Abstract
Alcohol drinking during adolescence is associated in adulthood with heavier alcohol drinking and an increased rate of alcohol dependence. Past research in our laboratory has indicated that peri-adolescent ethanol consumption can enhance the acquisition and reduce the rate of extinction of ethanol self-administration in adulthood. Caveats of the past research include reinforcer specificity, increased oral consumption during peri-adolescence, and a lack of quantitative assessment of the reinforcing properties of ethanol. The current experiments were designed to determine the effects of peri-adolescent ethanol or saccharin drinking on acquisition and extinction of oral ethanol self-administration and ethanol seeking, and to quantitatively assess the reinforcing properties of ethanol (progressive ratio). Ethanol or saccharin access by alcohol-preferring (P) rats occurred during postnatal day (PND) 30–60. Animals began operant self-administration of ethanol or saccharin after PND 85. After 10 weeks of daily operant self-administration, rats were tested in a progressive ratio paradigm. Two weeks later, self-administration was extinguished in all rats. Peri-adolescent ethanol consumption specifically enhanced the acquisition of ethanol self-administration, reduced the rate of extinction for ethanol self-administration, and quantitatively increased the reinforcing properties of ethanol during adulthood. Peri-adolescent saccharin consumption was without effect. The data indicate that ethanol consumption during peri-adolescence results in neuroadaptations that may specifically enhance the reinforcing properties of ethanol during adulthood. This increase in the reinforcing properties of ethanol could be a part of biological sequelae that are the basis for the effects of adolescent alcohol consumption on the increase in the rate of alcoholism during adulthood.
Keywords: alcoholism, adolescence, operant, self-administration, progressive ratio
Introduction
For the vast majority of Americans, the initiation of alcohol use begins during adolescence. A high percentage (12%) of adolescents begin using alcohol during middle school (8th grade), and prior to high school graduation the vast majority of Americans (80–90%) have consumed alcohol (Johnston, O'Malley, Bachman, & Schulenberg., 2004). In combination with moderate alcohol consumption, adolescents engage in binge drinking episodes in a U-shape pattern related to age. Self-reports have indicated that 22 and 28% of 10th and 12th grade students, respectively, reported an incident of binge drinking within the previous 2 weeks (Johnston et al., 2004). College students report a previous high level of binge drinking during high school (70%) and frequent on-going episodes of binge drinking during college (44%). A subset of college students are frequent binge drinkers (19–25% report more than 3 episodes of binge drinking per week; SAMHSA, 2008; Wechsler, Dowdall, Davenport, & Castillo, 1995; Wechsler, Lee, Kuo, & Lee, 2000).
Alcohol consumption during adolescence is associated with a number of deleterious consequences. Age of first drink and the propensity to have binge ethanol-drinking episodes during adolescence is associated with increased alcohol involvement, heavier drinking bouts, arrests for driving with ability impaired, and an increased rate of alcohol dependence during adulthood (Chou & Pickering, 1992; Hingson, Heeren, & Edwards, 2006; Hingson, Hereen, & Winter, 2008). Epidemiological studies have indicated a 1.3 to 1.6 times increased rate of alcohol dependence in individuals who initiate alcohol use prior to the age of 15 (Dawson, Goldstein, Chou, Ruan, & Grant, 2008). The deleterious effects of adolescent ethanol consumption on adult alcohol dependence are compounded in individuals with a family history of alcoholism (Agrawal et al., 2009; Jacobus et al., 2009).
The neurological remodeling of the adolescent brain is extensive and includes cortical and limbic regions (Spear, 2000). It is the interaction between adolescent ethanol drinking and the neurological flux of adolescence that is thought to produce the enduring deleterious consequences observed in adult alcoholics. Peri-adolescent ethanol intake in male alcohol-preferring (P) rats produces persistent neuroadaptations in the posterior ventral tegmental area (pVTA) that enhance the sensitivity, and prolong the response, to ethanol (Toalston et al., 2014). Repeated alcohol administration during adolescence alters the expression of histones in the nucleus accumbens (Pascual, Boix, Felipo, & Guerri, 2009). Recent electrophysiological data have indicated that adolescent ethanol intake has a tendency to affix a portion of the hippocampus in the adolescent state (Spear & Swartzwelder, 2014). Specifically, adolescent ethanol intake has been shown to produce persistent alterations in GABAA receptors in the dentate granule cells that result in a maintained higher sensitivity to ethanol (Fleming, Acheson, Moore, Wilson, & Swartzwelder, 2012). Adolescent ethanol intake, but not comparable adult exposure, results in long-term alterations in inactivating potassium channels in interneurons in the hippocampus that may be the basis of memory-related impairment produced by adolescent ethanol intake (Li et al., 2013). The neuroadaptations produced by adolescent ethanol intake are likely the biological components that mediate the alteration in behavior observed in these organisms.
Peri-adolescent alcohol drinking by P rats has been reported to produce long-lasting alterations in the reinforcing effects of ethanol, as indicated by P rats with access to ethanol, compared to the water control group, acquiring acquisition of ethanol operant responding sooner, showing a greater resistance to extinguishing of responding, and having a more prolonged elevated level of relapse responding for ethanol (Rodd-Henricks et al., 2002a). Adult ethanol intake for a comparable time period did not result in these effects (Rodd-Henricks et al., 2002b). In Sprague-Dawley rats, adolescent consumption of a sweetened ethanol solution increased adult consumption of the same solution, but not unsweetened ethanol (Broadwater, Varlinskaya, & Spear, 2013). Adolescent consumption of a sweetened solution increased intake of the sweetened solution, but not ethanol or a sweetened ethanol solution (Broadwater et al., 2013). In mice, adolescent ethanol intake can enhance adult ethanol consumption, but this is influenced by gender and genotype (Moore, Mariani, Linsenbardt, Melón, & Boehm, 2010; Strong et al., 2010).
The current experiments were designed to determine the effects of peri-adolescent ethanol and saccharin (SACC) consumption on the acquisition and extinction of ethanol self-administration, and to quantifiably assess the reinforcing properties of oral ethanol self-administration (progressive ratio). The overall hypothesis to be tested was that peri-adolescent ethanol consumption would enhance acquisition of ethanol self-administration, retard extinction training, and enhance the reinforcing properties of ethanol during adulthood.
Methods
Subjects
Female pups (n = 61) from the 60th and 61st generations of the selectively bred alcohol-preferring P line were weaned at 21 days of age and housed with littermates until the beginning of the experiment. At 28–29 days of age, subjects were transferred to and maintained in individual hanging wire mesh cages with access to ad libitum water and food. Body weights increased normally during the course of the experiment for all groups. Female subjects were chosen over male subjects due to general weight consistency desired over the long length of time used for operant testing. Subjects used in these experiments were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care. All research protocols were approved by the Institutional Care and Use Committee of the Indiana University School of Medicine (Indianapolis, IN), in accordance with guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, the NIH, and the Guide for the Care and Use of Laboratory Animals (2011).
Peri-adolescent ethanol or saccharin exposure procedure
Pups were single-housed in hanging stainless-steel cages (Allentown Caging Equipment Co., Allentown, New Jersey) on PND 28. Subjects were initially maintained on a 12-h light/dark cycle, with lights on at 9:00 AM. There were 3 adolescent exposure groups (water, ethanol, and SACC). On PND 30, subjects received either ad libitum water or continuous access to 15% v/v ethanol and water, or 0.0125% w/v SACC and water until PND 60 as previously described (Rodd-Henricks et al., 2002a). The concentrations of ethanol and SACC used were determined by previous research that indicated that under operant conditions, P rats self-administered these concentrations at equivalent levels (Nowak et al., 1999). Food was available ad libitum. Bottle and body weights for all subjects were recorded every other day.
On PND 60, ethanol or SACC access ceased, and subjects were pair-housed in standard shoebox cages, within the same treatment condition. Subjects were also immediately transferred to a 12-h reverse dark/light cycle, lights off at 10:00 AM, to optimize rats’ nocturnal activity levels for later procedures. After PND 60, subjects received no further oral ethanol or SACC intake experience in their home cage.
Adult operant self-administration procedure
Self-administration experiments were conducted in standard two-lever operant chambers (25 × 28 × 30 cm, Coulbourn Instruments, Allentown, PA), within ventilated sound-attenuated enclosures. Two levers, located on the same wall, were placed 15 cm above a grid floor and 13 cm apart. Directly beneath each lever was a trough from which a 0.1-mL dipper cup could rise (response-contingently, controlled by a desktop computer) to deliver fluid. When a reinforced response was reached and the dipper cup would rise, a small cue light illuminated the dipper cup (4 sec). Arrangement of water and reinforcement levers to the left or right position were counterbalanced among subjects, but levers remained the same for each subject throughout all experimental sessions. Chambers were illuminated by house lights during experimental sessions. Sessions (except the Progressive Ratio tests) were 60 min in duration, occurring daily.
Operant self-administration testing started on PND 75. Rats were allowed to self-administer water, and 15% ethanol or 0.0125% SACC (6 total groups: naïve in adolescence, ethanol operant; naïve in adolescence, SACC operant; ethanol in adolescence, ethanol operant; ethanol in adolescence, SACC operant; SACC in adolescence, ethanol operant; and SACC in adolescence, SACC operant). Rats were only tested for either ethanol or SACC self-administration during adulthood. Therefore, there were two separate groups of rats that were tested for ethanol or SACC self-administration during adulthood. During the initial 2 weeks of daily operant access, all solutions (water and ethanol/SACC) were reinforced on a fixed ratio of 1 (FR-1) schedule. The response requirement for ethanol and SACC was increased to an FR-3 schedule for 2 weeks, and then to FR-5 schedule for 2 weeks.
Progressive ratio test
Following the initial 6 weeks of operant self-administration, all rats were exposed to a progressive ratio procedure for the operant reinforcer that was assigned to that subject (ethanol or SACC). The progressive ratio test was a slightly modified version established in the laboratory that was able to readily detect group differences in the reinforcing properties of oral ethanol self-administration (Rodd et al., 2003). The response requirement in the progressive ratio was as follows: all rats began at a FR-1 schedule of reinforcement on the ethanol/SACC lever, and after 3 reinforcements (3 lever presses), the schedule was increased by 4 to FR-5; after receiving another 3 reinforcements (15 lever presses), the schedule was increased by 3 to FR-8. This pattern of increasing the response requirement by 3 after each 3 reinforcements was continued until the rat did not meet the FR requirement within 7 min. The lowest FR value that the rat could not attain was defined as the breakpoint. This progressive ratio schedule was selected in an attempt to balance the multiple factors involved in oral ethanol self-administration (i.e., slow delivery of the drug [0.1 mL/reinforcement], slow rate of absorption of ethanol, and the sedative effects of ethanol). Therefore, this progressive ratio paradigm was used in order to sufficiently increase the required workload before the sedative effects of ethanol began to interfere with operant performance. The SACC progressive ratio pattern was the same as used for ethanol. The water lever was maintained on a FR-1 throughout the progressive ratio paradigm.
Extinction testing
Following the initial progressive ratio test, all rats received 2 additional weeks of FR-5 operant access to the original assigned solutions (water, and ethanol or SACC). On PND 134, rats were placed into the operant chambers during the 60-min time period, but neither water nor ethanol or SACC was available. Water was also not available during the extinction procedure since water is a primary reinforcer and has been shown to influence responding during extinction testing (i.e., superstitious behaviors, Macintosh, 1977). The lever previously associated with the delivery of ethanol or SACC was maintained on a FR-5 schedule, and the lever previously associated with the delivery of water was maintained on a FR-1. With the exception of no fluid being presented, the delivery system operated exactly as during acquisition; rats still received the auditory stimulus of the dipper raising and the visual cue of the small light being illuminated above the dipper trough. Rats were exposed to the extinction sessions for 9 consecutive days.
Statistical analysis
The initial analysis examined the effects of peri-adolescent ethanol or SACC consumption on the acquisition of adult operant oral self-administration of ethanol or SACC. The analysis for each solution was a mixed-factor analysis of variance (ANOVA); between-subject factor of peri-adolescent drinking condition and within-subject factor of operant session. Responding during the first extinction period was analyzed for each solution in a similar manner. Tukey's b tests were used to perform the post hoc comparisons. A p value < .05 was considered significant.
The reinforcing properties of ethanol and SACC assessed through the progressive ratio procedure was analyzed using a multifactor between-subject (peri-adolescent drinking condition and solution) ANOVA. Post hoc comparisons were Tukey's b.
Results
Peri-adolescent ethanol consumption
Previous studies have found that adolescent P rats will consume ethanol within even the first 24 h of exposure (McKinzie et al., 1998; Rodd-Henricks et al., 2002a). The present study found similar results. The final week's daily intake average for SACC was 30.1 ± 1.7 g of the 0.0125% saccharin solution, and the final week's intake average for ethanol was 8.8 ± 1.1 g/kg (data not shown). The amount of ethanol consumed would be predicted to produce significant, pharmacologically relevant (>50 mg%) blood alcohol concentrations (Bell et al., 2003, 2004, 2006).
Acquisition of ethanol or SACC self-administration
The results indicated that only peri-adolescent consumption of ethanol enhanced the acquisition of adult ethanol self-administration (Fig. 1, left panel). The overall analysis revealed a significant heterogeneity of variance; therefore, the repeated-measure analysis F values were interpreted using the Hotelling's Trace correction analysis to compensate for the violation of the assumption of the repeated-measure ANOVA. The results indicated a significant effect of Day (F[6,20] = 4.564; p = 0.005), no effect of Adolescent Drinking Condition (F[2,25] = 1.3; p = 0.289), but a significant Day × Adolescent Drinking Condition interaction (F[12,38] = 2.0; p = 0.05). The significant interaction term was decomposed by holding Day constant and examining whether there were significant differences between the Adolescent Drinking Conditions groups for each separate Day. Individual ANOVAs performed on each Day revealed that there was only a significant effect of Adolescent Drinking Condition during the fourth acquisition session (F[2,25] = 4.2; p = 0.027, all other sessions p values > 0.164). Post hoc comparisons (Tukey's b) indicated that during the fourth acquisition session, rats that consumed ethanol during adolescence responded more for ethanol than the other two groups.
Fig. 1.
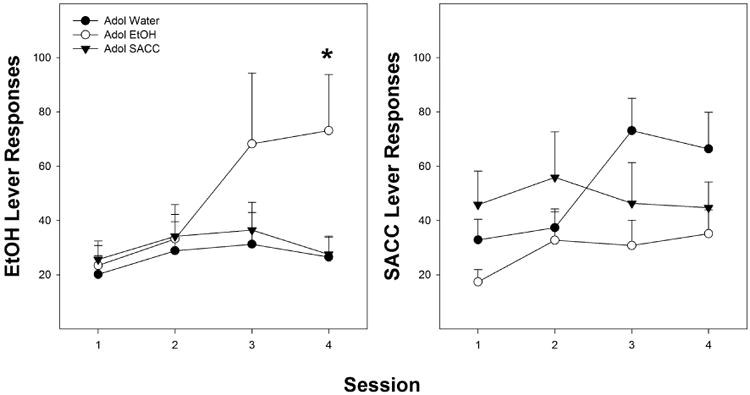
The left panel depicts the mean (± SEM) average number of responses on the ethanol lever during sessions 1–4 in peri-adolescent naïve, ethanol, and SACC drinkers (all at FR–1; n = 8, 9, and 11, respectively). * indicates significantly more responses in the peri-adolescent ethanol drinkers compared to water or SACC drinkers. The right panel depicts the mean (± SEM) average number of responses on the SACC lever during sessions 1–4 in peri-adolescent naïve, ethanol, and SACC drinkers (n = 8, 8, and 10, respectively). There were no statistically significant differences between the peri-adolescent groups for SACC responding.
In contrast, peri-adolescent consumption of ethanol or SACC failed to alter the acquisition of adult SACC self-administration (Fig. 1, right panel). The results indicated a significant effect of Day (F[6,8] = 4.3; p = 0.007), but no effect of Adolescent Drinking Condition (F[2,23] = 2.0; p = 0.158), and no significant Day × Adolescent Drinking Condition interaction (F[12,38] = 1.0; p = 0.467).
Progressive ratio testing
Peri-adolescent ethanol consumption increased the reinforcing properties of ethanol during adulthood (Fig. 2, left panel). Examining the Breakpoints obtained for ethanol self-administration, there was a significant effect of Peri-Adolescent Drinking Condition (F[2,28] = 3.9; p = 0.033). Post hoc comparisons indicated that P rats that consumed ethanol during adolescence expressed a higher breakpoint for ethanol than P rats that consumed only water during adolescence (Tukey's b).
Fig. 2.
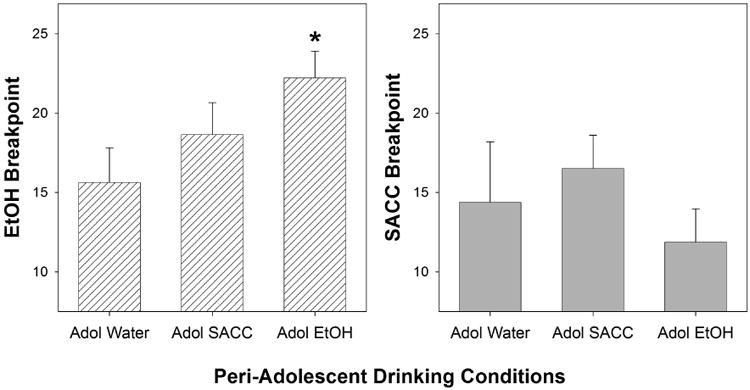
The left panel depicts the mean (± SEM) average breakpoint for the ethanol lever in peri-adolescent naïve, ethanol, and SACC drinkers (n = 8, 9, and 11, respectively). * indicates significantly higher breakpoint level in the peri-adolescent ethanol drinkers compared to water drinkers. The right panel depicts the mean (± SEM) average breakpoint for the SACC lever in peri-adolescent naïve, ethanol, and SACC drinkers (n = 8, 8, and 10, respectively). There were no statistically significant differences between the peri-adolescent groups for SACC responding.
In contrast, ethanol or SACC consumption during adolescence did not alter the breakpoint of SACC during adulthood (Fig. 2, right panel). Statistically, there was no significant effect of Adolescent Drinking Condition on SACC Breakpoint responding (F[2,28] = 0.3; p = 0.78), or SACC lever responses and total time of PR test (p = 1.0 and p = 0.83, respectively).
Extinction
Peri-adolescent ethanol consumption affected the rate of extinction responding for ethanol during adulthood (Fig. 3, left panel). A repeated-measure ANOVA (within-subject factor Day) indicated a significant effect of Day (F[9,20] = 86.7; p < 0.001) and a Day × Adolescent Drinking Condition interaction (F[18,42] = 2.1; p = 0.027). The interaction term was decomposed by examining the effects of Adolescent Drinking Condition on each individual Extinction session. During the first Extinction session, there was a significant effect of Adolescent Drinking Condition on the number of responses on the lever previously associated with the delivery of ethanol (F[2,28] = 5.8; p = 0.008). Post hoc comparisons confirmed that the P rats that consumed ethanol during adolescence responded more on the lever previously associated with the delivery of ethanol than P rats that consumed SACC or water only during adolescence. Similarly, during the second session of Extinction, P rats that consumed ethanol during adolescence responded significantly more than the other two groups (p = 0.028).
Fig. 3.
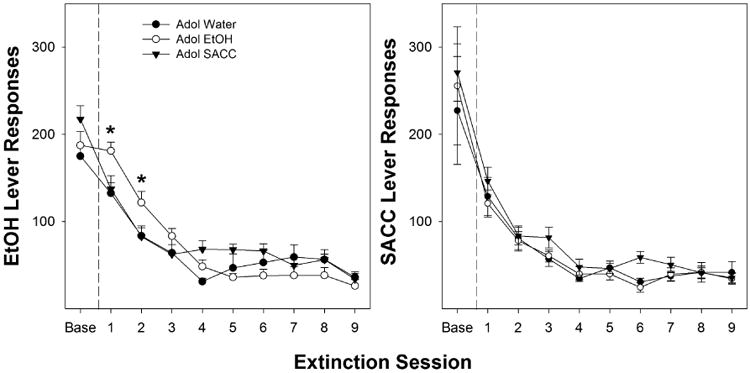
The left panel depicts the mean (± SEM) average number of responses on the ethanol lever during extinction training in peri-adolescent naïve, ethanol, and SACC drinkers (n = 8, 9, and 11, respectively). * indicates significantly more responses in the peri-adolescent ethanol drinkers compared to water or SACC drinkers. The right panel depicts the mean (± SEM) average number of responses on the SACC lever during extinction training in peri-adolescent naïve, ethanol, and SACC drinkers (n = 8, 8, and 10, respectively). There were no statistically significant differences between the peri-adolescent groups for the extinction of SACC responding.
In contrast, peri-adolescent consumption of ethanol or SACC did not alter SACC extinction learning (Fig. 3, right panel). A repeated-measure ANOVA (within-subject factor Day) indicated a significant effect of Day (F[9,15] = 10.9; p < 0.001), but no effect of Adolescent Drinking Condition (F[2,23] = 0.3; p = 0.78) or Day × Adolescent Drinking Condition interaction (F[18,32] = 1.3; p = 0.26). Although the interaction term was not significant, the effect of Adolescent Drinking Condition on each Extinction session was performed to parallel the analysis performed for adult ethanol operant self-administration. As confirmed by the overall ANOVA, there was no significant effect of Adolescent Drinking Condition on SACC Extinction responding for an individual session (F values [2,23] < 1.9; p values < 0.174).
Discussion
The findings of the current experiments indicate that peri-adolescent ethanol consumption by female P rats produces neuroadaptations that result in enhancement of the reinforcing properties of ethanol during adulthood (Fig. 1–3). Specifically, adolescent ethanol consumption results in an increased rate of the acquisition of ethanol self-administration (Fig. 1), an increase in the willingness to work to obtain ethanol (breakpoint; Fig. 2), and a reduction in the rate of extinction of responding for ethanol (Fig. 3). In addition, the data also indicate that these effects are specific for peri-adolescent ethanol consumption and are not observed following peri-adolescent SACC consumption.
The current findings indicate that peri-adolescent ethanol consumption can alter adult ethanol self-administration behaviors. Recently (Toalston et al., 2014), we have reported that comparable peri-adolescent consumption in P rats enhances the reinforcing properties of ethanol as measured by intracranial self-administration of ethanol within the pVTA. In addition, ethanol microinjected into the pVTA in P rats with a peri-adolescent history of ethanol consumption resulted in greater and longer DA release in the nucleus accumbens shell compared to rats that only consumed water during peri-adolescence (Toalston et al., 2014). Therefore, these current data sets indicate that peri-adolescent ethanol consumption enhances the reinforcing properties of ethanol in adulthood within the mesolimbic DA system, which could be the basis for the current findings.
The effects of ethanol consumption during adolescence on adult ethanol consumption are inconsistently reported in the literature. However, like every aspect of alcohol studies, the manner, amount, and duration of ethanol exposure is likely to affect the observed consequences of ethanol consumption. Non-physiologically relevant levels of alcohol consumption (ethanol intake levels that would produce no significant blood ethanol concentration) during adolescence have been shown to have no effect on adult ethanol consumption (Siegmund, Vengeliene, Singer, & Spanagel, 2005; Slawecki, 2002 Slawecki & Betancourt, 2002; Slawecki, Thorsell, & Ehlers, 2004). Significant consumption of sweetened ethanol in Sprague-Dawley adolescent rats increases adult consumption of sweetened ethanol but not unadulterated high ethanol concentration solutions (20%; Broadwater et al., 2013). Injections of ethanol during adolescence can result in a conditioned taste aversion to sweetened solutions and indeterminate effects on adult ethanol consumption (Gilpin, Karanikas, & Richardson, 2012). In contrast, adolescents injected with ethanol using procedures that do not produce taste aversion can enhance adult ethanol consumption (Maldonado-Devincci, Alipour, Michael, & Kirstein, 2010; Pascual et al., 2009; Pascual, Blanco, Cauli, Minarro, & Guerri, 2007). Voluntary ethanol drinking during adolescence at a level that produces pharmacologically relevant levels of ethanol has been shown to enhance adult ethanol consumption (O'Tousa, Matson, & Grahame, 2013; Strong et al., 2010; Walker & Ehlers, 2009). In P rats, adolescent ethanol consumption (oral free-choice) increases the acquisition of ethanol self-administration (operant), decreases the rate of extinction of ethanol self-administration, enhances relapse drinking, and enhances the ability of a priming dose of ethanol to increase ethanol-seeking (Rodd-Henricks et al., 2002a). These effects of adolescent ethanol consumption in P rats were not observed in adult P rats given similar drinking exposure (Rodd-Henricks et al., 2002b). The current data indicate that consumption of another reinforcer (SACC) during peri-adolescence does not result in parallel findings. In addition, the current studies indicate that peri-adolescent ethanol consumption quantitatively enhances the willingness to obtain ethanol during adulthood (increased breakpoint).
The higher responding on the ethanol lever by the peri-adolescent ethanol group compared to the peri-adolescent water or water and SACC groups during extinction sessions 1–2 replicates the previous study that indicated peri-adolescent ethanol drinking increases resistance to extinguish alcohol-seeking behavior (Rodd-Henricks et al., 2002a). A reduction in the rate of extinction has been linked with an increase in reward saliency to the reinforcer and/or an increase in the tolerance to the emotional aspects of extinction training (Azrin, Hutchinson, & Hake, 1966; Macintosh, 1977). Combined with the results for the progressive ratio test, the current data indicate that, in part, the reduction in the rate of extinction observed in peri-adolescent ethanol-consuming P rats is likely based upon an increase in the reward saliency of ethanol.
Overall, the data indicate that adolescent ethanol consumption in a rodent model of alcoholism produced persistent alterations in the propensity to self-administer ethanol and the rewarding properties of ethanol during adulthood. The increase in reward sensitivity during adulthood following adolescent ethanol consumption may be the biological basis for the deleterious effects that adolescent alcohol consumption has on adult alcoholism. Elucidating specific neuroadaptations within the mesolimbic DA system produced by adolescent ethanol consumption could lead to interventions/treatments to counter the consequences of this common human behavior.
Highlights.
EtOH consumption during adolescence increases EtOH intake during adulthood.
Saccharin consumption during adolescence has no effect on adult EtOH intake.
Adolescent EtOH consumption increases the reward value of EtOH during adulthood.
Acknowledgments
Sources of support: Grants AA07611, AA07462, AA012262, AA0200396 and AA013522
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agrawal A, Sartor CE, Lynskey MT, Grant JD, Pergadia ML, Grucza R, et al. Evidence for an interaction between age at first drink and genetic influences on DSM-IV alcohol dependence symptoms. Alcoholism: Clinical and Experimental Research. 2009;33:2047–2056. doi: 10.1111/j.1530-0277.2009.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azrin NH, Hutchinson RR, Hake DF. Extinction-induced aggression. Journal of the Experimental Analysis of Behavior. 1966;9:191–204. doi: 10.1901/jeab.1966.9-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Boutwell CL, Hsu CC, Lumeng L, Murphy JM, et al. Effects of long-term episodic access to ethanol on the expression of an alcohol deprivation effect in low alcohol-consuming rats. Alcoholism: Clinical and Experimental Research. 2004;28:1867–1874. doi: 10.1097/01.alc.0000148101.20547.0a. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks ZA, Kuc KA, Lumeng L, Li TK, Murphy JM, et al. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on the intake of ethanol by male and female periadolescent alcohol-preferring (P) rats. Alcohol. 2003;29:137–148. doi: 10.1016/s0741-8329(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, et al. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacology, Biochemistry, and Behavior. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Broadwater M, Varlinskaya EI, Spear LP. Effects of voluntary access to sweetened ethanol during adolescence on intake in adulthood. Alcoholism: Clinical and Experimental Research. 2013;37:1048–1055. doi: 10.1111/acer.12049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SP, Pickering RP. Early onset of drinking as a risk factor for lifetime alcohol-related problems. British Journal of Addiction. 1992;87:1199–1204. doi: 10.1111/j.1360-0443.1992.tb02008.x. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Goldstein RB, Chou SP, Ruan WJ, Grant BF. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorder. Alcoholism: Clinical and Experimental Research. 2008;32:2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming RL, Acheson SK, Moore SD, Wilson WA, Swartzwelder HS. In the rat, chronic intermittent ethanol exposure during adolescence alters the ethanol sensitivity of tonic inhibition in adulthood. Alcoholism: Clinical and Experimental Research. 2012;36:279–285. doi: 10.1111/j.1530-0277.2011.01615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilpin NW, Karanikas CA, Richardson HN. Adolescent binge drinking leads to changes in alcohol drinking, anxiety, and amygdalar corticotropin releasing factor cells in adulthood in male rats. PLoS One. 2012;7:e31466. doi: 10.1371/journal.pone.0031466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Edwards EM. Age at drinking onset, alcohol dependence, and their relation to drug use and dependence, driving under the influence of drugs, and motor-vehicle crash involvement because of drugs. Journal of Studies on Alcohol and Drugs. 2008;69:192–201. doi: 10.15288/jsad.2008.69.192. [DOI] [PubMed] [Google Scholar]
- Hingson RW, Heeren T, Winter MR. Age at drinking onset and alcohol dependence: age at onset, duration, and severity. Archives of Pediatrics & Adolescent Medicine. 2006;160:739–746. doi: 10.1001/archpedi.160.7.739. [DOI] [PubMed] [Google Scholar]
- Institute for Laboratory Animal Research ILAR. Guide for the Care and Use of Laboratory Animals. Vol. 8. Washington, D.C.: National Academies Press; 2011. [Google Scholar]
- Jacobus J, McQueeny T, Bava S, Schweinsburg BC, Frank LK, Yang TT, et al. White matter integrity in adolescents with histories of marijuana use and binge drinking. Neurotoxicology and Teratology. 2009;31:349–355. doi: 10.1016/j.ntt.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston LD, O'Malley PM, Bachman JG, Schulenberg JE. Monitoring the future; national survey results on drug use 1975–2004 Volume I: Secondary school students. Bethesda, MD: National Institute on Drug Abuse; 2004. NIH Publication No. 04-5507. [Google Scholar]
- Li Q, Fleming RL, Acheson SK, Madison RD, Moore SD, Risher ML, et al. Long-term modulation of A-type K(+) conductances in hippocampal CA1 interneurons in rats after chronic intermittent ethanol exposure during adolescence or adulthood. Alcoholism: Clinical and Experimental Research. 2013;37:2074–2085. doi: 10.1111/acer.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macintosh JJ. Stimulus control: Attentional factors. In: Honig WK, Staddon JER, editors. Handbook of operant behavior. Englewood Cliffs, NJ: Prentice-Hall; 1977. pp. 162–241. [Google Scholar]
- Maldonado-Devincci AM, Alipour KK, Michael LA, Kirstein CL. Repeated binge ethanol administration during adolescence enhances voluntary sweetened ethanol intake in young adulthood in male and female rats. Pharmacology, Biochemistry, and Behavior. 2010;96:476–487. doi: 10.1016/j.pbb.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Murphy JM, Li TK, Lumeng L, McBride WJ. Development of alcohol drinking behavior in rat lines selectively bred for divergent alcohol preference. Alcoholism: Clinical and Experimental Research. 1998;22:1584–1590. [PubMed] [Google Scholar]
- Moore EM, Mariani JN, Linsenbardt DN, Melón LC, Boehm SL., 2nd Adolescent C57BL/6J (but not DBA/2J) mice consume greater amounts of limited-access ethanol compared to adults and display continued elevated ethanol intake into adulthood. Alcoholism: Clinical and Experimental Research. 2010;34:734–742. doi: 10.1111/j.1530-0277.2009.01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak KL, McKinzie DL, McBride WJ, Murphy JM. Patterns of ethanol and saccharin intake in P rats under limited-access conditions. Alcohol. 1999;19:85–96. doi: 10.1016/s0741-8329(99)00028-2. [DOI] [PubMed] [Google Scholar]
- O'Tousa DS, Matson LM, Grahame NJ. Effects of intoxicating free-choice alcohol consumption during adolescence on drinking and impulsivity during adulthood in selectively bred high-alcohol preferring mice. Alcoholism: Clinical and Experimental Research. 2013;37:141–149. doi: 10.1111/j.1530-0277.2012.01857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual M, Blanco AM, Cauli O, Minarro J, Guerri C. Intermittent ethanol exposure induces inflammatory brain damage and causes long-term behavioural alterations in adolescent rats. The European Journal of Neuroscience. 2007;25:541–550. doi: 10.1111/j.1460-9568.2006.05298.x. [DOI] [PubMed] [Google Scholar]
- Pascual M, Boix J, Felipo V, Guerri C. Repeated alcohol administration during adolescence causes changes in the mesolimbic dopaminergic and glutamatergic systems and promotes alcohol intake in the adult rat. Journal of Neurochemistry. 2009;108:920–931. doi: 10.1111/j.1471-4159.2008.05835.x. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Murphy JM, Lumeng L, Li TK, et al. Effects of repeated alcohol deprivations on operant ethanol self-administration by alcohol-preferring (P) rats. Neuropsychopharmacology. 2003;28:1614–1621. doi: 10.1038/sj.npp.1300214. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, et al. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: I. Periadolescent exposure. Alcoholism: Clinical and Experimental Research. 2002a;26:1632–1641. doi: 10.1097/01.ALC.0000036301.36192.BC. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, Bell RL, Kuc KA, Murphy JM, McBride WJ, Lumeng L, et al. Effects of ethanol exposure on subsequent acquisition and extinction of ethanol self-administration and expression of alcohol-seeking behavior in adult alcohol-preferring (P) rats: II. Adult exposure. Alcoholism: Clinical and Experimental Research. 2002b;26:1642–1652. doi: 10.1097/01.ALC.0000036302.73712.9D. [DOI] [PubMed] [Google Scholar]
- Siegmund S, Vengeliene V, Singer MV, Spanagel R. Influences of age at drinking onset on long-term ethanol self-administration with deprivation and stress phases. Alcoholism: Clinical and Experimental Research. 2005;29:1139–1145. doi: 10.1097/01.alc.0000171928.40418.46. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ. Altered EEG responses in adult rats exposed to ethanol during adolescence. Alcoholism: Clinical and Experimental Research. 2002;26:246–254. [PubMed] [Google Scholar]
- Slawecki CJ, Betancourt M. Effects of adolescent ethanol exposure on ethanol consumption in adult rats. Alcohol. 2002;26:23–30. doi: 10.1016/s0741-8329(01)00192-6. [DOI] [PubMed] [Google Scholar]
- Slawecki CJ, Thorsell A, Ehlers CL. Long-term neurobehavioral effects of alcohol or nicotine exposure in adolescent animal models. Annals of the New York Academy of Sciences. 2004;1021:448–452. doi: 10.1196/annals.1308.062. [DOI] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Spear LP, Swartzwelder HS. Adolescent alcohol exposure and persistence of adolescent-typical phenotypes into adulthood: a mini-review. Neuroscience and Biobehavioral Reviews. 2014;45:1–8. doi: 10.1016/j.neubiorev.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong MN, Yoneyama N, Fretwall AM, Snelling C, Tanchuck MA, Finn DA. “Binge” drinking experience in adolescent mice shows sex differences and elevated ethanol intake in adulthood. Hormones and Behavior. 2010;58:82–90. doi: 10.1016/j.yhbeh.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration Office of Applied Studies SAMHSA. The NSDUH report: Quantity and frequency of alcohol use among underage drinkers. Rockville; Maryland: 2008. [Google Scholar]
- Toalston JE, Deehan GA, Jr, Hauser SR, Engleman EA, Bell RL, Murphy JM, et al. Reinforcing properties and neurochemical response of ethanol within the posterior ventral tegmental area are enhanced in adulthood by periadolescent ethanol consumption. The Journal of Pharmacology and Experimental Therapeutics. 2014;351:317–326. doi: 10.1124/jpet.114.218172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BM, Ehlers CL. Appetitive motivational experience during adolescence results in enhanced alcohol consumption during adulthood. Behavioral Neuroscience. 2009;123:926–935. doi: 10.1037/a0016002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Dowdall GW, Davenport A, Castillo S. Correlates of college student binge drinking. American Journal of Public Health. 1995;85:921–926. doi: 10.2105/ajph.85.7.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler H, Lee JE, Kuo M, Lee H. College binge drinking in the 1990s: a continuing problem. Results of the Harvard School of Public Health 1999 College Alcohol Study. Journal of American College Health. 2000;48:199–210. doi: 10.1080/07448480009599305. [DOI] [PubMed] [Google Scholar]


