Abstract
Background
In 2010, the World Health Organization recommended that all patients with suspected malaria are tested for malaria before treatment. In rural African settings light microscopy is often unavailable. Diagnosis has relied on detecting fever, and most people were given antimalarial drugs presumptively. Rapid diagnostic tests (RDTs) provide a point‐of‐care test that may improve management, particularly of people for whom the RDT excludes the diagnosis of malaria.
Objectives
To evaluate whether introducing RDTs into algorithms for diagnosing and treating people with fever improves health outcomes, reduces antimalarial prescribing, and is safe, compared to algorithms using clinical diagnosis.
Search methods
We searched the Cochrane Infectious Disease Group Specialized Register; CENTRAL (The Cochrane Library); MEDLINE; EMBASE; CINAHL; LILACS; and the metaRegister of Controlled Trials for eligible trials up to 10 January 2014. We contacted researchers in the field and reviewed the reference lists of all included trials to identify any additional trials.
Selection criteria
Individual or cluster randomized trials (RCTs) comparing RDT‐supported algorithms and algorithms using clinical diagnosis alone for diagnosing and treating people with fever living in malaria‐endemic settings.
Data collection and analysis
Two authors independently applied the inclusion criteria and extracted data. We combined data from individually and cluster RCTs using the generic inverse variance method. We presented all outcomes as risk ratios (RR) with 95% confidence intervals (CIs), and assessed the quality of evidence using the GRADE approach.
Main results
We included seven trials, enrolling 17,505 people with fever or reported history of fever in this review; two individually randomized trials and five cluster randomized trials. All trials were conducted in rural African settings.
In most trials the health workers diagnosing and treating malaria were nurses or clinical officers with less than one week of training in RDT supported diagnosis. Health worker prescribing adherence to RDT results was highly variable: the number of participants with a negative RDT result who received antimalarials ranged from 0% to 81%.
Overall, RDT supported diagnosis had little or no effect on the number of participants remaining unwell at four to seven days after treatment (6990 participants, five trials, low quality evidence); but using RDTs reduced prescribing of antimalarials by up to three‐quarters (17,287 participants, seven trials, moderate quality evidence). As would be expected, the reduction in antimalarial prescriptions was highest where health workers adherence to the RDT result was high, and where the true prevalence of malaria was lower.
Using RDTs to support diagnosis did not have a consistent effect on the prescription of antibiotics, with some trials showing higher antibiotic prescribing and some showing lower prescribing in the RDT group (13,573 participants, five trials, very low quality evidence).
One trial reported malaria microscopy on all enrolled patients in an area of moderate endemicity, so we could compare the number of patients in the RDT and clinical diagnosis groups that actually had microscopy confirmed malaria infection but did not receive antimalarials. No difference was detected between the two diagnostic strategies (1280 participants, one trial, low quality evidence).
Authors' conclusions
Algorithms incorporating RDTs can substantially reduce antimalarial prescribing if health workers adhere to the test results. Introducing RDTs has not been shown to improve health outcomes for patients, but adherence to the test result does not seem to result in worse clinical outcomes than presumptive treatment.
Concentrating on improving the care of RDT negative patients could improve health outcomes in febrile children.
17 April 2019
Update pending
Studies awaiting assessment
The CIDG is currently examining a new search conducted up to 16 Aug, 2018 for potentially relevant studies. These studies have not yet been incorporated into this Cochrane Review.
Keywords: Adult; Child; Humans; Algorithms; Point‐of‐Care Systems; Reagent Kits, Diagnostic; Africa; Anti‐Bacterial Agents; Anti‐Bacterial Agents/therapeutic use; Antimalarials; Antimalarials/therapeutic use; Fever; Fever/drug therapy; Fever/etiology; Malaria; Malaria/complications; Malaria/diagnosis; Malaria/drug therapy; Randomized Controlled Trials as Topic
Plain language summary
Rapid diagnostic tests versus clinical diagnosis for managing fever in settings where malaria is common
Cochrane Collaboration researchers conducted a review of the effects of introducing rapid diagnostic tests (RDTs) for diagnosing malaria in areas where diagnosis has traditionally been based on clinical symptoms alone. After searching for relevant trials, they included seven randomized controlled trials, which enrolled 17,505 people with fever.
What are RDTs and how might they improve patient care
RDTs are simple to use diagnostic kits which can detect the parasites that cause malaria from one drop of the patient's blood. They do not require laboratory facilities or extensive training, and can provide a simple positive or negative result within 20 minutes, making them suitable for use in rural areas of Africa where most malaria cases occur.
Improving malaria diagnosis by introducing RDTs is unlikely to improve the health outcomes of people with true malaria as they would probably have received antimalarials even if the health worker was relying on clinical symptoms alone. However, for patients with fever not due to malaria, RDTs could improve health outcomes by prompting the health worker to look for and treat the true cause of their fever earlier.
What the research says
In these trials, diagnosis using RDTs had little or no effect on the number of people remaining unwell four to seven days after treatment (low quality evidence).
However, using RDTs reduced the prescription of antimalarials by up to three‐quarters (moderate quality evidence), and this reduction was highest where health workers only prescribed antimalarials following a positive test, and where malaria was less common.
Using RDTs to support diagnosis did not have a consistent effect on the prescription of antibiotics, with some trials showing an increase in antibiotic prescription and some showing a decrease (very low quality evidence).
Use of RDTs did not result in more patients with malaria being incorrectly diagnosed as not having malaria and being sent home without treatment (low quality evidence).
Summary of findings
Summary of findings for the main comparison. Summary of findings table.
| RDT diagnosis versus clinical diagnosis for managing patients with fever in malaria endemic settings | |||||
| Patient or population: People with fever Settings: Malaria endemic settings Intervention: Algorithms that include malaria RDTs Control: Algorithms based on clinical symptoms and signs only | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (trials) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Clinical diagnosis | RDT diagnosis | ||||
| Patients still unwell at day 4 to 7 | 55 per 1000 | 50 per 1000 (38 to 64) | RR 0.90 (0.69 to 1.17) | 6990 (5 trials) | ⊕⊕⊝⊝ low1,2,3,4 |
| Patients prescribed antimalarials | 946 per 1000 | 587 per 1000 (492 to 691) | RR 0.62 (0.52 to 0.73) | 17,287 (7 trials) | ⊕⊕⊕⊝ moderate1,5,6,7 |
| Patients prescribed antibiotics | ‐ | ‐ | Not pooled | 13,573 (5 trials) | ⊕⊝⊝⊝ very low1,8,9 |
| Patients with microscopically confirmed malaria not receiving antimalarials | 27 per 1000 | 33 per 1000 (17 to 62) | RR 1.21 (0.64 to 2.28) | 1280 (1 trial) |
⊕⊕⊝⊝ low10,11,12 |
| The basis for the assumed risk is the median risk across control groups. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; HR: Hazard ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 No serious risk of bias: None of these trials adequately described allocation concealment, however this was not downgraded. 2 No serious inconsistency: Statistical heterogeneity was low. However, in one trial health worker compliance with the RDT protocol was very low, with a high prescription of antimalarials in both groups. This trial found no effect (RR 1.01), while in the remaining trials with good compliance there is a trend towards benefit with RDTs. 3 Downgraded by one for serious indirectness: The only patients who could feasibly benefit from the use of RDT are patients with a negative RDT whose fever is not due to malaria. The management protocol and advice given to health workers about how to manage these patients in these trials is unclear and the effect of RDT use on antibiotic prescribing was highly varied. These five trials were conducted in rural areas in Burkina Faso, Zambia, and Uganda (three trials). The health staff were community health workers, nurses or clinical officers. 4 Downgraded by one for serious imprecision: There is a trend towards benefit with RDTs, however this does not reach statistical significance, even when the trial with poor adherence to the RDT protocol was excluded. 5 Downgraded by one for serious inconsistency: The size of the reduction in antimalarial prescription varied according to HW compliance with RDT results. In one trial from Burkina Faso, where HW prescribed high levels of antimalarials to negative RDTs, no difference in antimalarial prescription was seen. In the remaining six trials HW compliance was much higher, and prescriptions lower 6 No serious indirectness; These trials were mainly conducted in rural settings in Africa, with a range of malaria endemicity. 7 No serious imprecision:. Statistically significant differences were seen in all six trials with moderate or high heathworker adherence 8 Downgraded by two for very serious inconsistency: There is a large range of effects both increasing and decreasing antibiotic use across trials. 9 Downgraded by one for serious indirectness: The only patients who could feasibly benefit from the use of RDT are patients with a negative RDT whose fever is not due to malaria. The management protocol and advice given to health workers about how to manage these patients in these trials is unclear 10 No serious risk of bias: This trial was individually randomized and at unclear risk of selection bias. 11 Downgraded by one for serious indirectness: Only one trial conducted microscopy on all participants. This trial was conducted in Ghana in an area of unclear endemicity. The number of missed diagnoses is likely to vary with malaria endemicity. In the three trials from Uganda which only conducted microscopy on participants in the RDT arm: the negative predictive value was 0.96 in the very high endemic setting, 0.97 in the high endemic setting, and 0.93 in the medium endemic setting. 12 Downgraded by one for serious imprecision: The 95% CI is wide including what may be clinically important increase in missed cases.
Background
Description of the condition
Malaria is a febrile illness, caused by infection with the Plasmodium parasite, and is spread from person to person by the bite of infected Anopheles mosquitoes. Five Plasmodium species infect humans, of which Plasmodium falciparum is the most common in Africa and responsible for most of the severe disease cases (WHO 2012).
'Uncomplicated' malaria is the mild form of the disease which commonly presents as a fever. Light microscopy is the gold standard for confirming the diagnosis by detecting parasites in the symptomatic person's blood (WHO 2010a). However, the vast majority of malaria episodes and deaths occur in rural parts of Africa where diagnostic services are limited. Consequently diagnosis of malaria has often relied on clinical symptoms alone (D'Acremont 2009; English 2009).
Description of the intervention
Rapid diagnostic tests (RDTs) are individual test kits that can detect Plasmodium‐specific antigens in a drop of fresh blood using lateral flow immunochromatography (WHO 2006; Wongsrichanalai 2007). RDTs offer a feasible alternative to microscopy, particularly for rural first‐level health facilities, as they do not require a laboratory or special equipment, are simple to use with relatively little training, and provide a positive or negative result within 20 minutes (Wongsrichanalai 2007).
Two RDT types are in common use; 1) HRP‐2; which detects a histidine‐rich protein produced by P. falciparum, and 2) pLDH; which detects the parasite lactate dehydrogenase (pLDH) enzymes produced by all species of Plasmodium that cause malaria in humans (WHO 2010b; Wongsrichanalai 2007). A Cochrane Review of the diagnostic test accuracy of RDTs concluded that both tests were highly sensitive (having few false negative results) and highly specific (having few false positive results); HRP‐2: sensitivity = 95.0%, specificity = 95.2%; pLDH: sensitivity = 93.2%, specificity = 98.5% (Abba 2011).
Interventions to introduce RDTs are usually multifactorial including: in‐service training and supervision of health workers, and dissemination of written guidelines or protocols, as well as introduction of the test itself. These supplementary interventions are necessary to assure adherence to diagnostic and treatment algorithms, and appropriate use of the RDT device under field conditions.
How the intervention might work
The clinical symptoms associated with malaria are poor predictors of the disease. Reliance on clinical signs alone results in significant overuse of antimalarials, with between 32% and 93% of patients being falsely diagnosed with malaria, dependent on the local malaria endemicity (Koram 2007; Rolland 2006; Zikusooka 2008). The introduction of RDTs to improve malaria diagnosis therefore has the potential to substantially reduce the over‐prescription of antimalarial drugs, by reducing the misclassification of fevers, especially in low prevalence areas (Lubell 2007; Zikusooka 2008; Zurovac 2008). However, for patients that have malaria as the true cause of their fever, RDT introduction is unlikely to improve their health outcomes, as they would receive antimalarials even under an algorithm based on clinical symptoms (see Figure 1). Instead, the potential health benefits of introducing RDTs are restricted to people whose fever is not due to malaria, for whom a negative RDT result should prompt the health worker to look for and treat the true cause of their fever. RDT introduction also has the potential for harm when false negative RDT results misclassify patients as not having malaria and consequently the appropriate antimalarial is not given or is delayed (D'Acremont 2009; Graz 2011; Talisuna 2007; WHO 2010a).
1.
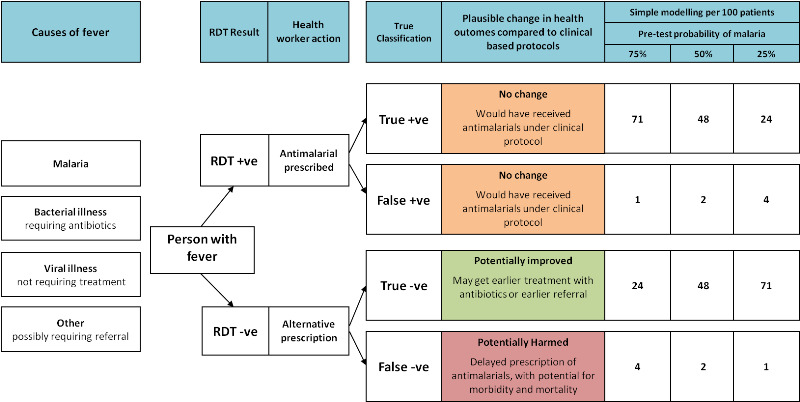
Logic framework for predicting the effect on health outcomes of using a HRP‐2 RDT with 95% sensitivity and 95.2% specificity (Abba 2011).
Basic modelling, using the sensitivity and specificity of the HRP‐2 RDT gained from a Cochrane Review (Abba 2011), predicts that areas of low malaria transmission have the greatest potential for health benefits as a result of introducing RDTs, and the lowest potential for harm from false negatives (see Figure 1). However, some suggest that in very low transmission settings where clinical malaria can occur at parasite densities lower than 100 parasites/µL of blood, RDTs may have lower sensitivity and lead to higher numbers of false negatives (English 2009; Murray 2008; Wongsrichanalai 2007).
Widespread overuse of antimalarials is also thought to contribute to the development and spread of antimalarial resistance, and reductions in overuse through the use of RDTs could contribute to limiting this risk (Shillcutt 2008).
Why it is important to do this review
The World Health Organization now recommends that all suspected malaria cases receive a parasitological diagnosis prior to treatment (WHO 2010a), and RDTs are the most feasible way of achieving this in rural areas of Africa (D'Acremont 2009; WHO 2006; WHO 2010a).
For policy makers seeking to introduce and improve malaria diagnosis in rural settings, this review aims to evaluate the effect of introducing RDTs into clinical algorithms on both the health outcomes for patients, and the unnecessary overuse of antimalarials. For health workers working in rural areas, this review also aims to evaluate the safety of RDT‐supported algorithms, and the potential for patients to be misdiagnosed as non‐malaria and sent home without appropriate treatment.
Objectives
To evaluate whether introducing RDTs into algorithms for diagnosing and treating people with fever improves health outcomes, reduces antimalarial prescribing, and is safe compared to algorithms using clinical diagnosis.
Methods
Criteria for considering studies for this review
Types of studies
Individual or cluster randomized controlled trials (RCTs).
Types of participants
Patients with fever, or a reported history of fever, living in malaria endemic areas. We excluded trials conducted in non‐endemic areas (for example, fever in travellers in Europe).
Types of interventions
Intervention
Diagnostic algorithms using RDTs to determine treatment for fever.
Control
Diagnostic algorithms based on clinical diagnosis to determine treatment for fever.
Types of outcome measures
Clinical
Patients still unwell at day 4+ follow‐up.
Prescribing
Patients with fever prescribed antimalarials.
Patients with fever prescribed antibiotics.
Safety
Microscopy‐positive patients not prescribed antimalarials.
Microscopy‐negative patients prescribed antimalarials.
Search methods for identification of studies
We attempted to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and ongoing).
Electronic searches
We searched the following databases up to 10 January 2014 using the search terms described in Appendix 1: Cochrane Infectious Disease Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; CINAHL; and LILACS.
In addition we searched the metaRegister of Controlled Trials (mRCT) and the WHO trials register using "malaria" AND "rapid diagnostic test*" OR "presumptive treatment" as search terms.
Searching other resources
Researchers and organizations
We contacted researchers in the field to identify additional trials that may have been eligible for inclusion.
Reference lists
We checked the reference lists of all selected trials identified by the search strategy described above.
Data collection and analysis
Selection of studies
John Odaga (JO) and Joseph A. Lokong (JAL) independently screened the abstracts in the search list for potentially relevant trials. We compared the list of potential articles independently identified by both authors. JO retrieved the full texts of the selected trials, which were made available to both authors. Both JO and JAL independently assessed each trial for inclusion, using an eligibility form based on the inclusion criteria. We included a trial if it satisfied all of the eligibility criteria. We resolved any disagreements by referring to the original articles or through discussions, or both, and where necessary by consulting Paul Garner (PG) and Sarah Donegan (SD).
Data extraction and management
JO and PG independently extracted outcomes data from the included trials, guided by a standard data extraction form. We resolved any disagreements by referring to the original paper and through discussions. Where necessary, we sought clarification from trial authors by contacting them directly to provide relevant data missing from the included trials (for example, number of participants by age group, number of health centres excluded from analysis).
Assessment of risk of bias in included studies
JO and PG independently assessed and judged the quality of the selected papers using the standard criteria (Higgins 2011). We assessed risk of bias against seven items: (1) how allocation sequence was generated (2) how allocation was concealed to participants, investigators and outcome assessors; (3) blinding of participants and investigators; (4) blinding of outcome assessors; (5) completeness of outcomes data (number analysed relative to number randomized) (6) selective reporting: whether all pre‐specified outcomes are reported; and (7) other sources of bias.
Measures of treatment effect
For all the included outcomes we calculated a risk ratio (RR) and presented the results alongside the 95% confidence interval (CI).
Unit of analysis issues
We performed analyses of all outcomes at individual levels using generic inverse variance method. Five of the included trials were cluster RCTs in which the unit of randomization were health facilities but analyses were performed at patient level.
Where trial authors had adjusted their results for the effect of clustering, we extracted the cluster adjusted RR and standard error and entered the natural log of these into Review Manager (RevMan) using the generic inverse variance method as recommended by Higgins 2011.
Where trial authors had not adjusted their results for the effect of clustering, we extracted the simple summary data for all relevant outcomes and calculated crude RR & 95% CI using Review Manager (RevMan). We adjusted for the effects of clustering using the approximate analysis method (as described in Higgins 2011). This involves inflating the standard error of the RR using an estimate of the design effect, and entering the natural logs of the adjusted RR and corresponding Standard Errors (SE) into Review Manager (RevMan) using the generic inverse variance method. For measures of antimalarial and antibiotic prescribing, we applied an external design effect of 3.8, as recommended by Rowe 2002 for health facility surveys assessing antimalarial treatment in Benin. For other measures we used the design effect stated by the trial authors when calculating their sample size.
When trial authors had correctly adjusted their results for the effect of clustering, but presented their results as Odds Ratio (OR) rather than Risk Ratio (RR), we again extracted the simple summary data and conducted our own approximate adjustment for clustering as described above.
One trial was a cluster RCT with three clusters per group (Hopkins 2008 UGA (Medium), Hopkins 2008 UGA (High), Hopkins 2008 UGA (V High)). However, we presented the data stratified by malaria endemicity where there was only one cluster per group. As a consequence, we could not make any adjustment for clustering. However, any clustering effect is likely to be very small, and unlikely to substantially affect the result or our interpretation.
Assessment of heterogeneity
We assessed heterogeneity among trials by inspecting the forest plots for overlapping CIs. We also applied the Chi2 test for heterogeneity with a 10% level of statistical significance, and an I2 statistic value greater than 40% to denote moderate levels of heterogeneity (Higgins 2011).
Data synthesis
We analysed the data using Review Manager (RevMan).
Where we had pooled data we used the generic inverse variance method which allows for meta‐analysis of both individually and cluster randomized trials. When we detected moderate levels of heterogeneity we combined trials using the random‐effects model which assumes the trials are estimating different, but related, intervention effects (Higgins 2011).
Quality of evidence
We assessed the quality of evidence across each outcome measure using the GRADE approach. The quality rating across trials has four levels: high, moderate, low, or very low. RCTs are initially categorized as high quality but can be downgraded after assessment of five criteria: risk of bias, consistency, directness, imprecision, and publication bias (Guyatt 2008).
Subgroup analysis and investigation of heterogeneity
Where we detected moderate heterogeneity, we performed subgroup analyses by stratifying results by the level of health worker adherence to the RDT result, the level of malaria endemicity, and the age group of the targeted population.
Results
Description of studies
Results of the search
From the search strategy, we identified a total 273 abstracts of trial reports (after removal of duplicates) and ten records of ongoing trials (see Figure 2 for the study flow diagram). We did not deem any of the ongoing trials relevant to this review.
2.
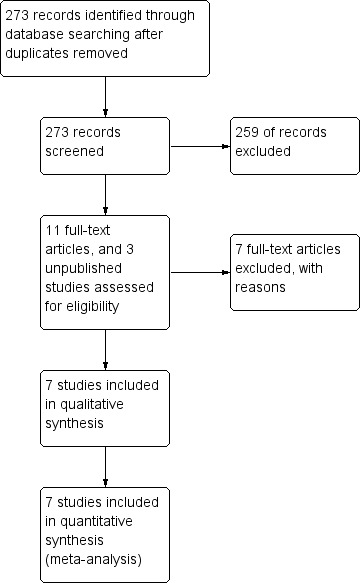
Study flow diagram.
Included studies
We included seven RCTs that enrolled 17,505 participants; two individually RCTs (Ansah 2010 GHA; Bisoffi 2009 BFA), two published cluster‐RCTs (Skarbinski 2009 KEN; Yeboah‐Antwi 2010 ZAM), and three unpublished cluster‐RCTs (Hopkins 2008 UGA (V High); Hopkins 2008 UGA (High); Hopkins 2008 UGA (Medium)). For a summary of the trial characteristics see Table 2, and for full details of individual trials see the 'Characteristics of included studies' tables.
1. Summary of characteristics of included studies.
| Characteristic | Trial ID | ||||||
| Ansah 2010 GHA | Bisoffi 2009 BFA | Yeboah‐Antwi 2010 ZAM | Skarbinski 2009 KEN | Hopkins 2008 UGA (V High) | Hopkins 2008 UGA (High) | Hopkins 2008 UGA (Medium) | |
| Setting | |||||||
| Country | Ghana | Burkina Faso | Zambia | Kenya | Uganda | Uganda | Uganda |
| Endemicity | Not indicated | Seasonal | High and low | High and low | Very high | High | Medium |
| Health facility, location | Health centres or dispensaries; rural | Dispensaries, rural & urban | Community posts | Health centres and hospitals | Health centres | Health centres | Health centres |
| Unit of randomization | Individuals | Individuals | Clusters | Clusters | Clusters | Clusters | Clusters |
| Proportion of RDTs positive | 63% | 53% | 28% | Not stated | 73% | 46% | 32% |
| Proportion of reference slides positive | 38% | Not stated | Not stated | 4% | 54% | 37% | 29% |
| Participants | |||||||
| Number of health facilities | 3 | 10 | 31 | 30 | 2 | 2 | 2 |
| Target population for malaria treatment | All | All | < 5 years | ≥ 5 years | All | All | All |
| Number randomized | 3452 | 2169 | 3125 | 2004 | 4197 | 2213 | 1550 |
| Number analysed for antimalarial prescribing | 3442 | 2169 | 3047 | 6691 | 4197 | 2213 | 1550 |
| Loss to follow‐up | 0.3% | 0.0% | 2.5% | ‐ | 0.0% | 0.0% | 0.0% |
| Outcomes reported | |||||||
| Clincal outcomes | No | Yes | Yes | No | Yes | Yes | Yes |
| Prescribing of antimalarials | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Prescribing of antibiotics | Yes | Yes | No | No | Yes | Yes | Yes |
1 Skarbinski 2009 KEN: 2004 participants were randomized but outcomes were collected through baseline and post intervention surveys. 669 participants were evaluated in teh post‐intervention survey.
Setting
All seven RCTs were conducted in Africa, in rural areas of Ghana, Burkina Faso, Zambia, Kenya, and Uganda. All trials were undertaken in basic healthcare facilities without microscopes. The health workers responsible for diagnosing and treating patients with fever were community health workers in one trial (Yeboah‐Antwi 2010 ZAM), nurses In two trials (Ansah 2010 GHA; Bisoffi 2009 BFA), and a mix of clinical officers and nurses in four trials (Hopkins 2008 UGA (V High); Hopkins 2008 UGA (High); Hopkins 2008 UGA (Medium); Skarbinski 2009 KEN).
Regarding malaria endemicity, Ansah 2010 GHA did not describe it, Bisoffi 2009 BFA described it as seasonal, and Skarbinski 2009 KEN and Yeboah‐Antwi 2010 ZAM described it as a mix of 'high and low'. The three trials from Uganda were conducted in areas of 'very high', 'high' and 'medium' endemicity respectively (Hopkins 2008 UGA (V High); Hopkins 2008 UGA (High); Hopkins 2008 UGA (Medium)). For subgroup analyses by endemicity we have used the proportion of RDTs testing positive as a surrogate marker for endemicity rather than these vague descriptors.
Interventions
The intervention consisted of training health workers to diagnose and treat patients with fever using clinical protocols incorporating RDTs. The duration of training was short (ranging from one half day in Kenya to five days in Zambia), and the level of ongoing supervision varied between trials (see Table 3). Supportive supervision (observation of tasks with feedback) was provided monthly in Zambia, and once in Kenya (two months after training). In Uganda, no formal supervision was provided, and in Ghana and Burkina Faso the level of supervision was unclear.
2. Description of the interventions.
| Characteristic | Trial ID | ||||||
| Ansah 2010 GHA | Bisoffi 2009 BFA | Yeboah‐Antwi 2010 ZAM | Skarbinski 2009 KEN | Hopkins 2008 UGA (V High) | Hopkins 2008 UGA (High) | Hopkins 2008 UGA (Medium) | |
| Training | |||||||
| Who was trained to follow the RDT algorithm? | Nurses and nursing assistants | Nurses | Community health workers | Nurses, clinical officers and doctors | Clinical officers and nurses | Clinical officers and nurses | Clinical officers and nurses |
| Who conducted the training? | Nurses, after a TOT course | Not described | Experienced IMCI trainers | Clinical officers and nurses, after a two‐ week TOT course | Experienced national trainers | Experienced national trainers | Experienced national trainers |
| How long was the training? (days) | 2 | 3 | 5 | Half‐day | 3 | 3 | 3 |
| Was a written guideline provided? | Unclear | Unclear | Unclear | Yes | Yes | Yes | Yes |
| What supervision was conducted? | Unclear | Unclear | Review of records and feedback each month | Observation and feedback once after two months | One day of supportive supervision two weeks after the training | One day of supportive supervision two weeks after the training | One day of supportive supervision two weeks after the training |
| Were staff incentives provided? | No | No | Bicycles | No | No | No | No |
| Who conducted the RDT tests? | Research staff | Research staff | Prescribers | Prescribers | Prescribers | Prescribers | Prescribers |
| Which RDT‐type | OptiMAL‐IT (pLDH) | Paracheck (HRP‐2) | ICT malaria Pf (HRP‐2) | Paracheck (HRP‐2) | Paracheck (HRP‐2) | Paracheck (HRP‐2) | Paracheck (HRP‐2) |
| Were the RDTs provided free? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the antimalarials provided free? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Were the antibiotics provided free? | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Algorithm | |||||||
| Test all cases of fever with RDTs | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Prescribe only if RDT is positive | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Do not prescribe if RDT is negative | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Treatment of RDT negative cases | Not described | Look for other causes and treat as per STG | Amoxicillin if signs of pneumonia; else refer | Not described | Look for other causes and treat as per STG | Look for other causes and treat as per STG | Look for other causes and treat as per STG |
| Guideline on prescribing antibiotics | Not mentioned | Not mentioned | Not mentioned | If pneumonia is suspected | Not mentioned | Not mentioned | Not mentioned |
In two trials, members of the research team conducted the RDT tests and then sent the results to the health workers for interpretation and treatment (Ansah 2010 GHA; Bisoffi 2009 BFA). The authors state that this approach aimed to optimise the quality of RDT results and minimise time pressure on the health workers. In the Zambian, Kenyan, and Ugandan trials, the clinical officers, nurses or community health workers carried out the test themselves.
Only four trials reported to have provided written guidelines to the intervention health facilities following training (Hopkins 2008 UGA (V High); Hopkins 2008 UGA (Medium); Hopkins 2008 UGA (High); Skarbinski 2009 KEN), and only five trials included in their training a clear message about the need for health workers to look for alternative causes of fever in patients with negative RDTs (Bisoffi 2009 BFA; Hopkins 2008 UGA (V High); Hopkins 2008 UGA (Medium); Hopkins 2008 UGA (High); Skarbinski 2009 KEN).
RDTs, antimalarials, and antibiotics were provided to patients free of charge in all trials.
Adherence to algorithm
Only the three trials from Uganda provide data on the extent to which RDTs were conducted in the intervention arm. In these trials, at least 97% of all fever cases were tested by RDTs to confirm the presence of malaria prior to treatment (Hopkins 2008 UGA (V High); Hopkins 2008 UGA (High); Hopkins 2008 UGA (Medium)). Six trials provide data on the proportion of RDT‐positive patients for whom antimalarials were prescribed (see Table 4). Health workers prescribed antimalarials to 98% to 100% of cases with positive RDTs, and to up to 81% of patients with negative RDTs. Where this is likely to have affected the outcome we conducted subgroup analyses by level of health worker adherence.
3. Assessment of endemicity and health worker adherence.
| Trial ID | Age group | Proportion of RDTs positive (%) | Proportion of reference slide positive (%) | Endemicity classification1 | Proportion of patients prescribed antimalarials (%) | Health worker adherence classification2 | |
| RDT arm | Clinical arm | ||||||
| Hopkins 2008 UGA (V High) | All | 73 | 54 | Very high | 72 | 95 | High |
| Bisoffi 2009 BFA | All | 55 | ‐ | High | 89 | 87 | Very low |
| Hopkins 2008 UGA (High) | All | 46 | 37 | High | 45 | 98 | High |
| Ansah 2010 GHA | All | 41 | 38 | High | 70 | 93 | Low |
| Hopkins 2008 UGA (Medium) | All | 32 | 29 | Moderate | 32 | 98 | High |
| Yeboah‐Antwi 2010 ZAM | < 5 | 28 | ‐ | Moderate | 28 | 99 | High |
| Skarbinski 2009 KEN | > 5 | ‐ | 4 | Low | 41 | 54 | Very low3 |
1 The endemicity classification is the Cochrane Review authors' judgement based on the proportion of RDTs testing positive: Very high = > 60%; High = 40% to 59%; Moderate = 6% to 39%. 2 The health worker adherence classification is the Cochrane Review authors' judgement and is based on the difference between the proportion of RDTs testing positive and the proportion of patients being prescribed antimalarials in the RDT arm: High = difference < 10%; Moderate = difference 11% to 20%; Low = difference 21% to 30%; Very low = difference > 30%. 3 For Skarbinski 2009 KEN, the proportion of RDTs testing positive was unavailable.
Participants
One trial targeted children under the age of five years (Yeboah‐Antwi 2010 ZAM), one trial targeted older children (Skarbinski 2009 KEN), and five trials targeted all age groups. All trials recruited participants with fever or history of fever except the three unpublished trials which recruited participants with any complaint. However, for these unpublished trials we restricted our analysis to only the subgroup of participants which presented with fever.
Excluded studies
We excluded seven trials and listed the reasons in the Characteristics of excluded studies table.
Risk of bias in included studies
We have presented a summary of the risk of bias assessment in Figure 3.
3.
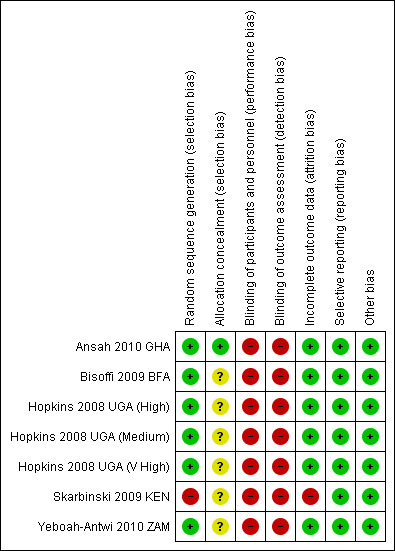
Risk of bias summary: review authors' judgements about each risk of bias item for each included trial. Green = low risk of bias, red = high risk of bias, yellow = unclear risk of bias.
Allocation
Only one trial adequately described both sequence generation and allocation concealment to be considered at low risk of selection bias (Ansah 2010 GHA). The remaining six trials were at unclear risk due to inadequate descriptions of allocation concealment.
Blinding
Blinding of participants and health workers to the use of RDTs was not be possible. However, blinding of the outcome assessment was possible in all trials but was not described in any of them.
Incomplete outcome data
Attrition bias was at low risk of bias (≤ 3%) in six trials, and in one trial the attrition rate was high (30%) and unequal in the two arms (Skarbinski 2009 KEN).
Selective reporting
All trials reported outcomes that were pre‐specified in the methods sections of their protocols and reports. The risk of selective reporting was low.
Other potential sources of bias
Three trials (Ansah 2010 GHA; Skarbinski 2009 KEN; Yeboah‐Antwi 2010 ZAM) acknowledged baseline imbalance in the number or quality of the health workers enrolled into the trial, which were adjusted for using different methods.
Effects of interventions
See: Table 1
See Table 1 for a summary of the results and GRADE appraisal of the quality of evidence.
Clinical outcomes
Patients still unwell at day 4+ follow‐up
Five trials from settings with very high, high, and moderate malaria endemicity, reported the proportion of patients who were still unwell four to seven days after treatment, and found no significant differences between clinical and RDT‐supported diagnosis (RR 0.90, 95% CI 0.69 to 1.17, 6990 participants, five trials, Analysis 1.1, Figure 4). The absolute numbers of participants remaining unwell ranged from 2.8 to 9.3% in those diagnosed with an RDT, and from 4.1 to 10.8% in those diagnosed clinically (see Appendix 2).
1.1. Analysis.
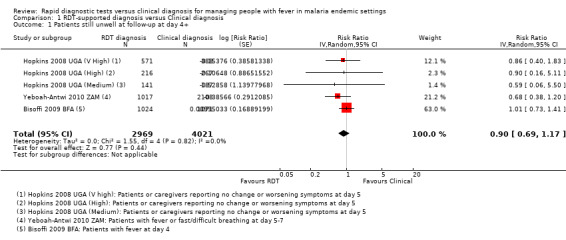
Comparison 1 RDT‐supported diagnosis versus Clinical diagnosis, Outcome 1 Patients still unwell at follow‐up at day 4+.
4.
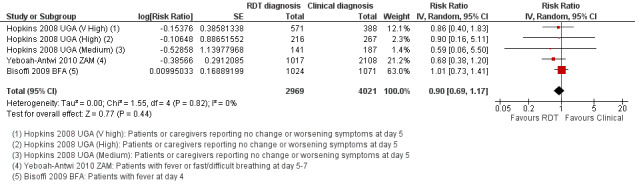
Forest plot of comparison: 1 RDT‐supported diagnosis versus Clinical diagnosis, outcome: 1.1 Patients still unwell at follow‐up at day 4+.
Statistical heterogeneity was low (I2 = 0%). However, in one trial health worker compliance with the RDT‐supported diagnosis was very low, with a high prescription of antimalarials in both groups regardless of the RDT result (Bisoffi 2009 BFA). This trial found no difference between the intervention arms in the proportion of cases who were still unwell at follow‐up (2095 participants, one trial, Analysis 1.2). In the remaining trials with improved health worker compliance, there is a trend towards a health benefit with using RDTs, although the CI is wide and includes the possibility of no difference between groups (4895 participants, four trials, Analysis 1.2).
1.2. Analysis.
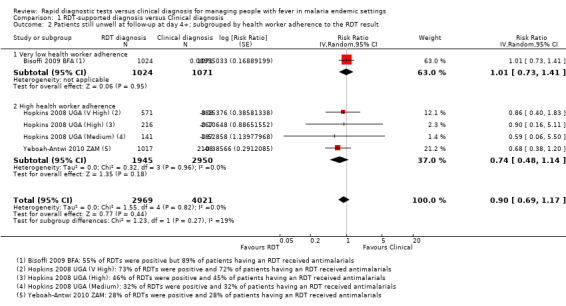
Comparison 1 RDT‐supported diagnosis versus Clinical diagnosis, Outcome 2 Patients still unwell at follow‐up at day 4+; subgrouped by health worker adherence to the RDT result.
Prescribing outcomes
Patients with fever prescribed antimalarials
Although fewer patients in the group with RDT‐supported diagnosis were prescribed antimalarials there is substantial heterogeneity between trials, with no impact on prescribing in one trial and moderate or large effects in the others (17,287 participants, seven trials, I² = 98%, Analysis 1.3). This variation seems most related to health worker adherence to the RDT‐supported protocol (Figure 5). In the trial from Burkina Faso health workers prescribed antimalarials to 81% of patients with negative RDT results, and consequently no difference in antimalarial prescribing was detected (RR 1.02, 95% CI 0.99 to 1.06, 2169 participants, one trial, Analysis 1.4). In the four trials in which health worker adherence was high, the reduction in prescribing of antimalarials was large (Analysis 1.4, Figure 5).
1.3. Analysis.
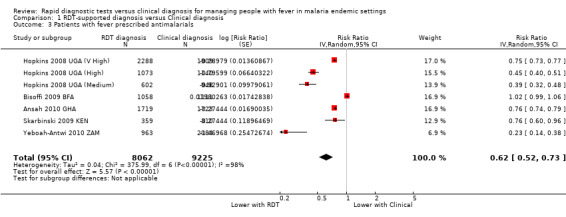
Comparison 1 RDT‐supported diagnosis versus Clinical diagnosis, Outcome 3 Patients with fever prescribed antimalarials.
5.
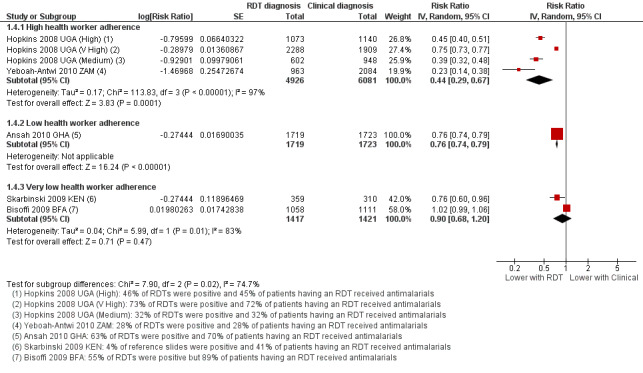
Forest plot of comparison: 1 RDT‐supported diagnosis versus Clinical diagnosis, outcome: 1.4 Patients with fever prescribed antimalarials; subgrouped by health worker adherence to the RDT result.
1.4. Analysis.
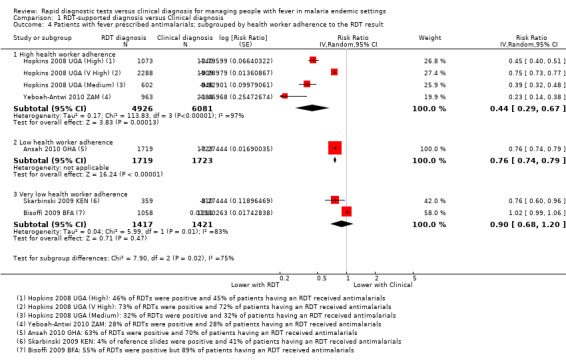
Comparison 1 RDT‐supported diagnosis versus Clinical diagnosis, Outcome 4 Patients with fever prescribed antimalarials; subgrouped by health worker adherence to the RDT result.
Within the subgroup of trials with high health worker adherence, the relative malaria endemicity also seems to influence the size of the reduction in antimalarial prescriptions. The biggest reductions were seen where less than 30% of people presenting with fever tested positive by RDT, and smaller reductions were seen where RDT positivity was greater than 40%, or greater than 70% (11,007 participants, four trials, Analysis 1.5).
1.5. Analysis.
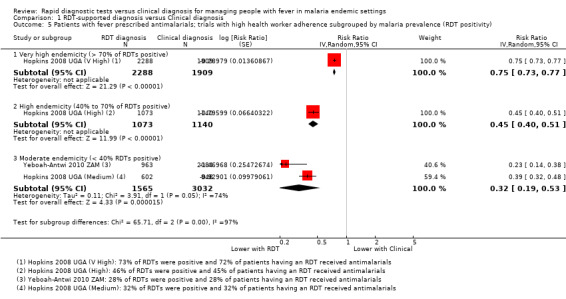
Comparison 1 RDT‐supported diagnosis versus Clinical diagnosis, Outcome 5 Patients with fever prescribed antimalarials; trials with high health worker adherence subgrouped by malaria prevalence (RDT positivity).
We also conducted a subgroup analysis by age of participants, and the reduction in antimalarial use appears largest in participants over the age of five (Analysis 1.6). We were unable to assess whether this difference was due to reduced health worker adherence when treating children aged less than five as the data were unavailable.
1.6. Analysis.
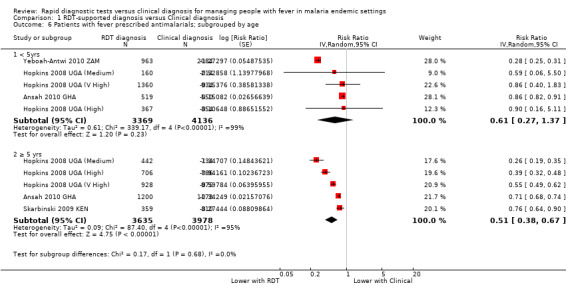
Comparison 1 RDT‐supported diagnosis versus Clinical diagnosis, Outcome 6 Patients with fever prescribed antimalarials; subgrouped by age.
Patients with fever prescribed antibiotics
Five trials reported the proportion of patients prescribed antibiotics with very variable results (13,573 participants, five trials, Analysis 1.7).
1.7. Analysis.
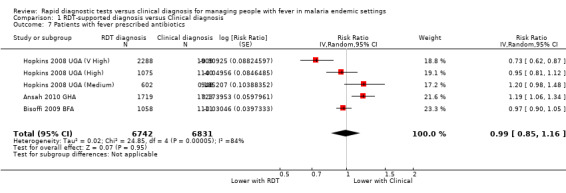
Comparison 1 RDT‐supported diagnosis versus Clinical diagnosis, Outcome 7 Patients with fever prescribed antibiotics.
In Burkina Faso, where compliance with the RDT result was very low and no difference was seen in antimalarial prescribing behaviour, there was also no difference in antibiotic prescribing (Bisoffi 2009 BFA, Analysis 1.7). In the two trials with the largest relative reduction in antimalarial prescribing, there was no significant difference in antibiotic prescribing between groups ((Hopkins 2008 UGA (Medium); Hopkins 2008 UGA (High), Analysis 1.7). In these trials, the RDT protocol did not recommend antibiotics for all RDT negative patients but instead advised the health worker to look for other causes of fever and treat appropriately.
In the Uganda setting with very high endemicity, where over 70% of RDTs were positive, and antimalarial prescribing was reduced by a quarter the RDT protocol also reduced antibiotic prescribing (Hopkins 2008 UGA (V High), Analysis 1.7). Conversely, in Ghana where 63% of RDTs were positive and antimalarial prescribing was also reduced by a quarter, antibiotic prescribing increased in the RDT group (Ansah 2010 GHA, Analysis 1.7).
Safety outcomes
Microscopy positive patients not prescribed antimalarials
Only one trial conducted microscopy on all participants in both intervention arms allowing identification of malaria cases 'missed' by the RDT‐supported protocol, or 'false negatives' (Ansah 2010 GHA). This trial was conducted in Ghana where 63% of RDTs were positive for malaria. The proportion of reference slide positive patients not prescribed antimalarials was higher with the use of RDTs but this did not reach statistical significance (1280 participants, one trial, Analysis 1.8).
1.8. Analysis.
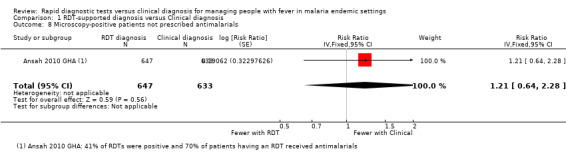
Comparison 1 RDT‐supported diagnosis versus Clinical diagnosis, Outcome 8 Microscopy‐positive patients not prescribed antimalarials.
In addition, the three trials from Uganda conducted microscopy on just the participants in the arms using RDT‐supported diagnosis. The proportion of microscopy positive patients not given antimalarials due to a negative RDT result was 2.0% in the very high endemic setting (95% CI 1.2% to 2.8%, 1187 participants), 4.5% in the high endemic setting (95% CI 2.3% to 6.6%, 357 participants), and 17.2% in the area of medium endemicity (95% CI 11.6% to 22.8%, 174 participant). Table 5 examines how these figures translate into negative and positive predictive values in the different settings. In the area of medium endemicity, the accuracy of RDTs performed by health workers was not as high as in settings of high and very high endemicity with a sensitivity of 82.8%, and specificity of 72.2%. Consequently, the negative predictive value of a negative RDT in this setting is 0.93, which means that for every 100 patients with a negative RDT result, seven patients will have malaria parasites demonstrated by microscopy (false negatives).
4. Negative and positive predictive values of RDTs in trials by Hopkins et al.
| Trial ID | Proportion of reference slides positive | Number of patients1 | Sensitivity6 (95% CI) | Specificity7 (95% CI) | NPV8 | PPV9 | |||
| TP2 | FP3 | TN4 | FN5 | ||||||
| Hopkins 2008 UGA (V High) | 55% | 1165 (51.1%) | 454 (19.9%) | 633 (27.8%) | 26 (1.1%) | 97.8% | 59.4% | 96.2% | 72.0% |
| Hopkins 2008 UGA (High) | 37% | 347 (33.7%) | 100 (9.7%) | 567 (55.0%) | 17 (1.7%) | 95.3% | 85.0% | 97.1% | 77.6% |
| Hopkins 2008 UGA (Medium) | 29% | 145 (24.6%) | 45 (7.5%) | 378 (63.2%) | 30 (5.0%) | 82.9% | 89.4% | 92.7% | 76.3% |
1 This data has been taken from the trial data of Hopkins 2008 UGA (V High), Hopkins 2008 UGA (High), & Hopkins 2008 UGA (Medium), and converted into a percentage. 2 TP = True positive = RDT positive and microscopy positive 3 FP = False positive = RDT positive and microscopy negative 4 TN = True negative = RDT negative and microscopy negative 5 FN = False negative = RDT negative and microscopy positive 6 Sensitivity = The proportion of people with fever due to malaria correctly identified with a positive RDT result = TP/(TP+FN) 7 Specificity = The proportion of people with fever due to non‐malaria illness correctly identified with a negative RDT result = TN/(TN+FP) 8 NPV = Negative predictive value = The proportion of people with a negative RDT result who have a non‐malaria cause of their fever = TN/(TN+FN) 9 PPV = Positive predictive value = The proportion of people with a positive RDT result who have malaria as a cause of their fever = TP/(TP+FP)
Microscopy negative patients prescribed antimalarials
The same trial from Ghana also allows identification of 'false positives'; the number of patients without malaria on microscopy who tested positive by RDT and received antimalarials (Ansah 2010 GHA). In this trial RDT‐supported diagnosis significantly reduced the over treatment of malaria (RR 0.60, 95% CI 0.57 to 0.64, 2162 participants, one trial, Analysis 1.9), but 53.9% of people with negative microscopy still received antimalarials.
1.9. Analysis.
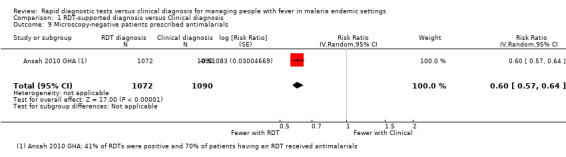
Comparison 1 RDT‐supported diagnosis versus Clinical diagnosis, Outcome 9 Microscopy‐negative patients prescribed antimalarials.
In the three trials from Uganda, which conducted microscopy on just the participants in the arms using RDT‐supported diagnosis, the proportions of slide negative participants who were prescribed antimalarials because they tested positive on RDT (false positives) was 34.4% in the medium transmission setting (95% CI 29.9% to 39.0%, 418 participants), 15.2% in the high transmission setting (95% CI 12.4% to 18.1%, 617 participants), and 43.9% in the very high transmission setting (95% CI 40.8 to 46.9%, 1028 participants).
Discussion
Summary of main results
Seven trials, enrolling 17,505 participants, are included in this review; two individually RCTs and five cluster RCTs.
In most trials the health workers diagnosing and treating malaria were nurses or clinical officers who had undergone less than one week of training in RDT supported diagnosis. Health worker adherence to the RDT result was highly variable, with the percentage of participants with a negative RDT result who received antimalarials ranging from 0% to 81%.
In these trials, RDT‐supported diagnosis had little or no effect on the number of participants remaining unwell at four to seven days after treatment (low quality evidence).
However, using RDTs reduced the prescription of antimalarials by up to three‐quarters (moderate quality evidence), and as would be expected reductions in prescribing of antimalarials were highest where health workers adherence to the RDT result was high, and where the true prevalence of malaria was lower.
Using RDTs to support diagnosis did not have a consistent effect on the prescription of antibiotics with some trials showing an increase in antibiotic prescription and some showing a decrease (very low quality evidence).
In a single trial from a setting with moderate endemicity, which reported microscopy results for all enrolled patients, RDT supported diagnosis did not result in a statistically significant excess of patients with microscopically confirmed malaria who did not receive antimalarials (low quality evidence).
Overall completeness and applicability of evidence
The included trials were all conducted in first‐level health facilities in rural areas of Africa, where the majority of malaria cases occur, and where RDTs offer the only feasible alternative to presumptive treatment of malaria based on clinical symptoms alone. These trials are from settings with a range of levels of malaria endemicity, and the findings could reasonably be applied to other similar African settings.
The main concern of health workers regarding the use of RDTs is the risk of missing malaria cases and sending children home without antimalarials when the result of the RDT is a false negative (D'Acremont 2009; English 2009; Murray 2008; Wongsrichanalai 2007). Reassuringly, this review found no difference in patient health outcomes when RDTs are introduced, and the trend is in the direction of benefit. However, only one trial adequately evaluated the risk of false negative results by applying the gold standard light microscopy to all fever patients in both treatment arms (Ansah 2010 GHA), and although the result did not reach statistical significance the trend was towards higher numbers of missed cases when RDTs were used.
The additional trial from Uganda, which reported decreased RDT sensitivity in the setting with lowest endemicity adds to this concern. In this setting, of 404 patients with negative RDTs, 30 (7%) had malaria parasites following microscopy analysis. This risk may be considered too high by some patients and health workers unless adequate measures are taken to ensure the safety of these patients, such as routine follow‐up at 24 or 48 hours and repeat testing if they remain unwell. These data also raise concerns about the performance of RDTs in real‐life clinical scenarios. The cause of the low sensitivity is not clear, and may be user‐dependent, but reassuringly this trial appears to be an outlier when seen in the context of all the observational data on RDT sensitivity and specificity (see Figure 6). Of the 71 trials of HRP‐2 RDTs included in the Cochrane diagnostic test accuracy review, 51 were conducted in areas of lower endemicity with a pooled sensitivity of 95.1% (95% CI 93.1 to 96.6) and specificity of 95.9% (95% CI 94.1 to 97.2) (Abba 2011).
6.
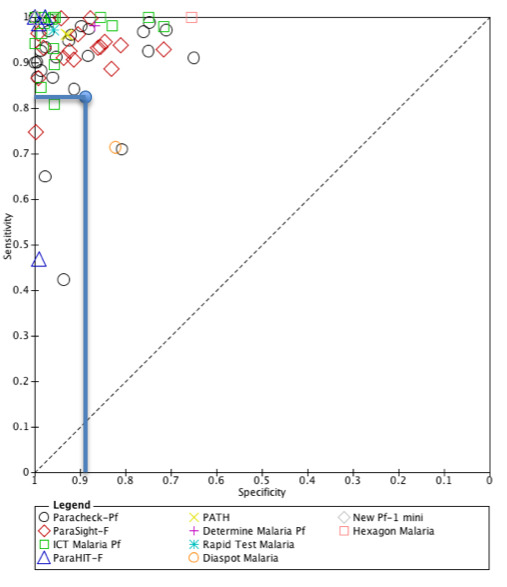
Sensitivity and specificity of 71 trials of HRP‐2 RDTs included in the Cochrane Review of RDTS for diagnosing P. falciparum malaria (Abba 2011). The data from Hopkins 2008 UGA (Medium) is represented with a blue circle at sensitivity 0.829 and specificity 0.894.
The five trials included in this review show no clear trend in prescribing of antibiotics which may indicate inconsistency in protocols for managing RDT negative results. This is surprising given that those with negative RDTs are the only people who will potentially benefit from the introduction of RDTs and also those who will potentially be harmed. Future research, and future programmes, should concentrate on improving health worker guidance and patient health outcomes in this group.
Quality of the evidence
We assessed the quality of the evidence using the GRADE approach and presented the basis for the judgements in Table 1.
The evidence that introducing RDTs has little or no effect on health outcomes is of low quality, meaning we can only have limited confidence in this result. Although there were minor concerns about risk of bias (with none of the trials adequately minimising the risk of selection bias), and inconsistency (with one trial with very poor health worker adherence finding no evidence of an effect), the main reasons for downgrading the evidence were 'indirectness' and 'imprecision'. The evidence is indirect because health benefits of introducing RDTs will only be seen with adequate treatment of the fevers not caused by malaria. In these trials, the management protocol for patients with negative RDTs was unclear, and the mixed effects on antibiotic prescribing may suggest that the management of these patients was erratic. Once we excluded the trial with poor health worker adherence (Bisoffi 2009 BFA), there was a consistent trend towards benefit with the use of RDTs although this did not reach statistical significance. Larger trials may be necessary to show statistically significant benefits if they exist.
The evidence that introducing RDTs can substantially reduce the overuse of antimalarials is of moderate quality, meaning we can have reasonable confidence in this result. We downgraded the evidence due to concerns about inconsistency between trials, with large effects in some and complete absence of effects in others. This inconsistency is best explained by the variation in adherence of health workers to negative RDT results. Consequently, to see the reductions in antimalarial use predicted by the known malaria prevalence in any setting, healthcare managers will need to ensure adequate training, support, and supervision for health workers in the use of RDTs, and in the management of patients who test negative.
The evidence that using RDTs does not increase the proportion of patients with malaria who are sent home without antimalarials is of low quality, meaning we can have only limited confidence in this result. This result is from a single trial setting, and was downgraded for serious indirectness as the result is poorly applicable to elsewhere. The 95% CI is also wide and includes the possibility of clinically important harms with RDTs and was downgraded for serious imprecision.
Potential biases in the review process
We have reported the RDT sensitivity and specificity of a single arm from a trial at three sites in Uganda because this data was available to us (Hopkins 2008 UGA (V High); Hopkins 2008 UGA (High); Hopkins 2008 UGA (Medium)). However, this information is observational, and in this review we did not search for all trials that would present this information. These data therefore should only be considered in the context of the wider body of evidence included in the Cochrane Review by Abba 2011.
Agreements and disagreements with other studies or reviews
The review results are supported by findings from several quasi‐experimental and observational studies excluded from this review.
For health outcomes, two weekly cross‐over trials from Tanzania found no change in mortality with the introduction of RDTs. However, in one of these studies RDTs were associated with a decrease in the proportion of patients remaining unwell two weeks after treatment (Msellem 2009), and in the other RDTs were associated with an increase in the proportion still unwell after seven days of treatment (Mubi 2011).
For prescribing outcomes, several non‐RCTs have found reductions in antimalarial prescribing following the introduction of RDTs, especially in low transmission areas (Yukich 2012; D'Acremont 2011; Kyabayinze 2010; Msellem 2009; Reyburn 2007). Routine data from a large scale implementation project in Senegal found similar results over a three year period providing some evidence that RDTs reduce prescribing of antimalarials in routine practice as well as under experimental conditions (Thiam 2011).
Authors' conclusions
Implications for practice.
Algorithms incorporating RDTs can substantially reduce antimalarial prescribing if health workers adhere to the test results. The introduction of RDTs has not been shown to improve health outcomes for patients but adherence to the test result does not seem to result in worse clinical outcomes than presumptive treatment. Concentrating on improving the care of RDT negative patients could improve health outcomes in febrile children.
These trials were performed as these new RDT technologies were being rolled out, so observational studies and audits of guideline implementation will help monitor adherence over time.
Implications for research.
Decision making around the use of RDTs could be further informed by:
Continued evaluation of RDT sensitivity under operational conditions in settings with moderate or low endemicity,
Better quantification of the risk of patient harm to those with false negative RDT results,
Better quantification of the causes of non‐malaria fevers in these settings,
Design and evaluation of interventions aimed at improving the care of RDT negative patients such as improved protocols which include routine follow‐up or repeat RDT testing at 24 to 48 hours if patients remain unwell.
What's new
| Date | Event | Description |
|---|---|---|
| 8 May 2014 | Amended | There were minor errors in the values for assumed and corresponding risk in Table 1 which we have corrected. In addition, we corrected references provided in some of the footnotes to forest plots from Hopkins 2010 to Hopkins 2008. |
Acknowledgements
The editorial base for the Cochrane Infectious Diseases Group is funded by the UK Department for International Development (DFID) for the benefit of low‐ and middle‐income countries.
We thank Katherine Abba for permission to reproduce Figure 7 from Abba 2011.
The data synthesised in this review is from John Odaga's PhD thesis, which was funded by the Commonwealth Scholarship Commision (UK).
Appendices
Appendix 1. Detailed search terms for electronic databases
| Search set | Search terms |
| 1 | Malaria |
| 2 | Fever |
| 3 | Febrile illness |
| 4 | 1 or 2 or 3 |
| 5 | Rapid diagnostic test |
| 6 | RDT |
| 7 | Presumptive treatment |
| 8 | Syndromic approach |
| 9 | Treatment practice |
| 10 | Management |
| 11 | Prescription behaviour |
| 12 | Definite diagnosis |
| 13 | 5‐12/or |
| 14 | randomized controlled trial |
| 15 | random allocation |
| 16 | double blind method |
| 17 | single blind method |
| 18 | randomly |
| 19 | Clinical trials |
| 20 | 14‐19/or |
| 21 | 4 and 13 and 20* |
| * Search terms 14‐19 will not be applied to CENTRAL |
Appendix 2. Summary statistics for outcomes assessed
| Trial | RDT algorithm | Clinical algorithm | ||
| Events (%) | Total | Events (%) | Total | |
| 1.0 Patients | ||||
|
1.1 Patients prescribed antimalarials | ||||
| Yeboah‐Antwi 2010 ZAM* | 27.5 | 963 | 99.1 | 2084 |
| Hopkins 2008 UGA (Medium) | 31.7 | 602 | 99.3 | 948 |
| Hopkins 2008 UGA (High) | 43.9 | 1073 | 98.0 | 1140 |
| Hopkins 2008 UGA (V High) | 70.8 | 2288 | 94.6 | 1909 |
| Skarbinski 2009 KEN | 40.9 | 359 | 54.2 | 310 |
| Ansah 2010 GHA | 70.0 | 1719 | 92.7 | 1723 |
| Bisoffi 2009 BFA | 89.3 | 1058 | 87.2 | 1111 |
|
1.2 Microscopy positive patients receiving antimalarials | ||||
| Ansah 2010 GHA | 96.8 | 647 | 97.3 | 633 |
|
1.2 Microscopy positive patients missing antimalarials |
||||
| Ansah 2010 GHA | 3.2 | 647 | 2.7 | 633 |
|
1.3 Microscopy negative patients receiving antimalarials | ||||
| Ansah 2010 GHA | 53.9 | 1072 | 90.1 | 1090 |
|
1.4 Number prescribed antibiotics | ||||
| Bisoffi 2009 BFA | 52.9 | 1058 | 54.8 | 1111 |
| Hopkins 2008 UGA (Medium) | 52.0 | 602 | 47.8 | 948 |
| Hopkins 2008 UGA (High) | 51.9 | 1073 | 57.1 | 1140 |
| Hopkins 2008 UGA (V High) | 42.5 | 2288 | 58.9 | 1909 |
| Ansah 2010 GHA | 26.6 | 1719 | 22.3 | 1723 |
|
1.5 Patients still unwell at follow‐up at day 4, or after |
||||
| Yeboah‐Antwi 2010 ZAM | 9.3 | 1017 | 10.0 | 2108 |
| Hopkins 2008 UGA (Medium) | 2.8 | 141 | 4.8 | 187 |
| Hopkins 2008 UGA (High) | 3.7 | 216 | 4.1 | 267 |
| Hopkins 2008 UGA (V High) | 9.3 | 571 | 10.8 | 388 |
| Bisoffi 2009 BFA | 5.6 | 1024 | 5.5 | 1071 |
| 2.0 Subgroups | ||||
|
2.1 Fever patients receiving antimalarials | ||||
|
2.1.1 < 5 years | ||||
| Yeboah‐Antwi 2010 ZAM* | 27.5 | 963 | 99.1 | 2084 |
| Ansah 2010 GHA | 80.0 | 519 | 92.5 | 550 |
| Hopkins 2008 UGA (Medium) | 46.9 | 160 | 99.1 | 214 |
| Hopkins 2008 UGA (High) | 55.3 | 367 | 98.3 | 354 |
| Hopkins 2008 UGA (V High) | 83.3 | 1360 | 93.4 | 934 |
|
2.1.2 ≥ 5 years | ||||
| Ansah 2010 GHA | 65.8 | 1200 | 92.8 | 1173 |
| Skarbinski 2009 KEN | 40.9 | 359 | 54.2 | 310 |
| Hopkins 2008 UGA (Medium) | 26.2 | 442 | 99.3 | 734 |
| Hopkins 2008 UGA (High) | 38.0 | 706 | 97.8 | 786 |
| Hopkins 2008 UGA (V High) | 52.4 | 928 | 95.8 | 975 |
| *RRs calculated from these summary statistics may be different from those in the analysis. In the analysis, the review authors extracted RRs which had been adjusted for clustering and baseline imbalance. | ||||
Data and analyses
Comparison 1. RDT‐supported diagnosis versus Clinical diagnosis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Patients still unwell at follow‐up at day 4+ | 5 | 6990 | Risk Ratio (Random, 95% CI) | 0.90 [0.69, 1.17] |
| 2 Patients still unwell at follow‐up at day 4+; subgrouped by health worker adherence to the RDT result | 5 | 6990 | Risk Ratio (Random, 95% CI) | 0.90 [0.69, 1.17] |
| 2.1 Very low health worker adherence | 1 | 2095 | Risk Ratio (Random, 95% CI) | 1.01 [0.73, 1.41] |
| 2.2 High health worker adherence | 4 | 4895 | Risk Ratio (Random, 95% CI) | 0.74 [0.48, 1.14] |
| 3 Patients with fever prescribed antimalarials | 7 | 17287 | Risk Ratio (Random, 95% CI) | 0.62 [0.52, 0.73] |
| 4 Patients with fever prescribed antimalarials; subgrouped by health worker adherence to the RDT result | 7 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 4.1 High health worker adherence | 4 | 11007 | Risk Ratio (Random, 95% CI) | 0.44 [0.29, 0.67] |
| 4.2 Low health worker adherence | 1 | 3442 | Risk Ratio (Random, 95% CI) | 0.76 [0.74, 0.79] |
| 4.3 Very low health worker adherence | 2 | 2838 | Risk Ratio (Random, 95% CI) | 0.90 [0.68, 1.20] |
| 5 Patients with fever prescribed antimalarials; trials with high health worker adherence subgrouped by malaria prevalence (RDT positivity) | 4 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 5.1 Very high endemicity (> 70% of RDTs positive) | 1 | 4197 | Risk Ratio (Random, 95% CI) | 0.75 [0.73, 0.77] |
| 5.2 High endemicity (40% to 70% of RDTs positive) | 1 | 2213 | Risk Ratio (Random, 95% CI) | 0.45 [0.40, 0.51] |
| 5.3 Moderate endemicity (< 40% RDTs positive) | 2 | 4597 | Risk Ratio (Random, 95% CI) | 0.32 [0.19, 0.53] |
| 6 Patients with fever prescribed antimalarials; subgrouped by age | 6 | Risk Ratio (Random, 95% CI) | Subtotals only | |
| 6.1 < 5yrs | 5 | 7505 | Risk Ratio (Random, 95% CI) | 0.61 [0.27, 1.37] |
| 6.2 ≥ 5 yrs | 5 | 7613 | Risk Ratio (Random, 95% CI) | 0.51 [0.38, 0.67] |
| 7 Patients with fever prescribed antibiotics | 5 | 13573 | Risk Ratio (Random, 95% CI) | 0.99 [0.85, 1.16] |
| 8 Microscopy‐positive patients not prescribed antimalarials | 1 | 1280 | Risk Ratio (Fixed, 95% CI) | 1.21 [0.64, 2.28] |
| 9 Microscopy‐negative patients prescribed antimalarials | 1 | 2162 | Risk Ratio (Random, 95% CI) | 0.60 [0.57, 0.64] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ansah 2010 GHA.
| Methods | Trial design: individually RCT Patients evaluated on day 28 Reference slides taken on all patients |
|
| Participants | Children and adults with suspected malaria Exclusion: pregnancy, illness requiring admission, non‐compliance with allocated test or treatment, not living locally Number of participants randomized: 3452 Number analysed for primary outcome (prescribing of antimalarials): 3442 (0.3% loss to follow‐up) |
|
| Interventions | RDT plus treatment versus clinical diagnosis plus treatment. (A second component examining RDT versus microscopy did not meet our entry criteria). Health workers in both groups received training and held guidelines RDT performed by research team Health workers complied with guidelines partially: 49.5% of participants with negative RDT results received antimalarials |
|
| Outcomes | Primary: Patients treated with antimalarials who did not have malaria based on reference slide. Secondary:
|
|
| Notes | Country: Ghana RDT: OptiMAL‐IT Setting: three health centres, of all referral levels Transmission: not indicated Dates: July 2007 to December 2008 Funding: Gates Malaria Partnership |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated blocks of 10. |
| Allocation concealment (selection bias) | Low risk | Numbers placed in sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Trial participants and staff were aware of allocated tests, the results, and prescriptions. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Both the trial participants and personnel were aware of the diagnostic outcomes and the medications prescribed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was low and comparable in both settings (≤ 3%). |
| Selective reporting (reporting bias) | Low risk | Reported on all ‐ outcomes specified in prospective trial register. |
| Other bias | Low risk | No other sources of bias identified. |
Bisoffi 2009 BFA.
| Methods | Trial design: individually RCT lasting two months, one month in rainy season, one month in dry season |
|
| Participants | Number of participants randomized: 2169 (1058 in RDT arm, 1111 in presumptive treatment arm) Number analysed for primary outcomes: (a) prescribing of antimalarials analysis 2169 (0% loss); (b) clinical outcomes: 2095 (3.4% loss) Inclusion: age ≥ 6 years; axillary temperature ≥ 37.5°C Exclusion: severe malaria |
|
| Interventions | Intervention: RDT‐based policy for fever Control: Presumptive treatment Both groups received training and held guidelines RDT performed by research team Health workers did not comply with guidelines most of the time: 81% of participants with negative RDT results received antimalarials |
|
| Outcomes | Primary: patients with fever on day 4 Secondary:
|
|
| Notes | Country: Burkina Faso RDT: paracheck (HRP2) Setting: peripheral health centres. Sampling: convenient selection of health centres to ensure rural/urban representativeness Transmission: stable with seasonal transmission Dates: 2006; end of dry season and rainy season Funding: UNIDEA‐UNICREDIT Foundation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random list. |
| Allocation concealment (selection bias) | Unclear risk | Not indicated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both the trial participants and personnel were aware of intervention allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Both the trial participants and personnel were aware of the diagnosis made and treatment prescribed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was generally low (95.4% dry season; 97.3% rainy season) but not differentiated by trial group. Performed available case analysis, although reported to have performed intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Low risk | Reported on all trial outcomes described in the methodology. |
| Other bias | Low risk | No other sources of bias identified. |
Hopkins 2008 UGA (High).
| Methods | Trial design: cluster randomized pre‐post open‐label trial; data included in this review are from the two months following introduction of RDTs to the intervention arm. | |
| Participants | Number of participants randomized: total fever cases 2213 (1073 in RDT arm, 1140 in presumptive treatment arm); Number of participants randomized for clinical outcomes was 25% of total fever cases, 553 (268 versus 285 respectively) Number of participants analysed for primary outcomes: (a) prescribing of antimalarials analysis 2213 (0% loss); (b) clinical outcomes: 483 (12.7% loss: 19.5% in intervention arm versus 6.3% in presumptive treatment arm) Inclusion: Any patient deemed eligible for RDT testing by the healthworker Exclusion: None |
|
| Interventions | Intervention: Training in fever case management based on RDTs. Control: Standard‐of‐care symptom‐based or empiric treatment of fever. The intervention group received training and RDTs; one‐day follow‐up support supervision was conducted two weeks after the initial three‐day training. Data collection commenced after the follow‐up support supervision visit. The control group continued usual symptom‐based care according to existing Uganda Ministry of Health guidelines. RDT performed by treating clinician (usually clinical officer or nursing staff). No other formal supervision was provided. |
|
| Outcomes | Primary: patients with fever on day 4 Secondary:
|
|
| Notes | Country: Uganda RDT: paracheck (HRP2) Setting: peripheral health centres Sampling: Lack of microscopy services, at least three full‐time clinical staff, estimated patient volume of at least 200 patients per week, willingness of health centre staff to participate in the trial, and location within 20 km of a sentinel health centre established by the Uganda Malaria Surveillance Project. Transmission: High; reference slide positivity in all participants with fever: 46% Dates: 2008; first half Funding: Exxon Mobil Corp. via the Academic Alliance Foundation; and NIH, USA, K23 AI065457‐01 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin flip in the presence of health centre leaders. |
| Allocation concealment (selection bias) | Unclear risk | Allocation of RDTs was decided by coin flip in the presence of study staff and representatives from each matched pair of health centers. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both the trial participants and personnel were aware of intervention allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Both the trial participants and personnel were aware of the diagnosis made and treatment prescribed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was low (0.0% for prescribing of antimalarials; 12.7% for clinical outcomes, differentiated by trial group). Performed available case analysis. |
| Selective reporting (reporting bias) | Low risk | Reported on all trial outcomes described in the methodology. |
| Other bias | Low risk | No other sources of bias identified. |
Hopkins 2008 UGA (Medium).
| Methods | Trial design: cluster randomized pre‐post open‐label trial; data included in this review are from the two months following introduction of RDTs in the intervention arm. | |
| Participants | Number of participants randomized: total fever cases 1550 (602 in RDT arm, 948 in presumptive treatment arm) Number of participants randomized for clinical outcomes was 25% of total fever cases, i.e.388 (151 versus 267 respectively) Number of participants analysed for primary outcomes: (a) prescribing of antimalarials analysis 1550 (0% loss); (b) clinical outcomes: 328 (15.4% loss: 6.3% in intervention arm versus 21.1% in presumptive treatment arm) Inclusion: Any patient deemed eligible for RDT testing by the healthworker Exclusion: None |
|
| Interventions | Intervention: Training in fever case management based on RDTs. Control: Standard‐of‐care symptom‐based or empiric treatment of fever. The intervention group received training and RDTs; one‐day follow‐up support supervision was conducted two weeks after the initial three‐day training. Data collection commenced after the follow‐up support supervision visit. The control group continued usual symptom‐based care according to existing Uganda Ministry of Health guidelines. RDT performed by treating clinician (usually clinical officer or nursing staff). No other formal supervision was provided. |
|
| Outcomes | Primary: patients with fever on day 4 Secondary:
|
|
| Notes | Country: Uganda RDT: paracheck (HRP2) Setting: peripheral health centres Sampling: Lack of microscopy services, at least 3 full‐time clinical staff, estimated patient volume of at least 200 patients per week, willingness of health centre staff to participate in the trial, and location within 20 km of a sentinel health centre established by the Uganda Malaria Surveillance Project. Transmission: Medium; reference slide positivity in all participants with fever 32% Dates: 2008; first half Funding: Exxon Mobil Corp. via the Academic Alliance Foundation; and NIH, USA, K23 AI065457‐01 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin flip in the presence of health centre leaders. |
| Allocation concealment (selection bias) | Unclear risk | Allocation of RDTs was decided by coin flip in the presence of study staff and representatives from each matched pair of health centers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both the trial participants and personnel were aware of intervention allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Both the trial participants and personnel were aware of the diagnosis made and treatment prescribed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was low (0.0% for prescribing of antimalarials; 15.4% for clinical outcomes, differentiated by trial group) Performed available case analysis, |
| Selective reporting (reporting bias) | Low risk | Reported on all trial outcomes described in the methodology |
| Other bias | Low risk | No other sources of bias identified |
Hopkins 2008 UGA (V High).
| Methods | Trial design: cluster randomized pre‐post open‐label trial; data included in this review are from the two months following introduction of RDTs in the intervention arm. | |
| Participants | Number of participants randomized: total fever cases 4197 (2288 in RDT arm, 1909 in presumptive treatment arm); Number of participants randomized for clinical outcomes was 25% of total fever cases, i.e. 1049 (572 versus 477 respectively) Number analysed for primary outcomes: (a) prescribing of antimalarials analysis 4197 (0% loss); (b) clinical outcomes: 959 (8.6% loss: 0.2% in intervention arm versus 17.2% in presumptive treatment arm) Inclusion: Any patient deemed eligible for RDT testing by the healthworker Exclusion: None |
|
| Interventions | Intervention: Training in fever case management based on RDTs Control: Standard‐of‐care symptom‐based or empiric treatment of fever The intervention group received training and RDTs; one‐day follow‐up support supervision was conducted two weeks after the initial three‐day training. Data collection commenced after the follow‐up support supervision visit. The control group continued usual symptom‐based care according to existing Uganda Ministry of Health guidelines. RDT performed by treating clinician (usually clinical officer or nursing staff). No other formal supervision was provided. |
|
| Outcomes | Primary: patients with fever on day 4 Secondary:
|
|
| Notes | Country: Uganda RDT: paracheck (HRP2) Setting: peripheral health centres Sampling: Lack of microscopy services, at least three full‐time clinical staff, estimated patient volume of at least 200 patients per week, willingness of health centre staff to participate in the trial, and location within 20 km of a sentinel health centre established by the Uganda Malaria Surveillance Project. Transmission: Very high; reference slide positivity in all participants with fever 73% Dates: 2008; first half Funding: Exxon Mobil Corp. via the Academic Alliance Foundation; and NIH, USA, K23 AI065457‐01 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin flip in the presence of health centre leaders. |
| Allocation concealment (selection bias) | Unclear risk | Allocation of RDTs was decided by coin flip in the presence of study staff and representatives from each matched pair of health centers |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both the trial participants and personnel were aware of intervention allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Both the trial participants and personnel were aware of the diagnosis made and treatment prescribed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was low (0.0% for prescribing of antimalarials; 8.6% for clinical outcomes, differentiated by trial group). Performed available case analysis. |
| Selective reporting (reporting bias) | Low risk | Reported on all trial outcomes described in the methodology. |
| Other bias | Low risk | No other sources of bias identified. |
Skarbinski 2009 KEN.
| Methods | Cluster RCT; randomized by health facilities Stratified random selection of facilities, by transmission settings (high/low) and facility type (hospitals, health centres and dispensaries) Took into account a design effect of two in sampling Reference slide taken. Results not reported Trial lasted four months |
|
| Participants | Inclusion: age ≥ 5 years, irrespective of condition Number of participants randomized: Intervention arm: 799 Number analysed for primary outcome (prescribing of antimalarials): 669 (16.3% loss) |
|
| Interventions | Intervention: RDTs for fever patients ≥ 5 years Control: Presumptive treatment of fever Boh groups received training and held guidelines RDT performed by health workers Health workers complied with guidelines partially: 41% of participants with negative RDT results received antimalarials |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
|
| Notes | Country: Kenya RDT: paracheck Setting: all referral levels of facilities, 60 in total, 30 in each arm Transmission: Hyperendemic or holoendemic at some sites and low and seasonal at others. Dates: June to September 2006 Funding: USAID |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Systematic allocation. |
| Allocation concealment (selection bias) | Unclear risk | Although probabilistic sampling was used in selecting the participating health facilities, the participants had foreknowledge of intervention assignments. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both the trial participants and personnel were aware of intervention allocation. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Both the trial participants and personnel were aware of the diagnoses and prescriptions. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Per protocol analysis; loss to follow‐up was high, more at the intervention facilities (20.2%) than at the control facilities (11.2%). |
| Selective reporting (reporting bias) | Low risk | Reported on all pre‐specified outcomes (prescribing of ACT); did not explicitly report on overall antimalarial prescribing, but the summary data were available for inclusion in the review. |
| Other bias | Low risk | Baseline imbalance minimised by stratifying facilities by level and randomly selecting within each level. Summary data were adjusted for baseline imbalance. Results could be biased towards the null, because some facilities in the comparison arms had RDT. Loss of complete clusters: not reported. |
Yeboah‐Antwi 2010 ZAM.
| Methods | Cluster randomized, by health posts. Pairs matched by distance from health centre then randomized. Patinets follow‐up and clinical status evaluated 5 to 7 days after initial contact. Estimates of measures of effects were adjusted for clustering & baseline imbalance using generalised estimating equations with exchangeable correlation matrix. |
|
| Participants | Inclusion: Children (6 months to 5 years); presenting with fever with or without other conditions Total enrolled and randomized: 3125 (1017 in the RDT arm and 2108 in the clinical diagnosis arm) Number analysed for primary outcomes: (a) prescribing of antimalarials: 3047 (2.5% loss); (b) clinical outcomes: 3125 (0% loss) |
|
| Interventions | Intervention: RDT‐aided algorithm Control: Clinical algorithm Both groups received training and held guidelines RDT performed by health workers; additional interventions provided to increase adherence with RDT results Health workers complied with guidelines most of the time: only 0.4% of participants with negative RDT results received antimalarials |
|
| Outcomes | 1. Children with fever who received AL 2. Children still experiencing symptoms at follow‐up (day 5 to 7) |
|
| Notes | Country: Zambia RDT: ICT Malaria Pf (ICT Diagnostics) Setting: Community health posts, manned by community health workers with six‐week training in basic clinical skills, rural and urban Sampling: 42 community health posts Transmission: High prevalence (valley) and low prevalence (plateau) areas Dates: Between December 2007 and November 2008 Funding: Not provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Authors report random allocation; numbers were generated by random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Participants were aware beforehand of the diagnostic procedures they were assigned to. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Both the trial participants and personnel were aware of the diagnostic procedures applied. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Both the trial participants and personnel were aware of the diagnostic outcomes, and the medications prescribed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss to follow‐up was low and comparable in both settings (2.5% in patients assessed for prescribing of antimalarials; 3% in patients assessed for clinical outcomes). |
| Selective reporting (reporting bias) | Low risk | Reported on all pre‐specified outcomes. |
| Other bias | Low risk | Recruitment bias was low‐pairs of aid posts were matched by distance then randomized. Baseline imbalance: selected clusters were similar and imbalance adjusted for. Loss of whole clusters: no loss reported. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chinkhumba 2010 | Not a RCT but a cross‐sectional survey, without a comparison group. |
| D'Acremont 2011 | Intervention facilities were selected purposively; there was no random assignment to comparison arms. |
| Faucher 2010 | Not a RCT; and examined the effect of withholding anti‐malaria to RDT‐positive children rather than comparing RDT‐based policy with presumptive treatment. |
| Kyabayinze 2010 | Not a RCT. |
| Msellem 2009 | Not a RCT (weekly cross‐over of intervention). |
| Mubi 2011 | Not a RCT(weekly cross‐over of intervention). |
| Reyburn 2007 | Comparison was policy based on microscopy rather than presumptive treatment. |
Differences between protocol and review
We included PG, DS and HH as authors.
Regarding outcomes:
We paraphrased measure of clinical outcomes to "patients still unwell at day 4+ of follow‐up" and considered it as a primary outcome. In the protocol we considered "persistence of fever at day 4+, based on self‐report‐‐not confirmed by means of a thermometer" as a secondary outcome.
We included "Parasitaemia on day 4+" as a primary outcome in the protocol. We deleted this in the review because the trials included are pragmatic in nature and did not assess parasitaemia.
"Microscopy positive patients not receiving antimalarials" was included as a secondary outcome because it is an issue of concern to decision‐makers.
We included "Microscopy negative patients prescribed antimalarials" as a secondary outcome measure because it is a measure of antimalarial wastage, which is one of the primary reasons for introducing RDT‐based policies.
Contributions of authors
JO wrote the protocol and the first draft of the review during his PhD programme at Liverpool School of Tropical Medicine, with input from PG and JAL. JO was responsible for data entry and analysis. JO and JAL independently screened studies for inclusion, assessed risk of bias from the included trials and extracted data from them. PG provided supervision throughout the review process. SD provided advice on the statistical methods. DS reviewed all sections of the review; in particular the trial outcomes, Summary of findings tables, main results, discussion, and conclusion. HH provided unpublished trial data and contributed to the interpretation, and discussion. All authors contributed to the final interpretation and results.
Sources of support
Internal sources
Liverpool School of Tropical Medicine, UK.
External sources
Department for International Development (DfID), UK.
Declarations of interest
The authors have no known conflicts of interest.
Unchanged
References
References to studies included in this review
Ansah 2010 GHA {published data only}
- Ansah EK, Narh‐Bana S, Epokor M, Akanpigbiam S, Quartey AA, Gyapong J, et al. Rapid testing for malaria in settings where microscopy is available and peripheral clinics where only presumptive treatment is available: a randomised controlled trial in Ghana. British Medical Journal 2010;340:c930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bisoffi 2009 BFA {published data only}
- Bisoffi Z, Sirima BS, Angheben A, Lodesani C, Gobbi F, Tinto H, et al. Rapid malaria diagnostic tests vs. clinical management of malaria in rural Burkina Faso: Safety and effect on clinical decisions. A randomized trial. Tropical Medicine & International Health 2009;14(5):491‐8. [DOI] [PubMed] [Google Scholar]
Hopkins 2008 UGA (High) {unpublished data only}
- Hopkins H, Ojaku A, Yeka A, Angutoko P, Ategeka J, Okiror R, Olwoch P, Ssekabira U, Asiimwe C, Nabakooza J, Rwakimari JB, Mpanga Sebuyira L, Wabwire Mangen F, Dorsey G. Effectiveness and safety of training in fever case management with rapid diagnostic tests (RDTs) for malaria at peripheral health centers in Uganda: randomized controlled trial. Unpublished data.
Hopkins 2008 UGA (Medium) {unpublished data only}
- Hopkins H, Ojaku A, Yeka A, Angutoko P, Ategeka J, Okiror R, Olwoch P, Ssekabira U, Asiimwe C, Nabakooza J, Rwakimari JB, Mpanga Sebuyira L, Wabwire Mangen F, Dorsey G. Effectiveness and safety of training in fever case management with rapid diagnostic tests (RDTs) for malaria at peripheral health centers in Uganda: randomized controlled trial. Unpublished data.
Hopkins 2008 UGA (V High) {unpublished data only}
- Hopkins H, Ojaku A, Yeka A, Angutoko P, Ategeka J, Okiror R, Olwoch P, Ssekabira U, Asiimwe C, Nabakooza J, Rwakimari JB, Mpanga Sebuyira L, Wabwire Mangen F, Dorsey G. Effectiveness and safety of training in fever case management with rapid diagnostic tests (RDTs) for malaria at peripheral health centers in Uganda: randomized controlled trial. Unpublished data.
Skarbinski 2009 KEN {published data only}
- Skarbinski J, Ouma PO, Causer LM, Kariuki SK, Barnwell JW, Alaii JA, et al. Effect of malaria rapid diagnostic tests on the management of uncomplicated malaria with artemether‐lumefantrine in Kenya: a cluster randomized trial. American Journal of Tropical Medicine and Hygiene 2009;80(6):919‐26. [PubMed] [Google Scholar]
Yeboah‐Antwi 2010 ZAM {published data only}
- Yeboah‐Antwi K, Pilingana P, Macleod WB, Semrau K, Siazeele K, Kalesha P, et al. Community case management of fever due to malaria and pneumonia in children under five in Zambia: a cluster randomized controlled trial. PLoS Medicine 2010;7(9):e1000340. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Chinkhumba 2010 {published data only}
- Chinkhumba J, Skarbinski J, Chilima B, Campbell C, Ewing V, San Joaquin M, et al. Comparative field performance and adherence to test results of four malaria rapid diagnostic tests among febrile patients more than five years of age in Blantyre, Malawi. Malaria Journal 2010;9:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Acremont 2011 {published data only}
- D'Acremont V, Kahama‐Maro J, Swai N, Mtasiwa D, Blaise Genton B, Lengeler C. Reduction of anti‐malarial consumption after rapid diagnostic tests implementation in Dar es Salaam: a before‐after and cluster randomized controlled study. Malaria Journal 2011;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Faucher 2010 {published data only}
- Faucher JF, Makoutode P, Abiou G, Béhéton T, Houzé P, Ouendo E, et al. Can treatment of malaria be restricted to parasitologically confirmed malaria? A school‐based study in Benin in children with and without fever. Malaria Journal 2010;9:104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kyabayinze 2010 {published data only}
- Kyabayinze DJ, Asiimwe C, Nakanjako D, Nabakooza J, Counihan H, Tibenderana JK. Use of RDTs to improve malaria diagnosis and fever case management at primary health care facilities in Uganda. Malaria Journal 2010;9:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Msellem 2009 {published data only}
- Msellem MI, Mårtensson A, Rotllant G, Bhattarai A, Strömberg J, Kahigwa E, et al. Influence of rapid malaria diagnostic tests on treatment and health outcome in fever patients, Zanzibar: a crossover validation study. PLoS Medicine 2009;6(4):e1000070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mubi 2011 {published data only}
- Mubi M, Janson A, Warsame M, Mårtensson A, Källander K, Petzold MG, et al. Malaria rapid testing by community health workers is effective and safe for targeting malaria treatment: randomised cross‐over trial in Tanzania. PLoS ONE 2011;6(7):e19753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Reyburn 2007 {published data only}
- Reyburn H, Mbakilwa H, Mwangi R, Mwerinde O, Olomi R, Drakeley C, et al. Rapid diagnostic tests compared with malaria microscopy for guiding outpatient treatment of febrile illness in Tanzania: randomised trial. British Medical Journal 2007;334(7590):403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abba 2011
- Abba K, Deeks JJ, Olliaro PL, Naing CM, Jackson SM, Takwoingi Y, et al. Rapid diagnostic tests for diagnosing uncomplicated P. falciparum malaria in endemic countries. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD008122.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
D'Acremont 2009
- D'Acremont V, Lengeler C, Mshinda H, Mtasiwa D, Tanner M, Genton B. Time to move from presumptive malaria treatment to laboratory‐confirmed diagnosis and treatment in African children with fever. PLoS Medicine 2009;6(1):e252. [DOI] [PMC free article] [PubMed] [Google Scholar]
English 2009
- English M, Reyburn H, Goodman C, Snow RW. Abandoning presumptive antimalarial treatment for febrile children aged less than five years: a case of running before we can walk?. PLoS Medicine 2009;6(1):e1000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Graz 2011
- Graz B, Willcox M, Szeless T, Rougemont A. "Test and treat" or presumptive treatment for malaria in high transmission situations? A reflection on the latest WHO guidelines. Malaria Journal 2011;10:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT Green S (Editors). Cochrane Handbook for Systematic Reviews of Interventions; Version 5.1.0 (updated March 2011); The Cochrane Collaboration. Available from www.cochrane‐handbook.org. Chichester: John Wiley and Sons, Ltd, 2011. [Google Scholar]
Koram 2007
- Koram KA, Molyneux ME. When is “malaria” malaria? The different burdens of malaria infection, malaria disease, and malaria‐like illnesses. American Journal of Tropical Medicine and Hygiene 2007;77(6 Suppl):1‐5. [PubMed] [Google Scholar]
Lubell 2007
- Lubell Y, Reyburn H, Mbakilwa H, Mwangi R, Chonya K, Whitty CJ, et al. The cost‐effectiveness of parasitologic diagnosis for malaria‐suspected patients in an era of combination therapy. American Journal of Tropical Medicine and Hygiene 2007;77(6 Suppl):128‐32. [PubMed] [Google Scholar]
Murray 2008
- Murray CK, Gasser RA Jr, Magill AJ, Miller RS. Update on rapid diagnostic testing for malaria. Clinical Microbiology Reviews 2008;21(1):97‐110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager (RevMan) [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Rolland 2006
- Rolland E, Checchi F, Pinoges L, Balkan S, Guthmann JP, Guerin PJ. Operational response to malaria epidemics: are rapid diagnostic tests cost‐effective?. Tropical Medicine and International Health 2006;11(4):398‐408. [DOI] [PubMed] [Google Scholar]
Rowe 2002
- Rowe AK, Lama M, Onikpo F, Deming MS. Design effects and intraclass correlation coefficients from a health facility cluster survey in Benin. International Journal for Quality in Health Care 2002;14(6):421‐3. [DOI] [PubMed] [Google Scholar]
Shillcutt 2008
- Shillcutt S, Morel C, Goodman C, Coleman P, Bell D, Whitty CJ, et al. Cost‐effectiveness of malaria diagnostic methods in sub‐Saharan Africa in an era of combination therapy. Bulletin of the World Health Organization 2008;86(2):101‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talisuna 2007
- Talisuna AO, Meya DN. Diagnosis and treatment of malaria. British Medical Journal 2007;334(7590):375‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thiam 2011
- Thiam S, Thior M, Faye B, Ndiop M, Diouf ML, Diouf MB, et al. Major reduction in anti‐malarial drug consumption in Senegal after nation‐wide introduction of rapid diagnostic tests. PLoS One 2011;6(4):e18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization. Towards quality testing of malaria rapid diagnostic tests: evidence and methods. Geneva: World Health Organization, 2006. [Google Scholar]
WHO 2010a
- World Health Organization. Guidelines for the treatment of malaria. 2nd Edition. Geneva: World Health Organization, 2010:1‐194. [Google Scholar]
WHO 2010b
- World Health Organization. Malaria rapid diagnostic test performance: results of the WHO product testing of malaria RDTs: Round 1 (2008). Geneva: World Health Organization, 2009:1‐4. [Google Scholar]
WHO 2012
- World Health Organization. World Malaria Report. Geneva: World Health Organization, 2012. [Google Scholar]
Wongsrichanalai 2007
- Wongsrichanalai C, Barcus MJ, Muth S, Sutamihardja A, Wernsdorfer WH. A review of malaria diagnostic tools: microscopy and rapid diagnostic test (RDT). American Journal of Tropical Medicine and Hygiene 2007;77(6 Suppl):119‐27. [PubMed] [Google Scholar]
Yukich 2012
- Yukich JO, Bennett A, Albertini A, Incardona S, Moonga H, Chisha Z, et al. Reductions in artemisinin‐based combination therapy consumption after the nationwide scale up of routine malaria rapid diagnostic testing in Zambia. American Journal of Tropical Medicine and Hygiene 2012;87(3):437‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zikusooka 2008
- Zikusooka CM, McIntyre D, Barnes KI. Should countries implementing an artemisinin‐based combination malaria treatment policy also introduce rapid diagnostic tests?. Malaria Journal 2008;7:176. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zurovac 2008
- Zurovac D, Larson BA, Skarbinski J, Slutsker L, Snow RW, Hamel MJ. Modeling the financial and clinical implications of malaria rapid diagnostic tests in the case‐management of older children and adults in Kenya. American Journal of Tropical Medicine and Hygiene 2008;78(6):884‐91. [PMC free article] [PubMed] [Google Scholar]


