1773–1783. doi: 10.1016/j.yexcr.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Xue YY, Lu SD, Wang Y, Zhang LM, Huang YL, Signore AP, Chen J, Sun FY. Bcl-2 enhances neurogenesis and inhibis apoptosis of new born neurons in adult rat brain following a transient middle cerebral artery occlision. Neurobiol Dis. 2006;24:345–356. doi: 10.1016/j.nbd.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Zhang X, Peng X, Fang M, Zhou C, Zhao F, Zhang Y, Xu Y, Zhu Q, Luo J, Chen G, Wang X. Up-regulation of apelin in brain tissue of patients with epilepsy and an epileptic rat model. Peptides. 2011;32:1793–1799. doi: 10.1016/j.peptides.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Zheng YT, Liu B, Lodato R, Li QL, Lan DH, Hong XY, Xian H. Amniotic cells protect and repair mouse brain cells following ischemia-reperfusion injury. Zhongguo Zuzhi Gongcheng Yanjiu. 2014;18:6024–6028. [Google Scholar]


