Abstract
Rationale
Effort-related motivational symptoms such as anergia and fatigue are common in patients with depression and other disorders. Research implicates pro-inflammatory cytokines in depression, and administration of cytokines can induce effort-related motivational symptoms in humans.
Objectives
The present experiments focused on the effects of the pro-inflammatory cytokine interleukin 1-beta (IL-1β) on effort-related choice behavior.
Methods
Rats were tested on a concurrent fixed ratio 5 lever pressing/chow feeding choice procedure, which assesses the tendency of rats to work for a preferred food (high carbohydrate pellets) in the presence of a concurrently available but less preferred substitute (laboratory chow).
Results
IL-1β (1.0–4.0 μg/kg IP) shifted choice behavior, significantly decreasing lever pressing and increasing intake of the freely available chow. The second experiment assessed the ability of the adenosine A2A antagonist MSX-3 to reverse the behavioral effects of IL-1β. MSX-3 attenuated the effort-related impairments produced by IL-1β, increasing lever pressing and also decreasing chow intake. In the same dose range that shifted effort-related choice behavior, IL-1β did not alter food intake or preference in parallel free-feeding choice studies, indicating that these low doses were not generally suppressing appetite or altering preference for the high carbohydrate pellets. In addition, IL-1β did not affect core body temperature.
Conclusions
These results indicate that IL-1β can reduce the tendency to work for food, even at low doses that do not produce a general sickness, malaise, or loss of appetite. This research has implications for the involvement of cytokines in motivational symptoms such as anergia and fatigue.
Keywords: accumbens, motivation, fatigue, depression, anergia, adenosine
Motivated behaviors can be characterized by a high degree of behavioral activation and work output, and organisms frequently make effort-related decisions based upon analyses of motivational value and work-related response costs (Salamone and Correa 2002, 2012). Effort-based choice behavior is studied using tasks that offer choices between high effort options leading to highly valued reinforcers vs. low effort/low reward options (Salamone et al. 2007, 2012). Exertion of effort in motivated behavior and effort-related choice behavior are regulated by neural circuits involving nucleus accumbens dopamine (DA), prefrontal/anterior cingulate cortex, ventral pallidum, and basolateral amygdala (Salamone et al. 1997, 2007; Walton et al. 2003; Caignard et al. 2006; Floresco and Ghods-Sharifi 2008; Mingote et al. 2008a; Font et al. 2008; Farrar et al. 2008, 2010; Hauber and Sommer 2009; Mai et al. 2012; Salamone and Correa 2012). It has been suggested that tasks measuring effort-related choice behavior could be used as animal models of motivational symptoms such as anergia and fatigue, which are seen in patients with major depression and other conditions (Salamone et al. 2006, 2007, 2012; Salamone and Correa 2012; Dantzer et al. 2012). This suggestion is consistent with recent human studies showing that decreased selection of high effort/high reward options is seen in patients with major depression (Treadway et al. 2012), and also in schizophrenics manifesting a high degree of negative symptoms (Gold et al. 2013), while the opposite effect is produced by administration of amphetamine (Wardle et al. 2011).
Cytokines are known to play a critical role in the regulation of the immune system, which coordinates physiological responses to infection, injury, and foreign antigens (Dantzer 2001). Evidence gathered over the last few decades has demonstrated that pro-inflammatory cytokines play a role in aspects of depression, including motivational symptoms such as psychomotor slowing, anergia, and fatigue (Bret-Dibat et al. 1995; Dantzer 2001; Raison et al. 2010; Felger and Miller 2012; Dantzer et al. 2012). Depressed patients have elevated levels of pro-inflammatory cytokines such as tumor necrosis factor alpha, interleukin 6 (IL-6), and interleukin 1β (IL-1β) as compared to the general population (Dowlati et al. 2010; Hiles et al. 2012), and high levels of interleukin IL-1β in depressed patients were predictive of a lack of therapeutic response to the antidepressants nortriptyline and escitalopram (Cattaneo et al. 2013). In addition, patients with cancer or viral infections are often treated with these cytokines, and side effects of this treatment can involve depressive-like symptoms, including fatigue/anergia (Capuron et al. 2002, 2009; Felger et al. 2012a). In fact, fatigue and loss of energy are reported to be the most common symptoms induced by interferon alpha (IFN-α); these symptoms occur in 80% of patients receiving treatment with IFN-α (Miller 2009; Felger et al. 2012a). Moreover, patients that received IFN-α treatment, when compared to medically healthy people with major depression, showed less agitation and suicidal ideation, but significantly greater psychomotor slowing (Capuron et al. 2009). Pro-inflammatory cytokines are thought to influence brain function in multiple ways, and there is evidence that they act on macrophage-like cells in the choroid plexus and circumventricular organs that induce the central synthesis of cytokines, which in turn diffuse into the brain tissue (Dantzer 2009). In addition, peripheral cytokines can act on afferent branches of cranial nerves such as the glossopharyngeal and vagus, instigating the central production of cytokines by microglia (Dantzer 2009). Centrally acting cytokines affect transmission of brain monoamine systems, including dopamine (DA), norepinephrine, and serotonin, all of which have been implicated in depression (Song et al. 2008; Miller 2009; Dantzer 2009). Recent evidence suggests that mesolimbic DA may be particularly sensitive to inflammatory cytokines, where they have been reported to interfere with vesicular DA storage and DA synthesis (Felger and Miller 2012).
In rats and mice, administration of IL-1β produced depressive-like effects in commonly used tasks such as the forced swim and tail suspension tests (Minor et al., 2003; Dunn and Swiergiel 2005; Hanff et al. 2010), and also reduced social exploration and locomotor activity (Castanon et al., 2001). Lipopolysaccaride (LPS), a component of the outer membrane of gram negative bacteria, increased the expression of inflammatory cytokines and induced depressive-like effects in mice tested on the tail suspension and forced swim tests (Frenois et al., 2007; O’Connor et al. 2009), and intracranial administration of IL-6 to rats produced depressive-like effects in the forced swim test (Wu and Lin 2008). In addition, some studies indicate that administration of cytokines to rodents can affect behavioral activation and exertion of effort in rats responding on instrumental tasks. Injection of the cytokine inducer polyriboinosinic: polyribocytidylic acid decreased spontaneous wheeling in rats (Katafuchi et al., 2005). Furthermore, Merali et al. (2003) reported that administration of IL-1β to rats reduced lever pressing on a progressive ratio schedule.
The present experiments characterized the ability of the pro-inflammatory cytokine IL-1β to alter effort-related choice behavior as assessed by the concurrent FR 5/chow feeding procedure (Salamone et al. 1991, 2007). This task assesses the tendency of rats to work for a preferred food (high carbohydrate pellets) in the presence of a concurrently available but less preferred substitute (laboratory chow). Thus, rats can either lever press on a fixed ratio 5 (FR 5) schedule of reinforcement for a more preferred food (high carbohydrate pellets), or approach and consume the less preferred laboratory chow that is freely available in the operant chamber. Rats trained on this procedure receive most of their food by responding on the lever for the more preferred sucrose pellet, and eat very little of the freely available chow. Previous research has shown that this task is sensitive to the effects of various pharmacological manipulations, including low doses of DA antagonists and accumbens DA depletions or antagonism (Salamone et al. 1991, 2002, 2007, 2009; Farrar et al. 2010), stimulation of accumbens core adenosine A2A receptors (Font et al. 2008), or stimulation of GABAA receptors in ventral pallidum (Farrar et al. 2008), all of which shift choice behavior by decreasing lever pressing and increasing chow intake. This shift in choice behavior is not dependent upon changes in food consumption or preference, and is not mimicked by pre-feeding or appetite suppressant drugs, which fail to increase chow intake at doses that suppress lever pressing (Salamone et al. 1991, 2002; Cousins et al. 1994; Koch et al. 2000; Sink et al. 2008; Nunes et al. 2013). In the present study, experiment 1 examined the ability of the pro-inflammatory cytokine IL-1β to produce effort-related impairments in rats tested on the concurrent FR 5/chow feeding procedure, and it was hypothesized that IL-1β would decrease lever pressing but increase intake of the concurrently available lab chow. Experiment 2 studied the ability of the adenosine A2A antagonist MSX-3 to reverse the effort-related effects of IL-1β. MSX-3 was studied because of research showing that adenosine A2A antagonists can attenuate the effort-related effects of DA antagonists (Farrar et al. 2007; Nunes et al. 2010; Santerre et al. 2012), and can produce effects that are consistent with the behavioral profile of an antidepressant in rodents tested on the swim test or tail suspension task (El Yacoubi et al. 2001; Hodgson et al. 2009; Hanff et al. 2010). Finally, control experiments assessed the effects of IL-1β on food intake and preference, and on core body temperature.
Materials and Methods
Subjects
Adult male, drug-naïve, Sprague Dawley rats (Harlan-Sprague Dawley, Indianapolis, IN, USA) were house in a colony maintained at 23 °C with 12-h light/dark cycles (lights on at 7:00h). Rats (n=32) weighed 290–340 g at the beginning of the study, and were initially food restricted to 85% of their free-feeding body weight for operant training. Rats were fed supplemental chow to maintain weight throughout the study, with water available ad libitum. Despite the food restriction, rats were allowed modest weight gain throughout the experiment. Different groups of rats were used for each experiment. Animal protocols were approved by the University of Connecticut animal care and use committee, and followed NIH guidelines.
Pharmacological agents and selection of doses
Recombinant rat IL-1β was obtained from R&D systems (Minneapolis, MN, USA) and was dissolved in 0.9% saline that also served as the vehicle control. The doses of IL-1β were based on previously published data (Merali et al., 2003) and on extensive pilot studies conducted to determine the time intervals and the precise dose range to be used (pilot studies showed that higher doses of IL-1β, such as 8.0–10.0 μg/kg, suppressed both lever pressing and food intake). MSX-3 ((E)-phosphoric acid mono-[3-[8-[2-(3-methoxyphenyl)vinyl]-7-methyl-2,6-dioxo-1-prop-2-ynyl-1,2,6,7-tetrahydropurin-3-yl] propyl] ester disodium salt) was provided by Christa Müller at the Pharmazeutisches Institut, Universität Bonn, in Bonn, Germany (Hockemeyer et al. 2004). To prepare the drug solution, MSX-3 (free acid) was dissolved in 0.9% saline, and pH was adjusted by titrating with microliter quantities of 1.0 N NaOH until the solid drug was in solution. The final pH was usually 7.5±0.2 and was not allowed to exceed 7.8.
Behavioral procedures
Behavioral sessions were conducted in operant conditioning chambers (28×23×23cm, Med Associates). Rats were initially trained to lever press on a continuous reinforcement schedule (30-min sessions, during 5 days) to obtain 45-mg pellets, (Bioserve, Frenchtown, NJ, USA), and then were shifted to the FR5 schedule (30-min sessions, 5 days/week) and trained for several additional weeks. Rats were then trained on the concurrent FR5/chow-feeding procedure. With this task weighed amounts of laboratory chow (Labortory diet, 5P00 Prolab RHM 3000, Purina Mills, St. Louis MO, USA; typically 15–20 g, three large pieces) were concurrently available on the floor of the chamber during the FR5 sessions. At the end of the session, rats were immediately removed from the chambers, and food intake was determined by weighing the remaining food (including spillage). Rats were trained until they attained stable levels of baseline lever pressing and chow intake (i.e. consistent responding over 1200 lever presses per 30 min), after which drug testing began. For most baseline days, rats did not receive supplemental feeding; however, over weekends and after drug tests, rats usually received supplemental chow in the home cage. On baseline and drug treatment days, rats normally consumed all the operant pellets that were delivered from lever pressing during each session. For the food preference study, rats were trained for several weeks in 30 min sessions in which both Bio-serv pellets and laboratory chow were concurrently and freely available for consumption. At the end of the session, rats were immediately removed from the chambers, and food intake was determined by weighing both of the remaining foods (including spillage).
Experimental Procedures
Rats were trained on the concurrent FR5/chow feeding procedure (as described above) before testing began, and each experiment employed different groups of rats. Experiments 1–4 used a within-groups design; with each rat receiving all intraperitoneal (IP) drug treatments in their particular experiment in a randomly varied order (one treatment per week, with none of the treatment sequences repeated across different animals in the same experiment). Baseline (i.e. non drug) sessions were conducted four additional days per week. Behavioral measures included both the number of lever presses and the amount of freely available lab chow that was consumed. The specific treatments and testing times for each experiment are listed below.
Experiment 1: Effects of systemic administration of IL-1β on the concurrent FR5/chow-feeding procedure
All animals were trained until stable baseline performance was achieved (i.e. lever presses consistently over 1200 per session). On drug test days, all animals (n=8) received IP injections of the following doses of IL-1β: saline vehicle, 1.0, 2.0, and 4.0 μg/kg. This experiment used a within-groups design, with all rats receiving all drug treatments in a randomly varied order. Baseline training (i.e. non-drug) sessions were conducted four additional days per week, and behavioral performance on these baseline days was unaffected by the previous injections. All injections were given 90 minutes before the beginning of the testing session.
Experiment 2: Effects of systemic administration of IL-1β on the concurrent FR5/chow-feeding procedure: reversal with MSX-3
All animals were trained until a stable baseline performance was achieved (i.e. lever presses over 1200). On drug test days, rats (n=8) received IP injections of the following doses of IL-1β plus MSX-3: saline vehicle plus saline vehicle, 4.0 μg/kg IL-1β plus saline vehicle, 4.0 μg/kg IL-1β plus 0.5 mg/kg MSX-3, 4.0 μg/kg IL-1β plus 1.0 mg/kg MSX-3, and 4.0 μg/kg IL-1β plus 2.0 mg/kg MSX-3. IL-1β injections were given 90 minutes before testing, and MSX-3 was injected 20 minutes before testing. Experiment 2 used a within-groups design, with all rats receiving all drug treatments in a randomly varied order. Baseline training (i.e. non-drug) sessions were conducted four additional days per week.
Experiment 3: Effects of systemic administration of IL-1β on food intake and preference
Rats were extensively trained until stable baseline performance was achieved (i.e. food consumption over 10 grams). Then, the rats (n=8) received IP injections of the following doses of IL-1β: saline vehicle, 1.0, 2.0, and 4.0 μg/kg. This experiment used a within-groups design, with all rats receiving all drug treatments in a randomly varied order. Baseline training (i.e. non-drug) sessions were conducted four additional days per week. All injections were given 90 minutes before the beginning of the testing session.
Experiment 4: Effects of IL-1β on body temperature
Untrained rats (n = 8) received the following injections in a randomly varied order once per week: vehicle saline or 4.0 μg/kg IL-1β IP, 105 minutes before testing (i.e., at a time that corresponded to the midpoint of the operant behavior tests). This experiment used a within-groups design, with all rats ultimately receiving both treatments. Rats were held loosely by an experimenter, while a thin, flexible thermistor probe (Fisher Scientific, Pittsburgh, PA) was then inserted 6 cm into the animal’s rectum. Temperature was recorded when it ceased to fluctuate for at least 5 s. These methods have previously been used to measure temperature changes induced by cannabinoid receptor agonists (McLaughlin et al. 2005, 2013).
Statistical Analyses
Number of lever presses and gram quantity of chow intake from the 30 min sessions in experiments 1–3 were analyzed with repeated measures of analysis of variance (ANOVA). When the overall ANOVA was significant, non-orthogonal planned comparisons using the overall error term was used to compare each treatment with the vehicle control group (Keppel 1991). For these comparisons, α level was kept at 0.05 because the number of comparisons was restricted to the number of treatments minus one (Keppel 1991). With this analysis, each condition that combined IL-1β plus MSX-3 was compared with the IL-1β plus vehicle condition.
Results
Experiment 1: Effects of systemic administration of IL-1β on the concurrent FR5/chow-feeding procedure
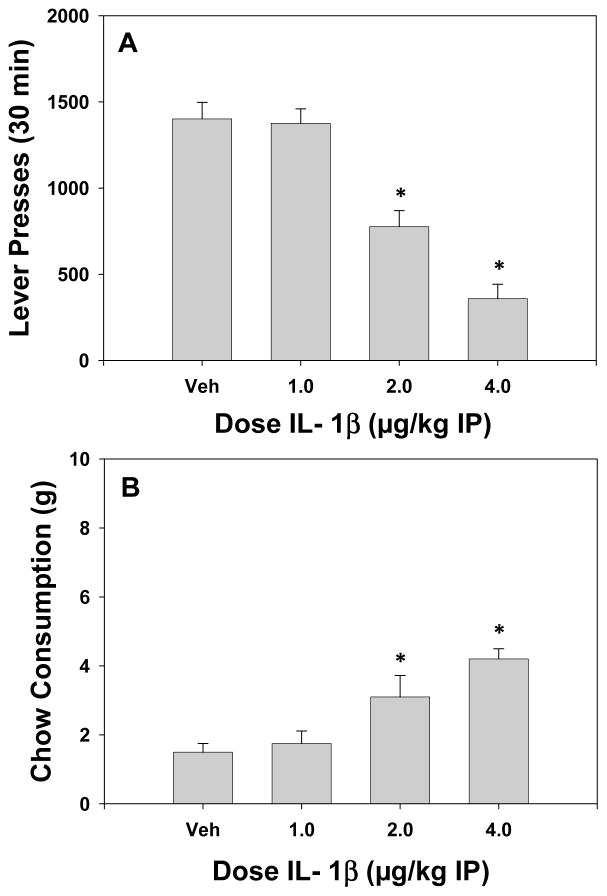
Systemic administration of IL-1β significantly decreased lever pressing and produced a concurrent increase in the consumption of the freely available lab chow as shown in figure 1A and B. The ANOVA revealed a significant effect of dose on lever pressing F(3, 21) = 31.5; p < 0.05. There was also an overall significant effect of dose on chow intake F(3, 21) = 11.3; p < 0.05. Planned comparisons were performed and showed that the two highest doses of IL-1β significantly decreased lever pressing and increased the consumption of the freely available lab chow relative to control (p < 0.05).
Figure 1.
This figure depicts the effects of IL-1β on FR5/chow feeding choice performance. A. Mean (± SEM) number of lever presses after treatment with vehicle and various doses of IL-1β (1.0–4.0 μg/kg IP). B. Mean (± SEM) intake of lab chow (in grams) after treatment with vehicle and various doses of IL-1β. * p < 0.05, different from vehicle, planned comparison.
Experiment 2: Effects of systemic administration of IL-1β on the concurrent FR5/chow-feeding procedure: reversal with MSX-3
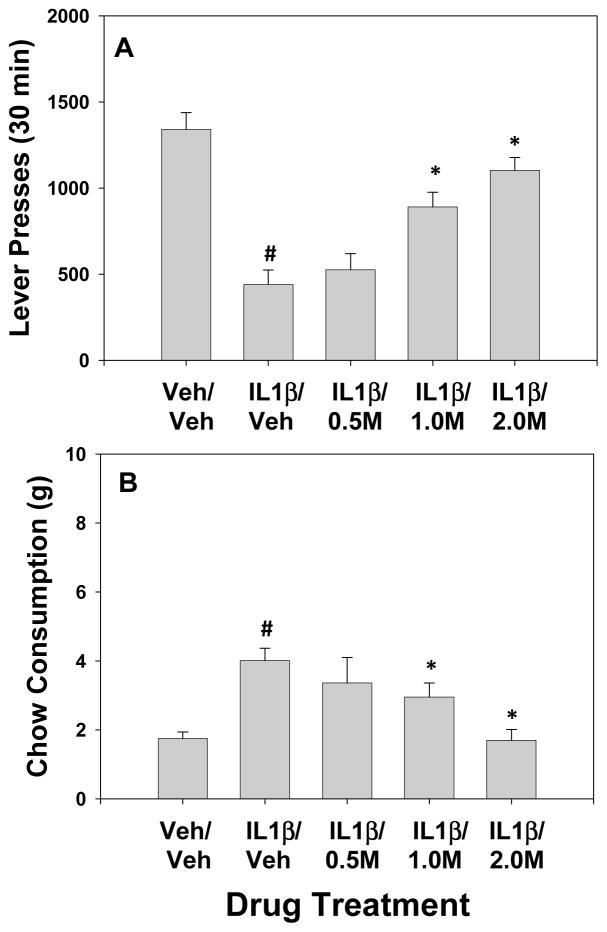
The results of experiment 2 are shown in figure 2A, B. MSX-3 was able to attenuate the behavioral effects of IL-1β. There was an overall significant effect of drug treatment on lever pressing F(4,28) = 22.2; p < 0.05). There was also an overall significant effect of drug treatment on chow intake F(4,28) = 6.7; p < 0.05). Planned comparisons were performed and showed that IL-β suppressed lever pressing and increased chow intake, and that the two highest doses of MSX-3 were able to attenuate the effects of IL-Iβ, both on lever pressing and consumption of the freely available lab chow, relative to the IL-1β alone condition (p < 0.05).
Figure 2.
The effects of the adenosine A2A antagonist MSX-3 on IL-1β-induced changes in performance on the FR5/chow feeding choice task are shown. Rats received IP injections of vehicle plus vehicle (Veh/Veh), 4.0 μg/kg IL-1β plus vehicle (IL-1β/Veh), and 4.0 μg/kg IL-1β plus 0.5, 1.0 or 2.0 mg/kg doses of MSX-3 (M). A. Mean (±SEM) number of lever presses during the 30 min session. B. Mean (±SEM) gram quantity of chow intake. # IL-1β plus vehicle significantly different from vehicle/vehicle, p<0.05; * MSX-3 plus IL-1β significantly different from IL-1β plus vehicle p < 0.05.
Experiments 3–4: IL-1β had no effect on food intake, food preference, or body temperature
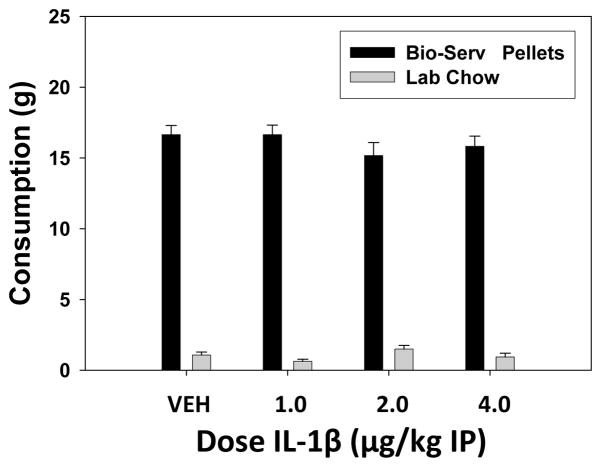
The results of the feeding study (experiment 3) are shown in figure 3. Administration of IL-1β had no effect on food intake, or preference of the high carbohydrate pellets vs. laboratory chow. There was a significant difference between food type consumed, with rats preferring the Bio-serv pellets over the standard lab chow (F(1, 14) = 958.2; p < 0.001). However, there was no significant effect of drug treatment on food intake (p > 0.05), and no significant drug treatment x food type interaction (p > 0.05). In experiment 4, injections of IL-1β had no effect on core body temperature. Mean (± SEM) body temperature (in decrees celsius) for each condition were as follows: saline vehicle, 38.19 ± 0.16; IL-1β, 37.91 ± 0.22 (t = 1.01, df = 7, n.s.).
Figure 3.
This figure shows the results of experiment 3, which assessed the effects of IL-1β on food intake and preference. Mean (± SEM) intake of Bio-serv pellets and lab chow (in grams) after treatment with vehicle and various doses (1.0–4.0 μg/kg IP) of IL-1β. There was an overall significant preference for Bio-serv pellets over chow, but no drug effects and no drug treatment by food type interaction.
Discussion
These experiments were conducted to characterize the behavioral effects of the pro-inflammatory cytokine interleukin 1 beta (IL-1β) using the concurrent FR5/chow feeding procedure (Salamone et al., 1991, 2009; Nunes et al., 2010). Administration of IL-1β shifted effort-related choice behavior as measured by the FR5/chow feeding choice task, decreasing lever pressing while producing a concomitant increase in the consumption of the concurrently available lab chow. Thus, like DA antagonism (Salamone et al., 1991, 2001; Sink et al., 2008), systemic and intra-accumbens injections of the DA depleting agent tetrabenazine (Nunes et al. submitted), and intra-accumbens injections of pilocarpine (Nunes et al. 2013), IL-1β can suppress food-reinforced lever pressing at doses that leave behavior directed towards the acquisition and consumption of food. These results are consistent with other data suggesting that IL-1β can affect instrumental responding reinforced by food. Larson et al. (2002) showed that administration of IL-1β reduced lever pressing on progressive ratio and FR32 schedules of reinforcement in mice, but had little effect on FR4 responding. Furthermore, in rats tested on a progressive ratio schedule, IL-1β decreased lever pressing and lowered the highest ratio achieved (Merali et al. 2003).
In order to produce a drug-induced shift in effort-related choice behavior, functions such as primary food motivation, appetite, and the ability to consume food must be relatively intact. This could have been problematic with injections of IL-1β, because cytokines produce behavioral effects that are collectively referred to as ‘sickness behavior’, which include decreased motor activity and loss of appetite, as well as psychomotor slowing and fatigue (Kent et al. 1994; Aubert et al. 1997; Harden et al. 2008; Dantzer 2009; Dantzer et al. 2012; Raedler et al. 2011; DellaGioia et al. 2012; Felger and Miller 2012). Thus, it could have been difficult to disentangle effects of IL-1β on food-reinforced lever pressing vs. those on food intake, since other drugs that suppress appetite or induce aversive food reactions, including amphetamine, fenfluramine, and cannabinoid CB1 antagonists/inverse agonists, all fail to increase chow intake at doses that suppress food-reinforced lever pressing (Cousins et al. 1994; Salamone et al. 2002; Sink et al. 2008). Also, drugs that produce substantial drowsiness/sedation (e.g. the adenosine A2A agonist CGS 21680) have been shown to decrease both lever pressing and chow intake (Mingote et al. 2008b), and did not induce the shift from lever pressing to chow intake at systemic doses that induced sedation (Font et al. 2008). Our own pilot studies indicated that higher doses of IL-1β could suppress food intake in addition to lever pressing, but the low doses used in experiments 1 above enabled us to observe a shift if choice from lever pressing to chow intake. Moreover, in the dose range tested, IL-1β did not affect intake of Bio-serv pellets or the preference for pellets over chow in free-feeding preference tests (experiment 3). Furthermore, although some studies have shown that IL-1β can increase body temperature 2–4 hours after IP injection (McLaughlin et al. 1992; Mouihate et al. 1998; Taylor et al. 2002), the results of experiment 4 demonstrated that IL-1β did not significantly affect body temperature 105 min after injection (i.e., a time period corresponding to the midpoint of the operant behavior session). Taken together, these results suggest that IL-1β-was not suppressing lever pressing for Bio-serv pellets because of changes in appetite, food preference, or a general sickness or malaise that was strong enough to reduce primary food motivation. Instead, the present results indicate that under the specific conditions studied in the present experiments, rats treated with IL-1β were still directed towards the acquisition and consumption of food, but were less likely to work for food by lever pressing.
Although the mechanism of action through which IL-1β can affect performance on the FR5/chow feeding choice task is unknown, pro-inflammatory cytokines are reported to reduce DA transmission (Kamata et al., 2000; Kitagami et al. 2003; Shuto et al. 1997; Felger et al. 2012b; Capuron et al. 2012; Felger and Miller 2012; but see also Song et al. 2007). Thus, in view of the literature implicating DA in effort-related choice behavior, it is possible that DAergic mechanisms may contribute to the effort-related effects of IL-1β. Recent studies indicate that cytokines can interfere with DA storage by reducing expression of the type 2 monoamine transporter (VMAT2), and also can suppress DA synthesis by reducing the availability of the essential cofactor tetrahydrobiopterin (Felger and Miller 2012). Patients treated with pro-inflammatory cytokines develop psychomotor slowing and fatigue and show reduced activation of nucleus accumbens as measured by fMRI (Capuron et al. 2012). Moreover, reductions in the presence of the DA metabolite homovanillic acid in cerebrospinal fluid were correlated with the degree of fatigue in patients treated with IFN-α (Felger and Miller 2012). These results suggest that cytokines may exert effects on DA that cause animals to reallocate their instrumental response selection based upon the response requirements of the task, and select lower cost alternatives to obtain food (Salamone et al. 2007, 2009). Furthermore, as described above, the effects of IL-1β in rats responding on the FR5/chow feeding choice procedure resembled those produced by DA antagonism or depletion (Salamone et al. 2007; Salamone and Correa 2012). Future research using microdialysis or measures of DA-related signal transduction (Segovia et al. 2011, 2012; Santerre et al. 2012; Randall et al. 2012) should be used to study the effects of IL-1β on DA transmission, and other transmitters also should be investigated.
Experiment 2 studied the ability of the selective adenosine A2A antagonist MSX-3 to reverse the effects of IL-1β on the concurrent FR5/chow choice task. Though previous work has shown that MSX-3 had no effect of FR5/chow feeding choice performance when administered on its own (Farrar et al. 2007), co-administration of MSX-3 significantly reversed the behavioral effects of IL-1β injections, restoring the baseline behavioral pattern by increasing lever pressing and decreasing chow intake in IL-1β-treated rats. These results are consistent with previous research demonstrating that adenosine A2A receptor blockade or genetic deletion can reverse the effort-related effects of DA antagonism and depletion (Farrar et al. 2007, 2010; Worden et al. 2009; Mott et al. 2009; Salamone et al. 2009; Nunes et al. 2010; Pardo et al. 2012). Furthermore, the present findings are consistent with reports showing that the adenosine A2A antagonists can produce effects that are consistent with the behavioral profile of an antidepressant in rodents tested on the swim test or tail suspension task (El Yacoubi et al. 2001; Hodgson et al. 2009; Hanff et al. 2010). The precise mechanism through which adenosine A2A antagonists exert their motivational effects is unknown, although anatomical and neurochemical evidence would indicate that a likely substrate is the enkephalin-positive medium spiny neurons in nucleus accumbens (Ferré et al. 1997; Rosin et al. 1998). In striatal regions, adenosine A2A receptors and DA D2 receptors are concentrated on enkephalin-positive GABAergic ventral striatopallidal neurons (Ferré et al. 1997, 2008; Rosin et al. 1998; Mingote et al. 2008), and adenosine A2A receptor antagonism has been shown to reverse the basic cellular effects of DA depletion or D2 antagonism on these neurons (Farrar et al. 2010; Santerre et al. 2012; Nunes et al. submitted). It has been suggested that adenosine A2A receptor blockade could be useful in treating motivational symptoms in humans such as psychomotor slowing, anergia, apathy and fatigue, which are evident in major depression and other disorders (Salamone et al. 2009, 2012; Farrar et al. 2007, 2010; Nunes et al. 2010; Santerre et al. 2012). Thus, it is possible that adenosine A2A receptor antagonists could also be useful for treating effort-related motivational symptoms produced by administration of cytokines.
In summary, these experiments focused on the behavioral effects of systemic administration of the pro-inflammatory cytokine IL-1β. IL-1β shifted effort-related choice, producing a significant decrease in lever pressing with a concurrent increase in the consumption of the freely available lab chow. The adenosine A2A antagonist MSX-3 attenuated the behavioral impairments produced by systemic administration of IL-1β. In the same dose range that shifted effort-related choice behavior, IL-1β did not alter food intake or preference in parallel free-feeding choice studies, indicating that these low doses were not generally suppressing appetite or altering preference for the high carbohydrate pellets. In addition, IL-1β did not affect core body temperature. These results indicate that IL-1β can reduce the tendency to work for food, even at low doses that do not produce a general food-related malaise, or a loss of appetite. This research has implications for the involvement of cytokines in motivational symptoms such as anergia and fatigue in depressed patients, as well as those receiving cytokine treatment (Dantzer et al. 2012). Cytokines also are thought to be involved in the anergia/fatigue-related symptoms seen in patients with infectious or inflammatory disease (Dantzer et al. 2008; Miller 2009; Harboe et al. 2009), multiple sclerosis (Lapierre and Hum 2008), and Parkinson’s disease (Katsarou et al. 2007). Thus, it should be recognized that tests of effort-related choice behavior are not intended to serve as animal models of depression, per se. Rather, they are being studied as potential models of a specific class of symptoms (i.e., effort-related motivational symptoms) that is characteristic of depression, but also spans multiple disorders and conditions (Treadway et al. 2012; Dantzer et al. 2012; Gold et al. 2013). This suggestion is consistent with the recent trend in mental health research that places less emphasis on traditional diagnostic categories, and instead focuses on the neural circuits mediating specific pathological symptoms (i.e., the Research Domain Criteria approach; Cuthbert and Insel 2013).
Acknowledgments
This work was supported by a grant to J.S. from the National Institute of Mental Health (MH094966), and to Merce Correa from Fundació Bancaixa/U. Jaume I. (P1.1B2010-43), and a SURF grant to B. Epling.
Footnotes
Disclosure/Conflict of Interest: J. Salamone has received grants from Merck-Serrono, Pfizer, and Roche, and is a consultant for Merz.
References
- Aubert A, Kelley KW, Dantzer R. Differential effect of lipopolysaccharide on food hoarding behavior and food consumption in rats. Brain Behav Immun. 1997;11(3):229–238. doi: 10.1006/brbi.1997.0503. [DOI] [PubMed] [Google Scholar]
- Bret-Dibat JL, Bluthé RM, Kent S, Kelley KW, Dantzer R. Lipopolysaccharide and interleukin-1 depress food motivated behavior in mice by a vagal-mediated mechanism. Brain Behav Immun. 1995;9(3):242–246. doi: 10.1006/brbi.1995.1023. [DOI] [PubMed] [Google Scholar]
- Cagniard B, Balsam PD, Brunner D, Zhuang X. Mice with chronically elevated dopamine exhibit enhanced motivation, but not learning, for a food reward. Neuropsychopharmacology. 2006;31:1362–1370. doi: 10.1038/sj.npp.1300966. [DOI] [PubMed] [Google Scholar]
- Capuron L, Hauser P, Hinze-Selch D, Miller AH, Neveu PJ. Treatment of cytokine-induced depression. Brain Behav Immun. 2002;16(5):575–580. doi: 10.1016/s0889-1591(02)00007-7. [DOI] [PubMed] [Google Scholar]
- Capuron L, Fornwalt FB, Knight BT, Harvey PD, Ninan PT, Miller AH. Does cytokine-induced depression differ from idiopathic major depression in medically healthy individuals? J Affect Disord. 2009;119:181–185. doi: 10.1016/j.jad.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Drake DF, Woolwine BJ, Spivey JR, Crowe RJ, Votaw JR, Goodman MM, Miller AH. Dopaminergic mechanisms of reduced basal ganglia responses to hedonic reward during interferon alpha administration. Arch Gen Psychiatry. 2012;69(10):1044–1053. doi: 10.1001/archgenpsychiatry.2011.2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon N, Bluthé RM, Danzter R. Chronic treatment with the atypical antidepressant tianeptine attenuates sickness behavior induced by peripheral but not central lipopolysaccharide and interleukin-1beta in the rat. Psychopharmacology (Berl) 2001;154(1):50–60. doi: 10.1007/s002130000595. [DOI] [PubMed] [Google Scholar]
- Cattaneo A, Gennarelli M, Uher R, Breen G, Farmer A, Aitchison KJ, Craig IW, Anacker C, Zunsztain PA, McGuffin P, Pariante CM. Candidate Genes Expression Profile Associated with Antidepressants Response in the GENDEP Study. Differentiating between Baseline ‘Predictors’ and Longitudinal ‘Targets’. Neuropsychopharmacology. 2013;38:377–385. doi: 10.1038/npp.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cousins MS, Wei W, Salamone JD. Pharmacological characterization of performance on a concurrent lever pressing/feeding choice procedure: effects of dopamine antagonists, cholinomimetic, sedative and stimulant drugs. Psychopharmacology (Berl) 1994;116(4):529–537. doi: 10.1007/BF02247489. [DOI] [PubMed] [Google Scholar]
- Cuthbert BN, Insel TR. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Ann N Y Acad Sci. 2001;933:222–234. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine, sickness behavior, and depression. Immunol Allergy Clin North Am. 2009;29:247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Meagher MW, Cleeland CS. Translational approaches to treatment-induced symptoms in cancer patients. Nat Rev Clin Oncol. 2012;9:414–426. doi: 10.1038/nrclinonc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dellagioia N, Devine L, Pittman B, Hannestad J. Bupropion pre-treatment of endotoxin-induced depressive symptoms. Brain Behav Immun. 2013;31:197–204. doi: 10.1016/j.bbi.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, Lanctôt KL. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- Dunn AJ, Swiergiel AH. Effects of interleukin-1 and endotoxin in the forced swim and tail suspension tests in mice. Pharmacol Biochem Behav. 2005;81(3):688–693. doi: 10.1016/j.pbb.2005.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Costentin J, Vaugeois JM. Adenosine A2A receptors and depression. Neurology. 2003;61(Suppl 6):S82–87. doi: 10.1212/01.wnl.0000095220.87550.f6. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Pereira M, Velasco F, Hockemeyer J, Muller CE, Salamone JD. Adenosine A(2A) receptor antagonism reverses the effects of dopamine receptor antagonism on instrumental output and effort-related choice in the rat: implications for studies of psychomotor slowing. Psychopharmacology (Berl) 2007;191:579–586. doi: 10.1007/s00213-006-0554-5. [DOI] [PubMed] [Google Scholar]
- Farrar AM, Font L, Pereira M, Mingote S, Bunce JG, Chrobak JJ, Salamone JD. Forebrain circuitry involved in effort-related choice: Injections of the GABAA agonist muscimol into ventral pallidum alter response allocation in food-seeking behavior. Neuroscience. 2008;152:321–330. doi: 10.1016/j.neuroscience.2007.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar AM, Segovia KN, Randall PA, Nunes EJ, Collins LE, Stopper CM, Port RG, Hockemeyer J, Müller CE, Correa M, Salamone JD. Nucleus accumbens and effort-related functions: behavioral and neural markers of the interactions between adenosine A2A and dopamine D2 receptors. Neuroscience. 2010;166:1056–1067. doi: 10.1016/j.neuroscience.2009.12.056. [DOI] [PubMed] [Google Scholar]
- Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33(3):315–327. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Cole SW, Pace TW, Hu F, Woolwine BJ, Doho GH, Raison CL, Miller AH. Molecular signatures of peripheral blood mononuclear cells during chronic interferon-α treatment: relationship with depression and fatigue. Psychol Med. 2012a;42:1591–1603. doi: 10.1017/S0033291711002868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felger JC, Li L, Marvar PJ, Woolwine BJ, Harrison DG, Raison CL, Miller AH. Tyrosine metabolism during interferon-alpha administration: association with fatigue and CSF dopamine concentrations. Brain Behav Immun. 2012b;31:153–160. doi: 10.1016/j.bbi.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferré S, Fredholm BB, Morelli M, Popoli P, Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20:482–487. doi: 10.1016/s0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- Ferré S, Quiroz C, Woods AS, Cunha R, Popoli P, Ciruela F, Lluis C, Franco R, Azdad K, Schiffmann SN. An update on adenosine A2A-dopamine D2 receptor interactions: Implications for the function of G-protein coupled receptors. Curr Pharm Des. 2008;14:1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Ghods-Sharifi S. Amygdala-prefrontal contrical circuitry regulates effort-based decision making. Cereb Cortex. 2007;17(2):251–260. doi: 10.1093/cercor/bhj143. [DOI] [PubMed] [Google Scholar]
- Font L, Mingote S, Farrar AM, Pereira M, Worden L, Stopper C, Port RG, Salamone JD. Intra-accumbens injections of the adenosine A2A agonist CGS 21680 affect effort-related choice behavior in rats. Psychopharmacology (Berl) 2008;199:515–526. doi: 10.1007/s00213-008-1174-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenois F, Moreau M, O’Connor J, Lawson M, Micon C, Lestage J, Kelley KW, Dantzer R, Castanon N. Liopolysaccharide induces delayed FosB/DeltaFosB immunostaining within the mouse extended amygdala, hippocampus and hypothalamus, that parallel the expression of depressive-like behavior. Psychoneuroendocrinology. 2007;32:516–531. doi: 10.1016/j.psyneuen.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JM, Strauss GP, Waltz JA, Robinson BM, Brown JK, Frank MJ. Negative symptoms of schizophrenia are associated with abnormal effort-cost computations. Biol Psychiatry. 2013;74:130–136. doi: 10.1016/j.biopsych.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanff TC, Furst SJ, Minor TR. Biochemical and anatomical substrates of depression and sickness behavior. Isr J Psychiat Rel Sci. 2010;47:64–71. [PubMed] [Google Scholar]
- Harboe E, Tjensvoll AB, Vefring HK, Goransson LG, Kvaloy JT, Omdal R. Fatigue in primary Sjogren’s syndrome-A link to sickness behaviour in animals? Brain, Behav Immunity. 2009;23:1103–1108. doi: 10.1016/j.bbi.2009.06.151. [DOI] [PubMed] [Google Scholar]
- Harden LM, du Plessis I, Poole S, Laburn HP. Interleukin (IL)-6 and IL-1 beta act synergistically within the brain to induce sickness behavior and fever in rats. Brain Behav Immun. 2008;22(6):838–849. doi: 10.1016/j.bbi.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Hauber W, Sommer S. Prefrontostriatal circuitry regulates effort-related decision making. Cereb Cortex. 2009;19(10):2240–2247. doi: 10.1093/cercor/bhn241. [DOI] [PubMed] [Google Scholar]
- Hiles SA, Baker AL, de Malmanche T, Attia J. A meta-analysis of difference in IL-6 and IL-10 between people with and without depression: exploring the causes of heterogeneity. Brain Behav Immun. 2012;26(7):1180–1188. doi: 10.1016/j.bbi.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Hockemeyer J, Burbiel JC, Müller CE. Multigram-scale syntheses, stability, and photoreactions of A2A adenosine receptor antagonists with 8-styrylxanthine structure: potential drugs for Parkinson’s disease. J Org Chem. 2004;69:3308–3318. doi: 10.1021/jo0358574. [DOI] [PubMed] [Google Scholar]
- Hodgson RA, Bertorelli R, Varty GB, Lachowicz JE, Forlani A, Fredduzzi S, Cohen-Williams ME, Higgins GA, Impagnatiello F, Nicolussi E, Parra LE, Foster C, Zhai Y, Neustadt BR, Stamford AW, Parker EM, Reggiani A, Hunter JC. Characterization of the potent and highly selective A2A receptor antagonists preladenant and SCH 412348 [7-[2-[4-2,4-difluorophenyl]-1 piperazinyl]ethyl]-2-(2-furanyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine]in rodent models of movement disorders and depression. J Pharmacol Exp Ther. 2009;330:294–303. doi: 10.1124/jpet.108.149617. [DOI] [PubMed] [Google Scholar]
- Kamata M, Higuchi H, Yoshimoto M, Yoshida K, Shimizu T. Effect of single intracerebroventricular injection of alpha-interferon on monoamine concentrations in the rat brain. Eur Neuropsychopharmacol. 2000;10(2):129–132. doi: 10.1016/s0924-977x(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Katafuchi T, Kondo T, Take S, Yoshimura M. Enhanced expression of brain interferon-alpha and serotonin transporter in immunologically induced fatigue in rats. Eur J Neurosci. 2005;22:2817–2826. doi: 10.1111/j.1460-9568.2005.04478.x. [DOI] [PubMed] [Google Scholar]
- Katsarou Z, Bostantjopoulou S, Hatzizisi O, Giza E, Soler-Cardona A, Kyriazis G. Immune factors or depression? Fatigue correlates in Parkinson’s disease. Rev Neurol. 2007;45:725–728. [PubMed] [Google Scholar]
- Kent S, Bluthé RM, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends Pharmacol Sci. 1992;13:24–28. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- Keppel . Design and Analysis: A Researcher’s Handbook. 3. Prentice-Hall; 1991. [Google Scholar]
- Kitamura Y, Yagi T, Kitagawa K, Shinomiya K, Kawasaki H, Asanuma M, Gomita Y. Effects of bupropion of the forced swim test and release of dopamine in the nucleus accumbens in ACTH-treated rats. Naunyn Schmiedebergs Arch Pharmacol. 2010;382(2):151–158. doi: 10.1007/s00210-010-0521-x. [DOI] [PubMed] [Google Scholar]
- Koch M, Schmid A, Schnitzler HU. Role of nucleus accumbens (DA) D1 and D2 receptors in instrumental and Pavlovian paradigms of conditioned reward. Psychopharmacology. 2000;152:67–73. doi: 10.1007/s002130000505. [DOI] [PubMed] [Google Scholar]
- Lapierre Y, Hum S. Treating fatigue. Internat MS J. 2007;14:64–71. [PubMed] [Google Scholar]
- Larson SJ, Romanoff RL, Dunn AJ, Glowa JR. Effects of interleukin-1beta on food-maintained behavior in the mouse. Brain Behav Immun. 2002;16(4):398–410. doi: 10.1006/brbi.2001.0634. [DOI] [PubMed] [Google Scholar]
- Mai B, Sommer S, Hauber W. Motivational states influence effort-based decision making in rats: the role of dopamine in the nucleus accumbens. Cogn Affect Behav Neurosci. 2012;12:74–84. doi: 10.3758/s13415-011-0068-4. [DOI] [PubMed] [Google Scholar]
- McLaughlin CL, Rogan GJ, Tou J, Baile CA, Joy WD. Food intake and body temperature responses of rats to recombinant human interleukin-1 beta and a tripeptide interleukin-1 beta antagonist. Physiol Behav. 1992;52:1155–1160. doi: 10.1016/0031-9384(92)90475-h. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Lu D, Winston KM, Thakur G, Swezey LA, Makriyannis A, Salamone JD. Behavioral effects of the novel cannabinoid full agonist AM 411. Pharmacol Biochem Behav. 2005;81:78–88. doi: 10.1016/j.pbb.2005.02.005. [DOI] [PubMed] [Google Scholar]
- McLaughlin PJ, Thakur GA, Vemuri VK, McClure ED, Brown CM, Winston KM, Wood JT, Makriyannis A, Salamone JD. Behavioral effects of the novel potent cannabinoid CB1 agonist AM 4054. Pharmacol Biochem Behav. 2013;109:16–22. doi: 10.1016/j.pbb.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merali Z, Brennan K, Brau P, Anisman H. Dissociating anorexia and anhedonis elicited by interleukin-1beta: anti-deprssant and gender effects on responding for “free chow” and “earned” sucrose intake. Psychopharmacology (Berl) 2003;165(4):413–418. doi: 10.1007/s00213-002-1273-1. [DOI] [PubMed] [Google Scholar]
- Miller AH. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav Immunity. 2009;23:149–158. doi: 10.1016/j.bbi.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote S, Font L, Farrar AM, Vontell R, Worden LT, Stopper CM, Port RG, Sink KS, Bunce JG, Chrobak JJ, Salamone JD. Nucleus accumbens adenosine A2A receptors regulate exertion of effort by acting on the ventral striatopallidal pathway. J Neurosci. 2008a;28:9037–9046. doi: 10.1523/JNEUROSCI.1525-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mingote S, Pereira M, Farrar AM, McLaughlin PJ, Salamone JD. Systemic administration of the adenosine A(2A) agonist CGS 21680 induces sedation at doses that suppress lever pressing and food intake. Pharmacol Biochem Behav. 2008b;89:345–351. doi: 10.1016/j.pbb.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minor TR, Huang Q, Foley EA. Cytokine-purine interactions in behavioral depression in rats. Integr Physiol Behav Sci. 2003;38:189–202. doi: 10.1007/BF02688853. [DOI] [PubMed] [Google Scholar]
- Mott AM, Nunes EJ, Collins LE, Port RG, Sink KS, Hockemeyer J, Müller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effects of the dopamine antagonist haloperidol on effort-related decision making in a T-maze cost/benefit procedure. Psychopharmacology (Berl) 2009;204(1):103–112. doi: 10.1007/s00213-008-1441-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouihate A, Chen X, Pittman QJ. Interleukin-1beta fever in rats: gender difference and estrous cycle influence. Am J Physiol. 1998;275(5 Pt 2):R1450–1454. doi: 10.1152/ajpregu.1998.275.5.R1450. [DOI] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Santerre JL, Given AB, Sager TN, Correa M, Salamone JD. Differential effects of selective adenosine antagonists on the effort-related impairments induced by dopamine D1 and D2 antagonism. Neuroscience. 2010;170:268–280. doi: 10.1016/j.neuroscience.2010.05.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes EJ, Randall PA, Podurgiel S, Correa M, Salamone JD. Nucleus accumbens neurotransmission and effort-related choice behavior in food motivation: Effects of drugs acting on dopamine, adenosine, and muscarinic acetylcholine receptors. Neurosci Biobehav Rev. 2013 doi: 10.1016/j.neubiorev.2013.04.002. http://dx.doi.org/10.1016/j.neubiorev.2013.04.002epub before print. [DOI] [PubMed]
- O’Connor JC, André C, Wang Y, Lawson MA, Szegedi SS, Lestage J, Castanon N, Kelley KW, Dantzer R. Interferon-gamma and tumor necrosis factor-alpha mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guerin. J Neurosci. 2009;29:4200–4209. doi: 10.1523/JNEUROSCI.5032-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo M, Lopez-Cruz L, Valverde O, Ledent C, Baqi Y, Muller CE, Salamone JD, Correa M. Adensoine A2A receptor antagonism and genetic deletion attenuate the effects of dopamine D2 antagonism on effort-related decision making in mice. Neuropharmacology. 2012;62(5–6):2068–2077. doi: 10.1016/j.neuropharm.2011.12.033. [DOI] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raedler TJ. Inflammatory mechanisms in major depressive disorder. Curr Opin Psychiatry. 2011;24(6):519–525. doi: 10.1097/YCO.0b013e32834b9db6. [DOI] [PubMed] [Google Scholar]
- Randall PA, Pardo M, Nunes EJ, López Cruz L, Vemuri VK, Makriyannis A, Baqi Y, Müller CE, Correa M, Salamone JD. Dopaminergic modulation of effort-related choice behavior as assessed by a progressive ratio chow feeding choice task: pharmacological studies and the role of individual differences. PLoS One. 2012;7(10):e47934. doi: 10.1371/journal.pone.0047934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosin DL, Robeva A, Woodard RL, Guyenet PG, Linden J. Immunohistochemical localization of adenosine A2A receptors in the rat central nervous system. J Comp Neurol. 1998;401:163–186. [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76(3):470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Steinpreis RE, McCullough LD, Smith P, Grebel D, Mahan K. Haloperidol and nucleus accumbens (DA) depletion suppress lever pressing for food but increase free food consumption in a novel food choice procedure. Psychopharmacology (Berl) 1991;104:515–521. doi: 10.1007/BF02245659. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Cousins MS, Snyder BJ. Behavioral functions of nucleus accumbens dopamine: empirical and conceptual problems with the anhedonia hypothesis. Neurosci Biobehav Rev. 1997;21:341–359. doi: 10.1016/s0149-7634(96)00017-6. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Wisniecki A, Carlson BB, Correa M. Nucleus accumbens dopamine depletions make animals highly sensitive to high fixed ration requirements but do not impair primary food reinforcement. Neuroscience. 2001;105(4):863–870. doi: 10.1016/s0306-4522(01)00249-4. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Arizzi MN, Sandoval MD, Cervone KM, Aberman JE. (DA) antagonists alter response allocation but do not suppress appetite for food in rats: contrast between the effects of SKF 83566, raclopride, and fenfluramine on a concurrent choice task. Psychopharmacology (Berl) 2002;160:371–380. doi: 10.1007/s00213-001-0994-x. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM, Farrar AM. Nucleus Accumbens (DA) and the forebrain circuitry involved in behavioral activation and effort-related decision making: implications for understanding anergia and psychomotor slowing in depression. Curr Psychiat Rev. 2006;2:267–280. [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Farrar AM, Font L, Patel V, Schlar DE, Nunes EJ, Collins LE, Sager TN. Differential actions of adenosine A1 and A2A antagonists on the effort-related effects of (DA) D2 antagonism. Behav Brain Res. 2009;201:216–222. doi: 10.1016/j.bbr.2009.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Nunes EJ, Randall PA, Pardo M. The behavioral pharmacology of effort-related choice behavior: dopamine, adenosine, and beyond. J Exp Anal Behav. 2012;97(1):125–146. doi: 10.1901/jeab.2012.97-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santerre JL, Nunes EJ, Randall PA, Baqi Y, Müller CE, Salamone JD. Behavioral studies with the novel adenosine A2A antagonist MSX-4: reversal of the effects of (DA) D2 antagonism. Pharamcol Biochem Behav. 2012;102(4):477–487. doi: 10.1016/j.pbb.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Segovia KN, Correa M, Salamone JD. Slow phasic changes in nucleus accumbens dopamine release during fixed ratio acquisition: a microdialysis study. Neuroscience. 2011;196:178–188. doi: 10.1016/j.neuroscience.2011.07.078. [DOI] [PubMed] [Google Scholar]
- Segovia KN, Correa M, Lennington JB, Conover JC, Salamone JD. Changes in nucleus accumbens and neostriatal c-Fos and DARPP-32 immunoreactivity during different stages of food-reinforced instrumental training. Eur J Neurosci. 2012;35(8):1354–1367. doi: 10.1111/j.1460-9568.2012.08036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto H, Kataoka Y, Korikawa T, Fujihara N, Oishi R. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. 1997;747(2):348–351. doi: 10.1016/s0006-8993(96)01371-6. [DOI] [PubMed] [Google Scholar]
- Sink KS, Vemuri VK, Olszewska T, Makriyannis A, Salamone JD. Cannabinoid CB1 antagonists and dopamine antagonists produce different effects on a task involving response allocation and effort-related choice in food-seeking behavior. Psychopharmacology. 2008;196(4):565–574. doi: 10.1007/s00213-007-0988-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Li X, Kang Z, Kadotomi Y. Omega-3 fatty acid ethyl-eicosapentaenoate attenuates IL-1beta-induced changes in dopamine and metabolites in the shell of the nucleus accumbens: involved with PLA2 activity and corticosterone secretion. Neuropsychopharmacology. 2007;32(2):736–744. doi: 10.1038/sj.npp.1301117. [DOI] [PubMed] [Google Scholar]
- Song C, Manku MS, Horrobin DF. Long-chain polyunsaturated fatty acids modulate interleukin-1beta-induced changes in behavior, monoaminergic neurotransmitters, and brain inflammation in rats. J Nutr. 2008;138(5):954–963. doi: 10.1093/jn/138.5.954. [DOI] [PubMed] [Google Scholar]
- Taylor AN, Tio DL, Heng NS, Yirmiya R. Alcohol consumption attenuates febrile responses to lipopolysaccharide and interleukin-1 beta in male rats. Alcohol Clin Exp Res. 2002;26:44–52. [PubMed] [Google Scholar]
- Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol. 2012;121(3):553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton ME, Bannerman DM, Alterescu K, Rushworth MFS. Functional specialization within medial frontal cortex of the anterior cingulate for evaluating effort-related decisions. J Neurosci. 2003;23:6475–6479. doi: 10.1523/JNEUROSCI.23-16-06475.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle MC, Treadway MT, Mayo LM, Zald DH, de Wit H. Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci. 2011;31(46):16597–16602. doi: 10.1523/JNEUROSCI.4387-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden LT, Shahriari M, Farrar AM, Sink KS, Hockemeyer J, Müller CE, Salamone JD. The adenosine A2A antagonist MSX-3 reverses the effort-related effects of dopamine blockade: differential interaction with D1 and D2 family antagonists. Psychopharmacology (Berl) 2009;203(3):489–499. doi: 10.1007/s00213-008-1396-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu TH, Lin CH. IL-6 mediated alterations on immobile behavior of rats in the forced swim test via ERK1/2 activation in specific brain regions. Behav Brain Res. 2008;193:183–191. doi: 10.1016/j.bbr.2008.05.009. [DOI] [PubMed] [Google Scholar]