Abstract
Purpose.
Amino-amide or amino-ester local anesthetics, which are currently used for topical ocular anesthesia, are short acting and may delay corneal healing with long-term use. In contrast, site 1 sodium channel blockers (S1SCBs) are potent local anesthetics with minimal adverse tissue reaction. In this study, we examined topical local anesthesia with two S1SCBs, tetrodotoxin (TTX) or saxitoxin (STX) individually or in combination with α2-adrenergic receptor agonists (dexmedetomidine or clonidine), and compared them with the amino-ester ocular anesthetic proparacaine. The effect of test solutions on corneal healing was also studied.
Methods.
Solutions of TTX ± dexmedetomidine, TTX ± clonidine, STX ± dexmedetomidine, dexmedetomidine, or proparacaine were applied to the rat cornea. Tactile sensitivity was measured by recording the blink response to probing of the cornea with a Cochet-Bonnet esthesiometer. The duration of corneal anesthesia was calculated. Cytotoxicity from anesthetic solutions was measured in vitro. The effect on corneal healing was measured in vivo after corneal debridement followed by repeated drug administration.
Results.
Addition of dexmedetomidine to TTX or STX significantly prolonged corneal anesthesia beyond that of either drug alone, whereas clonidine did not. Tetrodotoxin or STX coadministered with dexmedetomidine resulted in two to three times longer corneal anesthesia than did proparacaine. S1SCB-dexmedetomidine formulations were not cytotoxic. Corneal healing was not delayed significantly by any of the test solutions.
Conclusions.
Coadministration of S1SCBs with dexmedetomidine provided prolonged corneal anesthesia without delaying corneal wound healing. Such formulations may be useful for the management of acute surgical and nonsurgical corneal pain.
Keywords: tetrodotoxin, saxitoxin, dexmedetomidine, clonidine, corneal anesthesia
Prolonged duration corneal anesthesia from coadministration of site 1 sodium channel blockers and dexmedetomidine.
Conventional amino-ester and amino-amide local anesthetics are used to reduce ocular pain related to corneal injury and ophthalmic surgery.1,2 They act by binding to an intracellular domain of the sodium channel and blocking sodium influx.3 They produce corneal anesthesia for 15 to 20 minutes when applied topically, with return of normal sensation after 60 minutes4 and so require repeated administration. Brief ophthalmic procedures, such as cataract extraction, are routinely performed under local anesthesia with conventional local anesthetics, which are administered by a variety of techniques, including topical application onto the cornea, injection into or around the muscle cone, and injection under the Tenon's capsule.5 Given frequently, these agents may delay epithelial healing, cause anterior segment inflammation,6 corneal ulceration, and occasionally neurotrophic keratopathy.1 Their short durations of action and the potential for tissue toxicity exclude their use in lengthier ophthalmic procedures, and limit their use for other causes of corneal pain such as traumatic abrasions and recurrent erosions.1,6 An ocular anesthetic formulation with prolonged effect and minimal toxicity is needed. Such a formulation could be used to prevent pain more effectively during longer surgical procedures and for outpatient management of minor corneal injury during the period when ocular pain is most intense.
Tetrodotoxin (TTX) and saxitoxin (STX) are potent local anesthetics that act by binding to site 1 on the extracellular part of the sodium channel and blocking sodium influx.7 Tissue toxicity from site 1 sodium channel blockers (S1SCBs) after injection at peripheral nerves is minimal,8 even when delivered for prolonged periods.9 Site 1 sodium channel blockers also produce corneal analgesia with minimal toxicity to the corneal epithelium,2,6,10 although systemic toxicity from ocular application has been reported.11
Coadministration of local anesthetics with adjuvant agents can enhance anesthetic effect and/or (in the case of S1SCBs) prevent potential systemic toxicity by reducing the dose required for a given effect. For example, we have shown that corneal analgesia can be prolonged by combining S1SCBs with conventional local anesthetics.12
Similarly, addition of the α2-adrenergic receptor (α2-AR) agonists clonidine or dexmedetomidine to conventional amino-amide or amino-ester local anesthetics extends the duration of peripheral nerve block13–17 and ocular anesthesia.18 However, in both of those cases, the conventional local anesthetic is believed to be toxic to the cornea, which could limit usefulness.
Here, we hypothesize that coadministration of S1SCBs and α2-AR agonists for ocular anesthesia will prolong corneal block. This hypothesis is supported by the fact that clonidine can enhance the duration of sciatic nerve blockade from TTX.19 Moreover, α2-AR agonists are likely to have minimal toxicity to the cornea. In fact, dexmedetomidine has been shown to reduce local tissue inflammation from conventional local anesthetics.17,20
Here, we report prolonged corneal anesthesia from local anesthetic formulations comprised of dexmedetomidine and TTX or STX, and compare them with the widely used amino-ester ocular anesthetic, 0.5% (wt/vol) proparacaine. We also characterize in vitro cytotoxicity to corneal cells and in vivo corneal healing in the setting of repeated drug administration of those compounds.
Materials and Methods
Materials
Tetrodotoxin (>98% purity; Abcam, Cambridge, MA, USA), saxitoxin, clonidine hydrochloride (>99% purity; Sigma-Aldrich Corp., St. Louis, MO, USA), and dexmedetomidine hydrochloride (>99% purity; Tocris Bioscience, Ellisville, MO, USA) formulations were prepared in 20 mM citrate solution (pH 4.5). Saxitoxin was a generous gift from Sherwood Hall (Food and Drug Administration, College Park, MD, USA). Proparacaine (pharmaceutical grade; Sigma-Aldrich Corp.) was prepared in 0.9% (wt/vol) saline. All drug solutions were prepared immediately before use. Thirty microliters of each formulation was topically applied to the cornea. For in vitro cell viability assays, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and phenazine methosulfate were purchased from Promega Corp. (Madison, WI, USA). All chemicals were used as provided by the manufacturer without additional purification.
Cell Viability Assay
Immortalized human corneal limbal epithelial (HCLE) cells were cultured in keratinocyte serum-free medium (KSFM; Invitrogen, Carlsbad, CA, USA) supplemented with epidermal growth factor (EGF) and bovine pituitary extract until cells reached 50% confluence. Culture medium was then changed to 1:1 unsupplemented low-calcium Dulbecco's modified Eagle's medium (DMEM) and F12 Ham's nutrient mixture (Invitrogen) mixed with KSFM in a 1:1 ratio. Differentiation was induced by exposing the cells to a 1:1 mixture of DMEM/F12 medium (Invitrogen) supplemented with EGF and newborn calf serum. All cells were incubated at 37°C in a 5% CO2 environment. HCLE cells were then incubated in 96-well tissue culture plates with 150 μL of media containing either 3.1 mM TTX + 0.21 mM dexmedetomidine, 3.1 mM TTX alone, or 0.21 mM dexmedetomidine alone. All drug solutions were dissolved in 20 mM citrate buffer (pH 4.5) so that they would be in the same buffer in which TTX was dissolved. Media was prepared by adding 10× drug solution to fresh media in a 1:9 ratio so that each cell culture well contained 133.4-μL fresh media and 16.6 μL of test solution (20 mM citrate buffer with or without drugs). Solutions were filtered aseptically using a 0.2-μm syringe filter. Cellular viability was measured after 4, 8, 16, and 24 hours using the MTS colorimetric assay normalized to cells that were not exposed to drug solutions.
Animals
Male Sprage-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) weighing 280 to 320 grams were housed in groups, in a 6 AM to 6 PM light-dark cycle. Animals were cared for in accordance with protocols approved by the Animal Care and Use Committee at Boston Children's Hospital (Boston, MA, USA), as well as the Guide for the Care and Use of Laboratory Animals of the US National Research Council, and the ARVO statement for the Use of Animals in Ophthalmic and Vision Research.
Application of Corneal Medications
Rats were gently restrained by wrapping them in a towel, leaving the head exposed for drug application. Animals then received local anesthetic solutions in the form of topical drops to the left eye. The right eye remained untreated to serve as a control for systemic anesthetic effect on the cornea. In studies of topical anesthetic efficacy, animals received a single dose of test solution in a volume of 30 μL. Animals in studies of healing after corneal debridement were given 30 μL of test solution immediately after creation of the corneal lesion and then every 12 hours until the epithelium was completely healed.
Drug Preparation
Tetrodotoxin formulations (Table) were prepared in 20 mM citrate solution (pH 4.5) with or without 50 μg/mL (0.21 mM) dexmedetomidine hydrochloride so that each 30-μL drop delivered 1.5 μg of dexmedetomidine (5 μg/kg for a 300-g rat). That dose was derived from the published observation that dexmedetomidine doses above 6 μg/kg perineurally produce undesired sedation in rats.16 Therefore, to avoid systemic side effects we selected a total dexmedetomidine dose of 5μg/kg. (Although drug permeation across the cornea is minimal [<10% for most drugs],21 systemic absorption after passage into the nasolarrimal duct can produce toxicity.22) Tetrodotoxin formulations were also prepared with the same concentration of clonidine (0.21 mM) to facilitate comparison to dexmedetomidine.
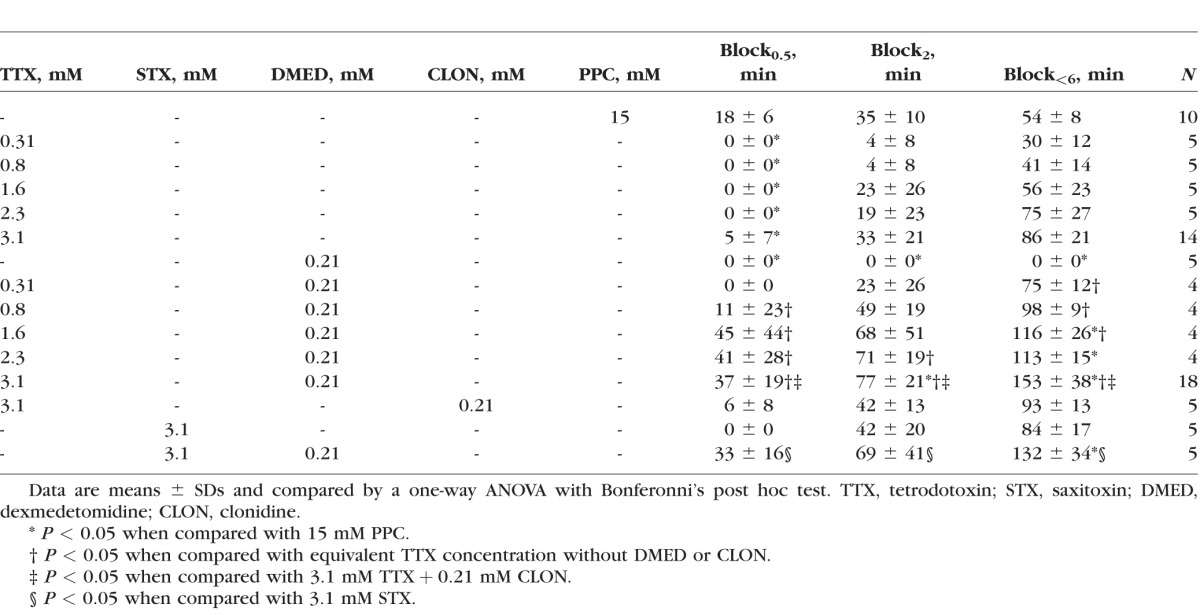
Table.
Effect of Agents on the Duration of Corneal Anesthesia
Assessment of Corneal Nociceptive Blockade
Corneal tactile sensitivity was tested, using a Cochet-Bonnet esthesiometer (Luneau Ophthalmologie, Chartres, France) as previously described.12,23 Briefly, the esthesiometer consists of an adjustable length nylon monofilament that exerts pressure inversely proportional to its length and can be adjusted from 0.5 to 6 cm. A longer, more flexible filament length is least painful, whereas a shorter, stiffer filament length is most painful. Testing began by gently placing the tip of the fully extended monofilament perpendicularly onto the cornea and applying enough pressure to cause the filament to bend. Eyes were probed with the monofilament in this fashion three times to determine presence or absence of a blink response, starting with the filament at 6 cm. Care was taken to avoid contact with eyelashes, which could also elicit a blink. In the event of a partial blink, the cornea was probed three additional times to confirm the presence or absence of a blink response. If no blink was elicited, the filament length was reduced by 0.5-cm increments and testing repeated until a blink was elicited. Testing started 15 minutes after anesthetic drops were administered and repeated every 15 minutes until complete return to baseline. The operator testing corneal nociception was not aware of which anesthetic treatment was assigned to any given rat (i.e., she/he was “blinded”). The filament length required to elicit a blink response is a measure of the degree of analgesia. Intensity of corneal nociceptive block was described as complete for rats that failed to blink in response to a 0.5-cm filament (block0.5), dense for absence of response to a 2-cm filament (block2), and partial for absence of response to a 6-cm filament (block<6). The duration of corneal block for each parameter of block intensity (block0.5, block2, and block<6) was calculated as the time elapsed after application of anesthetic drops for which the blink response was absent with stimulation by a given filament length.
Assessment for Sedation
Rats were assessed for sedation from systemically absorbed α2-AR agonists immediately after application of anesthetic solutions and every 15 minutes thereafter (immediately prior to testing of corneal sensation) until ocular sensation returned to baseline. We used the Sedation Rating Scale for rats,24 which is a 6-point scale ranging from 0 (asleep, eyes fully closed, loss of righting reflex) to 5 (fully awake, eyes wide open, grooming, feeding, and ambulating).
Corneal Epithelial Debridement Studies
Under isoflurane anesthesia, a 2-mm diameter circular defect was made in the central corneal epithelium of the right eye with a 2-mm trephine as described.25 Under a stereomicroscope, the corneal epithelium within the area demarcated by the defect was removed by gentle brushing with an Algerbrush II corneal rust ring remover fitted with a 1.0-mm burr (Ambler Surgical, Exton, PA, USA), leaving the basement membrane intact. Thirty microliters of drug solution were administered to the debrided eye immediately after creation of the corneal lesion and then every 12 hours until the epithelium was completely healed. Fluorescein (FULGLO strips; Akron, Inc., Lake Forest, IL, USA) was instilled and photographs of the cornea were taken with a Nikon D90 camera (Nikon, Tokyo, Japan) fitted with a 40-mm AF-S Micro NIKKOR f/2.8 lens (Nikon) every 12 hours until complete re-epithelialization. An external light source with a cobalt-blue filter was used to illuminate the fluorescein filled corneal defect. Images were analyzed with Fiji (ImageJ2) software (http://imagej.nih.gov/ij/; provided in the public domain by the National Institutes of Health, Bethesda, MD, USA) to measure the wound area at each time point and calculate the rate of corneal re-epithelialization. The rate of corneal re-epithelialization was calculated by subtracting the wound area (mm2) at 24 hours from the wound area (mm2) immediately after debridement and dividing by 24.
Statistical Analysis
For corneal block durations and wound healing assays, data were reported as means and SDs of N observations and were compared using one-way ANOVA with Bonferonni's post hoc test. Data from in vitro analysis were presented as means and SDs of N observations and were compared using two-way ANOVA with Bonferonni's post hoc test. All data analyses were performed using SPSS version 19 (SPSS, Inc., Chicago, IL, USA).
Results
Corneal Nerve Block Studies
Animals received a single 30-μL eye drop of 0.31 to 3.1 mM TTX, or 15 mM (0.5% wt/vol) proparacaine (Table).12,26,27 The S1SCB concentrations studied were within the range of those studied previously.2,6,10–12,27,28 We used a validated rat model12,23 to measure the tactile sensitivities of corneas after anesthetic solutions were applied. Animals treated with 15 mM proparacaine achieved maximal corneal block (block0.5) for 18 minutes, deep block (block2) for 35 minutes, and partial block (block<6) for 54 minutes (Table). Animals treated with TTX alone demonstrated concentration-dependent corneal analgesia (Table). Only the highest TTX concentration (3.1 mM) yielded block0.5 of any duration, but only in 5 of 14 animals, whereas block0.5 occurred in all animals treated with 15 mM proparacaine (P = 0.013, χ2 test). That concentration of TTX (3.1 mM) yielded an average block<6 of 86 minutes compared with 54 minutes for 15 mM proparacaine, although this difference was not statistically significant (P = 0.136). In the range 0.8 to 2.3 mM, TTX produced block<6 durations comparable to those from 15 mM proparacaine (P > 0.05 for all comparisons).
To test the hypothesis that dexmedetomidine would prolong the duration of corneal block from TTX, we administered solutions of TTX alone or in combination with 0.21 mM dexmedetomidine and compared the resulting corneal nerve block durations. The dexmedetomidine concentration studied here was 2-15,16 to 5-fold19 greater than that which has been studied in peripheral nerve, yet 25 to 50 times less than what has been studied for topical application to the cornea21,29 (see Methods for further discussion of the selection of doses). Dexmedetomidine alone did not produce any degree of corneal anesthesia, but enhanced the effect of TTX and STX for all three measures of corneal block intensity (Table). For example, 3.1 mM TTX produced block0.5 for 5 minutes, block2 for 33 minutes, and block<6 for 86 minutes, compared with 37, 77, and 153 minutes, respectively for 3.1 mM TTX + 0.21 mM dexmedetomidine (P < 0.001 for all comparisons; these represented 2- to 7-fold prolongations of nerve blockade from TTX). Corneal block durations for the drug combination increased in proportion to TTX concentration.
Block0.5 and block2 durations from 1.6 to 2.3 mM TTX coadministered with dexmedetomidine were not statistically significantly different from the same measures for proparacaine alone, but block<6 durations were more than twice as long as for proparacaine (P < 0.05 for all block<6 comparisons). In contrast, 3.1 mM TTX + 0.21 mM dexmedetomidine produced block0.5 for 37, block2 for 77, and block<6 for 153 minutes, which were 2 to 3 times longer than the corresponding blocks from proparacaine (P = 0.316, 0.001 and < 0.001, respectively).
To determine whether block prolongation by dexmedetomidine applies to other S1SCBs, we administered topical formulations of 3.1 mM saxitoxin alone or in combination with 0.21 mM dexmedetomidine. The durations of block0.5, block2, and block<6 for saxitoxin were similar to those for TTX (P = 1.000 for all comparisons) and those for 3.1 mM saxitoxin + 0.21 dexmedetomidine were similar to those for 3.1 mM TTX + 0.21 mM dexmedetomidine (P = 1.000 for all comparisons).
To determine whether other α2-adrenergic receptor agonists would enhance corneal anesthesia from TTX in a manner similar to dexmedetomidine, we administered topical formulations of 3.1 mM TTX + 0.21 mM clonidine. Surprisingly, given the effect of dexmedetomidine, the durations of block0.5, block2, and block<6 from 3.1 mM TTX + 0.21 mM clonidine were similar to those from TTX alone (P = 1.000 for all comparisons).
We used the blink response in the contralateral (untreated) eye as a measure of the degree of systemically distributed anesthetic drug. Sensory deficits were not detected in contralateral eyes for any drug formulations. We used the Sedation Rating Scale for rats24 to measure sedation from clonidine or dexmedetomidine. All groups, regardless of treatment, received scores of 5 ± 0 (i.e., no sedation was observed).
In Vitro Cytotoxicity Studies
To determine the cytotoxicity of dexmedetomidine and TTX, HCLE cells were incubated with media containing 0.21 mM dexmedetomidine, 3.1 mM TTX, or 0.21 mM dexmedetomidine + 3.1 mM TTX (Fig. 1). Cell viability was measured by the MTS assay over a 24-hour period. HCLE cell survival was not reduced compared with cells that were not exposed to drug (P > 0.05 at all time points).
Figure 1.
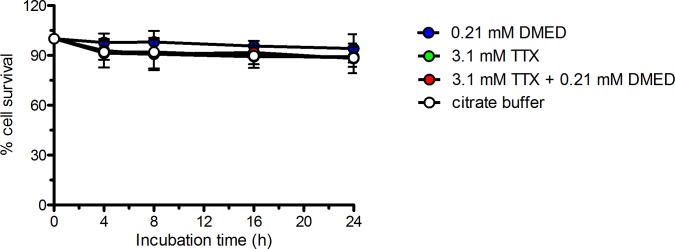
Effect of dexmedetomidine (DMED) and tetrodotoxin (TTX) on survival of HCLE cells was determined using the MTS assay. Data are means ± SD, n = 4. Comparisons at each time point were performed using two way ANOVA with Bonferonni post hoc correction. One group received a volume of 0.2 M citrate buffer equal to that of the drug solutions added because it was the buffer in which drug solutions were dissolved. All P > 0.05.
Effect on the Rate of Corneal Healing
Proparacaine30 and TTX + proparacaine12 formulations have been reported to delay corneal wound healing. To assess whether the combination of TTX + dexmedetomidine alters corneal healing, we measured the rate of corneal re-epithelialization following debridement of 2 mm-diameter area of the corneal epithelium. Drug solutions were applied to the cornea after the debridement procedure and then every 12 hours thereafter until the epithelium was completely healed. In total, three doses of drug solution were applied to animals in each group. Thirty minutes prior to applying drug solutions, the size of the corneal defect was measured as follows. Fluorescein was instilled, eyes were illuminated with an external light source using a cobalt-blue filter, and photographs were taken for digital analysis to measure the size of the defect. The rate of re-epithelialization was not decreased in any treatment group when compared with the saline control (Fig. 2; P > 0.05 for all comparisons, n = 5). All defects were healed by 36 hours.
Figure 2.
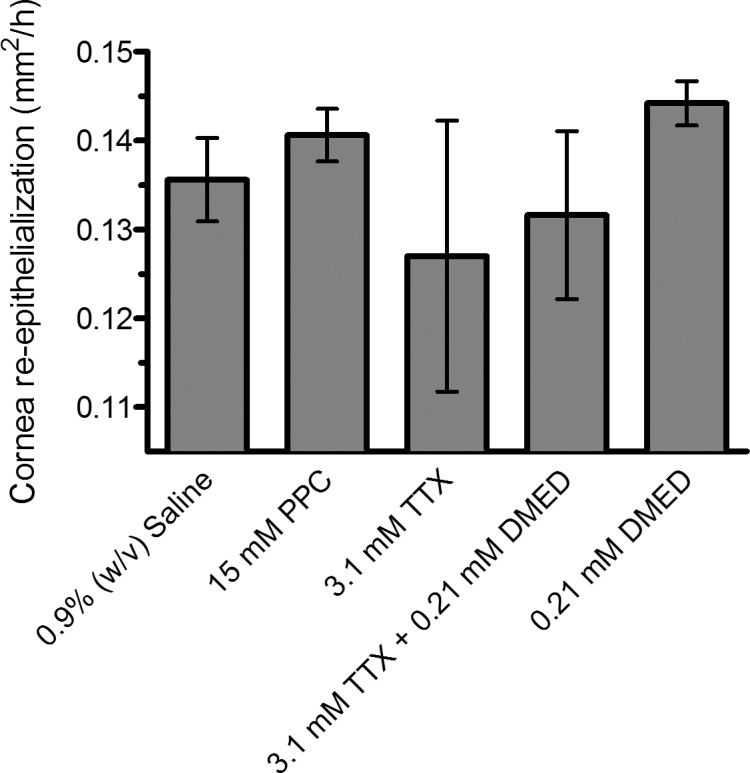
Effect of test compounds on the average rate of corneal re-epithelialization after surgical debridement of a 2-mm2 area of corneal epithelium. Data are means ± SD, n = 5. Groups were compared with saline-treated controls using one-way ANOVA with Bonferonni post hoc correction (n = 5, P = 0.320). PPC, proparacaine; TTX, tetrodotoxin; DMED, dexmedetomidine.
Discussion
The α2-AR agonist dexmedetomidine prolonged corneal analgesia from two different S1SCBs, TTX and STX, and those combinations yielded durations of corneal analgesia 2 to 3 times longer than that of the widely used ocular anesthetic proparacaine. The durations of block achieved by coadministration of S1SCBs and dexmedetomidine, and the apparent lack of corneal toxicity of that combination suggest that such formulations may be useful treatments for acute corneal pain from a variety of conditions, including corneal abrasions28 or procedures such as excimer laser keratectomy2 and photorefractive keratectomy.28
Given frequently, conventional local anesthetics are believed to produce severe corneal injury.1 Here, there was no apparent toxic effect of the TTX + dexmedetomidine combination in vitro or in vivo, even with prolonged or repeated administration. Also, there was no relation between the duration of nerve blockade and the rate of corneal healing (Fig. 3). It is possible that an adverse effect on healing might be seen in a more severe model of injury, or in a different injury model. These data suggest that it might be possible to use formulations of this kind for repeated and sustained corneal analgesia.
Figure 3.
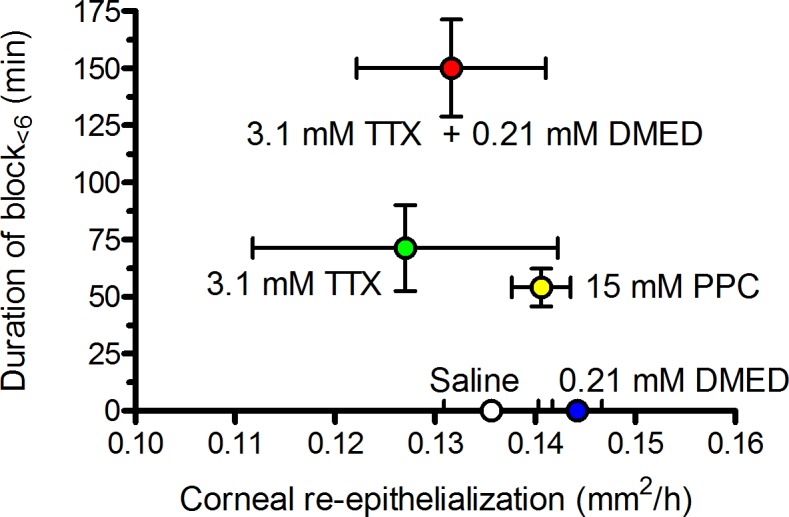
Relationship between duration of corneal anesthesia and rate of corneal re-epithelialization after topical application of anesthetics. Data are means ± SD for both parameters. Duration of corneal anesthesia and rate of re-epithelialization were studied in separate cohorts. n = 5 for all re-epithelialization groups; n = 18 for 3.1 mM TTX + 0.21 mM DMED; n = 14 for 3.1 mM TTX; n = 10 for 15 mM PPC; n = 5 for saline and 0.21 mM DMED. PPC, proparacine; TTX, tetrodotoxin; DMED, dexmedetomidine.
The high concentrations of TTX (and hence doses) used here (≤30 μg, compared to ≤5 μg used in peripheral nerve in rats8,19,31) raises the issue of potential systemic toxicity if absorbed into a wound or after passage into the nasolacrimal duct.12,22 Others have reported systemic toxicity in animals from topically applied TTX at concentrations much higher than were used here.11 We did not observe any analgesia in the contralateral eyes of animals, which would have suggested systemic toxicity. Systemic toxicity would become increasingly unlikely when used in larger species, including man, because the S1SCB concentration and drop volume required for local effect would be similar to that used here (30 μL), while the volume of distribution, which determines systemic toxicity, would be 200 to 300 times larger.32 Moreover, dexmedetomidine could be used to decrease the amount of S1SCB necessary to achieve a given duration of block: 0.31 mM TTX with dexmedetomidine produced similar block durations to that from 3.1 mM TTX alone (P = 1.000 for all comparisons).
There were dissimilarities between the pharmacology on the cornea and at the sciatic nerve. On the cornea, dexmedetomidine enhanced corneal block, while clonidine did not, even at concentrations much higher than those at which the latter markedly prolonged sciatic nerve blockade with TTX.19 This may be due to unexplored differences in drug permeability between peripheral nerve sheaths and the cornea. (In that regard, note also the large difference in the TTX concentrations required for effect in peripheral nerve and on the cornea). Differences between clonidine and dexmedetomidine could also be attributable to differences in α-AR subtype specificity. Clonidine, which binds both α1 and α2-ARs, is eight times less selective for the α2-AR than dexmedetomidine.33 In addition, the α2-AR has been divided into three pharmacologic subtypes,34 α2A, α2B, and α2C, of which the α2A and α2c subtypes are expressed on the corneal epithelium.35 While dexmedetomidine and clonidine both act on all three AR subtypes, dexmedetomidine is a more potent agonist.36 It may be that at higher concentrations clonidine would also potentiate corneal block from S1SCBs. However, if that were the case, one would have expected the concentration of clonidine used here to have had some effect because the concentrations of clonidine (and dexmedetomidine) studied here were two orders of magnitude greater than effective concentrations in other peripheral nerves (0.21 mM here compared with 2.7 μM for dexmedetomidine16 and 10 μM for clonidine in peripheral nerve19).
The lack of effect by clonidine suggests that it may be premature to conclude that dexedetomidine's effect on TTX at the cornea is due to local α2-AR agonist activity and its consequences. Vasoconstriction has been invoked to explain α2-AR agonists' prolongation of peripheral nerve blockade by both S1SCBs19 and conventional local anesthetics.37 It is not clear that vasoconstriction would play a major role in drug clearance from the ocular surface because the cornea is avascular,38 and there are other mechanisms of elimination (tearing, drainage) that would not be affected by vasoconstriction. Moreover, the effects of clonidine and dexmedetomidine on peripheral nerve blockade by conventional local anesthetics are reported to be due to blockade of the current (Ih) produced by hyperpolarization-activated cation channels, and not effects on α-adrenergic receptors.13,15 Hyperpolarization-activated cation channels are expressed throughout the nervous system.39,40 Clonidine's lack of effect on the duration of corneal analgesia from TTX raises the possibility that Ih blockade does not play a role in dexmedetomidine's prolongation of analgesia from TTX in the cornea. At this time, we cannot discern the mechanism by which dexmedetomidine prolongs corneal analgesia from TTX.
In conclusion, dexmedetomidine greatly enhances the analgesic effect of S1SCBs on the cornea, and dexmedetomidine-S1SCB combinations provide better corneal anesthesia than the commonly used corneal anesthetic proparacaine. Dexmedetomidine-S1SCB combinations do not cause corneal toxicity, even after repeated administration for up to 36 hours. Dexmedetomidine-S1SCB combinations may present an appealing analgesic option for corneal procedures and acute corneal pain.
Acknowledgments
Supported by a National Institutes of Health Grant GM073626 (Bethesda, MD, USA).
Disclosure: J.B. McAlvin, P; C. Zhan, P; J.C. Dohlman, None; P.E. Kolovou, None; B. Salvador-Culla, None; D.S. Kohane, P
References
- 1. Ting JY,, Barns KJ,, Holmes JL. Management of Ocular Trauma in Emergency (MOTE) Trial: a pilot randomized double-blinded trial comparing topical amethocaine with saline in the outpatient management of corneal trauma. J Emerg Trauma Shock. 2009; 2: 10–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Schwartz DM,, Duncan KG,, Duncan JL. Experimental use of tetrodotoxin for corneal pain after excimer laser keratectomy. Cornea. 1998; 17: 196–199. [DOI] [PubMed] [Google Scholar]
- 3. Ragsdale DS,, McPhee JC,, Scheuer T,, Catterall WA. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994; 265: 1724–1728. [DOI] [PubMed] [Google Scholar]
- 4. Nomura K,, Singer DE,, Aquavella JV. Corneal sensation after topical anesthesia. Cornea. 2001; 20: 191–193. [DOI] [PubMed] [Google Scholar]
- 5. Kumar C,, Dowd T. Ophthalmic regional anaesthesia. Curr Opin Anaesthesiol. 2008; 21: 632–637. [DOI] [PubMed] [Google Scholar]
- 6. Schwartz DM,, Fields HL,, Duncan KG,, Duncan JL,, Jones MR. Experimental study of tetrodotoxin a long-acting topical anesthetic. Am J Ophthalmol. 1998; 125: 481–487. [DOI] [PubMed] [Google Scholar]
- 7. Zhang MM,, Gruszczynski P,, Walewska A,, Bulaj G,, Olivera BM,, Yoshikami D. Cooccupancy of the outer vestibule of voltage-gated sodium channels by micro-conotoxin KIIIA and saxitoxin or tetrodotoxin. J Neurophysiol. 2010; 104: 88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Padera RF,, Tse JY,, Bellas E,, Kohane DS. Tetrodotoxin for prolonged local anesthesia with minimal myotoxicity. Muscle Nerve. 2006; 34: 747–753. [DOI] [PubMed] [Google Scholar]
- 9. Epstein-Barash H,, Shichor I,, Kwon AH,, et al. Prolonged duration local anesthesia with minimal toxicity. Proc Natl Acad Sci U S A. 2009; 106: 7125–7130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwartz DM,, Duncan KG,, Fields HL,, Jones MR. Tetrodotoxin: anesthetic activity in the de-epithelialized cornea. Graefes Arch Clin Exp Ophthalmol. 1998; 236: 790–794. [DOI] [PubMed] [Google Scholar]
- 11. Ogura Y,, Mori Y. Mechanism of local anesthetic action of crystalline tetrodotoxin and its derivatives. Eur J Pharmacol. 1968; 3: 58–67. [DOI] [PubMed] [Google Scholar]
- 12. Wang L,, Shankarappa SA,, Tong R,, et al. Topical drug formulations for prolonged corneal anesthesia. Cornea. 2013; 32: 1040–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kroin JS,, Buvanendran A,, Beck DR,, Topic JE,, Watts DE,, Tuman KJ. Clonidine prolongation of lidocaine analgesia after sciatic nerve block in rats is mediated via the hyperpolarization-activated cation current not by alpha-adrenoreceptors. Anesthesiology. 2004; 101: 488–494. [DOI] [PubMed] [Google Scholar]
- 14. Kettner SC. Dexmedetomidine as adjuvant for peripheral nerve blocks. Br J Anaesth. 2013; 111: 123. [DOI] [PubMed] [Google Scholar]
- 15. Brummett CM,, Hong EK,, Janda AM,, Amodeo FS,, Lydic R. Perineural dexmedetomidine added to ropivacaine for sciatic nerve block in rats prolongs the duration of analgesia by blocking the hyperpolarization-activated cation current. Anesthesiology. 2011; 115: 836–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Brummett CM,, Padda AK,, Amodeo FS,, Welch KB,, Lydic R. Perineural dexmedetomidine added to ropivacaine causes a dose-dependent increase in the duration of thermal antinociception in sciatic nerve block in rat. Anesthesiology. 2009; 111: 1111–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brummett CM,, Norat MA,, Palmisano JM,, Lydic R. Perineural administration of dexmedetomidine in combination with bupivacaine enhances sensory and motor blockade in sciatic nerve block without inducing neurotoxicity in rat. Anesthesiology. 2008; 109: 502–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cabral SA,, Carraretto AR,, Brocco MC,, Abreu Baptista JF,, Gomez RS. Effect of clonidine added to lidocaine for sub-Tenon's (episcleral) anesthesia in cataract surgery. J Anesth. 2014; 28: 70–75. [DOI] [PubMed] [Google Scholar]
- 19. Kohane DS,, Lu NT,, Cairns BE,, Berde CB. Effects of adrenergic agonists and antagonists on tetrodotoxin-induced nerve block. Reg Anesth Pain Med. 2001; 26: 239–245. [DOI] [PubMed] [Google Scholar]
- 20. Tufek A,, Kaya S,, Tokgoz O,, et al. The protective effect of dexmedetomidine on bupivacaine-induced sciatic nerve inflammation is mediated by mast cells. Clin Invest Med. 2013; 36: E95–E102. [DOI] [PubMed] [Google Scholar]
- 21. Chiang CH,, Schoenwald RD. Ocular pharmacokinetic models of clonidine-3H hydrochloride. J Pharmacokinet Biopharm. 1986; 14: 175–211. [DOI] [PubMed] [Google Scholar]
- 22. Zimmerman TJ,, Kooner KS,, Kandarakis AS,, Ziegler LP. Improving the therapeutic index of topically applied ocular drugs. Arch Ophthalmol. 1984; 102: 551–553. [DOI] [PubMed] [Google Scholar]
- 23. Lawrenson JG,, Ruskell GL. Investigation of limbal touch sensitivity using a Cochet-Bonnet aesthesiometer. Br J Ophthalmol. 1993; 77: 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salamone JD,, Cousins MS,, Maio C,, Champion M,, Turski T,, Kovach J. Different behavioral effects of haloperidol, clozapine and thioridazine in a concurrent lever pressing and feeding procedure. Psychopharmacology (Berl). 1996; 125: 105–112. [DOI] [PubMed] [Google Scholar]
- 25. Ksander BR,, Kolovou PE,, Wilson BJ,, et al. Frank NY: ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature. 2014; 511: 353–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bartfield JM,, Holmes TJ,, Raccio-Robak N. A comparison of proparacaine and tetracaine eye anesthetics. Acad Emerg Med. 1994; 1: 364–367. [DOI] [PubMed] [Google Scholar]
- 27. Liu JC,, Steinemann TL,, McDonald MB,, Thompson HW,, Beuerman RW. Topical bupivacaine and proparacaine: a comparison of toxicity onset of action, and duration of action. Cornea. 1993; 12: 228–232. [DOI] [PubMed] [Google Scholar]
- 28. Duncan KG,, Duncan JL,, Schwartz DM. Saxitoxin: an anesthetic of the deepithelialized rabbit cornea. Cornea. 2001; 20: 639–642. [DOI] [PubMed] [Google Scholar]
- 29. Harrison R,, Kaufmann CS. Clonidine. Effects of a topically administered solution on intraocular pressure and blood pressure in open-angle glaucoma. Arch Ophthalmol. 1977; 95: 1368–1373. [DOI] [PubMed] [Google Scholar]
- 30. Lu L,, Reinach PS,, Kao WW. Corneal epithelial wound healing. Exp Biol Med (Maywood). 2001; 226: 653–664. [DOI] [PubMed] [Google Scholar]
- 31. Kohane DS,, Yieh J,, Lu NT,, Langer R,, Strichartz GR,, Berde CB. A re-examination of tetrodotoxin for prolonged duration local anesthesia. Anesthesiology. 1998; 89: 119–131. [DOI] [PubMed] [Google Scholar]
- 32. Kohane DS,, Sankar WN,, Shubina M,, Hu D,, Rifai N,, Berde CB. Sciatic nerve blockade in infant, adolescent, and adult rats: a comparison of ropivacaine with bupivacaine. Anesthesiology. 1998; 89: 1199–1208, discussion 10A. [DOI] [PubMed] [Google Scholar]
- 33. Virtanen R,, Savola JM,, Saano V,, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2-adrenoceptor agonist. Eur J Pharmacol. 1988; 150: 9–14. [DOI] [PubMed] [Google Scholar]
- 34. Leiphart JW,, Dills CV,, Levy RM. Alpha2-adrenergic receptor subtype specificity of intrathecally administered tizanidine used for analgesia for neuropathic pain. J Neurosurg. 2004; 101: 641–647. [DOI] [PubMed] [Google Scholar]
- 35. Woldemussie E,, Wijono M,, Pow D. Localization of alpha 2 receptors in ocular tissues. Vis Neurosci. 2007; 24: 745–756. [DOI] [PubMed] [Google Scholar]
- 36. Jasper JR,, Lesnick JD,, Chang LK,, et al. Ligand efficacy and potency at recombinant alpha2 adrenergic receptors: agonist-mediated [35S]GTPgammaS binding. Biochem Pharmacol. 1998; 55: 1035–1043. [DOI] [PubMed] [Google Scholar]
- 37. Yabuki A,, Higuchi H,, Yoshitomi T,, et al. Locally injected dexmedetomidine induces vasoconstriction via peripheral alpha-2A adrenoceptor subtype in guinea pigs. Reg Anesth Pain Med. 2014; 39: 133–136. [DOI] [PubMed] [Google Scholar]
- 38. Ambati BK,, Nozaki M,, Singh N,, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006; 443: 993–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Biel M,, Wahl-Schott C,, Michalakis S,, Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009; 89: 847–885. [DOI] [PubMed] [Google Scholar]
- 40. Orio P,, Parra A,, Madrid R,, Gonzalez O,, Belmonte C,, Viana F. Role of Ih in the firing pattern of mammalian cold thermoreceptor endings. J Neurophysiol. 2012; 108: 3009–3023. [DOI] [PubMed] [Google Scholar]


