Abstract
OBJECTIVE:
China is the only country where nerve growth factor is approved for large-scale use as a clinical medicine. More than 10 years ago, in 2003, nerve growth factor injection was listed as a national drug. The goal of this article is to evaluate comprehensively the efficacy and safety of nerve growth factor for the treatment of neurological diseases.
DATA RETRIEVAL:
A computer-based retrieval was performed from six databases, including the Cochrane Library, PubMed, EMBASE, Sino Med, CNKI, and the VIP database, searching from the clinical establishment of nerve growth factor for treatment until December 31, 2013. The key words for the searches were “nerve growth factor, randomized controlled trials” in Chinese and in English.
DATA SELECTION:
Inclusion criteria: any study published in English or Chinese referring to randomized controlled trials of nerve growth factor; patients with neurological diseases such as peripheral nerve injury, central nerve injury, cranial neuropathy, and nervous system infections; patients older than 7 years; similar research methods and outcomes assessing symptoms; and measurement of nerve conduction velocities. The meta-analysis was conducted using Review Manager 5.2.3 software.
MAIN OUTCOME MEASURES:
The total effective rate, the incidence of adverse effects, and the nerve conduction velocity were recorded for each study.
RESULTS:
Sixty-four studies involving 6,297 patients with neurological diseases were included. The total effective rate in the group treated with nerve growth factor was significantly higher than that in the control group (P < 0.0001, RR: 1.35, 95%CI: 1.30–1.40). The average nerve conduction velocity in the nerve growth factor group was significantly higher than that in the control group (P < 0.00001, MD: 4.59 m/s, 95%CI: 4.12–5.06). The incidence of pain or scleroma at the injection site in the nerve growth factor group was also higher than that in the control group (P < 0.00001, RR: 6.30, 95%CI: 3.53–11.27), but such adverse effects were mild.
CONCLUSION:
Nerve growth factor can significantly improve nerve function in patients with nervous system disease and is safe and effective.
Keywords: nerve regeneration, neurological diseases, nerve growth factor, randomized controlled trials, meta-analysis, adverse effects, nerve conduction velocity, neural regeneration
Introduction
Nerve growth factor is the first neurotrophic factor that was discovered and demonstrates the functions of maintaining the survival of central and peripheral neurons and facilitating their growth, differentiation, and regeneration (Ebendal, 1989). Nerve growth factor has generated strong interest as a potential target for the treatment of Alzheimer’s disease. The dysfunction of basal forebrain cholinergic neurons is a basic feature of Alzheimer’s disease. Nerve growth factor is synthesized and secreted by cells in the cortex and hippocampus, and high-affinity (TrkA) and low-affinity (p75NTR) neurotrophin receptors are produced within the basal forebrain cholinergic neurons (Eriksdotter Jonhagen et al., 1998). Nerve growth factor released from target cells activates TrkA on axon terminals and triggers the activation of the PI3K/Akt, MEK/ERK, and phospholipase Cγ signaling pathways. The signal then retrogradely travels along axons to the cell body and promotes neuronal survival. The dysfunction of nerve growth factor and its receptors can induce selective degeneration of the basal forebrain cholinergic neurons during end-stage Alzheimer’s disease. The potential benefits treating neurological diseases with nerve growth factor has greatly motivated both clinicians and investigators (Olson et al., 1992; Eriksdotter Jonhagen et al., 1998). Nerve growth factor clearly promotes the regeneration of damaged nerves (Aloe et al., 2008; Chiaretti et al., 2008; Lambiase et al., 2009), and shows a large potential for other applications. However, the worldwide application of nerve growth factor has only recently started, and the appropriate combination nerve growth factor therapy, the best administration route and dosage, the efficacy, and the potential side effects all require further investigation. Careful basic and clinical research should be performed to support the wider application of nerve growth factor for the treatment of cerebrovascular disease and neurodegenerative diseases and for the repair of damaged nerves.
China is the first country to apply nerve growth factor as a clinical therapy and has accumulated a large amount of research data since nerve growth factor was listed as a national drug (Xia et al., 2009; Hu et al., 2010). Although hundreds of articles on nerve growth factor have been published in China, those results have not been widely appreciated throughout the world because of language restrictions. The present review is a meta-analysis of randomized controlled trials of nerve growth factor during the past ten years with the goal of comprehensively evaluating the efficacy and safety of nerve growth factor for the treatment of neurological diseases.
Data and Methods
Literature retrieval
Six databases were searched, including the Cochrane Library, PubMed, EMBASE, Sino Med, CNKI, and VIP databases, starting from the clinical establishment of nerve growth factor treatment until December 31, 2013. The subject headings and text of the articles were searched for the key words “nerve growth factor” or “NGF” and “randomized controlled trials” or “RCTs”.
Inclusion and exclusion criteria
Inclusion criteria
Any study published in English or Chinese referring to the randomized controlled trials of nerve growth factor; patients with neurological diseases; patients older than 7 years; similar research methods and outcomes assessing symptoms; and measurement of nerve conduction velocities.
Exclusion criteria
Duplicated articles, reviews, those involving animal experiments, those not published in English or Chinese, and those where the full text was unavailable were excluded.
Study selection and data extraction
Eligible studies were selected in two stages: first by screening the title and abstracts for relevance, and then by reviewing the full-text. The following data were extracted from each selected study: basic information, including the title, author, date of publication, and funding; participant information, including age, gender, diagnosis, number of participants in each group, and baseline comparisons; intervention measures information, including drugs, dosages, routes of administration, courses of treatment, and follow-up times in the treatment and control groups; and results information, including the results reported and criteria applied for measuring efficacy and safety. Two of the authors reviewed each citation at both stages. Conflicts were resolved between reviewers or by group consensus.
Quality assessment
The quality of all randomized controlled trials was assessed based on five categories: statistical analysis, outcomes, exposure, study population, and the specific domain of randomization for randomized controlled trial studies. The key elements of these categories for assessing the quality of citations were adapted from the Jadad scale (Jadad et al., 1996) for randomized controlled trial studies. Each quality item was rated as met (yes), unmet (no), or unsure.
Main outcome measurements
The main outcome measures were the total effective rate and the incidence of adverse effects. The secondary outcome measure was the nerve conduction velocity.
Statistical analysis
To reduce the heterogeneity of the studies, the nervous system diseases were divided into four groups: peripheral nerve injury, central nerve injury, cranial neuropathy, and nervous system infections. Each group was further divided into several subgroups, and meta-analyses were conducted within the subgroups. When the heterogeneity in the subgroups could not be explained, a sensitivity analysis was used to determine the impact of excluding specific studies on the overall estimate of the effect.
From each primary study, the effect estimates were extracted for the relationship between nerve growth factor treatment and the neurological disease. The heterogeneity was assessed using a test based on the deviations of the individual study estimates from the summary estimate of the effect and quantified with I2, which describes the proportion of the variance due to heterogeneity among studies rather than due to sampling error. An I2> 50% represents substantial heterogeneity. The random effects meta-analyses were conducted with RevMan 5.2.3 software (The Cochrane Collaboration, Australia) to determine the effect estimates, and the origin of the heterogeneity was discussed. For values of I2< 50%, fixed effect models were used to perform the meta-analysis.
Results
Data retrieval
The selection of studies is described in Figure 1. A total of 644 articles were retrieved, and 64 randomized controlled trial studies were finally selected, including two in English using recombinant human nerve growth factor (Apfel et al., 1998; Apfel et al., 2000) and 62 in Chinese using mouse nerve growth factor. Of these 64 articles, 22 (Apfel et al., 1998, 2000; Liu et al., 2007; Peng et al., 2009; Xia et al., 2009; Huang et al., 2010; Li et al., 2010a; Meng et al., 2010; Wang et al., 2010a, 2011a; Guo and Liu, 2011; Zhang et al., 2011b; Zhao, 2011; Fang et al., 2012a, b; Jiang et al., 2012; Ye et al., 2012; Chen, 2013; Chi and Zhai, 2013; Feng et al., 2013; Shen, 2013; Shu et al., 2013) examined peripheral nerve injury, 16 (Chen et al., 2004; Yuan and Lei, 2005; Li, 2006; Tang et al., 2008; Zhang et al., 2008, 2009, 2011a, 2012; Li and Yang, 2009; Wang and Liu, 2010; Wang et al., 2010b, c; Hou et al., 2012; Qi et al., 2012; Yan et al., 2012; Zheng et al., 2013) examined central nerve injury, 23 (Yang et al., 2006; Wang et al., 2007, 2012b; Peng et al., 2008; Tang and Wang, 2008; Huang and Li, 2010; Sun, 2010; Wang and Zhang, 2010; Zhang, 2010; Zhang et al., 2010; Chen et al., 2011; Li and Yuan, 2011; Mo et al., 2011; Xia and Pan, 2011; Lin et al., 2012; Ma et al., 2012; Shen, 2012; Zhao and Li, 2012; Li et al., 2013; Lu et al., 2013; Tang et al., 2013; Tian and Dong, 2013; Yu, 2013) examined cranial neuropathy, and three (Xia et al., 2010; Li et al., 2012; Shan et al., 2013) examined nervous system infections (Table 1). There were 6,297 patients in those 64 studies, including 3,346 patients in the experimental groups and 2,951 patients in the control groups. The case numbers in the studies ranged from a maximum of 948 (Apfel et al., 2000) to a minimum of 15 (Jiang et al., 2012). The ages of the patients ranged from 7 to 87 years old. The experimental group was treated with nerve growth factor and the control group received conventional treatments. The mouse nerve growth factor doses were 4–30 μg in 2 mL by intramuscular injection, once per day. The course of the treatment ranged from 7 to 112 days.
Figure 1.
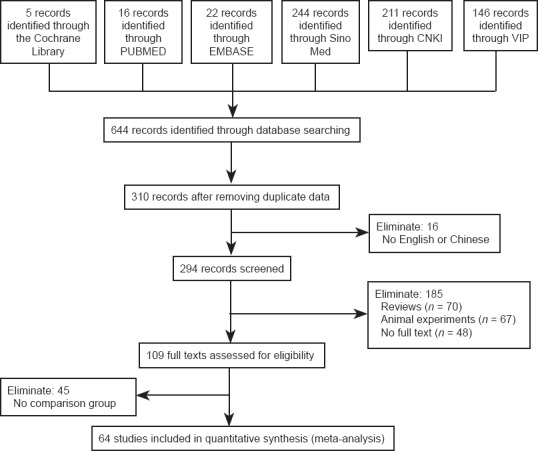
Flow diagram of study screening.
A total of 644 articles were retrieved, and 64 randomized controlled trials were finally selected for inclusion. Sino Med: China biomedical literature service system; CNKI: China National Knowledge Infrastructure; VIP: Chinese VIP network.
Table 1.
Basic data for the 62 included Chinese randomized controlled trial studies*
The total effective rate of treatment was 82.22% (2,751/ 3,346) in the nerve growth factor group and 62.69% (1,850/ 2,951) in the control group. The total effective rate of treatment was significantly higher in the nerve growth factor group than in the control group (P < 0.0001, RR: 1.35, 95%CI: 1.30–1.40).
Quality assessment
The commonly found sources of bias based on the study design are summarized in Figure 2. All of the studies described their statistical methods and addressed the potential confounding factors. All of the studies also clearly described the population, case participants, control participants, inclusion and exclusion criteria, and that the groups were recruited over the same time period. The most common unmet or unclear item was blinding, as it was often unclear whether the researchers encountered difficulties in implementing blinding. Of the 62 Chinese studies, only three (Wang et al., 2007; Li and Yang, 2009; Tang et al., 2013) mentioned blinding without providing details. Forty-nine studies mentioned randomization, four (Wang and Liu, 2010; Xia et al., 2010; Qi et al., 2012; Li et al., 2013) reported sequence generation using a random number table, and three (Liu et al., 2007; Jiang et al., 2012; Shen, 2012) used the hospitalization sequence number. However, 59 studies reported no significant difference before treatment between the case and control groups with regard to patient gender, age, disease severity, and course of the disease.
Figure 2.
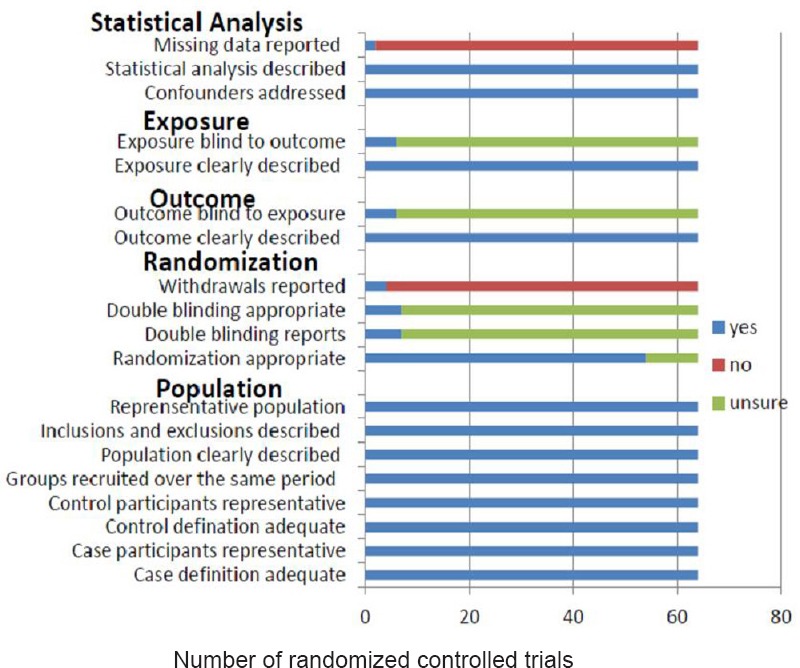
Quality assessment for the 62 random controlled trials.
The assessment was conducted based on five categories: statistical analysis, exposure, outcome, randomization, and population.
The Chinese patients were from 25 provinces and 59 hospitals, which included 45 provincial or municipal tertiary hospitals and 14 district or county secondary hospitals. The patients distributed in those hospitals represented the general case population, to a certain extent.
Meta-analysis results
Nerve growth factor and peripheral nerve injury
Twenty studies (Liu et al., 2007; Peng et al., 2009; Xia et al., 2009; Huang and Li, 2010; Li et al., 2010b; Meng et al., 2010; Wang and Liu, 2010; Guo and Liu, 2011; Wang et al., 2011a; Zhang et al., 2011b; Zhao, 2011; Fang et al., 2012a, b; Jiang et al., 2012; Ye et al., 2012; Chen, 2013; Chi and Zhai, 2013; Feng et al., 2013; Shen, 2013; Shu et al., 2013) reported the effect of nerve growth factor for the treatment of peripheral nerve injury. The peripheral nerve injuries were divided into three subgroups: diabetic peripheral neuropathy, polyneuropathy, and Guillain-Barre Syndrome. A RevMan forest plot detailing the effects of nerve growth factor on peripheral nerve injury is shown in Figure 3. The test of heterogeneity showed significant differences among the studies (x2= 98.57, df: 21, P < 0.00001, I2= 79% > 50%). Therefore, a random effect model was applied to determine the effect sizes. The total effective rate of treatment on peripheral nerve injury was significantly higher in the nerve growth factor group than in the control group (P < 0.00001, RR: 1.38, 95%CI: 1.26–1.62; Figure 3).
Figure 3.
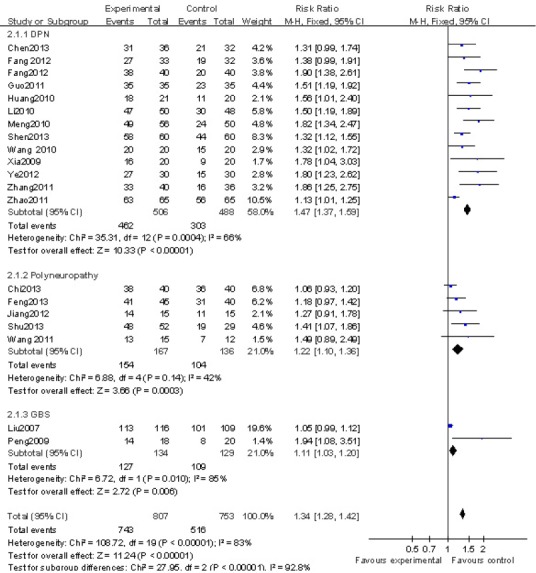
A RevMan forest plot of the effect of mouse nerve growth factor for the treatment of peripheral nerve injury.
Mantel-Haenszel risk ratios for dichotomous data are shown. DPN: Diabetic peripheral neuropathy; GBS: Guillain-Barre syndrome.
The heterogeneity in subgroup diabetic peripheral neuropathy (I2= 66%, P = 0.0004) was explained by the combined treatments used. The heterogeneity among the 8 studies (Xia et al., 2009; Li et al., 2010a; Meng et al., 2010; Wang et al., 2010a; Zhang et al., 2011b; Fang et al., 2012a; Ye et al., 2012; Shen, 2013) that combined nerve growth factor with other therapies (I2= 36%, P = 0.14) was lower than the heterogeneity among the 5 studies (Huang et al., 2010; Guo and Liu, 2011; Zhao, 2011; Fang et al., 2012b; Chen, 2013) that used only nerve growth factor treatments (I2= 57%, P = 0.05). The effect of the combined use of nerve growth factor and other therapies (RR: 1.57, 95%CI: 1.38–1.78) was higher than that of those using only nerve growth factor treatments (RR: 1.32, 95%CI: 1.11–1.57).
Twelve studies (Xia et al., 2009; Huang et al., 2010; Li et al., 2010b; Meng et al., 2010; Wang and Liu, 2010; Guo and Liu, 2011; Fang et al., 2012a, b; Ye et al., 2012; Chen, 2013; Feng et al., 2013; Shen, 2013) reported the nerve conduction velocities of 994 patients with peripheral nerve injury (Figure 4). The nerve conduction velocities included the median nerve motor conduction velocity, the median nerve sensory conduction velocity, the peroneal nerve motor conduction velocity, and the sural sensory conduction velocity. Because the test of heterogeneity showed significant differences among the studies (x2= 224.91, df: 39, I2= 83% > 50%, P < 0.00001), a random effect model was used to determine the effect sizes. The nerve conduction velocity was significantly higher in the nerve growth factor group than in the control group (P < 0.00001, MD: 4.59 m/s, 95%CI: 4.12–5.06; Figure 4).
Figure 4.
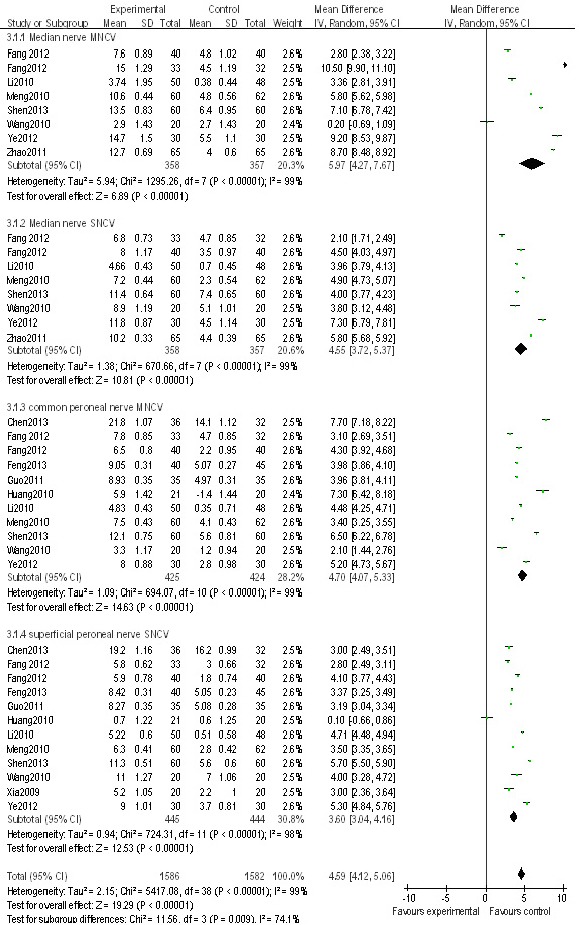
A RevMan forest plot of the mean difference estimates after treatment of peripheral never injury with mouse nerve growth factor.
MNCV: Motor nerve conduction velocity; SNCV: sensory nerve conduction velocity.
The subgroup analyses could not eliminate the heterogeneity in motor nerve conduction velocity among the studies (I2= 99%, P < 0.00001). A statistically significant heterogeneity (I2= 99%, P < 0.00001) remained when the analysis was restricted to studies of the combined use of nerve growth factor and other therapies. Similarly, when the studies were divided into those published before 2011 (Li et al., 2010a; Meng et al., 2010; Wang et al., 2010a; Zhao, 2011) and after 2012 (Fang et al., 2012a, b; Ye et al., 2012; Shen, 2013), the heterogeneities of each group were still statistically significant (I2= 99%, P < 0.00001; I2= 100%, P < 0.0001). In addition, the analysis of dosing for 30 μg doses (Meng et al., 2010; Wang et al., 2010a; Fang et al., 2012a; Ye et al., 2012; Shen, 2013) and 4–20 μg doses (Li et al., 2010a; Zhao, 2011; Fang et al., 2012b), showed that the heterogeneities were still statistically significant (I2= 99%, P < 0.00001; I2= 100%, P < 0.0001). Finally, the analysis of treatment course of 4 weeks (Li et al., 2010a; Zhao, 2011; Fang et al., 2012a; Ye et al., 2012) and 2–3 weeks (Meng et al., 2010; Wang et al., 2010a; Fang et al., 2012b; Shen, 2013) also showed that the heterogeneities were still statistically significant (I2= 100%, P < 0.00001; I2= 99%, P < 0.0001). There was no difference in the age of patients with peripheral nerve injury among the studies, and the age of the patients ranged from 40 to 87 years old. Therefore, age was not a major factor contributing to the heterogeneity. The origin of the heterogeneities may be from the different measurement methods used, the measurement error from the instruments or user, and the different sites where the electrodes were inserted into the muscles when conducting the electromyography testing.
The sensitivity analysis showed that the positive effect was persistent. The overall mean difference in nerve conduction velocity between the nerve growth factor group and the control group was 4.59 m/s (95%CI: 4.12–5.06, P < 0.00001; Figure 4). After removing a low-weight study (Ye et al., 2012), the overall mean difference became 4.41 m/s (95%CI: 3.93–4.89, P < 0.00001).
Nerve growth factor and central nerve injury
Sixteen studies (Chen et al., 2004; Yuan and Lei, 2005; Li, 2006; Tang et al., 2008; Zhang et al., 2008, 2009, 2011a, 2012; Li and Yang, 2009; Wang and Liu, 2010; Wang et al., 2010b, c; Hou et al., 2012; Qi et al., 2012; Yan et al., 2012; Zheng et al., 2013) reported the effect of nerve growth factor on central nerve injury. To reduce the clinical heterogeneity, the studies were divided into five groups by affliction: Alzheimer’s disease, cerebral infarction, spinal cord injury, traumatic brain injury, and CO poisoning. The test of heterogeneity showed significant differences among the studies (x2= 34.78, df: 15, P = 0.003, I2= 57% > 50%), and thus a random effect model was applied to determine the effect size. The total effective rate of treatment on central nerve injury was significantly higher in the nerve growth factor group than in the control group (RR: 1.22, 95%CI: 1.12–1.32, P < 0.00001). Because there was only one study in the Alzheimer’s disease subgroup, a sensitivity analysis was conducted. After removing the Alzheimer’s disease subgroup (Li and Yang, 2009), the heterogeneity remained (I2= 53% > 50%, P = 0.009) and the positive effect of nerve growth factor was unchanged (RR: 1.24, 95%CI: 1.14–1.34, P < 0.00001).
Nerve growth factor and cranial neuropathy
Twenty-three studies (Yang et al., 2006; Wang et al., 2007, 2010c, 2012b; Peng et al., 2008; Tang and Wang, 2008; Huang and Li, 2010; Sun, 2010; Zhang, 2010, 2011a; Chen et al., 2011; Mo et al., 2011; Xia and Pan, 2011; Ma et al., 2012; Shen, 2012; Zhao and Li, 2012; Li et al., 2013; Lu et al., 2013; Tang et al., 2013; Tian and Dong, 2013; Yu, 2013) reported the effect of nerve growth factor for the treatment of cranial neuropathy, including 14 for optic neuropathy, seven for facial paralysis, and two for hearing loss. The test of heterogeneity showed significant differences among the studies (x2= 65.47, df: 22, I2= 66% > 50%, P = 0.003), and therefore a random effect model was applied to determine the effect size. The total effective rate of treatment on cranial neuropathy was significantly higher in the nerve growth factor group than in the control group (RR: 1.31, 95%CI: 1.21–1.42, P < 0.00001). There were significant differences between the nerve growth factor group and control group in the treatment of optic neuropathy (RR: 1.38, 95%CI: 1.24–1.53, P < 0.00001), facial paralysis (RR: 1.19, 95%CI: 1.07–1.33, P = 0.002), and hearing loss (RR: 1.31, 95%CI: 1.00–1.70, P = 0.05).
Nerve growth factor and nervous system infection
Three studies reported the effect of nerve growth factor treatment on nervous system infections, including two studies of postherpetic neuralgia (Xia et al., 2010; Shan et al., 2013) and one study of meningitis in HIV (Li et al., 2012). The test of heterogeneity in the postherpetic subgroup showed no significant differences between the two studies (x2= 0.12, df: 2, I2= 0 < 50%, P = 0.94). Therefore, a fixed effect model was applied to determine the effect size. The total effective rate of treatment on nervous system infections was significantly higher in the nerve growth factor group than in the control group (RR: 1.28, 95%CI: 1.10–1.49, P < 0.00001).
Nerve growth factor safety analysis
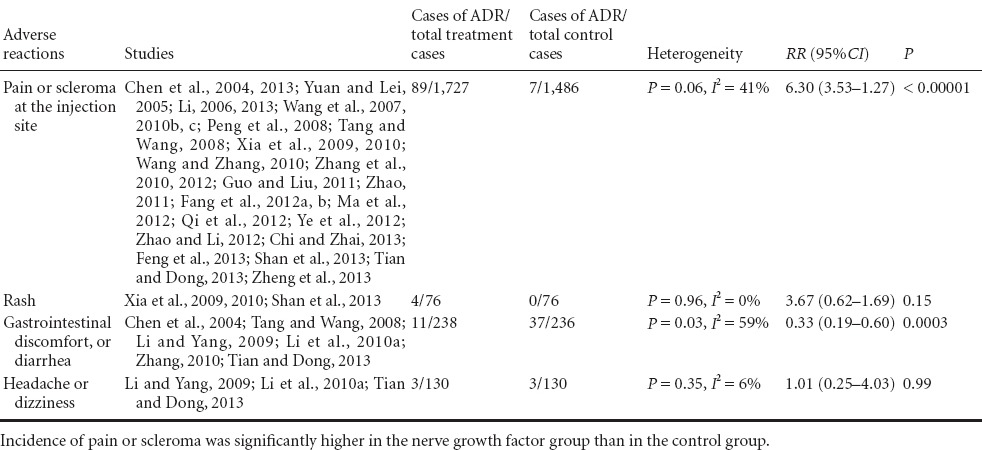
Of the 64 studies included, 38 studies reported the adverse effects of the nerve growth factor therapy (Table 2). The test of heterogeneity showed no significant differences in adverse effects among the studies (x2= 46.54, df: 25, I2= 46% < 50%, P = 0.006), and thus a fixed effect model was applied to determine the effect size. The most common side effect was pain or scleroma at the injection site. The incidence of pain or scleroma was significantly higher in the nerve growth factor group (5.23%, 89/1,727) than in the control group (0.54%, 7/1,486) (RR: 6.30, 95%CI: 3.53–11.27, P < 0.00001). However, the adverse effects were mild and could be relieved without specific treatment or with symptomatic treatment. The incidence of gastrointestinal discomfort or diarrhea was significantly lower in the nerve growth factor group (4.61%, 11/236) than in the control group (15.74%, 37/238) (RR: 0.33, 95%CI: 0.19–0.60, P = 0.0003). There were no significant differences between the nerve growth factor and control groups in the incidences of rash or headache (P = 0.15, P = 0.99).
Table 2.
Meta-analysis of adverse drug reactions (ADR) related to nerve growth factor treatment
Analysis of publication bias
The symmetry of the funnel plot (Figure 5) showed that there was no evidence of publication bias among the studies using mouse nerve growth factor and reporting adverse reactions.
Figure 5.
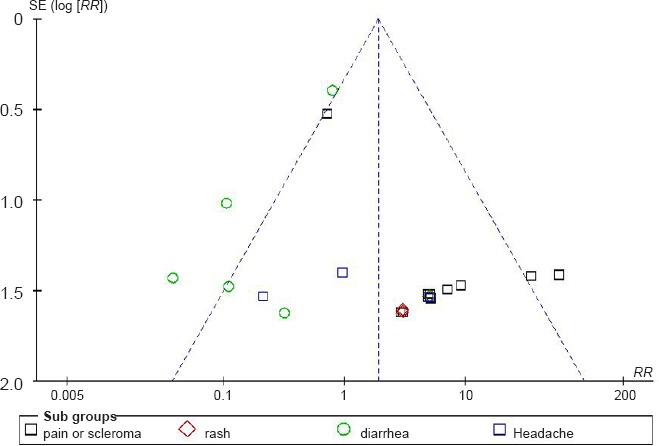
Funnel plot of publication bias among the studies using mouse nerve growth factor and reporting adverse reactions.
The symmetry of the funnel plot shows there was no evidence of publication bias among the studies using mouse nerve growth factor and reporting adverse reactions.
Discussion
This systematic review summarized studies to determine the efficacy and safety of nerve growth factor for the treatment of neurological diseases. The meta-analyses showed that the nerve growth factor therapy was effective and safe in patients with neurological diseases. Treatment with nerve growth factor clearly improved the nerve conduction velocity of patients. The average nerve conduction velocity increased by 4.59 m/s in the nerve growth factor group compared with the control group, which met the effectiveness criteria according to the American Diabetes Association standard (2006). The most common side effect was pain or scleroma at the injection site, but such adverse effects were mild and could be relieved without specific treatment.
Nerve growth factor was also used effectively to treat peripheral nerve injury. The combined use of nerve growth factor and other therapies, such as methylcobalamin, tanshinone II A, lipoic acid, and hyperbaric oxygen therapy, was even more effective than nerve growth factor alone. The effect of injecting Danhong Chinese medicine was better than that of nerve growth factor treatment for diabetic peripheral neuropathy. Diabetic peripheral neuropathy is a common chronic complication of diabetes with a prevalence rate of 32.7% among diabetes patients over 40 years old in the United States (Candrilli et al., 2007). There are no effective treatment methods for diabetic peripheral neuropathy (Brownlee, 2005), and the pathophysiology of diabetic peripheral neuropathy remains unclear. One hypothesis suggested was that diabetic peripheral neuropathy may be associated with a deficiency of nerve growth factor (Palacka et al., 2010). The level of nerve growth factor in the tissue and blood from both animal models of diabetes and patients with diabetic neuropathy is very low. This may be caused by disorders of glucose metabolism that occur with an increased generation of intracellular reactive oxygen species, increased production of oxygen free radicals, and increased NADH oxidase activity. All of those factors together may deplete the amount of neurotrophic factor in the tissue and blood (Chyun et al., 2006). If this hypothesis is true, exogenous nerve growth factor may be able to help relieve peripheral neuropathy.
Several case reports have suggested that the administration of nerve growth factor may cause certain potentially beneficial effects. The results reported by Olson et al. (1992) indicated that nerve growth factor may counteract the cholinergic deficits in Alzheimer’s disease. Nerve growth factor treatment can result in a marked transient increase in the uptake and binding of 11C-nicotine in the frontal and temporal cortex, improving verbal episodic memory. Eriksdotter Jonhagen et al. (1998) concluded that the long-term intracerebroventricular administration of nerve growth factor may induce potentially beneficial effects, and lower doses of nerve growth factor can decrease shooting pain. Considerable accumulated evidence has shown that nerve growth factor is a peripheral pain mediator, particularly in states of inflammatory pain (Pezet and McMahon, 2006). Nerve growth factor is upregulated in various inflammatory conditions, and, in many persistent pain models, nerve growth factor neutralizing molecule is an effective analgesic agent.
Treatment with recombinant human nerve growth factor for patients with diabetic peripheral neuropathy had been thought to herald a new type of treatment approach to such hitherto largely untreatable disorders (Zochodne and Said, 1998; Riggs, 1999). Unfortunately, further clinical trials failed to demonstrate significant beneficial effects (Apfel et al., 2000). The author concluded that side effects and lower doses (0.1 μg/kg) may explain why the trails were unsuccessful (Apfel, 2002).
The results presented here are consistent with another systematic review of nerve growth factor treatment for peripheral nerve injury (Liu and Liu, 2012). That review also suggested that nerve growth factor therapy was effective and safe for peripheral nerve injury. However, the authors of that review used the OR instead of the RR as an effect indicator, which resulted in I2= 0, and therefore obscured the heterogeneity among the studies.
Finding effective drugs that can effectively penetrate the blood-brain barrier is one of the most difficult challenges in the treatment of central nerve diseases. One published study determined the permeability of I125-labeled β-nerve growth factor (13 kDa) extracted from aborted fetuses across the blood-brain barrier in rats. β-nerve growth factor with 4% I125-β-nerve growth factor was able to cross the blood-brain barrier 30 minutes after injection (Zhu et al., 2002). The molecular weight of nerve growth factor is 13.5 kDa, which is similar to that of β-nerve growth factor. Both in vitro and in vivo studies have shown that nerve growth factor encapsulated in liposomes can also penetrate the blood-brain barrier (Xie et al., 2005).
One case report that was not included in the present review reported the successful application of mouse nerve growth factor (Enjingfu, Xiamen Beida Road Bioengineering, Xiamen, Fujian Province, China) for the treatment of a Chinese patient with radiation-induced temporal lobe necrosis (Wang et al., 2011b). Late temporal lobe necrosis is a severe complication of radiation treatment for nasopharyngeal carcinoma. After a continuous injection of mouse nerve growth factor for 2 months, the necrotic disease completely disappeared from the bilateral temporal lobes. The first author of that paper reported a total of 10 temporal lobe necrosis cases treated with mouse nerve growth factor therapy in another paper (Eriksdotter Jonhagen et al., 1998; Wang et al., 2012a). The results of those cases showed that the neurological symptoms or signs disappeared completely in four patients, improved in four patients, and there was no change in two patients.
The present review has several limitations. The majority of articles had unclear descriptions of the randomization procedures and lacked blinding, which may have created performance biases and detection biases. Because of treatment of adverse events, the patients and researchers may have been aware of the therapeutic interventions. In addition, some difficulties may be cause by an incomplete retrieval of the identified research. These limitations contribute to both the uncertainty in the results of the primary studies and to that in our meta-analyses.
All 62 of the studies included for meta-analysis were based on randomized controlled trials on neurological diseases in China. To our knowledge, China is the only state where nerve growth factor is approved for use as a clinical medicine for randomized controlled trials. Nerve growth factor has not caused any serious adverse reactions, such as heart, liver, or kidney problems or severe allergic reactions since it was classified as a national category I new drug in China over 10 years ago. Nerve growth factor has been used to treat almost every neurological disease in a diverse population across a wide range of Chinese health care practice settings. All of this use in clinical practices highlights the important role played by nerve growth factor for the treatment of neurological diseases and provides strong evidence for the wider application of nerve growth factor in the future.
Acknowledgments
We acknowledged Yan-yan Ma from Chinese PLA General Hospital for the assistance in writing the manuscript, Xiu-yu Shi from Chinese PLA General Hospital for searching literature, and Lin-yan Hu from Chinese PLA General Hospital for providing supply of materials.
Footnotes
Funding: This study was financially supported by the National Science and Technology Major Projects for “Major New Drugs Innovation and Development”, No. 2012ZX09201-301-005.
Conflicts of Interest: None declared.
Copyedited by McCarty W, Norman C, Wang J, Yang Y, Li CH, Song LP, Zhao M
References
- Aloe L, Tirassa P, Lambiase A. The topical application of nerve growth factor as a pharmacological tool for human corneal and skin ulcers. Pharmacol Res. 2008;57:253–258. doi: 10.1016/j.phrs.2008.01.010. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association standard. Standards of medical care in diabetes-2006. Diabetes Care. 2006;29(Suppl 1):S4–42. [PubMed] [Google Scholar]
- Apfel SC. Nerve growth factor for the treatment of diabetic neuropathy: what went wrong, what went right, and what does the future hold? Int Rev Neurobiol. 2002;50:393–413. doi: 10.1016/s0074-7742(02)50083-0. [DOI] [PubMed] [Google Scholar]
- Apfel SC, Kessler JA, Adornato BT, Litchy WJ, Sanders C, Rask CA. Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. NGF Study Group. Neurology. 1998;51:695–702. doi: 10.1212/wnl.51.3.695. [DOI] [PubMed] [Google Scholar]
- Apfel SC, Schwartz S, Adornato BT, Freeman R, Biton V, Rendell M, Vinik A, Giuliani M, Stevens JC, Barbano R, Dyck PJ. Efficacy and safety of recombinant human nerve growth factor in patients with diabetic polyneuropathy: a randomized controlled trial. rhNGF Clinical Investigator Group. JAMA. 2000;284:2215–2221. doi: 10.1001/jama.284.17.2215. [DOI] [PubMed] [Google Scholar]
- Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54:1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- Candrilli SD, Davis KL, Kan HJ, Lucero MA, Rousculp MD. Prevalence and the associated burden of illness of symptoms of diabetic peripheral neuropathy and diabetic retinopathy. J Diabetes Complications. 2007;21:306–314. doi: 10.1016/j.jdiacomp.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Chen J. Clinical observation of mouse nerve growth factor for injection for treatment of diabetic peripheral neuropathy. Zhongguo Yiyao Zhinan. 2013;11:571–572. [Google Scholar]
- Chen YW, Zi XH, Tu QY, Song Z, Li XB. Role of mouse nerve growth factor in the treatment of cerebrovascular disease with diabetes mellitus. Zhongguo Linchuang Yisheng. 2004;6:1573–1574. [Google Scholar]
- Chen YY, Zheng F, Chen Q. Combined treatment with mouse nerve growth factor and compound anisodine for optic nerve contusion. Haixia Yaoxue. 2011;23:102–103. [Google Scholar]
- Chi YL, Zhai Y. Clinical researches of mouse nerver growth factor for the treatment of 80 cases with polyneuropathy. Zhongguo Yiyao Zhinan. 2013;11:534. [Google Scholar]
- Chiaretti A, Antonelli A, Genovese O, Fernandez E, Giuda D, Mariotti P, Riccardi R. Intraventricular nerve growth factor infusion improves cerebral blood flow and stimulates doublecortin expression in two infants with hypoxic-ischemic brain injury. Neurol Res. 2008;30:223–228. doi: 10.1179/016164107X247948. [DOI] [PubMed] [Google Scholar]
- Chyun DA, Melkus GD, Katten DM, Price WJ, Davey JA, Grey N, Heller G, Wackers FJ. The association of psychological factors, physical activity, neuropathy, and quality of life in type 2 diabetes. Biol Res Nurs. 2006;7:279–288. doi: 10.1177/1099800405285748. [DOI] [PubMed] [Google Scholar]
- Ebendal T. NGF in CNS: experimental data and clinical implications. Prog Growth Factor Res. 1989;1:143–159. doi: 10.1016/0955-2235(89)90008-2. [DOI] [PubMed] [Google Scholar]
- Eriksdotter Jonhagen M, Nordberg A, Amberla K, Backman L, Ebendal T, Meyerson B, Olson L, Seiger, Shigeta M, Theodorsson E, Viitanen M, Winblad B, Wahlund LO. Intracerebroventricular infusion of nerve growth factor in three patients with Alzheimer's disease. Dement Geriatr Cogn Disord. 1998;9:246–257. doi: 10.1159/000017069. [DOI] [PubMed] [Google Scholar]
- Fang XM, Wang YR, Ye WC, Gao L. Effect of mouse nerve growth factor and Danhong injection in the treatment of elderly diabetic peripheral neuropathy. Xibu Yixue. 2012a;24:2059–2061. [Google Scholar]
- Fang XM, Wang YR, Ye WC, Gao L. Clinical observation of mouse nerve growth factor treating diabetic peripheral neuropathy. J Med Theor Proc. 2012b;25:627–628. [Google Scholar]
- Feng T, Xu XZ, Yang Y, Zhu HY, Fan YW, Wang LK. Clinical researches of combination nerver growth factor and methycobal for the treatment of 45 cases with peripheral neuropathy. Zhongwai Yiliao. 2013:128–129. [Google Scholar]
- Guo ZY, Liu YA. Clinical observation of mouse nerve growth factor for treatment of 35 cases with diabetic peripheral neuropathy. Zuzhong yu Shenjing Jibing. 2011;18:364–366. [Google Scholar]
- Hou LP, Lou M, Ying JQ. Combined treatment with mouse nerve growth factor and hyperbaric oxygen for acute carbon monoxide poisoning. Renmin Junyi. 2012;55:519–521. [Google Scholar]
- Hu HT, Lin YY, Hu H. Injection site pain of mouse nervous growth factor. A review of randomized, double-blinded, parallei placebo controlled phase i to iii clinical trials. Zhongguo Yaowu Jingjie. 2010;7:397–400. [Google Scholar]
- Huang X, Li QM. Efficacy of mouse nerve growth factor in treating traumatic optic neuropathy. Shenjing Sunshang yu Gongneng Chongjian. 2010;5:433–435. [Google Scholar]
- Huang ZX, Yin XY, Huang ZS, Liang M. Therapy of diabetic peripheral neuropathy with extraneous nerve growth factor. Linchuang Junyi Zazhi. 2010;38:573–575. [Google Scholar]
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhang QJ, Zhao R, Cong R, Tian Y. Effect of mouse nerve growth factor in the treatment of peripheral nerve entrapment syndrome. Zhejiang Linchuang Yixue Zazhi. 2012;14:399–402. [Google Scholar]
- Lambiase A, Aloe L, Centofanti M, Parisi V, Mantelli F, Colafrancesco V, Manni GL, Bucci MG, Bonini S, Levi-Montalcini R. Experimental and clinical evidence of neuroprotection by nerve growth factor eye drops: Implications for glaucoma. Proc Natl Acad Sci U S A. 2009;106:13469–13474. doi: 10.1073/pnas.0906678106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xu SJ, Gao JX. Clinical observation of mouse nerve growth factor for treatment of 50 cases with diabetic peripheral neuropathy. Zhongguo Yixue Chuangxin. 2010a;7:65–66. [Google Scholar]
- Li JM, Li GX, Hu HT, Jiang CM, Zhao CY, Liu XS. A phase I tolerance study ofmouse nerve growth factor for injection. Zhongguo Xinyao Zazhi. 2010b;19:1337–1341. [Google Scholar]
- Li LC, Yang ZY. Clinical observation of mouse nerve growth factor for injection for treatment of 68 cases with Alzheimer disease. Guizhou Yiyao. 2009;33:1062–1064. [Google Scholar]
- Li P, Wang XQ, Wang SZ. Nutritional effects of mouse nerve growth factor on optic nerve in patients with eye trauma. Zhongguo Xiangcun Yiyao. 2013;20:45–46. [Google Scholar]
- Li R, Yuan HM. Clinical evaluation of mouse nerve growth factor and methyIprednisolone on acute optic neuritis. Int J Ophthalmol. 2011;11:2230–2231. [Google Scholar]
- Li SL. Effect of mouse nerve growth factor in the treatment of acute cerebral infarction. World Health Digest. 2006;3:44. [Google Scholar]
- Li W, Zhang XD, Tong HF, Huang XJ, Hao W, Wang W. Mouse nerve growth factor in the treatment of HIV infection complicated with crypetococcal meningitis:retrospective analysis of clinical features. Zhongguo Bingdubing Zazhi. 2012;2:277–280. [Google Scholar]
- Lin YH, Wang JS, Dai HY, Sun LH. Clinical evaluation of mouse nerve growth factor and acupuncture on oculomotor nerve palsy. Zhongguo Shiyong Shenjing Jibing Zazhi. 2012;15:74–75. [Google Scholar]
- Liu HB, Zhang BQ, Fang SY, Teng JF, Lian YJ, Yu XM, Zhang BA. Repair role of mouse nerve growth factor to peripheral nerve in Giullain-Barre syndrome. Zhongguo Xinyao yu Linchuang Zazhi. 2007;26:343–344. [Google Scholar]
- Liu YR, Liu Q. Meta-analysis of mNGF therapy for peripheral nerve injury: a systematic review. Chin J Traumatol. 2012;15:86–91. [PubMed] [Google Scholar]
- Lu JH, Ma WB, Zhao MZ. The investigation of therapeutic effects of combined mouse nerve growth factor and methylprednisolone on acute retrobulbar neuritis. Linchuang Yanke Zazhi. 2013;21:255–257. [Google Scholar]
- Ma JL, Sun XY, Zhang J, Lou HD. Eficacy of mouse nerve grow th factor in treating diabetic optic neuropathy. Int Eye Sci. 2012;12:1958–1960. [Google Scholar]
- Meng XQ, Qu XB, Liu W. Effect of tanshinone II A and mouse nerve growth factor in the treatment of elderly diabetic peripheral neuropathy. J Clin Res. 2010;27:806–809. [Google Scholar]
- Mo JN, Huang Y, Zhou WK. The eficacy of mouse nerve growth factor for injection in idiopathic facial paralysis. Zhongguo Yiyao Zhinan. 2011;9:32–33. [Google Scholar]
- Olson L, Nordberg A, von Holst H, Backman L, Ebendal T, Alafuzoff I, Amberla K, Hartvig P, Herlitz A, Lilja A. Nerve growth factor affects 11C-nicotine binding, blood flow, EEG, and verbal episodic memory in an Alzheimer patient (case report) J Neural Transm Park Dis Dement Sect. 1992;4:79–95. doi: 10.1007/BF02257624. [DOI] [PubMed] [Google Scholar]
- Palacka P, Kucharska J, Murin J, Dostalova K, Okkelova A, Cizova M, Waczulikova I, Moricova S, Gvozdjakova A. Complementary therapy in diabetic patients with chronic complications: a pilot study. Bratisl Lek Listy. 2010;111:205–211. [PubMed] [Google Scholar]
- Peng C, Shu XM, Yang BZ, Li J. Observation on therapeutic effects of IVIG and mNGF cure for the children with Guillain-Barre syndrome. Zunyi Yixueyuan Xuebao. 2009;32:35–37. [Google Scholar]
- Peng H, Chen H, Li H. Study on mouse nerve growth factor (NGF) in treatment of facial nerve injury. J Clin Exp Med. 2008;7:3–4. [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Qi XW, Wang ZJ, An RZ. Combined treatment with mouse nerve growth factor and traction physiotherapy for 31 nerve-root type cervical spondylosis patients. Yiyao Daobao. 2012;31:1585–1587. [Google Scholar]
- Riggs JE. Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. Neurology. 1999;52:1517–1518. doi: 10.1212/wnl.52.7.1517-a. [DOI] [PubMed] [Google Scholar]
- Shan BH, Wu Y, Zheng YJ, Zhu L. Efficacy of mouse nerve growth factor in the treatment of elderly pationts with shingles postherpetic neuralgia. Zhongguo Laonianxue Zazhi. 2013;33:1395–1396. [Google Scholar]
- Shen GL. Changchun: Changchun University of Chinese Medicine, China; 2012. Acupoint injection of mouse nerve growth factor for treatment of ischemic optic neuropathy. [Google Scholar]
- Shen W. Improvment of rheology and nerve conduction velocity with mouse nerve growth factor and Danhong Chinese medicine injection in the treatment of diabetic peripheral neuropathy. Zhongguo Laonianxue Zazhi. 2013;33:2749–2750. [Google Scholar]
- Shu LW, Hu XH, Liu DL. Acupuncture point injection treatment with mouse nerve growth factor for peripheral nerve injury. Zhongguo Shangcan Yixue. 2013;21:90–91. [Google Scholar]
- Sun LJ. Combined treatment with mouse nerve growth factor and vinpocetine for acute angle-closure glaucoma optic nerve damage. Xiandai Zhongxiyi Jiehe Zazhi. 2010;19:3283–3284. [Google Scholar]
- Tang JF, Guo ZQ, Yang J, Lu Y. Combined treatment with intratympanic mouse nerve growth factor and dexamethasone for sudden deafness. Zhongguo Gaodeng Yixue Jiaoyu. 2013;4:145–146. [Google Scholar]
- Tang MY, Gong C, Wu WC. Coordinated therapy of methylprednisolone and mouse nerve growth factor for injection on acute spinal cord injury. Linchuang Yixue Xuekan. 2008;17:90–92. [Google Scholar]
- Tang XM, Wang Y. Outcome of treatment with nerve growth factor in patients with Bell's palsy. Linchuang Yixue Zazhi. 2008;28:23–24. [Google Scholar]
- Tian JW, Dong C. Clinical observation of mouse nerve growth factor for idiopathic facial paralysis. Jiankang Dashiye. 2013;1:46–47. [Google Scholar]
- Wang D, Liu N. Nerve growth factor in the treatment of cerebral contusion and laceration. Zhonghua Shenjing Yixue Zazhi. 2010;9:416–418. [Google Scholar]
- Wang DS, Huang LT, Yang XT, Lan GH. Clinical observation of local injection treatment with mouse nerve growth factor for peripheral nerve injury. Qiqihar Yixueyuan Xuebao. 2011a;32:369–370. [Google Scholar]
- Wang LB, Wang Y, Guo L. Combined treatment with mouse nerve growth factor and hyperbaric oxygen for diabetic peripheral neuropathy. Linchuang Huicui. 2010a;25:1813–1815. [Google Scholar]
- Wang X, Ying H, Zhou Z, Hu C, Eisbruch A. Successful treatment of radiation-induced temporal lobe necrosis with mouse nerve growth factor. J Clin Oncol. 2011b;29:e166–168. doi: 10.1200/JCO.2010.31.7081. [DOI] [PubMed] [Google Scholar]
- Wang XS, Zhang X, Zhou RL, Chen MF, Chen XY. Clinical observations about treating patients of cerebral infarction with exogenous nerve growth factor. Zhongguo Bingdubing Zazhi. 2010b;27:675–677. [Google Scholar]
- Wang XS, Ying HM, Zhou ZR, He XY, Hu CS. Mouse nerve growth factor in the treatment of radiation-induced cerebral necrosis. Zhongguo Shenjing Zhongliu Zazhi. 2012a;10:147–151. [Google Scholar]
- Wang YC, Sun HM, Dong ZX. Mouse nerve growth factor for injection. Zhongguo Xinyao Zazhi. 2007;16:1538–1539. [Google Scholar]
- Wang YG, Zhang DD. Clinical efficacy of mouse nerve growth factor for treatment of optic nerve contusion. Hangkong Hangtian Yiyao. 2010;21:435–436. [Google Scholar]
- Wang YG, Wei GQ, Chen J. Mouse nerve growth factor in the treatment of delayed encephalopathy after carbon monoxide poisoning. Huabei Guofang Yiyao. 2010c;22:227–229. [Google Scholar]
- Wang Z, Ma MJ, Han Q. Analysis the efficacy of nerve growth factor in the treatment of facial paralysis. Shenjing Sunshang yu Gongneng Chongjian. 2012b;7:286–288. [Google Scholar]
- Xia F, Liu N, Han JL, Wang JC. Combined treatment with mouse nerve growth factor and mecobalamin for diabetic peripheral neuropathy. Zhonghua Shenjing Waike Jibing Yanjiu Zazhi. 2009;8:179–180. [Google Scholar]
- Xia QY, Pan XB. Clinical observation of mouse nerve growth factor for retrobulbar injection in the treatment of optic atrophy. Yixue Xinxi. 2011;7:3255–3256. [Google Scholar]
- Xia YH, Liu D, Li SJ, Guo JM, Li M, Fu DD, Li ZG, Tian ZW. Efficacy of mouse nerve growth factor in the treatment of shingles postherpetic neuralgia. Shiyong Yixue Zazhi. 2010;26:3227–3228. [Google Scholar]
- Xie Y, Ye L, Zhang X, Cui W, Lou J, Nagai T, Hou X. Transport of nerve growth factor encapsulated into liposomes across the blood-brain barrier: in vitro and in vivo studies. J Control Release. 2005;105:106–119. doi: 10.1016/j.jconrel.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Yan JY, Li JF, Zhang X, Liu GN. 24 Cases with Spinal Cord Injury after fractures of thoracolumbar segment treated by mouse nerve growth factor, electromyographic biofeedback and electro-acupuncture. Neimenggu Yixue Zazhi. 2012;44:7–10. [Google Scholar]
- Yang K, Song GT, Hu JH, Zhan H, Li J. Clinical observation of mouse nerve growth factor in treatment of 26 cases with facial nerve injury. Zhejiang Chuangshang Waike. 2006;11:109. [Google Scholar]
- Ye WC, Fang XM, Wang YR, Gao L. Clinical observation of mouse nerve growth factor combining with a lipoic acid for treatment of diabetic peripheral neuropathy. Sichuan Yixue. 2012;33:2098–2100. [Google Scholar]
- Yu F. Clinical observation of mouse nerve growth factor for optic atrophy. Qiuyi Wenyao. 2013;11:80–81. [Google Scholar]
- Yuan P, Lei TT. Efficacy of nerve growth factor in patients with diffuse axonal injury. Chongqing Yixue. 2005;34:1877–1878. [Google Scholar]
- Zhang GF. Clinical observation of mouse nerve growth factor for treatment of 30 cases with facial paralysis. Linchuang Heli Yongyao. 2010;3:42. [Google Scholar]
- Zhang KF, Guo ZW, Song HJ. Effect of mouse nerve growth factor on neural function of patients after operation for protrusion of the lumbar lntervertebral disc. Zhongguo Kangfu Lilun yu Shijian. 2012;18:84–86. [Google Scholar]
- Zhang PL, Wang Y, Du ZY, Zhu YQ, Gu YF, Wu CG. Combined treatment with mouse nerve growth factor and interventional procedures for lumbar intervertebral disc. Zhongguo Kangfu Lilun yu Shijian. 2008;7:740–741. [Google Scholar]
- Zhang X, Yang LJ, Lu JM. Clinical observation of mouse nerve growth factor for optic atrophy. Zhongguo Linchuang Yanjiu. 2010;23:463–464. [Google Scholar]
- Zhang Y, Wei GK, Zhang JL. Combined treatment with mouse nerve growth factor and Shuxuetong chinese medicine injection for acute cerebral infarction. Zhongguo Zhongyi Jizheng. 2011a;20:535–536. [Google Scholar]
- Zhang YP. Clinical observation of mouse nerve growth factor for the treatment of acute cerebral infarction. Zhongguo Shiyong Shenjing Jibing Zazhi. 2009;12:35–37. [Google Scholar]
- Zhang ZL, Wei F, Zhao SG. The clinical researches of combination nerver growth factor and methycobal on the treatment of diabetic peripheral neuropathy. Neimenggu Yixueyuan Xuebao. 2011b;33:37–39. [Google Scholar]
- Zhao CH, Li YJ. Curative effect of mouse nerve growth factor for sudden deafness. Zhongguo Shiyong Shenjing Jibing Zazhi. 2012;15:17–18. [Google Scholar]
- Zhao JW. Clinical observation of mouse nerve growth factor for treatment of 65 cases with diabetic peripheral neuropathy. Zhongguo Xiandai Yaowu Yingyong. 2011;5:22–23. [Google Scholar]
- Zheng SH, Chen HJ, Luo WL. Clinical researches of combination nerver growth factor and methycobal for the treatment of pseudobulbar palsy. Qiuyi Wenyao. 2013;11:90. [Google Scholar]
- Zhu SP, Han XL, Zhang LS. I125 labeled β-NGF cross the blood-brain barrier. Tongweisu. 2002;15:65–68. [Google Scholar]
- Zochodne DW, Said G. Recombinant human nerve growth factor and diabetic polyneuropathy. Neurology. 1998;51:662–663. doi: 10.1212/wnl.51.3.662. [DOI] [PubMed] [Google Scholar]


