Abstract
Thalidomide is an effective drug for the treatment of ankylosing spondylitis but might induce peripheral neuropathy. This major adverse reaction has attracted much concern. The current study aimed to observe the incidence of thalidomide-induced peripheral neuropathy among ankylosing spondylitis patients for 1 year after treatment. In this study, 207 ankylosing spondylitis cases received thalidomide treatment, while 116 ankylosing spondylitis cases received other treatments. Results showed that the incidence of thalidomide-induced peripheral neuropathy in the thalidomide group was higher than that in the non-thalidomide group. There was no significant difference in the incidence of neuropathy between the < 6 months medication and ≥ 6 months medication groups. There were no differences in the mean age, gender, or daily dose between the two groups. The incidence of peripheral neuropathy among patients receiving 25, 50, 75, or 100 mg thalidomide per day was 4.6%, 8.5%, 17.1%, 21.7%, respectively. The incidence was significantly different between the groups receiving 25 mg and 100 mg thalidomide. In conclusion, thalidomide can induce peripheral neuropathy within 1 year after treatment of ankylosing spondylitis; however, age and gender have no obvious impact on the incidence of peripheral neuropathy. The incidence of peripheral neuropathy is associated with increasing daily doses of thalidomide.
Keywords: nerve regeneration, peripheral nerve injury, thalidomide, ankylosing spondylitis, adverse reactions, peripheral neuropathy, prospective study, treatment, dose, treatment time, age, sex, neural regeneration
Introduction
Thalidomide, a drug previously used to treat vomiting during pregnancy was withdrawn from the global market because it caused birth defects including severe fetal limb malformations in approximately 12,000 children (Matthews et al., 2003). Since the mid-1960s, thalidomide has shown potential for the treatment of erythema nodosum leprosy and was approved by the FDA in 1998 and re-marketed for the treatment of moderate and severe erythema nodosum leprosy (Hall et al., 2003). Thalidomide has immunomodulatory (Haslett et al., 1998; Yang et al., 2010), anti-inflammatory and antiangiogenic effects (Direskeneli et al., 2008; Liu et al., 2009), and inhibits tumor necrosis factor-α (Sampmo et al., 1991; Majumdar et al., 2002). In addition, it has been used to treat a variety of diseases, such as refractory multiple myeloma (Tosi et al., 2005; Mark et al., 2014), systemic onset juvenile rheumatoid arthritis (García-Carrasco et al., 2007), Behçet’s disease (Direskeneli et al., 2008) and immune diseases.
Thalidomide has been used to treat ankylosing spondylitis (AS) for over ten years (Huang et al., 2002; Zhu et al., 2010) and achieved good outcomes. However, thalidomide-induced peripheral neuropathy has attracted much concern because some patients suffering peripheral neuropathy discontinue medication. Previous studies have reported the neurological toxicity of thalidomide in patients with multiple myeloma (Tosi et al., 2005) and cutaneous lupus erythematosus (Frankel et al., 2013), but the conclusions remain controversial. Increasing evidence has indicated the probability and onset time of thalidomide-induced peripheral neuropathy in AS patients, as well as a correlation between peripheral neuropathy and patient age and sex, as well as with the dose and duration of treatment. The present study aimed to observe the incidence of thalidomide-induced peripheral neuropathy in AS patients, and explore the relevant factors of peripheral neuropathy pathogenesis.
Subjects and Methods
Subjects
A total of 367 AS patients were recruited from the Department of Rheumatology and Immunology, Shengjing Hospital of China Medical University (Shenyang, Liaoning Province, China) between January 2007 and June 2012.
Inclusion criteria
Diagnosis of AS was defined according to the modified New York criteria (1984) for AS. Patients were unable to accept biological therapy. In the thalidomide group, 238 AS cases were treated with thalidomide alone or as a combination of thalidomide with other antirheumatic drugs (Sulfasalazine, Shanghai Zhongxi Pharmaceutical (Group) Co., Ltd., Shanghai, China; Methotrexate, Shanghai Sine Pharmaceutical Co., Ltd., Shanghai, China; Leflunomide, Cinkate Corporation, USA), and also received intermittent nonsteroidal anti-inflammatory medication. In the non-thalidomide group, 129 AS cases were treated with non-steroidal anti-inflammatory drugs alone or a combination of non-steroidal anti-inflammatory drugs with other antirheumatic drugs (the same antirheumatic drugs as the thalidomide group). The maximum dose of methotrexate was 15 mg/wk, sulfasalazine was 2.25 g/d, and leflunomide was 20 mg/d. All patients were forced to take effective contraceptive measures during thalidomide treatment. All patients were required to meet the following inclusion criteria: Bath AS Disease Activity Index (BASDAI) values ≥ 4 and a spondylalgia visual analogue scale score ≥ 4 points. Written informed consent and an agreement to use contraceptive measures during the period of study were obtained from all patients.
Exclusion criteria
Patients with a history of severe diseases of the heart, liver, kidneys and other vital organs, or blood, diabetes mellitus and other endocrine system diseases; nulliparous young women, pregnant women and lactating women; or patients with peripheral neuropathy history and performance were excluded from the study. All patients or their relatives were informed of and agreed to the experimental regimen.
Methods
Drug interventions
Thalidomide (25 mg/tablet; Changzhou Pharmaceutical Factory Co., Ltd., China) was orally administered as a draught before sleep. The initial dose of thalidomide was 25 mg per day, and was then increased by 25–50 mg per day every 7 days, to a maximum dose of 100 mg per day. The medication was withdrawn when peripheral neuropathy developed, and was then changed to neurotrophic treatment.
Evaluation index
General information of patients in the thalidomide group and non-thalidomide group was analyzed and compared. General information included the mean age, gender, mean disease duration, human leukocyte antigen-B27 (HLA-B27) positive rate (HLA-B27 is an indicator of AS laboratory tests, HLA-B27 positive rate is 6–8% in normal people and 90% in AS patients), mean Bath Ankylosing Spondylitis Disease Activity Index (BASDAI; a range of 0–10 points, a higher score indicates more severe disease symptoms), and mean visual analogue scale (VAS) score (a range of 0–10 points, a higher score indicates more obvious pain). The incidence of thalidomide-induced peripheral neuropathy in the thalidomide and non-thalidomide groups was calculated. The number of AS patients suffering peripheral neuropathy in the first 6 months after treatment (medication time < 6 months) and in those at 6 months after treatment (medication time ≥ 6 months) was compared. The mean age, gender composition and daily dose of AS patients with or without peripheral neuropathy in the thalidomide group was compared. The incidence of peripheral neuropathy among different doses of thalidomide treatment was calculated.
Statistical analysis
Measurement data are expressed as the mean ± SD. Count data are expressed as a percentage. Data were analyzed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Differences between the two groups were compared using the two-sample t-test and chi-square test. P < 0.05 was considered statistically significant.
Results
Quantitative analysis and baseline information of subjects
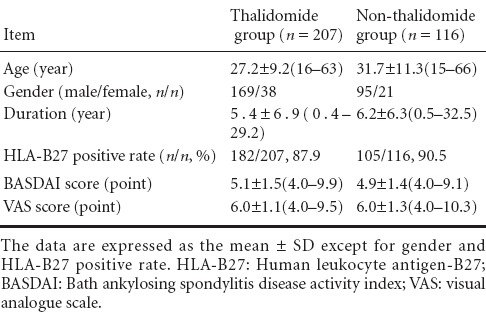
A total of 367 AS patients were included in this study between 2007 and 2012; 44 cases were lost in the 1-year course of treatment due to poor efficacy, while the remaining 323 cases completed the whole course of treatment and were divided into two groups. There were 207 AS patients in the thalidomide group, including 53 cases receiving thalidomide alone and 154 cases receiving a combined treatment of thalidomide and other antirheumatic drugs (sulfasalazine, methotrexate and leflunomide). There were 116 AS patients in the non-thalidomide group, including 22 cases receiving non-steroidal anti-inflammatory drugs alone and 94 cases receiving a combined treatment of non-steroidal anti-inflammatory drugs and other antirheumatic drugs. General information of patients in the thalidomide and non-thalidomide groups is shown in Table 1. There was no significant difference in the mean age, gender, disease duration, HLA-B27 positive rate, BASDAI score and VAS score between the two groups (P > 0.05).
Table 1.
General information of patients in the thalidomide and non-thalidomide groups
Incidence of thalidomide-induced peripheral neuropathy in AS patients
AS patients developed distal limb numbness, dysesthesia, muscle weakness, hypotonia, and tendon reflex decline after thalidomide treatment. Peripheral neuropathy was detected by electromyogram. The incidence of peripheral neuropathy was 10.61% (22/207) in the thalidomide group, which was significantly higher than in the non-thalidomide group, 1.72% (2/116) (P < 0.01). Thalidomide treatment was discontinued immediately after peripheral neuropathy occurred, and patients were changed to neurotrophic therapy and symptoms were reversed.
Onset time of peripheral neuropathy in the thalidomide group
In the thalidomide group, 22 AS patients developed peripheral neuropathy and were further assigned to two subgroups. Among them, 14 cases developed neuropathy after medication for < 6 months (< 6 months subgroup) and 8 cases after medication for ≥ 6 months (≥ 6 months subgroup). The percentages of AS patients in the two subgroups were calculated and the < 6 months subgroup had a lower percentage of AS patients than the ≥ 6 months subgroup (36.4% vs. 63.6%), although the difference was not statistically significant (P > 0.05).
The incidence of peripheral neuropathy in the thalidomide group
Twenty-two of 185 AS patients developed peripheral neuropathy after thalidomide treatment. There was no significant difference in the mean age, gender, or daily dose between the two groups (P > 0.05; Table 2).
Table 2.
Comparison of the age, gender, or daily dose of patients with or without peripheral neuropathy after thalidomide treatment
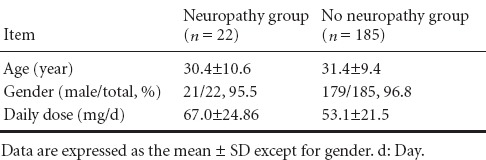
As shown in Table 2, there was no significant difference in patient mean age or gender composition between the two groups. The dose of thalidomide in the AS patients with peripheral neuropathy was higher than that without peripheral neuropathy, but the difference was not statistically significant. Therefore, we calculated the incidence of peripheral neuropathy after different doses of thalidomide treatment (Table 3).
Table 3.
Comparison of the incidence [n(%)] of peripheral neuropathy after different doses of thalidomide treatment
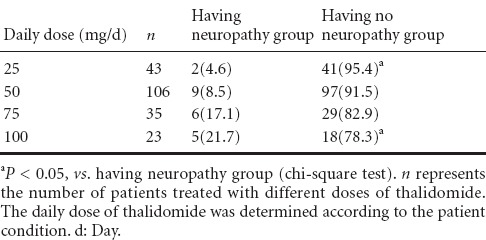
Results showed that as the daily dose of thalidomide increased, the incidence of thalidomide-induced peripheral neuropathy increased. Statistical analysis demonstrated a significant difference in the incidence of peripheral neuropathy between those receiving a dose of 25 mg/d and 100 mg/d of thalidomide treatment (P < 0.05), while no difference was found among the other groups.
Discussion
Nonsteroidal anti-inflammatory drugs combined with functional exercise contribute to mitigate AS symptoms (Dougados et al., 2001; Van der Heijde et al., 2005), but are ineffective in controlling severe AS. Sulfasalazine has therapeutic effects for the treatment of peripheral joints and enthesitis, but fails to cure the central axial joint (Clegg et al., 1996). Biological agents can rapidly alleviate AS disease conditions (Brandt et al., 2000; Van den Bosch et al., 2000), but their high cost is unacceptable for long-term continuous medication. Huang et al. (2002) reported the potent efficacy of thalidomide for the treatment of refractory AS and its biological mechanisms were associated with the inhibition of tumor necrosis factor-α gene expression. Subsequent studies suggested that thalidomide reduced the recurrence of AS in patients following the discontinuation of etanercept (Deng et al., 2013). A long-term thalidomide intervention was shown to be effective and safe for the treatment of refractory AS, and the therapeutic effect was enhanced along with the medication time (Zhu et al., 2010).
Thalidomide-induced peripheral neuropathy is one of the most common adverse reactions during thalidomide treatment. However, the neuropathy-related association with thalidomide remains controversial. Little evidence has been reported for the occurrence and risk factor of thalidomide-induced peripheral neuropathy in AS patients.
In this study, 323 AS patients who were unable to receive biological therapy were included, and were treated with nonsteroidal anti-inflammatory drugs and antirheumatic drugs. Among them, 207 cases received thalidomide treatment while 116 cases did not. The thalidomide-treated cases had a high incidence of peripheral neuropathy compared with the untreated cases, indicating that thalidomide intervention might cause peripheral neuropathy, consistent with previous findings (Briani et al., 2005; Coelho et al., 2005). Therefore, peripheral neuropathy symptoms such as distal limb numbness, dysesthesia, muscle weakness, hypotonia, and tendon reflex decline should be carefully monitored during thalidomide treatment. The thalidomide intervention should be withdrawn immediately after the above symptoms are detected, to avoid irreversible damage to peripheral nerves.
There are some controversies in the incidence of thalidomide-induced peripheral neuropathy during the treatment of autoimmune diseases. Studies have shown that 25% of 135 dermatologic patients developed symptomatic peripheral neuropathy after treatment with < 125 mg thalidomide (Bastuji-Garin et al., 2002). Briani et al. (2005) found that 36% of 14 patients with refractory cutaneous lupus erythematosus developed symptomatic peripheral neuropathy after thalidomide treatment with < 100 mg per day. Ochonisky et al. (1994) observed that of 42 dermatologic patients treated with 28–110 mg thalidomide per day, 21% of cases developed symptomatic peripheral neuropathy. In the present study, 207 SD patients were treated with 25–100 mg thalidomide per day, and 10.6% of patients developed symptomatic peripheral neuropathy. This evidence indicated that thalidomide treatment at a daily dose of < 100 mg yielded a lower incidence of peripheral neuropathy and was a safer option compared with a similar dose of thalidomide treatment for dermatologic diseases and lupus erythematosus., A higher incidence of thalidomide neuropathy in patients with dermatologic diseases and lupus erythematosus compared with AS patients receiving a similar dose of thalidomide might be related to the disease-specific pathogenesis mechanisms. The pathological basis of lupus erythematosus is vasculitis; therefore the vessels are more susceptible to drugs that aggravate hypoxic-ischemic conditions in nerve cells and induce adverse reactions of peripheral nerves. Large-scale studies are needed to compare the incidence of peripheral neuropathy among different autoimmune diseases after the same dose of thalidomide treatment, and to explore the pathogenesis mechanisms of thalidomide-induced peripheral neuropathy.
A prospective study of thalidomide neuropathy demonstrated that dermatologic patients were most vulnerable to peripheral neuropathy within 1 year after the initiation of thalidomide treatment (Bastuji-Garin et al., 2002). In the present study, we analyzed the onset time of thalidomide-induced peripheral neuropathy in AS patients, and revealed no significant difference between the < 6 months of medication and the ≥ 6 months medication subgroups; therefore, it is critical to monitor peripheral neuropathy during the first 1 year of thalidomide treatment. After 1 year or even longer, the incidence of peripheral neuropathy still requires further clinical observation.
Studies reported no significant difference in the mean age of 65 patients with refractory cutaneous lesions of lupus erythematosus with or without peripheral neuropathy after long-term thalidomide use (Coelho et al., 2005), or in 135 dermatologic patients with or without peripheral neuropathy (Bastuji-Garin et al., 2002). In this study, we compared the mean age of AS patients developing peripheral neuropathy after thalidomide treatment and found no difference, which was consistent with previous reports. Therefore patient age has no impact on the pathogenesis of thalidomide-induced peripheral neuropathy. Similarly, we compared the gender composition of AS patients developing peripheral neuropathy after thalidomide treatment, which also indicated no significant difference between the two groups. This finding is supported by the results of Bastuji-Garin et al. (2002). Therefore, patient gender also has no impact on the pathogenesis of peripheral neuropathy. Furthermore, our findings indicated that AS patients with peripheral neuropathy received a higher dose of thalidomide than AS patients without peripheral neuropathy, but there was no statistically significant difference between the two groups. A comparison of daily doses (25, 50, 75, and 100 mg per day) showed that the incidence of peripheral neuropathy in the 100 mg/g group (21.7%) was significantly higher than in the 25 mg/g group (4.6%). However, there was no significant difference among the other groups. This is similar to the findings of Bastuji-Garin et al. (2002), suggesting that a higher daily dose of thalidomide therapy might increase the risk of peripheral neuropathy. An increasing daily dose is highly associated with the risk of peripheral neuropathy, and a low dose of thalidomide is suggested to reduce adverse reactions.
Peripheral neuropathy is rarely seen in AS patients. In this study, two patients in the non-thalidomide group developed peripheral neuropathy. A female case with a history of swelling and pain in the ankle and wrist joints was negative for rheumatoid factor and anti-cyclic citrullinated peptide antibodies and her MRI revealed articular facet damage in the ankle joint. This indicated the possibility of AS accompanied with rheumatoid arthritis, which might induce peripheral neuropathy lesions. Another case received oral traditional Chinese medicine prescriptions prior to enrollment, which might influence the development of adverse events. The mechanisms of thalidomide-induced peripheral neuropathy are potentially related to antiangiogenic effects, and the inhibition of blood vessel formation may trigger secondary ischemia and hypoxia of nerve fibers, thus promoting the occurrence of neuropathy. In addition, this mechanism can be explained by the down-regulation of tumor necrosis factor-α, a reduction of nuclear factor κB, the acceleration of nerve cell death, and a decreased quantity of nerve cells. The underlying mechanism of thalidomide-induced peripheral neuropathy needs further exploration.
In summary, thalidomide treatment significantly increased the incidence of peripheral neuropathy in AS patients. Peripheral neuropathy is a major adverse reaction caused by thalidomide, and therefore should be carefully monitored. Thalidomide intervention should be terminated immediately after peripheral neuropathy occurs. Our findings suggest the incidence of thalidomide-induced peripheral neuropathy was similar within the first 6 months and after 6 months of treatment, so the whole 1-year post-treatment timepoint should be closely monitored. In addition, a higher daily dose of thalidomide increased the risk of peripheral neuropathy. Large-scale, long-term observations are needed to analyze the risk of thalidomide-induced peripheral neuropathy, as well as the effects of daily dosing on the pathogenesis of peripheral neuropathy.
Acknowledgments
We appreciate all staff from Department of Rheumatology and Immunology, Shengjing Hospital of China Medical University for providing valuable support in the data collection process.
Footnotes
Funding: This study was financially supported by the Natural Science Foundation of Liaoning Province of China, No. 2014021081.
Conflicts of Interest: None declared.
Copyedited by Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- Bastuji-Garin S, Ochonisky S, Bouche P, Gherardi RK, Duguet C, Derradine Z, Poli F, Revuz J. Incidence and risk factors for thalidomide neuropathy: a prospective study of 135 dermatologic patients. J Invest Dermatol. 2002;119:987–1020. doi: 10.1046/j.1523-1747.2002.19502.x. [DOI] [PubMed] [Google Scholar]
- Brandt J, Haibel H, Cornely D, Golder W, Gonzalez J, Reddig J, Thriene W, Sieper J, Braun J. Successful treatment of active ankylosing spondylitis with the anti-tumor necrosis factor alpha monoclonal antibody infliximab. Arthritis Rheum. 2000;43:1346–1352. doi: 10.1002/1529-0131(200006)43:6<1346::AID-ANR18>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Briani C, Zara G, Rondinone R, Ruggero S, Toffanin E, Ermani M, Ghirardello A, Zampieri S, Sarzi-Puttini P, Doria A. Positive and negative effects of thalidomide on refractory cutaneous lupus erythematosus. Autoimmunity. 2005;38:549–555. doi: 10.1080/08916930500285790. [DOI] [PubMed] [Google Scholar]
- Clegg DO, Reda DJ, Weisman MH, Blackburn WD, Cush JJ, Cannon GW, Mahowald ML, Schumacher HR, Taylor T, Budiman-Mak E, Cohen MR, Vasey FB, Luggen ME, Mejias E, Silverman SL, Makkena R, Alepa FP, Buxbaum J, Haakenson CM, Ward RH, Manaster BJ, Anderson RJ, Ward JR, Henderson WG. Comparison of sulfasalazine and placebo in the treatment of ankylosing spondylitis: a department of veterans affairs cooperative study. Arthritis Rheum. 1996;39:2004–2012. doi: 10.1002/art.1780391209. [DOI] [PubMed] [Google Scholar]
- Coelho A, Souto MJ, Cardoso CR, Salgado DR, Schmal TR, Waddington CM, de Souza Papi JA. Long-term thalidomide use in refractory cutaneous lesions of lupus erythematosus, a 65 series of Brazilian patients. Lupus. 2005;14:434–439. doi: 10.1191/0961203305lu2124oa. [DOI] [PubMed] [Google Scholar]
- Deng X, Zhang J, Zhang J, Huang F. Thalidomide reduces recurrence of ankylosing spondylitis in patients following discontinuation of etanercept. Rheumatol Int. 2013;33:1409–1413. doi: 10.1007/s00296-012-2571-5. [DOI] [PubMed] [Google Scholar]
- Direskeneli H, Ergun T, Yavuz S, Hamuryudan V, Eksioglu-Demiralp E. Thalidomide has both anti-inflammatory and regulatory effects in Behcet's disease. Clin Rheumatol. 2008;27:373–375. doi: 10.1007/s10067-007-0786-8. [DOI] [PubMed] [Google Scholar]
- Dougados M, Behier JM, Jolchine I, Calin A, van der HD, Olivieri I, Zeidler H, Herman H. Efficacy of celecoxib, a cyclooxygenase 2-specific inhibitor, in the treatment of ankylosing spondylitis: a six-week controlled study with comparison against placebo and against a conventional nonsteroidal antiinflammatory drug. Arthritis Rheum. 2001;44:180–185. doi: 10.1002/1529-0131(200101)44:1<180::AID-ANR24>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Fernyhough P, Smith DR, Schapansky J, Van Der PR, Gardiner NJ, Tweed CW, Kontos A, Freeman L, Purves-Tyson TD, Glazner GW. Activation of nuclear factor-kappa B via endogenous tumor necrosis factor alpha regulates survival of axotomized adult sensory neurons. J Neurosci. 2005;25:1682–1690. doi: 10.1523/JNEUROSCI.3127-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel HC, Sharon VR, Vleugels RA, Merola JF, Qureshi AA. Lower-dose thalidomide therapy effectively treats cutaneous lupus erythematosus but is limited by neuropathic toxicity. Int J Dermatol. 2013;52:1407–1409. doi: 10.1111/j.1365-4632.2011.05200.x. [DOI] [PubMed] [Google Scholar]
- García-Carrasco M, Fuentes-Alexandro S, Escárcega RO, Rojas-Rodriguez J, Escobar LE. Efficacy of thalidomide in systemic onset juvenile rheumatoid arthritis. Joint Bone Spine. 2007;74:500–503. doi: 10.1016/j.jbspin.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Hall VC, El-Aghary RA, Bouwhuis S, Rajkumar SV. Dermatlolgic side effects of thalidomide in patients with multiple myeloma. J Am Acad Dermatol. 2003;48:548–552. doi: 10.1067/mjd.2003.87. [DOI] [PubMed] [Google Scholar]
- Haslett PA, Corral LG, Albon M, Kaplan G. Thalidomide eostimulates primary human T lymphocytes, preferentially inducing proliferation, eytokine production, and eytotoxie responses in the CD8+ subset. J Exp Med. 1998;187:1885–1892. doi: 10.1084/jem.187.11.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Gu JR, Zhao W, Zhu J, Zhang JL, Yu DT. One-year open-label trial of thalidomide in ankylosing spondylitis. Arthritis Rheum. 2002;47:249–254. doi: 10.1002/art.10396. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li H, Matsui O. The antiangiogenic effect of thalidomide on occult liver metastases: an in vivo study in mice. J Gastroenterol Hepatol. 2009;24:1077–1081. doi: 10.1111/j.1440-1746.2008.05748.x. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Lamothe B, Aggarwal BB. Thalidomide suppresses NF-kappa B activation induced by TNF and H2O2, but not that activated by eeramide, lipopolyaaceharides, or phorbol ester. J Immunol. 2002;168:2644–2651. doi: 10.4049/jimmunol.168.6.2644. [DOI] [PubMed] [Google Scholar]
- Mark TM, Bowman IA, Rossi AC, Shah M, Rodriguez M, Quinn R, Pearse RN, Zafar F, Pekle K, Jayabalan D, Ely S, Coleman M, Chen-Kiang S, Niesvizky R. Thalidomide, clarithromycin, lenalidomide and dexamethasone therapy in newly diagnosed, symptomatic multiple myeloma. Leuk Lymphoma. 2014;55:2842–2849. doi: 10.3109/10428194.2014.896005. [DOI] [PubMed] [Google Scholar]
- Morawska M, Grzasko N, Kostyra M. Therapy-related peripheral neuropathy in multiple myeloma patients. Hematol Oncol. 2014 doi: 10.1002/hon.2149. doi: 10.1002/hon.2149. [DOI] [PubMed] [Google Scholar]
- Matthews SJ, McCoy C. Thalidominde: review of approved and investigational uses. Clin Ther. 2003;25:342–395. doi: 10.1016/s0149-2918(03)80085-1. [DOI] [PubMed] [Google Scholar]
- Ochonisky S, Verroust J, Bastuji-Garin S, Gherardi R, Revuz J. Thalidomide neuropathy incidence and clinico-electrophysiologic findings in 42 patients. Arch Dermatol. 1994;130:66–69. [PubMed] [Google Scholar]
- Sampmo EP, Samo EN, GaliUy R, Cohn ZA, Kaplan G. Thalidomide selectively inhibits tumor necrosis factor alpha production by stimulated human monocytes. J Exp Med. 1991;173:699–703. doi: 10.1084/jem.173.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi P, Zamagni E, Cellini C, Plasmati R, Cangini D, Tacchetti P, Perrone G, Pastorelli F, Tura S, Baccarani M, Cavo M. Neurological toxicity of long-term (>1 yr) thalidomide therapy in patients with multiple myeloma. Eur J Haematol. 2005;74:212–216. doi: 10.1111/j.1600-0609.2004.00382.x. [DOI] [PubMed] [Google Scholar]
- Van den Bosch F, Kruithof E, Baeten D, De KF, Mielants H, Veys EM. Effects of a loading dose regimen of three infusions of chimeric monoclonal antibody to tumour necrosis factor alpha (infliximab) in spondyloarthropathy: an open pilot study. Ann Rheum Dis. 2000;59:428–433. doi: 10.1136/ard.59.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der HD, Baraf HSB, Ramos-Remus C, Calin A, Weaver AL, Schiff M, James M, Markind JE, Reicin AS, Melian A, Dougados M. Evaluation of the efficacy of etoricoxib in ankylosing spondylitis: results of a 52-week randomized controlled study. Arthritis Rheum. 2005;52:1205–1215. doi: 10.1002/art.20985. [DOI] [PubMed] [Google Scholar]
- Yang PT, Xiao WG, Qin L, Zhao LJ, He LM, Ito M. A pilot study on changes of macrophage colony stimulating factor and transforming growth factor beta1 in male patients with ankylosing spondylitis taking thalidomide. Ann Rheum Dis. 2010;69:781–782. doi: 10.1136/ard.2009.114397. [DOI] [PubMed] [Google Scholar]
- Zhu J, Huang F, Zhang JL. The efficacy and safety of long-term thalidomide in the treatment of ankylosing spondylitis. Zhonghua Nei ke Za zhi. 2010;49:667–670. [PubMed] [Google Scholar]


