Abstract
Background
Adult anopheline mosquitoes transmit Plasmodium parasites that cause malaria. Some fish species eat mosquito larvae and pupae. In disease control policy documents, the World Health Organization includes biological control of malaria vectors by stocking ponds, rivers, and water collections near where people live with larvivorous fish to reduce Plasmodium parasite transmission. The Global Fund finances larvivorous fish programmes in some countries, and, with increasing efforts in eradication of malaria, policy makers may return to this option. We therefore assessed the evidence base for larvivorous fish programmes in malaria control.
Objectives
Our main objective was to evaluate whether introducing larvivorous fish to anopheline breeding sites impacts Plasmodium parasite transmission. Our secondary objective was to summarize studies evaluating whether introducing larvivorous fish influences the density and presence of Anopheles larvae and pupae in water sources, to understand whether fish can possibly have an effect.
Search methods
We attempted to identify all relevant studies regardless of language or publication status (published, unpublished, in press, or ongoing). We searched the following databases: the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL), published in The Cochrane Library; MEDLINE; EMBASE; CABS Abstracts; LILACS; and the metaRegister of Controlled Trials (mRCT) until 18 June 2013. We checked the reference lists of all studies identified by the above methods. We also examined references listed in review articles and previously compiled bibliographies to look for eligible studies.
Selection criteria
Randomized controlled trials and non-randomized controlled trials, including controlled before-and-after studies, controlled time series and controlled interrupted time series studies from malaria-endemic regions that introduced fish as a larvicide and reported on malaria in the community or the density of the adult anopheline population. In the absence of direct evidence of an effect on transmission, we carried out a secondary analysis on studies that evaluated the effect of introducing larvivorous fish on the density or presence of immature anopheline mosquitoes (larvae and pupae forms) in community water sources to determine whether this intervention has any potential in further research on control of malaria vectors.
Data collection and analysis
Three review authors screened abstracts and examined potentially relevant studies by using an eligibility form. Two review authors independently extracted data and assessed risk of bias of included studies. If relevant data were unclear or were not reported, we wrote to the trial authors for clarification. We presented data in tables, and we summarized studies that evaluated the effects of fish introduction on anopheline immature density or presence, or both. We used GRADE to summarize evidence quality. We also examined whether the authors of included studies reported on any possible adverse impact of larvivorous fish introduction on non-target native species.
Main results
We found no reliable studies that reported the effects of introducing larvivorous fish on malaria infection in nearby communities, on entomological inoculation rate, or on adult Anopheles density.
For the secondary analysis, we examined the effects of introducing larvivorous fish on the density and presence of anopheline larvae and pupae in community water sources. We included 12 small studies, with follow-up from 22 days to five years. Studies were conducted in a variety of settings, including localized water bodies (such as wells, domestic water containers, fishponds, and pools; six studies), riverbed pools below dams (two studies), rice field plots (three studies), and water canals (two studies). All studies were at high risk of bias.
The research was insufficient to determine whether larvivorous fish reduce the density of Anopheles larvae and pupae (nine studies, unpooled data, very low quality evidence). Some studies with high stocking levels of fish seemed to arrest the increase in immature anopheline populations, or to reduce the number of immature anopheline mosquitoes, compared with controls. However, this finding was not consistent, and in studies that showed a decrease in immature anopheline populations, the effect was not consistently sustained. Larvivorous fish may reduce the number of water sources withAnopheles larvae and pupae (five studies, unpooled data, low quality evidence).
None of the included studies reported effects of larvivorous fish on local native fish populations or other species.
Authors' conclusions
Reliable research is insufficient to show whether introducing larvivorous fish reduces malaria transmission or the density of adult anopheline mosquito populations.
In research examining the effects on immature anopheline stages of introducing fish to potential malaria vector breeding sites (localized water bodies such as wells and domestic water sources, rice field plots, and water canals) weak evidence suggests an effect on the density or presence of immature anopheline mosquitoes with high stocking levels of fish, but this finding is by no means consistent. We do not know whether this translates into health benefits, either with fish alone or with fish combined with other vector control measures. Our interpretation of the current evidence is that countries should not invest in fish stocking as a larval control measure in any malaria transmission areas outside the context of carefully controlled field studies or quasi-experimental designs. Research could also usefully examine the effects on native fish and other non-target species.
PLAIN LANGUAGE SUMMARY
Fish that feed on mosquito larvae for preventing malaria transmission
Plasmodium parasites cause malaria and are transmitted by adult Anopheles mosquitoes. Programmes that introduce fish into water sources near where people live have been promoted. The theory is that these fish eat the Anopheles mosquito larvae and pupae, thus decreasing the adult mosquito population and reducing the number of people infected with Plasmodium parasites.
In this review, we examined the research that evaluated introducing larvivorous fish to Anopheles mosquito breeding sites in areas where malaria was common, published up to 18 June 2013. We did not find any studies that looked at the effects of larvivorous fish on adult Anopheles mosquito populations or on the number of people infected with Plasmodium parasites. We included 12 studies that examined the effects of larvivorous fish on Anopheles larvae and pupae in different breeding sites, including localized water bodies (such as wells, domestic water containers, fishponds, and pools; six studies), riverbed pools below dams (two studies), rice field plots (three studies), and water canals (two studies). Research evidence is insufficient to show whether introduction of larvivorous fish reduces the number of Anopheles larvae and pupae in water sources (nine studies, unpooled data, very low quality evidence). However, larvivorous fish may reduce the number of water sources withAnopheles mosquito larvae and pupae (five studies, unpooled data, low quality evidence). None of the included studies examined the effects of introducing larvivorous fish on other native species present, but these studies were not designed to do this. Before much is invested in this intervention, better research is needed to determine the effect of introducing larvivorous fish on adult Anopheles populations and on the number of people infected with malaria. Researchers need to use robust controlled designs with an adequate number of sites. Also, researchers should explore whether introducing these fish affects native fish and other non-target species.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON [Explanation]
| Larvivorous fish for preventing malaria transmission | ||||||
| Patient or population: people living in malaria-endemic areas | ||||||
| Settings: malaria-endemic areas | ||||||
| Intervention: larvivorous fish | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect(95% CI) | No of studies | Quality of the evidence(GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Larvivorous fish | |||||
| Effects on malaria transmission | ||||||
| Clinical malaria (Incidence) | - | - | - | 0 studies | - | No trials |
| Entomological inoculation rate | - | - | - | 0 studies | - | No trials |
| Density of adult malaria vectors | - | - | - | 0 studies | - | No trials |
| Effects on larvae at potential mosquito breeding sites | ||||||
| Density of immature vector stages in water bodies Quasi-experimental studies | - | - | Not pooled | Nine studies | ⊕⊕○○ very low 1-8 | Variable effects reported |
| Breeding sites positive for immature vector stages Quasi-experimental studies | - | - | Not pooled | Five studies | ⊕⊕○○ low 1,9-11 | Positive effects reported |
| *The basis for the assumed risk (for example, the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). | ||||||
| CI: Confidence interval. | ||||||
| GRADE Working Group grades of evidence. | ||||||
| High quality: Further research is very unlikely to change our confidence in the estimate of effect. | ||||||
| Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. | ||||||
| Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. | ||||||
| Very low quality: We are very uncertain about the estimate. | ||||||
1No serious risk of bias: All studies suffered from additional problems such as a small number of sites sampled, but these were not deemed adequate to further downgrade the evidence.
2No serious inconsistency: All four studies (Howard 2007; Kim 2002; Sitaraman 1976; Yu 1989) found substantial reductions in immature vector density at the intervention sites.
3No serious indirectness: These four studies introduced larvivorous fish into household water sources in India (Sitaraman 1976), ponds in Kenya (Howard 2007), and rice fields in Korea (Kim 2002; Yu 1989) The longest follow-up was in Kenya and still showed benefit at five months. In one study from India, the duration of effect seemed to be influenced by the number of fish introduced.
4No serious imprecision: Although statistical significance was not reported, the effects in some studies (Howard 2007; Kim 2002; Sitaraman 1976; Yu 1989) appear large.
5Downgraded by one for inconsistency: Effects were variable. Large effects were observed in water canals in Sudan (Mahmoud 1985), but only until nine months' post intervention. Effects on immature vector populations in Central Java were dependent on vector species (Nalim 1988). No effect in ponds in Kenya stocked once with fish or restocked every two weeks with fish at follow-up (13 weeks). Some effect in water canals in Kenya restocked with fish every two weeks at follow-up (13 weeks) (Imbahale 2011a).
6No serious indirectness: These three studies introduced larvivorous fish into ponds in Kenya (Imbahale 2011a), ponds in Sudan (Mahmoud 1985), and rice fields in Central Java (Nalim 1988). The longest follow-up was in Central Java (six years) but showed different effects upon different vector species. In one study from Kenya, the effect seemed to be influenced by the type of site, as an effect was observed in water canal sites but not in pond sites.
7Downgraded by one for inconsistency: Effects were variable. In one study, no major difference between control and experimental groups was detected at final follow-up (120 days), but area under the curve suggested more rapid decline in larvae in experimental group (Kusumawathie 2008a). In one study, control and experimental groups were not matched at baseline (experimental group higher). However, substantively lower values were detected in the intervention arm at follow-up (one year) (Kusumawathie 2008b).
8No serious indirectness: Two studies introduced larvivorous fish into riverbed pools below dams in Sri Lanka (Kusumawathie 2008a; Kusumawathie 2008b). The longest follow-up still showed benefit at one year post-intervention in one study. However, control and experimental groups were not matched at baseline (experimental group higher) in all studies.
9No serious indirectness: This study introduced larvivorous fish into household water sources in Ethiopia (Fletcher 1992). Benefit was still shown at follow-up (one year).
Background
Description of the condition
Malaria is the most common vector-borne disease worldwide and is endemic in 104 countries. In 2011, an estimated 3.3 billion people globally were at risk of malaria, with people living in sub-Saharan Africa at highest risk of contracting the disease. An estimated 219 million cases of malaria (range 154 to 289 million) and 660,000 deaths (range 610,000 to 971,000) were reported in 2010 (WHO 2012). Plasmodium spp. parasites cause malaria in humans and are transmitted by female mosquitoes of the genus Anopheles. Of approximately 430 Anopheles species, between 30 and 50 species act as dominant vectors. The main strategies for preventing and controlling malaria include the following:
Prevention through vector control, mainly using long-lasting insecticidal nets (LLINs) (Lengeler 2004), or indoor residual spraying (IRS) (Tanser 2007), or both.
Early diagnosis and effective treatment of people with malaria (Sinclair 2009; Sinclair 2011; Sinclair 2012), chemoprevention in high-risk groups (Garner 2006), and seasonal chemoprophylaxis (Meremikwu 2012).
LLINs and IRS were developed against the most effective vectors, which share the attributes of feeding late at night and being anthropophilic (preferring to feed on humans), endophagic (preferring to feed indoors), and endophilic (preferring to rest indoors) (Lengeler 2004; Tanser 2007). However, many vectors, particularly in Asia and South America (but also in Africa), prefer animals to humans for their blood meals (are zoophilic) or feed early in the evening or outside of houses, where they will be less likely to encounter LLINs or IRS. The two main vector control strategies may be less effective in regions where vectors have these behavioural attributes. These factors have led some agencies and governments to propose other strategies for vector control, and interest in larviciding as a potential means of malaria control has been renewed (WHO 2006a; WHO-GMP 2012).
Description of the intervention
Larviciding attempts to control malaria by seeking to reduce the size of the immature vector population. Strategies include the following:
Permanently or temporarily reducing the availability of larval habitats (habitat modification and habitat manipulation).
Adding to standing water microbial or chemical substances that kill or inhibit the development of aquatic immature mosquito stages (Lacey 1990; Tusting 2013).
Providing biological control by introducing fish (Pyke 2008; Walton 2007), frogs (Raghavendra 2008), or invertebrate predators (such as dragonfly nymphs).
A separate Cochrane Review summarizes larviciding for strategies (1) and (2) (Tusting 2013). The review authors examined cluster-randomized controlled trials (cluster-RCTs), controlled before-and-after trials with at least one year of baseline data, and randomized cross-over trials that compared larval source management (LSM) with no LSM for malaria control. The review authors found some large effects in some studies but not in others. They concluded that when larval habitats are not too extensive, and when a sufficient proportion of these habitats can be targeted, LSM probably reduces the number of people who will develop malaria and probably reduces the proportion of the population infected with the Plasmodium parasite at any one time (moderate quality evidence). In the included studies, the intervention appeared to be effective in reducing the malaria transmission in a variety of countries where larviciding was implemented at a wide variety of sites. In a study from The Gambia, where mosquitoes were breeding in large swamps and rice paddies, spraying of swamps with larvicide by ground teams did not lead to any benefit. In this review, we evaluate the most common strategy for biological control: the use of fish that attack mosquito larvae and pupae.
The potential of the larvivorous fish Gambusia (Gambusia affinis and G. holbrooki; Pyke 2005) to ingest large numbers of mosquito larvae led to a series of laboratory-based studies on mosquito larval prey preferences and the optimization of systems to propagate these fish. Subsequently, field evaluations of Gambusia were undertaken to assess their impact on larval prevalence and density in mosquito breeding sites.G. affinis and G. holbrooki are native to the south-eastern United States but have been transported and released in multiple countries globally, so that today, these species are collectively the most widely geographically dispersed freshwater fishes in the world (Pyke 2008).
Gambusia may adversely affect native fishes and other organisms besides mosquitoes when introduced into new areas. Specialists are now examining the use of native fish species for larval control. Currently, approximately 315 larvivorous fish species belonging to 32 genera under seven families are used for mosquito control, and the family Cyprinodontidae contribute the highest number of genera (15) and species (300) (Goutam 2013). Other promising species for mosquito control belong to the genera Aphanius,Valencia,Aplocheilus,Oryzias,Epiplatys,Aphyosemion,Roloffia,Nothobranchius,Pachypanchax,Rivulus,Fundulus, and Cynolebias (Walton 2007).
How the intervention might work
As adult female Anopheles mosquitoes transmit malaria, the intensity of transmission is partly dependent on (1) whether Anopheles are infected with the Plasmodium sporozoite stage; and (2) how many Anopheles feed on humans during the transmission season or year. The percentage of infected mosquitoes multiplied by the biting rate is a common parameter by which to estimate the force of infection, called the entomological inoculation rate (EIR).
Anopheles mosquitoes lay their eggs in water sources in which they develop into larvae and then pupae. Anopheles larvae are found in a wide range of habitats, including fresh- or salt-water marshes, rice fields, mangrove swamps, edges of streams and rivers, grassy ditches, and small, temporary rain pools. Most species prefer clean, unpolluted water. Some mosquitoes may prefer specific sites in which to lay eggs, whilst others use a wide variety of breeding sites (such as temporary ground water pools, including footprints and ditches, as well as more permanent water sources, such as swamps and wells). The abundance of adult mosquitoes is dependent on a variety of factors. These include the number and size of suitable oviposition sites and the density of the immature mosquito stages at these sites. Several other ecological and environmental factors may influence the adult anopheline population, including temperature, rainfall patterns, and availability of bloodmeal sources.
The larger the mosquito population, the greater is the potential number of bites by vectors on humans, unless people take measures to avoid mosquito bites, such as sleeping under a LLIN. For a given sporozoite rate, increases in the human-biting rate or in mosquito density, or in both, will result in higher inoculation rates and greater malaria transmission. If the size of the vector population is limited by interventions that reduce the number of breeding sites or the density of vector larvae per breeding site, then malaria transmission to humans (with all other factors remaining the same) might potentially be reduced (Figure 1). Conversely, reducing the density of anopheline immature mosquitoes at a breeding site might have little or no effect on adult numbers because adult numbers may be determined largely or entirely by other factors. Reductions in the density of immature vectors could result in larger, more robust, longer-lived adults through reduced competition between immature Anopheles for resources (density-dependent effects), thereby minimizing the potential reduction in malaria transmission. However, Bond 2005 demonstrated that Anopheles pseudopunctipennis larvae had significantly prolonged developmental times in the presence of Poecilia sphenops fish and emerged as smaller adults. Smaller adult females can have reduced host-seeking responses (Takken 1998) and may produce smaller egg batches (Lyimo 1993).
Figure 1.
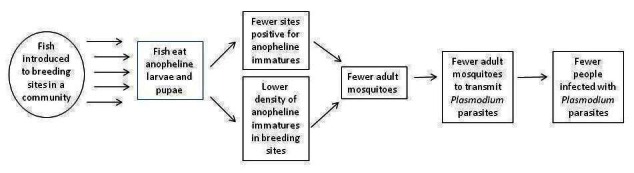
Larvivorous fish for preventing malaria transmission: conceptual framework.
Why it is important to do this review
The World Health Organization (WHO) recommendations from 2012 state that antilarval measures are likely to be cost-effective for control of malaria in areas where the breeding sites are limited in number, permanent, and easily found (that is, they are "fixed, finite and findable") (WHO-GMP 2012). The WHO has stated that environmental factors that increase the likelihood that larval control will be effective include a short transmission season, cool temperatures that extend for the duration of the immature stages, and breeding sites that are man-made and homogeneous in nature. In Africa, larviciding is thought to have the best potential to be effective in urban and arid areas and possibly in the East African highlands (WHO-GMP 2012). Indeed, the Cochrane Review of mosquito LSM indicated that the intervention often appeared to impact transmission when implemented in areas where it was feasible to do so (Tusting 2013).
Whether larvivorous fish are an option for LSM is the subject of this review. For at least 35 years, the WHO has promoted the use of larvivorous fish as an environmentally friendly alternative to insecticide-based interventions for malaria control. A WHO-sponsored interregional conference on malaria control in 1974 reported that "the utilization of larvivorous fish, mainly Gambusia or suitable local species, is the only practical measure that can be recommended where applicable, as in lakes, ponds, pools, wells, rice fields" (WHO 1974). A 2001 regional meeting in Kazakhstan recommended that more studies on larger numbers of local larvivorous and phytophagous fish be undertaken in different eco-epidemiological settings in that region, and that the search for effective larvivorous fish should continue (WHO 2001).
More recently, momentum has gathered in efforts to eliminate malaria, resulting in the 2006-2015 WHO-EURO regional strategy, which included larval control by introduction of larvivorous fish preferentially over other forms of larviciding (WHO 2006a). Currently, the use of fish is included among the recommended vector control strategies for elimination of malaria vectors, which tend to breed in permanent or semi-permanent water bodies that can be identified and treated, and where the density of the human population to be protected is sufficiently high to justify this intervention at all breeding sites (WHO 2006b; WHO 2007).
WHO recommendations for larviciding as a general strategy are guarded and conditional, but the use of fish is often included in listings of options, alongside clearly established effective measures such as LLINs. For example, the WHO integrated vector management plan to control malaria includes the "effective use of biologically-based agents such as bacterial larvicides and larvivorous fish" (HELI 2005). Fish were one of the traditional means of malaria control in the ex-Soviet Republics of Central Asia, where their use continues. For example, the Global Fund currently provides money for implementation of larvivorous fish against malaria in Tajikistan, although this investment appears modest (UNDP 2013).
Thus there appear to be differing views on whether introducing larvivorous fish is an effective larvicidal approach; some are strong advocates, whilst others question whether sufficient evidence exists to demonstrate its effectiveness, and whether the strategy can achieve the large reductions in larval numbers required to impact the size of the adult population. In addition, problems are associated with finding and treating all anopheline mosquito breeding sites within a specific area, and some breeding sites may be unsuitable for treatment. Dissemination of larvivorous fish as a control strategy for malaria has the potential for adverse effects on local ecosystems by reducing or eliminating indigenous fish, amphibians, and other invertebrates (Walton 2007).
We therefore carried out a systematic review of reliable research examining whether evidence shows that this form of larviciding has an impact on malaria. We also sought evidence of the potential to affect transmission, by summarizing studies on the effects of introducing fish on the density and presence of immature anopheline mosquitoes at potential breeding sites.
Objectives
Our main objective was to evaluate whether introducing larvivorous fish to anopheline breeding sites impacts Plasmodium parasite transmission. Our secondary objective was to summarize studies evaluating whether introducing larvivorous fish influences the density and presence of Anopheles larvae and pupae in water sources, to understand whether fish can possibly have an effect.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled trials (RCTs) and non-RCTs, including before-and-after controlled studies, controlled time series, and controlled interrupted time series designs (Figure 2). Comparison groups were geographically defined areas, and thus for RCTs, cluster-randomized designs were used. To be included, intervention and control groups needed to have:
Figure 2.
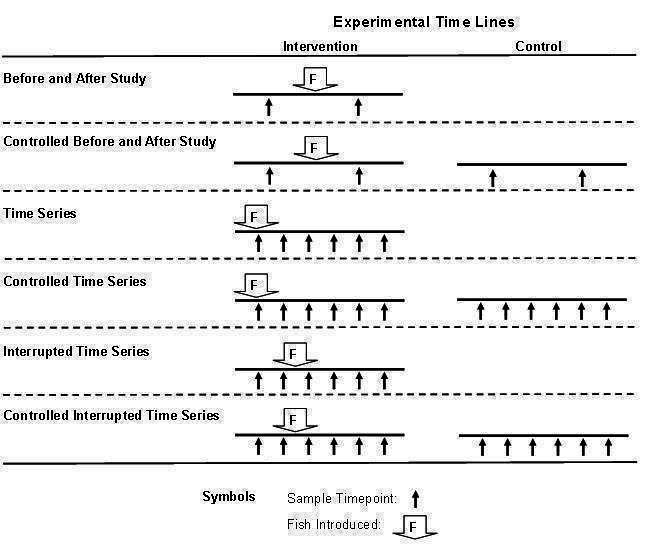
Experimental designs that have been used to attempt to evaluate the impact of fish on the larvae of vectors in malaria-endemic countries. In this figure, we depicted either two or six sample time points (shown by the arrows) as examples. Studies may sample at more time points, or at fewer time points in the case of time series studies.
equivalent accompanying antimalarial interventions;
baseline information;
contemporaneous data collection;
same locality (within the same regional area of the country);
comparable resident populations in relation to ethnic groups, housing, and wealth, based on baseline data provided within the study;
similar intensities of malaria transmission, based on baseline data provided within the study; and
sufficient geographic size to minimize masking of the impact of the intervention by immigrating vectors.
In studies of malaria transmission, we specified that intervention and control sites were at least a kilometre apart with a human population sample size adequate to detect = 25% reduction in Plasmodium parasite–positive people.
Types of participants
Children and adults living in rural and urban malaria-endemic areas.
Types of interventions
Interventions
Introduction of larvivorous fish of any species, either adults or juveniles, into anopheline mosquito breeding sites. This may have been done as a single intervention or as part of a more comprehensive vector control programme that included access to and use of LLINs, IRS, larvicides (including microbial larvicides and insect growth regulators), polystyrene beads, and environmental management.
Due to seasonal, climatic, and random variations at both immature (larvae and pupae) and adult stages, we included studies that monitored for one or more full years before fish were introduced and those that monitored at one or more time points at least 12 months after fish were introduced into intervention areas. For studies of immature anopheline mosquito populations, we included only studies with a follow-up period longer than three weeks, so that several generations of immature anophelines were monitored.
Controls
No larvivorous fish were introduced into control areas. All other vector control measures were the same in intervention and control arms. Thus, for example, we excluded studies that examined introduction of larvivorous fish combined with IRS and those that did not use IRS in the control arm.
Types of outcome measures
In the main analysis:
Number of confirmed episodes of malaria among community members. We defined malaria infections as laboratory-confirmed cases of malaria (Plasmodium parasitaemia detected by microscopy or by rapid diagnostic tests in active or passive case detection).
Entomological inoculation rate (EIR). This is defined as the estimated number of bites by infectious mosquitoes per person per unit of time (the product of the number of bites per person per day during the transmission season or per year by vector mosquitoes (the "human-biting rate") and the fraction of vector mosquitoes that are infectious (the "sporozoite rate").
Density of adult vector mosquitoes. This included measures in which sampling techniques appropriate for these vectors were used, including counting adult anopheline mosquitoes that either landed on exposed body parts of humans acting as bait or were collected resting inside buildings with the use of knockdown spray catches.
In the secondary analysis examining the effects on immature anopheline mosquitoes at potential mosquito breeding sites:
Density of immature vector stages at breeding sites, as measured by larval dipping (Silver 2008).
Percentage of breeding sites positive for immature anopheline mosquitoes.
In any studies that met the inclusion criteria for the main or the secondary analysis, we sought reporting on native fish populations or other effects on the local ecosystem.
Search methods for identification of studies
Methods used sought all relevant studies regardless of language or publication status (published, unpublished, in press, or ongoing).
Electronic searches
We examined the following databases up to 18 June 2013 using the search terms detailed in Appendix 1: the Cochrane Infectious Diseases Group Specialized Register; the Cochrane Central Register of Controlled Trials (CENTRAL) published in The Cochrane Library; MEDLINE; EMBASE; CABS Abstracts; and LILACS; as well as the metaRegister of Controlled Trials (mRCT) using 'malaria' and 'larvicide* or fish' as search terms; and the literature database of the Armed Forces Pest Management Board using the search terms ('frogs' and 'fish') and 'malaria'.
Searching other resources
Reference lists
We checked the reference lists of all studies identified by the above methods, references listed in review articles (Beltran 1973; Chandra 2008; Pyke 2008; Walker 2007), and previously compiled bibliographies (Gerberich 1968) to identify potential studies.
Data collection and analysis
Selection of studies
We screened the abstract of each title obtained from the search for potentially relevant studies. TB and DPW retrieved the corresponding full articles of these identified studies, and TB, DPW, and PG assessed inclusion by using an eligibility form. We independently screened each search result, assessed each article, and resolved any discrepancies between eligibility results through discussion. If studies did not meet the methods specified, we did not scrutinize further, and if eligibility was unclear, we sought clarification from the study authors.
Data extraction and management
DPW and TB independently extracted data from each study report onto a predesigned data extraction form. We discussed any discrepancies with a third review author (PG).
For the secondary analysis of the effect of introducing larvivorous fish on immature anopheline mosquitoes in water sources, we extracted information on study characteristics and study methods, including setting, comparability between sites, details of the fish intervention, and outcomes, and we examined how study authors measured these. We extracted descriptions of the epidemiology and intensity of transmission from each study, using the terms used by the study authors; co-interventions and whether both control and intervention arms experienced the same co-interventions; and, when study authors presented outcome data in graph or table format, the raw data when possible.
Design quality
We assessed the study design quality of each included study by examining whether study authors also reported on four specific factors: (1) pupae numbers (as larvivorous fish may preferentially eat particular instars of larvae or pupae) (Bence 1986; Homski 1994; Wurtsbaugh 1980); (2) distance between control and intervention sites; (3) whether other larvivorous species were present; and (4) whether vegetation was cleared or removed from the sites.
Assessment of risk of bias in included studies
For trials examining effects on malaria transmission that may be available for future updates of this review, we used standard Cochrane criteria to evaluate the risk of bias.
For studies examining effects on larvae, we assessed risk of bias on the basis of six factors: (1) study design; (2) site selection; (3) site allocation; (4) blinding of assessors; (5) baseline values comparable between sites; and (6) the number of sites. In Table 1, we have shown the exact criteria that we used to assess the risk of bias. DPW and PG independently assessed the risk of bias for each study, and resolved any discrepancies by discussion with a third review author (TB).
Table 1.
Risk of bias assessment
| Risk of bias factor | Risk of bias | ||
|---|---|---|---|
| High | Low | Unclear | |
| 1. Study design | Non-RCT | RCT | Not clearly reported or not reported |
| 2. Site selection | Method of selection of sites within study area not described | Method of selection of sites within study area described | Not clearly reported or not reported |
| 3. Site allocation | Allocation of treatment not performed by random allocation | Allocation of treatment performed by random allocation | Not clearly reported or not reported |
| 4. Blinding of assessors | Not blinded | Blinded | Not clearly reported or not reported |
| 5. Baseline values comparable between sites | Not comparable | Comparable | Not clearly reported or not reported |
| 6. Number of sites | May be inadequate (five to < 20 sites per group) Probably inadequate (< five sites per group or number of sites unknown) | Adequate number of sites (20 or more sites per group) | Not clearly reported or not reported |
Data synthesis
We carried out individual critical appraisal of each study on the possible effects of introduction of larvivorous fish on immature mosquitoes. The large variation in study design, outcomes, and reporting precluded any data synthesis. We tried to draw patterns of effect by grouping studies by habitat as follows:
Localized water bodies, including (a) wells, (b) domestic water containers, (c) fishponds and man-made pools, and (d) pools in a riverbed below a dam.
Rice field plots.
Water canals.
We described each study in a short narrative and presented the outcome results in table format. We reported results at baseline and at pre-specified time points at follow-up, and used GRADE to assess the quality of evidence.
Results
Description of studies
Within the Characteristics of included studies and Characteristics of excluded studies sections, we have given a description of included and excluded studies.
Results of the search
We identified 1286 titles and abstracts from the electronic search of databases and 12 additional articles after contacting researchers and screening reference lists. After we removed duplicates, 915 records remained. Of these, we obtained 117 potentially eligible articles. No studies were identified that fulfilled the selection criteria and reported on primary outcomes. None of the 117 potentially relevant articles were eligible in terms of design and interventions, and they did not report any outcomes relevant to our protocol or objectives. Of the 117 potentially eligible articles, we identified 12 studies that fulfilled the selection criteria for the secondary outcomes only and 105 studies that did not meet the eligibility criteria, did not report any outcomes, or did not do either. We have listed the reasons for exclusion of these studies in the Characteristics of excluded studies section. The strategy used for search and selection of studies is illustrated in Figure 3.
Figure 3.
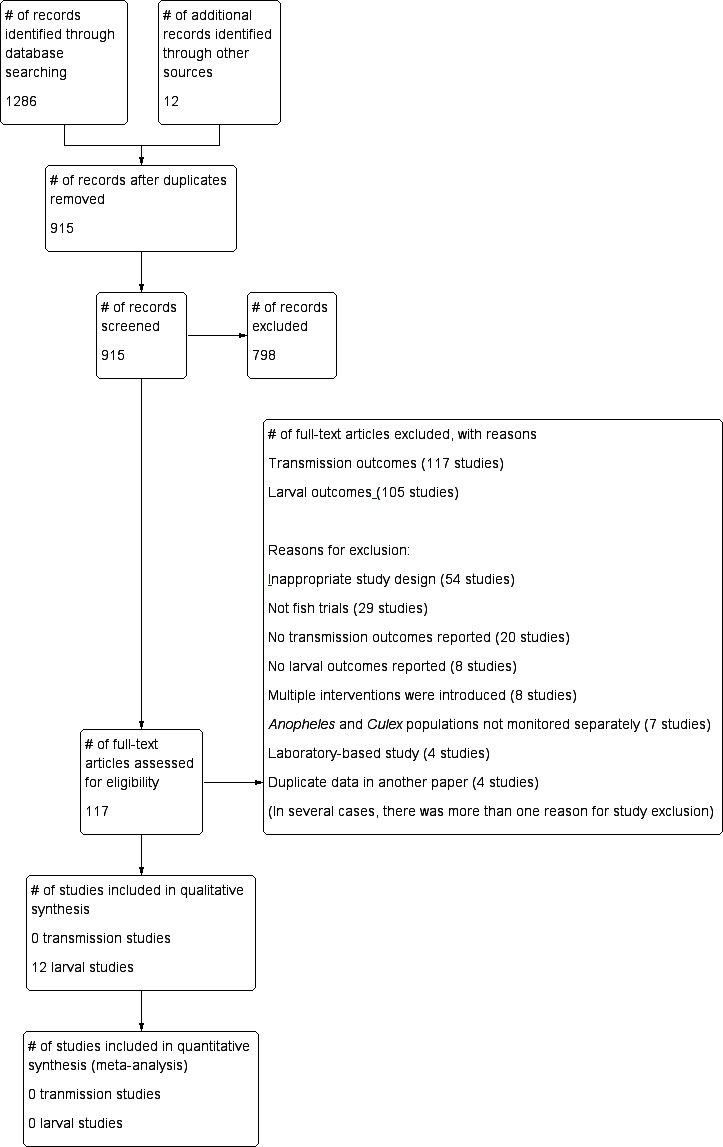
Study flow diagram.
Included studies
No studies reported on cases of malaria, EIR, or density of adult vector mosquitoes. There is thus no direct evidence this intervention impacts malaria transmission. Therefore, our analysis focuses only on the effects of fish stocking on the presence or density of immature mosquitoes in water sources.
Sites
We summarized the sites by type of water sources stocked, number of sites stocked, and site size (Table 2). Ecological sites included:
Table 2.
Ecological sites classified by site type, with a description of number of sites and their size
| Group | Site type | Study | Sites stocked | Unstocked | Site size |
|
|---|---|---|---|---|---|---|
| Surface area | Depth | |||||
| 1. Localized water bodies1 | (a) Wells | Sitaraman 1976 | 10 | Four | 1.5 m2 | 1.5 to 2.5 m |
| Menon 1978 | 3402 to 3438 | 317 | Not stated | Not stated | ||
| (b) Domestic water containers | Fletcher 19922 | 68 | 60 | Not stated | Not stated | |
| Sabatinelli 19913 | 1204 | 20 | Not stated | Not stated | ||
| (c) Fishponds and man-made pools | Howard 20075 | Two | One | 72 m2 to 128 m2 | Not stated | |
| Imbahale 2011a6 | 25 | Five | Average 1 m2 | 1 m | ||
| (d) Riverbed pools below dams | Kusumawathie 2008a | 29 | 31 | 0.25 to 1 m2 | < 1 m | |
| Kusumawathie 2008b | Two areas. Site number unknown | Two areas. Number of sites unknown | Not stated | Not stated | ||
| 2. Rice field plots | Rice field plots | Nalim 1988 | Not specified | Not specified | 23.9 ha in total | Not stated |
| Kim 2002 | Three | One | 300 m2 to 600 m2 | Not stated | ||
| Yu 1989 | Four | Two | 45 m3 | 0.01 m | ||
| 3. Water canals | Water canals | Imbahale 2011a | 25 | Five | Average 15 m2 | 0.3 m |
| Mahmoud 1985 | 20 | Five | 4 km to 10 km × 2 m wide | 1 m | ||
1Includes (a) wells, (b) domestic water containers, (c) fishponds and man-made pools, and (d) riverbed pools below dams.
2Included barrels, cisterns, wells, and washbasins.
3Included ablution basins and tanks.
4The number of sites at follow-up in November 1987; Sabatinelli 1991 did not specify the number sampled at the April 1988 follow-up.
5Included fishponds only.
6Included man-made pools only.
localized water bodies such as (a) wells; (b) domestic water containers (Fletcher 1992; Menon 1978; Sabatinelli 1991; Sitaraman 1976); (c) fishponds and man-made pools (Howard 2007; Imbahale 2011a); and (d) riverbed pools below dams (Kusumawathie 2008a; Kusumawathie 2008b);
The number and size of habitat sites chosen by the trial authors varied (see Table 2). For example, Fletcher 1992 introduced fish to 68 habitat sites and maintained 60 habitat sites as controls. Menon 1978 stocked fish in 3438 wells and left 317 wells without fish as controls. However, Howard 2007 used two fishponds as experimental sites and one fishpond as a control. Habitat sizes ranged from small, 1 m × 1 m × 1 m man-made ponds (Howard 2007) to 24.8 hectare plots of land (Nalim 1988). Notably, Nalim 1988 recorded the number of adult mosquitoes collected in emergence traps, and we used these data to determine the effects of larvivorous fish on the immature mosquito population.
Design
Of the 12 larval studies that we identified, one was a quasi-RCT (Fletcher 1992), six were controlled interrupted time series (Howard 2007; Kim 2002; Menon 1978; Sabatinelli 1991; Sitaraman 1976; Yu 1989), three were controlled time series (Imbahale 2011a; Mahmoud 1985; Nalim 1988), and two were controlled before-and-after studies (Kusumawathie 2008a; Kusumawathie 2008b). Two studies were undertaken in Sri Lanka (Kusumawathie 2008a, Kusumawathie 2008b), two in India (Menon 1978; Sitaraman 1976), one in Ethiopia (Fletcher 1992), two in Kenya (Howard 2007; Imbahale 2011a), one in Sudan (Mahmoud 1985), one in Grande Comore Island (Sabatinelli 1991), two in Korea (Kim 2002; Yu 1989), and one in Indonesia (Nalim 1988).
Intervention
We summarized in Table 3 the key details of the fish intervention provided for each study.
Table 3.
Details of the fish intervention
| Study | Fish species introduced | Stocking density | Type of site | Size of site | Size (maturity) of fish | Sex ratio Male: female | Time of year fish introduced | Restocked |
|---|---|---|---|---|---|---|---|---|
| Fletcher 1992 | Aphanius dispar | Five fish per barrel, 10 fish per cistern, 20 fish per well, 60 fish per washbasin; later, 10 fish per barrel and 40 fish per well | Domestic water containers | Not stated | Not stated | Not stated | February | Yes |
| Howard 2007 | Oreochromis niloticus | Two fish per m2 pond surface area | Abandoned fishponds | 104 m2 (Pond A), 128 m2 (Pond C), 72 m2 (Pond D) | One to two months old | Not stated | January | No |
| Imbahale 2011a | G. affinis | Total number based on feeding rate of four mosquito fish per 60 mosquito larvae per day | Man-made pools or water canals | Pools (average 1 m × 1 m × 1 m deep) or water canals (15 m × 1 m × 0.3 m deep) | 4 cm to 7 cm | Not stated | February | No (treatment arm: ponds fish once). Yes, fortnightly (treatment arms: pond fish only or water canal fish only) |
| Kim 2002 | (1) A. latipes with T. m. niloticus or (2) Aphyocypris chinensis + T. m. niloticus | (1) One pair T. m. niloticus/10 m2 water surface + 0.8 A. latipes/m2 water surface (2) One A. chinensis/m2 + two T. m. niloticus/10 m2 | Rice fields | Rice fields (1) 500 m2, (2) 300m2, or 600 m2 | Not stated | Not stated | June | No |
| Kusumawathie 2008a | P. reticulata | Five fish per m2 surface area | Riverbed pools below dams | 0.25 to 1 m2 surface area and < 1 m depth | Not stated | 2:3 | May | No |
| Kusumawathie 2008b | P. reticulata | Five fish per m2 surface area | Riverbed pools below dams | Not stated | Not stated | 2:3 | August | Yes |
| Mahmoud 1985 | G. holbrooki | Unclear. Authors state a total of 8000 to 12,000 fish per canal depending on length and 1000 fish | Canals | 1 m depth, 2 m width, 4 to 10 km length | Not stated | Not stated | October | Yes |
| Menon 1978 | G. affinis and A. blockii | 20 fish per negative well, 50 fish per positive well | Wells | Not stated | Not stated | Not stated | January | Yes |
| Nalim 1988 | P. reticulata and C. carpio | Nine C. carpio/10 m2 and two P. reticulata/m2 | Rice fields | 23.9 ha in total, but size of individual ponds not specified | Not stated | Not stated | Not stated | Yes |
| Sabatinelli 1991 | P. reticulata | Three to five fish per m3 | Domestic water containers | Size of domestic water containers (ablution basins and tanks) not clearly indicated | Not stated | Not stated | November | Not clearly indicated |
| Sitaraman 1976 | P. reticulata | Either 50 or 100 fish per well | Wells | 1.5 to 2.5 m depth, average square area 1.5 m2 | Not stated | Not stated | Not stated | No |
| Yu 1989 | A. latipes and T. m. niloticus | Two A. latipes/m2 and two T. m. niloticus/10 m2 or two A. latipes/m2 only | Rice fields | Each plot was 10 × 15 × 0.3 m, depth 10 cm | Not stated | Not stated | June | No |
The study authors used the following fish species in larval studies: Aphanius dispar (Fletcher 1992); Poecilia reticulata (Kusumawathie 2008a; Kusumawathie 2008b; Nalim 1988; Sabatinelli 1991; Sitaraman 1976); Cyprinus carpio (Nalim 1988); G. affinis (Imbahale 2011a; Menon 1978); G. holbrooki (Mahmoud 1985); Aplocheilus blockii (Menon 1978); Aplocheilus latipes (Kim 2002; Yu 1989); Aphyocypris chinensis (Kim 2002); Oreochromis niloticus (formerly Tilapia nilotica) (Howard 2007); and Tilapia mossambicus niloticus (Kim 2002; Yu 1989). Two studies also used the herbivorous species T. m. niloticus (Kim 2002; Yu 1989) to control aquatic weeds but they did not directly use this fish species for immature mosquito predation. Six studies introduced fish species that were indigenous to the area (Fletcher 1992; Howard 2007; Kim 2002; Menon 1978 (A. blockii only); Nalim 1988 (C. carpio only); Yu 1989 (A. latipes only)). Ten studies used non-indigenous fish species (Imbahale 2011a; Kim 2002 (T. m. niloticus only); Kusumawathie 2008a; Kusumawathie 2008b; Mahmoud 1985; Menon 1978 (G. affinis only); Nalim 1988 (P. reticulata only); Sabatinelli 1991; Sitaraman 1976; Yu 1989 (T. m. niloticus only)).
The number of fish introduced to sites varied, and stocking density depended primarily on the size of the water body treated (Table 3). Ten studies did not state the size or maturity of the fish introduced (Fletcher 1992; Kim 2002; Kusumawathie 2008a; Kusumawathie 2008b; Mahmoud 1985; Menon 1978; Nalim 1988; Sabatinelli 1991; Sitaraman 1976; Yu 1989). Only two studies reported the size (Imbahale 2011a) or the maturity (Howard 2007) of the larvivorous fish introduced to the sites. Only two studies reported the sex ratio of fish introduced (Kusumawathie 2008a; Kusumawathie 2008b), but the remaining ten studies did not. Ten studies reported the time of year that fish were introduced to the intervention site (Fletcher 1992; Howard 2007; Imbahale 2011a; Kim 2002; Kusumawathie 2008a; Kusumawathie 2008b; Mahmoud 1985; Menon 1978; Sabatinelli 1991; Yu 1989), but two studies did not (Nalim 1988; Sitaraman 1976). Six studies monitored fish survival (Fletcher 1992; Kusumawathie 2008a; Mahmoud 1985; Menon 1978; Sabatinelli 1991; Sitaraman 1976). Six studies performed restocking of fish after regular monitoring of the fish population (Fletcher 1992; Kusumawathie 2008b; Menon 1978) or at pre-specified time points (Imbahale 2011a; Mahmoud 1985; Nalim 1988).
Design quality
We evaluated the following study design quality factors of the included studies and summarized the results in Table 4.
Table 4.
Design quality
| Study ID | Pupae numbers reported | Distance between sites | Other larvivorous species present | Vegetation cleared |
|---|---|---|---|---|
| Fletcher 1992 | Recorded but not reported | < 1 km | Not reported | Not reported |
| Howard 2007 | Only larvae and pupae combined reported | < 1 km | Not reported | Three ponds cleared of vegetation on a weekly basis |
| Imbahale 2011a | Not reported | Not reported | Not reported | Not reported |
| Kim 2002 | Not reported | < 1 km | Not reported for control site. For treatment site, no other larvivorous fish found. | Herbivorous fish T. m. niloticus used at experimental but not control sites |
| Kusumawathie 2008a | Recorded but not reported | < 1 km | Not reported | Not reported |
| Kusumawathie 2008b | Not reported | Not reported | Not reported | Not reported |
| Mahmoud 1985 | Not reported | Not reported | Not reported | Not reported |
| Menon 1978 | Not reported | Not reported | Not reported | Not reported |
| Nalim 1988 | Not reported | Not reported | Not reported | Not reported |
| Sabatinelli 1991 | Not reported | 3 km | Not reported | Not reported |
| Sitaraman 1976 | Yes | Not reported | Not reported | Not reported |
| Yu 1989 | Not reported | < 1 km | Not reported | Herbivorous fish T. m. niloticus used in one treatment arm only |
Pupae numbers reported: Larvivorous fish may preferentially eat particular instars of mosquito larvae or pupae (Walker 2007). Therefore, we checked whether studies monitored both larvae and pupae populations. Sitaraman 1976 reported both larvae and pupae numbers. Howard 2007 reported both larvae and pupae numbers combined. Fletcher 1992 recorded but did not report pupae numbers. The remaining nine studies did not report pupae numbers (Imbahale 2011a; Kim 2002; Kusumawathie 2008a; Kusumawathie 2008b; Mahmoud 1985; Menon 1978; Nalim 1988; Sabatinelli 1991; Yu 1989).
Distance between sites: One study had a distance of greater than 1 km between control and experimental sites (Sabatinelli 1991). Five studies had control and experimental sites < 1 km from each other (Fletcher 1992; Howard 2007; Kim 2002; Kusumawathie 2008a; Yu 1989). Six studies did not report the distance between these sites (Imbahale 2011a; Kusumawathie 2008b; Mahmoud 1985; Menon 1978; Nalim 1988; Sitaraman 1976).
Other larvivorous species present: None of the included studies reported whether other larvivorous species were present at the control and experimental sites. Kim 2002 reported that no other larvivorous fish species were present at the fish intervention site but did not monitor the control site.
Vegetation cleared: The vegetation coverage can also affect immature mosquito numbers. Nine studies did not report whether vegetation was cleared at the study sites (Fletcher 1992; Imbahale 2011a; Kusumawathie 2008a; Kusumawathie 2008b; Mahmoud 1985; Menon 1978; Nalim 1988; Sabatinelli 1991; Sitaraman 1976). Howard 2007 stated that at all sites, vegetation was cleared on a weekly basis. Two studies used the herbivorous fish, T. m. niloticus, to clear vegetation. However, Kim 2002 used this fish species at the experimental sites but not at the control sites, and Yu 1989 used this fish species in one treatment arm only.
Outcomes
Of the 12 larval studies that we included, nine studies examined the effects of larvivorous fish on the density of vector larvae (Howard 2007; Imbahale 2011a; Kim 2002; Kusumawathie 2008a; Kusumawathie 2008b; Mahmoud 1985; Sitaraman 1976; Yu 1989) or vector adults collected using emergence traps as a measure of larval density (Nalim 1988). Four of these studies were controlled interrupted time series (Howard 2007; Kim 2002; Sitaraman 1976; Yu 1989), three studies were controlled time series (Imbahale 2011a; Mahmoud 1985; Nalim 1988), and two studies were controlled before-and-after studies (Kusumawathie 2008a; Kusumawathie 2008b). Five studies recorded the percentage of sites positive for larvae of the vector (Fletcher 1992; Kusumawathie 2008a; Kusumawathie 2008b; Menon 1978; Sabatinelli 1991). Of these five studies, one study was a quasi-RCT (Fletcher 1992), two studies were controlled interrupted time series (Menon 1978; Sabatinelli 1991), and two studies were controlled before-and-after studies (Kusumawathie 2008a; Kusumawathie 2008b).
Excluded studies
We excluded 105 studies from the review because they did not meet the eligibility criteria, or they did not report any outcome of interest, or both. We have given the following reasons for exclusion in the Characteristics of excluded studies section: Anopheles and Culex populations were not monitored separately (seven studies); studies were not fish studies (29 studies); no primary outcomes were reported (20 studies); no secondary outcomes were reported (eight studies); multiple interventions were introduced, meaning that the effect of fish alone could not be determined (eight studies); the study was laboratory-based, not field-based (four studies); inappropriate study design was applied (54 studies); or the outcome data were already presented in another paper (four studies). In several cases, we excluded a study for more than one reason.
Risk of bias in included studies
We listed in Table 1 the criteria used to assess the risk of bias in included studies and presented our findings in the risk of bias tables in the Characteristics of included studies section. We have summarized the risk of bias results in Figure 4 and Figure 5.
Figure 4.
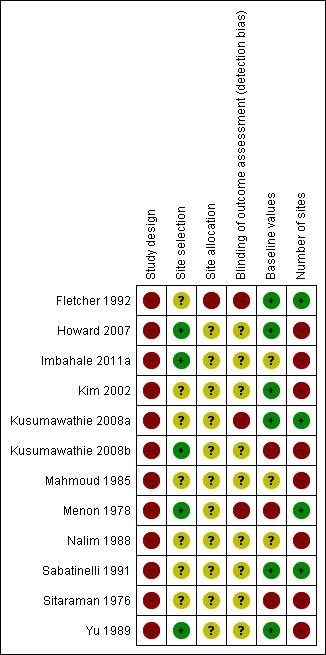
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Figure 5.
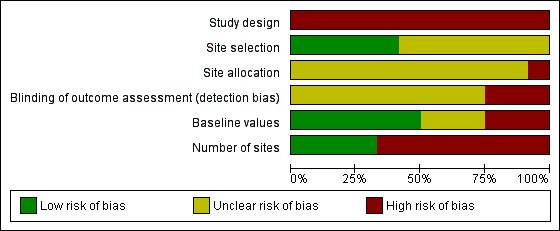
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Study design: None of the studies included randomized comparisons, and all were at high risk of bias.
Site selection: Seven studies did not state how they selected sites (Fletcher 1992; Kim 2002; Kusumawathie 2008a; Mahmoud 1985; Nalim 1988; Sabatinelli 1991; Sitaraman 1976) and were at unclear risk of bias. Five studies stated clearly how the sites were selected within the study area (Howard 2007; Imbahale 2011a; Kusumawathie 2008b; Menon 1978; Yu 1989) and were at low risk of bias.
Site allocation: Study authors did not give information about how they chose the comparator sites in eleven studies (Howard 2007; Imbahale 2011a; Kim 2002; Kusumawathie 2008a; Kusumawathie 2008b; Mahmoud 1985; Menon 1978; Nalim 1988; Sabatinelli 1991; Sitaraman 1976; Yu 1989), and the studies were at unclear risk of bias. One study was at high risk of bias (Fletcher 1992).
Blinding of assessors: Study authors did not blind outcome assessors to the intervention in three studies (Fletcher 1992; Kusumawathie 2008a; Menon 1978), and the studies were at high risk of bias. In the nine remaining studies, the risk of bias was unclear (Howard 2007; Imbahale 2011a; Kim 2002; Kusumawathie 2008b; Mahmoud 1985; Nalim 1988; Sabatinelli 1991; Sitaraman 1976; Yu 1989).
Baseline values comparable between sites: In three studies, baseline values before the intervention was introduced were not comparable between control and experimental sites, and the studies were classified as having high risk of bias (Kusumawathie 2008b; Menon 1978; Sitaraman 1976). In Kusumawathie 2008b, baseline values were comparable for two outcomes: (1) mean number of Anopheles larvae per 100 dips; and (2) average monthly percentage of sites positive for Anopheles larvae. However, baseline values were not comparable for the two other outcomes: (1) average monthly number of anopheline larvae per 100 pools; and (2) total number of Anopheles larvae; this study was at high risk of bias. Three studies were at unclear risk of bias (Imbahale 2011a; Mahmoud 1985; Nalim 1988). Six studies were at low risk of bias (Fletcher 1992; Howard 2007; Kim 2002; Kusumawathie 2008a; Sabatinelli 1991; Yu 1989).
Number of sites: Four studies were at low risk of bias, as they had an adequate number of sites (20 or more) per group (Fletcher 1992; Kusumawathie 2008a; Menon 1978; Sabatinelli 1991). We judged eight studies to be at high risk of bias, as three studies may have had an inadequate number of sites (5 to < 20) per group (Imbahale 2011a; Mahmoud 1985; Sitaraman 1976), and five studies probably had an inadequate number of sites (< 5) per group (Howard 2007; Kim 2002; Kusumawathie 2008b; Nalim 1988; Yu 1989).
Effects of interventions
See: Summary of findings for the main comparison
Primary analysis
We did not identify any studies that reported on any of the primary outcomes that we defined (number of malaria cases, EIR, or density of adult anopheline mosquitoes). Thus no direct evidence indicates that this intervention impacts malaria transmission.
Secondary analysis
For the secondary analysis of whether introduction of larvivorous fish impacts immature anopheline mosquitoes, all studies were at high risk of bias and provided only indirect evidence of the potential effectiveness of this intervention. As each study was very different, we have given a full critical appraisal of each study in Appendix 2 and a summary in the table below. We included 12 studies, which were conducted in localized water bodies, including wells, domestic water containers, and fishponds and pools (six studies); pools in a riverbed below a dam (two studies); rice field plots (three studies); or water canals (two studies).
Overall, some evidence from studies that ranged in size suggested that larvivorous fish could sometimes prevent increases in immature anopheline mosquito densities compared with control sites, and some studies provided evidence of sustained reductions in immature anopheline numbers during up to 11 months of follow-up, but these findings were not consistent. Despite stratification by site and careful critical analysis of each individual study, clear patterns were not evident, although stocking density seemed to have some impact on whether introducing larvivorous fish influenced immature anopheline density.
None of the studies reported on other ecosystem effects, including densities of endogenous fish.
| Site type | Study | Intervention | Outcome | Result | |||
|---|---|---|---|---|---|---|---|
| 1. Localized water bodies | (a) Wells | Sitaraman 1976 | 100 P. reticulata per well Experimental: 10 wells Control: four wells 50 P. reticulata per well Experimental: 12 wells Control: five wells | An. stephensi larval and pupal densities up to 28 days (100 fish per well) or 22 days (50 fish per well) | At high fish stocking levels, larvae were eliminated in the first four days in wells but reappeared at lower levels from day 24 onwards With lower fish stocking levels, a partial effect was noted for two weeks only, with rebound | ||
| Menon 1978 | Experimental: Gambusia or Aplocheilus fish to 3438 wells; 50 fish per well if anopheline larvae present; 20 fish per well if no larvae present Control: 317 wells | An. stephensi larval density up to four months' follow-up | This study appears to provide evidence of a larvicidal effect of fish in wells using relatively high fish stocking levels | ||||
| (b) Wells and domestic water containers | Fletcher 1992 | Experimental: Aphanius dispar (60 sites) Control: 51 sites | An. culicifacies adanensis larval density for 11 months' follow-up | This study provides evidence that fish introduction prevents an increase in the number of domestic water container sites with larvae compared with control up to 11 months' follow-up | |||
| Sabatinelli 1991 | Experimental: P. reticulata fish (59 sites in November 1987, total number of sites not specified) Control: 20 ablution basins | Percentage of containers positive for An. gambiae larvae for 11 months' follow-up | This study appears to show that fish reduce the number of domestic wash basins with larvae when added to these sites for up to 11 months | ||||
| (c) Fishponds and pools | Howard 2007 | Experimental: Oreochromis niloticus fish (two ponds) Control: one pond | Number of immature An. gambiae and An. funestus mosquitoes for five months' follow-up | Based on trends in the study authors' graph, data that we extracted from the graph, and the study authors' analysis, this study appears to provide limited evidence of a possible larvicidal effect of fish in ponds | |||
| Imbahale 2011a | See the water canals section below | ||||||
| (d) Riverbed pools below dams | Kusumawathie 2008a | Experimental: P. reticulata (29 riverbed pools) Control: 31 pools | Percentage of pools with Anopheles larvae, mean number of Anopheles larvae per pool, and mean number of Anopheles larvae per 100 dips up to 120 days' follow-up | At follow-up, the experimental group had greater reductions than the control group for the outcomes of percentage of pools with Anopheles larvae, mean number of larvae per pool, and mean number of larvae per 100 dips | |||
| Kusumawathie 2008b | Experimental: P. reticulata to all riverbed pools in Laxapana and Kotmale 1 study sites Control: all riverbed pools in Kotmale 2 and Nilambe | Percentage of pools with Anopheles larvae, mean number of Anopheles larvae per pool, and mean number of Anopheles larvae per 100 dips up to one year's follow-up | At follow-up, riverbed pools stocked with fish had larger reductions in terms of presence and density of larvae | ||||
| 2. Rice field plots | Nalim 1988 | Experimental: 23.9 hectares of rice fields with P. reticulata and C. carpio fish Control: did not specify the size of the control area used Total numbers of control and experimental field plots not specified | Number of An. aconitus, An. barbirostris, and An. annularis newly emerged adult mosquitoes collected/m2/day (trap area = 0.25 m2) up to six years' follow-up | Effects were mixed, with some indication of an effect of fish on An. aconitus and An. annularis, but not on An. barbirostris | |||
| Kim 2002 | Experimental: Tilapia mossambicus and A. latipes (Treatment A, one rice field plot) or Aphyocypris chinensis and Tilapia mossambicus (Treatment B and Treatment C, one rice field plot each) Control: three rice field plots of similar size | Number of An. sinensis larvae up to 13 weeks' (Treatment A) or seven weeks' (Treatment B and C) follow-up | In the control group and with Treatments B and C, the number of An. sinensis larvae was higher at two weeks' pre-intervention than at six weeks' pre-intervention. At two weeks' follow-up, the An. sinensis larval population in the control group was the same at two weeks' follow-up but decreased at six weeks' follow-up. Larvae were clearly reduced at the two sites where fish were introduced For treatment A, the number of An. sinensis larvae was higher at five weeks' follow-up than at one week's follow-up, and the number decreased at nine weeks' and 13 weeks' follow-up. This shows an average difference in larvae density between control and intervention over the entire period of observation. However, these data were less strong, as no baseline density in the intervention arm was noted, and any difference with the control could be due to chance | ||||
| Yu 1989 | Experimental: two plots treated with two species of fish (A. latipes and Tilapia mossambicus), two plots treated with one species alone (A. latipes) Control: two plots | Number of An. sinensis larvae up to four weeks' (one fish) or seven weeks' (two fish) follow-up | At four weeks, larvae had increased against baseline in both control and intervention plots, but the size of the increase was lower in the two one-fish intervention plots Follow-up at four weeks and at seven weeks showed considerably lower values in the two two-fish intervention plots than in the control | ||||
| 3. Water canals | Imbahale 2011a | Ponds Experimental: single (six ponds) and multiple stocking of G. affinis (six ponds) Control: six ponds Canals Experimental: G. affinis (six canals) Control: six canals | Estimated marginal mean values of younger (L1 and L2) and older (L3 and L4) An. gambiae s.l. larvae up to 13 weeks' follow-up | No difference was demonstrated between control and experimental groups at follow-up, apart from the fact that numbers of older larvae were lower in the canal intervention group | |||
| Mahmoud 1985 | Experimental: 20 canals treated with G. holbrooki Control: five canals | Density of a late larval stage of An. arabiensis (L4) up to 13 months' follow-up | An. arabiensis density was lower in intervention canals for two months (five months' and six months' post-intervention) just before and at the beginning of the dry season. Larval densities dropped in both intervention and control in the dry season (seven months' post-intervention) and at the end of the rainy season (13 months' post-intervention). Fish numbers failed to increase after the rainy season and during the last six months of the study. According to the authors, control of the flow of water from large to branch canals by gates deprived the fish of free movement. Also, during the rainy season, rainwater pools act as suitable breeding sites for An. arabiensis | ||||
Discussion
Summary of main results
We found no randomized trials or quasi-experimental studies that examined the direct impact of the use of larvivorous fish on malaria in people living in malaria-endemic communities; or on outcomes related to transmission, including the EIR and the density of adult vector mosquitoes. Therefore, we do not know whether larvivorous fish have an effect on adult anopheline mosquito populations or on malaria transmission in endemic communities.
We explored whether any evidence suggested that this form of vector control had any potential for an effect on malaria by examining the effect of larvivorous fish stocking on the density of immature vector stages and the percentage of breeding sites positive for immature vector stages compared with controls in studies ranging from three weeks up to five years in duration. These outcomes were examined in 12 small-scale studies undertaken in a variety of settings, including localized water bodies (wells, domestic water containers, fishponds or pools, and riverbed pools below dams; eight studies), rice field plots (three studies), and water canals (two studies). Evidence of an effect of larvivorous fish on the density of immature vector stages in water bodies was variable. We do not know from the available evidence whether larvivorous fish reduce the density of immature anopheline stages (nine studies, unpooled data,very low quality evidence). Larvivorous fish may cause a reduction in the percentage of breeding sites positive for immature vector stages (five studies, unpooled data, low quality evidence).
Due to the poor quality of the studies and the absence of any consistent effect, this is not an intervention that could sensibly be used in malaria control given this current evidence base. Whether these data can guide future research on which larvivorous fish species should be evaluated and which categories of breeding sites should be tested also is not entirely clear. Some reports describe almost 100% reduction of the immature Anopheles population (Fletcher 1992; Kusumawathie 2008a; Menon 1978; Sitaraman 1976). Effects of the fish intervention on immature anopheline populations were mainly reported in studies that used high stocking densities of fish in localized water bodies with short follow-up periods (< four months), although one study suggested that increasing larval numbers were inhibited for the 11 months' follow-up in domestic water sources (Fletcher 1992).
Monitoring of the immature mosquito population did not appear to influence decisions regarding implementation, such as fish restocking or increase in fish stocking density. None of the studies we identified that met the inclusion criteria examined the impact, if any, of larvivorous fish introduction on the environment or on native species present apart from the target mosquito species.
Overall completeness and applicability of evidence
The review demonstrates that evidence is currently insufficient regarding whether larviciding with fish impacts cases of human malaria or malaria transmission. The review shows that in some circumstances, the intervention leads to a reduction in immature mosquitoes in the water sources stocked with fish. This does not show an effect on malaria transmission but simply shows that the intervention may have a potential benefit worthy of further research.
Quality of the evidence
No evidence was found for the primary review outcome of examining the effects of introducing larvivorous fish on malaria transmission. The quality of evidence exploring the larvicidal effect of fish was mixed, and overall study design was poor.
Potential biases in the review process
Our search strategy was comprehensive, and it was not limited by language or publication status. Many of the older studies contained anecdotal evidence, and in many studies, fish were combined with other antimalarial interventions in uncontrolled designs, so attribution of an effect was not possible.
Agreements and disagreements with other studies or reviews
A Cochrane Review of larvicides (Tusting 2013) excludes fish. This review indicated that larviciding could be effective for preventing malaria transmission, but questions were raised about whether it was feasible to carry this out in many areas of Africa.
The current WHO regional strategy for the WHO European Region 2006-2015 recommends the use of larvivorous fish "in all existing or potential reservoirs where Anopheles species breed with particular attention to rice fields" (WHO 2006a). In addition, the WHO recommends this intervention for elimination of malaria in low and moderate endemic countries (WHO 2007). The use of larvivorous fish as part of an integrated programme to control malaria has been advocated, subject to further vector biology studies to ensure that the actual vector is targeted (Ghosh 2007). However, further high-quality evidence is required before these recommendations can be supported. Although this review demonstrates that use of larvivorous fish can cause a significant reduction in the number of immature mosquitoes, particularly in fixed breeding sites as opposed to temporary breeding sites, a direct correlation between reduction of immature mosquito numbers and reduction of the adult vector population or the number of cases of malaria in people needs to be demonstrated.
Authors' conclusions
Implications for practice
There is no reliable research evidence that introducing larvivorous fish has any effect on outcomes of transmission of human malaria. Whilst sometimes presented as biologically friendly compared with chemical larvicides, some authors have raised the possibility that larvivorous fish may harm indigenous species, including frogs and other fish species.
Implications for research
This review provides some research evidence that larvivorous fish, in some specific circumstances, can decrease immature mosquito populations in water bodies. However, this evidence is insufficient to support investing in the intervention as a policy without further reliable research.
If researchers judge that this is a potentially effective intervention, then well-designed quasi-experimental studies to examine the effects on malaria in humans or, at the very least, on the EIR or the density of adult vector mosquitoes are required. It is important to note that researchers should carefully consider the design of the studies and should randomly allocate interventions to sites to minimize the risk of bias. Also, researchers should undertake power calculations to decide the size of the study.
These studies should consider in the study design any factors that could influence or bias the results (study design, baseline values, number of sites, pupae numbers reported, distance between sites, other larvivorous species present, vegetation cleared). Several effect modifiers had dramatic effects on immature forms, both within and between studies.
This research needs to be undertaken in a variety of ecological zones and settings, including household water sources, ponds, water canals, riverbed pools below dams, and rice fields, and should take into account the seasonality of malaria transmission in these study areas. Ideally within these studies, the fish intervention should not be combined with other interventions, so the effect of larvivorous fish introduction alone on the adult mosquito population, or on the incidence of malaria, or on both, can be discerned. This is necessary before use of larvivorous fish can be recommended as a tool for malaria control, to be used either alone or in combination with other vector control methods. Furthermore, research studies should assess the environmental impact of larvivorous fish, particularly non-native introduced species, on the habitats into which they are released.
Apart from efficacy, questions remain regarding whether it is practical to deliver this method with the requisite quality and completeness of coverage on a larger scale than in experimental settings, whether it is cost-effective, whether it should be delivered as a stand-alone intervention or as an addition to IRS or LLINs, and whether this can be sustained for years.
Acknowledgments
Hellen Gelband was the academic editor for this review.
We are grateful to our affiliated institutions and organizations, and we thank the referees and editors for their constructive comments. The editorial base for the Cochrane Infectious Disease Group is funded by the Department for International Development (DFID), UK, for the benefit of developing countries. DPW was supported by a grant from the DFID. The findings and conclusions in this report have not been formally disseminated by the Centers for Disease Control and Prevention and should not be construed as representing any agency determination or policy. We are grateful to David Sinclair for his help with the GRADE assessment.
APPENDICES
Appendix 1. Search methods: detailed search strategies
| Search set | CIDG SRa | CENTRAL | MEDLINE | EMBASE | LILACS | CAB ABSTRACTS |
|---|---|---|---|---|---|---|
| 1 | mosquito* | mosquito* | mosquito* | mosquito$ | mosquito$ | mosquito* |
| 2 | control* OR breeding* OR larva* Or predat* | control* OR breeding* OR larva* OR predat* | control* OR breeding* OR larva* OR predat* | control$ OR breeding$ OR larva$ Or predat$ | control$ OR breeding$ OR larva$ OR predat$ | control* OR breeding* OR larva* Or predat* |
| 3 | 1 and 2 | 1 and 2 | PEST CONTROL, BIOLOGICAL | VECTOR CONTROL | 1 and 2 | 1 and 2 |
| 4 | (fish* or frog*) | MOSQUITO CONTROL/METHODS | 2 OR 3 | 2 OR 3 | (fish$ OR frog$) | (fish* or frog*) |
| 5 | larvivorous | 3 or 4 | 1 AND 4 | 1 AND 4 | larvivorous | larvivorous |
| 6 | 4 or 5 | (fish* OR frog*) | MOSQUITO CONTROL/METHODS | (fish$ OR frog$) | 4 or 5 | “Gambusia”OR “Poecilia”OR “Aphanius”OR “Oreochromis”OR “Tilapia”OR “Aplocheilus”OR “Cyprimus”OR “Ctenopharyngodon”OR “Rasbora”OR “Aphyocypris” |
| 7 | 3 and 6 | larvivorous | 5 OR 6 | larvivorous | 3 and 6 | 4 or 5 or 6 |
| 8 | — | 6 OR 7 | (fish* OR frog*) | “Gambusia”OR “Poecilia”OR “Aphanius”OR “Oreochromis”OR “Tilapia”OR “Aplocheilus”OR “Cyprimus”OR “Ctenopharyngodon”OR “Rasbora”OR “Aphyocypris” | — | 3 and 7 |
| 9 | — | 5 and 8 | larvivorous | 6 or 7 or 8 | — | — |
| 10 | — | — | “Gambusia”OR “Poecilia”OR “Aphanius”OR “Oreochromis”OR “Tilapia”OR “Aplocheilus”OR “Cyprimus”OR “Ctenopharyngodon”OR “Rasbora”OR “Aphyocypris” | 5 and 9 | — | — |
| 11 | — | — | 8 OR 9 OR 10 | — | — | — |
| 12 | — | — | 7 AND 11 | — | — | — |
aCochrane Infectious Diseases Group Specialized Register.
Appendix 2. Descriptive analysis of included studies
None of the included studies reported on cases of malaria, EIR, or the density of adult vector mosquitoes. Therefore, we did not find any direct evidence that this intervention impacts malaria transmission. We performed a descriptive analysis of the 12 included studies that examined the effect of fish stocking on immature anopheline mosquito presence or density, or both. We analysed the studies by the habitat type that study authors introduced for the larvivorous fish. Eight studies evaluated larvivorous fish in localized water bodies (including wells, domestic water containers, fishponds and pools, and riverbed pools created after dam construction), three studies used rice field plots, and two studies used water canals; see Table 2.
Section 1: Localized water bodies
(a) Wells
Two studies from India evaluated larviciding in wells (Sitaraman 1976; Menon 1978).
Sitaraman and colleagues introduced fish (100 P. reticulata) to 10 wells and maintained four wells as controls. The authors measured An. stephensi larval and pupal densities by taking five dips per well every four days until 28 days' post-intervention. They measured baseline values immediately before the introduction of larvivorous fish to the 10 wells. We examined the raw data reported by the authors for evidence of an effect of larvivorous fish on the immature An. stephensi population.
Baseline values in the control (four wells) and experimental groups (10 wells) were comparable before fish were introduced (assuming that these are the numerical totals across the 10 intervention and four control wells; Table 1A). In the experimental wells, immature mosquito numbers decreased rapidly after fish were introduced. This decrease in immature mosquito numbers was greater than in the control group. The study authors did not detect any immature mosquitoes in the 10 wells at four days' follow-up. They measured only 15 and 40 larvae at 24 and 28 days' post-intervention, respectively. At 28 days, the immature mosquito numbers (L1 to L4 stages) increased, and the study authors introduced fish into the control wells.
Sitaraman and colleagues also released 50 fish per well into 12 wells, with five wells in the same ward serving as controls, and followed immature mosquito numbers for 22 days (Table 2A). A dramatic drop in larvae from daily dips (50 per well) was seen early, with a 69% reduction in larvae and a 82% reduction in pupae by day 2; no such change was seen in the control wells. However, recovery of relatively immature larvae (L1 and L2 instars) was relatively rapid and baseline values were restored by day 10; although recovery of mature larvae (L3 and L4) was slower and less complete, with average density still 60% lower than baseline after three weeks (Table 1, page 317 of the paper).
With high fish stocking levels, larvae are eliminated in the first four days in wells but reappear at lower levels from day 24 onwards. With lower stocking levels, a partial effect was noted for two weeks only, with rebound.
Table 1A.
Sitaraman 1976: An. stephensi immature numbers before and after introduction of fish (100 guppies per well)
| Intervention | Immature stages | Pre-intervention | Follow-up (days) |
||
|---|---|---|---|---|---|
| 4 | 24 | 28 | |||
| Control (four wells) | L1 + L2 L3 + L4 Pupae | 296 346 44 | 236 254 64 | 94 36 24 | 240 156 16 |
| Intervention (10 wells) | L1 + L2 L3 + L4 Pupae | 890 960 205 | 0 0 0 | 15 0 0 | 40 0 0 |
Table 2A.
Sitaraman 1976 : An. stephensi immature numbers before and after introduction of fish (50 guppies per well)
| Intervention | Immature stages | Pre-intervention | Follow-up (days) |
||
|---|---|---|---|---|---|
| 4 | 16 | 22 | |||
| Control (five wells) | L1 + L2 L3 + L4 Pupae | 275 330 40 | 455 255 40 | 525 245 30 | 300 255 40 |
| Intervention (12 wells) | L1 + L2 L3 + L4 Pupae | 384 546 102 | 156 156 84 | 498 204 42 | 486 222 48 |
In a second study from India, Menon and colleagues introduced Gambusia or Aplocheilus fish to 3438 wells but kept 317 wells as controls. In experimental sites, if they found mosquito larvae, they stocked with 50 fish per well; if no larvae were present, they stocked with 20 fish per well. They measured An. stephensi larval density at baseline and monthly for four months. The proportion of wells with larvae was greater in the experimental group (32.8%) than in the control group (7.7%) at baseline (Table 3A). At follow-up, the proportion of wells with larvae dropped markedly in the experimental arm (< 1%) but not in the control arm. In the control group, percentage of wells with larvae increased to a maximum of 9.6% during follow-up. This study appears to provide evidence of a larvicidal effect of fish in wells using relatively high stocking levels.
Table 3A.
Menon 1978: percentage of wells with An. stephensi larvae in wells immediately before and after introduction of fish
| Intervention | Pre-intervention (percentage) | Follow-up (months) |
||
|---|---|---|---|---|
| 1 | 2 | 4 | ||
| Control | 7.7 | 8.0 | 8.6 | 9.6 |
| Intervention | 32.8 | 0.97 | 0.49 | 0.47 |
(b) Domestic water containers
Two studies examined larviciding in domestic water containers (Fletcher 1992; Sabatinelli 1991). In Ethiopia, Fletcher and colleagues introduced fish to wells, barrels, cisterns, and washbasins. On the Comoro Islands, located off the south-east coast of Africa, Sabatinelli and colleagues introduced fish to ablution basins and tanks.
Fletcher and colleagues introduced Aphanius dispar to 60 domestic water containers and kept 51 water containers as controls. They measured the An. culicifacies adanensis larval population using a standard dipping procedure pre-intervention and then either every two weeks (May to August 1987) or monthly for a total of 11 months. Control and experimental values were identical at baseline (0%). Sites allocated to the fish intervention had fewer An. culicifacies adanensis larvae at one year post-intervention compared with control sites (see Table 4A).
Fish introduction appears to prevent an increase in the number of domestic water container sites with larvae compared with controls up to 11 months' follow-up.
Table 4A.
Fletcher 1992: percentage of sites with An. culicifacies adanensis larvae before and after introduction of fish
| Intervention | Pre-intervention (percentage of sites) | Follow-up (months) |
|||
|---|---|---|---|---|---|
| 1 | 4 | 7 | 11 | ||
| Control | 0 | 0 | 2.0 | 13.7 | 4.2 |
| Intervention | 0 | 0 | 0.9 | 0 | 0 |
Sabatinelli and colleagues introduced P. reticulata to domestic water containers in Hantsambou village (59 ablution basins sites in November 1987, total number of sites not specified) and kept 20 ablution basins in Bandamadji village as control sites. They measured the percentage of containers positive for An. gambiae larvae by examining the surface and bottom of containers (at least 15 cm in diameter) in both experimental and control groups four times during the 11 months' follow-up. Control and experimental values were identical at baseline. At follow-up, the proportion of sites positive for An. gambiae larvae decreased at fish-treated sites but not at control sites (see Table 5A).
This study appears to show fish that reduce the number of domestic wash basins with larvae when added to these sites for up to 11 months.
Table 5A.
Sabatinelli 1991: percentage of sites with An. gambiae larvae before and after introduction of fish
| Intervention | Pre-intervention (percentage of sites) | Follow-up (months) |
||
|---|---|---|---|---|
| 1 | 5 | 11 | ||
| Control | 40 | 75 | 45 | 50 |
| Intervention | 41 | 7 | 1 | 8 |
(c) Fishponds and pools
Two studies based in Kenya examined use of larvivorous fish in ponds (Howard 2007; Imbahale 2011a).
Howard and colleagues compared two intervention ponds and one control pond, all located within 150 m of each other. They measured the number of immature An. gambiae and An. funestus mosquitoes by taking larval dips five to seven days per week. We explored the evidence for an effect, if any, in three ways: we made a simple description of trends in the graph; we extracted data carefully from the graph; and we examined the authors' analysis.
Trends in the graph: The authors provide a detailed graph showing An. gambiae immature populations over time in the three ponds. They used a 15-week baseline period before the fish were introduced into two of the three ponds. The control pond had much lower densities of An. gambiae immatures in the baseline period, with none present in the first 1.5 months; then followed a gradual increase in density month by month over the intervention period, with wide week-by-week and, at certain time points day-by-day, variations. At six months' post-intervention, larvae numbers peaked and the authors introduced fish to the control pond.
For the first experimental pond, densities were much higher than for the control pond at baseline. When fish were introduced, the density remained low, or possibly attenuated. For the second intervention pond, the intervention did not appear to be associated with any substantive visual pattern of reduction in density, although it could be argued that some attenuation was evident in the first five months. Thus critical appraisal of Figure 2 in Howard 2007 indicated increasing immatures in the control pond but did not provide convincing evidence of substantial and sustained decline in the two experimental ponds.
Extracting data from the graph: We took fixed time points before and after the intervention. Table 6A shows these data, which we estimated using a ruler against the y-axis. We chose the one- and three-month time points as standard normal values. We did not include the end time point of the experiment—when the study authors introduced fish to the control pond—as this will introduce bias as it is defined by an increase in larvae. Our analysis below supports evidence of reduction in the immature An. gambiae population in the first experimental but not in the second experimental pond.
Table 6A.
Howard 2007: An. gambiae immatures in three ponds before and after the introduction of fish
| Intervention | Pre-intervention (months) | Follow-up (months) | ||
|---|---|---|---|---|
| 3 | 1 | 1 | 3 | |
| Control pond | 0 | 7 | 7 | 4 |
| First experimental pond1 | 3 | 7 | 0 | 0 |
| Second experimental pond2 | 2 | 4 | 2 | 2 |
Authors' analysis: The authors used Mulla's formula to calculate percentage reduction in An. gambiae and An. funestus immatures, with estimates of 95.8% reduction in An. gambiae immatures in experimental pond 1 and 94.1% for experimental pond 2; and similar high reductions for An. funestus (98.3% in experimental pond 1, 97.5% in experimental pond 2). However, Mulla's formula depends on rates in the control arm, in which an increase in immature numbers was clearly seen over time. So one interpretation of these data is that fish are effective; the other is that these large effects are the result of particular ecological changes happening in the control pond. This study appears to provide limited evidence of a possible larvicidal effect of fish in ponds.
For the Imbahale 2011a study, refer to the water canals section below.
(d) Riverbed pools below dams
Two studies in Sri Lanka evaluated fish introduced to riverbeds pools located below dams (Kusumawathie 2008a; Kusumawathie 2008b).
In the Kusumawathie 2008a study, authors introduced P. reticulata to 29 riverbed pools below Kotmale dam and used 31 pools as controls. They measured the number of immature Anopheles using a 100 mL larval dipper at a frequency of six dips per m2 at baseline (day before fish were introduced) and up to 120 days' follow-up. Control and experimental groups had similar baseline values. At follow-up, the experimental group had greater reductions than the control group for the outcomes of percentage of pools with Anopheles larvae, mean number of larvae per pool, and mean number of larvae per 100 dips (Table 7A).
This study appears to provide evidence of a larvicidal effect of fish in riverbed pools below dams sustained up to four months.
Table 7A.
Kusumawathie 2008a: average percentage of pools with Anopheles larvae, mean number of larvae per pool, and mean number of larvae per 100 dips before and after introduction of larvivorous fish
| Outcome | Intervention | Pre-intervention | Follow-up |
|---|---|---|---|
| Percentage of pools with Anopheles larvae | Control Experimental | 100 100 | 31.03 0 |
| Mean number of larvae per pool | Control Experimental | 3.03 3.17 | 0.52 0 |
| Mean number of larvae per 100 dips | Control Experiment | 114.63 109.52 | 20 0 |
In the second study (Kusumawathie 2008b), Kusumawathie and colleagues introduced P. reticulata to all riverbed pools in Laxapana and Kotmale 1 study sites. They used riverbed pools in Kotmale 2 and Nilambe as control sites. They measured immature Anopheles densities using a 100 mL larval dipper at a frequency of six dips per m2 for one year pre-intervention and one year post-intervention. Baseline values at control and experimental sites were similar for the outcomes percentage pools with Anopheles larvae and mean number of larvae per 100 dips, but not for mean number of larvae per 100 pools. At follow-up, the riverbed pools stocked with fish had larger reductions in terms of presence and density of larvae (Table 8A).
This study indicates a partial effect of fish on presence and density of larvae in riverbed pools below dams for up to a year.
Table 8A.
Kusumawathie 2008b: average percentage of pools with Anopheles larvae, mean number of larvae per 100 pools, and mean number of larvae per 100 dips before and after introduction of larvivorous fish
| Outcome | Intervention | Pre-intervention | Follow-up |
|---|---|---|---|
| Percentage of pools with Anopheles larvae | Control Experimental | 15.95 17.39 | 12.52 5.79 |
| Mean number of larvae per 100 pools | Control Experimental | 28.78 142.94 | 27.44 11.25 |
| Mean number of larvae per 100 dips | Control Experiment | 8.52 11.84 | 9.02 3.4 |
Section 2: Rice field plots
Three studies, one in Central Java (Nalim 1988) and two in South Korea (Kim 2002; Yu 1989), evaluated fish introduced to rice fields;. In Central Java, Nalim and colleagues stocked 23.9 hectares of rice fields with P. reticulata and C. carpio fish. They did not specify the size of the control area that they used or the total number of control and experimental field plots. Using 80 emergence traps randomly located in the treated and control areas, they reported the numbers of An. aconitus, An. barbirostris, and An. annularis newly emerged adult mosquitoes collected/m2/day (trap area = 0.25 m2) over six years. Effects were mixed, with some evidence of an impact of fish on An. aconitus and An. annularis, but not on An. barbirostris (Table 9A).
This study indicates a partial effect of fish on the density of newly emerged An. aconitus and An. annularis, but not An. barbirostris, in rice field plots below dams for up to six years.
Table 9A.
Nalim 1988: average number of adult mosquitoes collected per m2 per day
| Species | Intervention | Year |
||
|---|---|---|---|---|
| 1 | 3 | 6 | ||
| An. aconitus 1 | Control Experimental | 2.4 3.35 | 4.2 0.2 | 1.2 0.01 |
| An. barbirostris1 | Control Experimental | 7.6 6.0 | 6.0 4.7 | 3.2 2.9 |
| An. annularis 1 | Control Experiment | 3.0 3.35 | 4.2 1.13 | 2.2 0.7 |
1We discarded two years of data (1982, 1983), as the study authors reported that the control area was sprayed with fenitrothion at the end of 1982.
In the South Korean study, Kim and colleagues introduced three slightly different interventions to three rice field plots measuring about 300 m2 to 600 m2. They compared these with a control area of three rice field plots of similar size. They introduced either Tilapia mossambicus and A. latipes (Treatment A) or Aphyocypris chinensis and Tilapia mossambicus (Treatment B and Treatment C) to rice field plots and took two dips, with between two and four replicates per rice field, every two weeks, to examine the average number of An. sinensis larvae.
We extracted data for specific time points before and after the intervention. The study authors used a six-week baseline period for Treatments B and C but no baseline for Treatment A before the fish were introduced into two plots.
The results provide a robust controlled before-and-after study (Treatments B and C), with four time points in the control period (Table 10A). Baseline measurements appeared similar at control and intervention sites. In the control group and for Treatments B and C, the number of An. sinensis larvae was higher at two weeks' pre-intervention than at six weeks' pre-intervention. After fish were introduced to the intervention sites, the An. sinensis larval population in the control group was the same at two weeks' follow-up but decreased at six weeks' follow-up. Larvae were clearly reduced at the two sites where fish were introduced.
The study also affords a controlled time series comparison between the control group and a third intervention site, where the fish were introduced at the start of observations (Treatment A; Table 11A). The number of An. sinensis larvae increased between one week and five weeks' follow-up at both control and experimental sites. However, the number of larvae decreased by 13 weeks' follow-up at both control and experimental sites. This shows an average difference in larvae density between control and intervention over the entire period of observation. However, these data are weaker, as no baseline density was noted in the intervention arm, and any difference from the control could be due to chance.
This study appears to provide limited evidence of a possible larvicidal effect of fish on An. sinensis larvae in rice paddy plots.
Table 10A.
Kim 2002: An. sinensis larvae at control (three plots) and experimental sites (two plots) before and after introduction of fish
| Intervention | Pre-intervention (weeks) | Follow-up (weeks) | ||
|---|---|---|---|---|
| 6 | 2 | 2 | 6 | |
| Control | 2.0 | 4.5 | 4.5 | 2.5 |
| Treatment B | 2.5 | 3.5 | 2.25 | 0.4 |
| Treatment C | 1.75 | 4.13 | 2.25 | 0.38 |
Table 11A.
Kim 2002: An. sinensis larvae at control plots (three plots) and at an experimental plot (one plot) after introduction of fish
| Intervention | Follow-up (weeks) |
|||
|---|---|---|---|---|
| 1 | 5 | 9 | 13 | |
| Control | 2.0 | 4.5 | 4.5 | 2.5 |
| Treatment A | 1.25 | 2.5 | 2.0 | 0.5 |
In South Korea, Yu and colleagues compared ponds treated with two species of fish (A. latipes and Tilapia mossambicus), one species alone (A. latipes), and a control group. The researchers selected six plots, 45 m2 in size and 0.3 m in depth, located within a confined rice field of 1000 m2. They randomly assigned two plots to each treatment group. They took measurements of the An. sinensis larval population every week, using a 500 mL dipper (two to four dips per rice field plot) or a nylon net (eight to 10 sweepings per sample). The study authors monitored the An. sinensis larval population for eight weeks before they introduced fish, and pre-intervention values were comparable between sites. In the first two intervention plots, they introduced one fish species: at four weeks, larvae had increased against baseline in both control and intervention ponds, but the size of the increase was smaller in the one-fish intervention pond (7.00 compared with 16.00, 56% lower; Table 12A).
In the next two intervention plots, they introduced two fish species, and follow-up at four weeks and seven weeks showed considerably lower values in the two-fish intervention pond than in the control pond (4.21 compared with 16.13, 74% lower; Table 12A). This study provides some evidence that larvivorous fish can constrain the rapid increases in larvae populations seen in untreated ponds.
Table 12A.
Yu 1989: average number of An. sinensis larvae in ponds before intervention and after introduction of fish
| Intervention | Pre-intervention1 | Follow-up (weeks) |
|
|---|---|---|---|
| 4 | 7 | ||
| Control | 4.56 | 16.0 | 16.13 |
| One-fish | 4.19 | 7.00 | Bacteria introduced |
| Two-fish | 4.50 | 4.87 | 4.21 |
1We recalculated the average pre-intervention values that the study authors reported in control and intervention groups, as the study authors incorrectly reported these values.
Section 3: Water canals
Two studies introduced fish to irrigation canals — one in Kenya (Imbahale 2011a) and one in Sudan (Mahmoud 1985).
In Kenya, Imbahale and colleagues compared the effects of G. affinis introduced to ponds or water canals versus control sites. The water sources were discrete; 18 ponds were 1 m2 in size and 1 m depth, and 12 canals were 15 m2 in size and 0.3 m in depth. For ponds, the authors evaluated the effects of single stocking and multiple stocking of fish by measuring An. gambiae s. l. larvae twice a week for 13 weeks; and for canals, they compared controls with a single stocking of fish. The study authors divided outcomes by younger larvae (L1 and L2) and older larvae (L3 and L4), and reported estimated marginal mean values. No difference was demonstrated between control and experimental groups at follow-up, apart from the fact that the numbers of older larvae were smaller in the canal intervention group (Table 13A).
This study provides some evidence of an effect of larvivorous fish up to 13 weeks in water canals but not in ponds.
Table 13A.
Imbahale 2011a: estimated marginal mean values of immature anopheline numbers after introduction of fish
| Intervention | Follow-up |
||
|---|---|---|---|
| Younger larvae (L1 and L2) 1 | Older larvae (L3 and L4) 1 | ||
| Ponds | Control | 2.667 (2.217 to 3.117) | 0.758 (0.551 to 0.964) |
| Fish (stocked once) | 2.667 (2.217 to 3.117) | 0.964 (0.757 to 1.170) | |
| Fish (multiple stocking) | 3.067 (2.604 to 3.505) | 0.903 (0.697 to 1.109) | |
| Canal | Control | 3.417 (2.896 to 3.937) | 1.177 (0.974 to 1.380) |
| Fish (stocked once) | 1.906 (1.386 to 2.427) | 0.547 (0.344 to 0.750) | |
1The study authors reported the estimated marginal mean ± 95% confidence interval (CI).
In Sudan, Mahmoud and colleagues introduced G. affinis to Gezira irrigation canals (4 km to 10 km in length, 2 m in width, 1 m in depth). They used 20 canals in the experimental group and five canals in the control group. In experimental canals, they released fish at 1 km intervals. They measured the density of a late larval stage of An. arabiensis (L4) larvae in both experimental and control canals by performing larval dips at two spots per kilometre in each canal, reporting averages by month from weekly dipping of 10 dips per spot for 14 months.
No baseline was provided, but An. arabiensis density was less in intervention canals for two months (five months' and six months' post-intervention) just before and at the beginning of the dry season (Table 14A). Larval densities dropped in both intervention and control groups in the dry season (seven months' post-intervention) and at the end of the rainy season (13 months' post-intervention). Fish numbers failed to increase after the rainy season and during the last six months of the study. According to the authors, control of the flow of water from large to branch canals by gates deprived the fish of free movement. Also, during the rainy season, rainwater pools act as suitable breeding sites for An. arabiensis.
Introducing larvivorous fish appears to partly constrain An. arabiensis larval density increases at the beginning of the dry season.
Table 14A.
Mahmoud 1985:density of An. arabiensis L4 larvae after introduction of fish
| Intervention | Follow-up (months) | |||
|---|---|---|---|---|
| 3 | 5 | 7 | 13 | |
| Control canals | 42 | 153 | 7 | 125 |
| Experimental canals | 25 | 24 | 1 | 124 |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fletcher 1992
| Methods | Study design: quasi-RCT Study location: Assab Sekir and Negado Sefer, Assab, Ethiopia Study dates: February 1987 to January 1988 Transmission intensity: endemic Malaria parasite species: not specified Primary vectors: An. culicifacies adanensis Breeding sites: domestic water containers Baseline data: February 1987 | |
| Participants | Not applicable | |
| Interventions | Fish species: Aphanius dispar Indigenous fish species used: yes Fish source: Gibdo River, 26 km from Assab Populated sites: domestic water containers and wells; 68 stocked (32 barrels, 11 cisterns, 24 wells, one washbasin), 60 unstocked (33 barrels, 10 cisterns, 16 wells, one washbasin) Restocked: yes, as necessary during monthly/biweekly surveys Co-interventions: none | |
| Outcomes | Percentage of breeding sites positive for anopheline larvae Method: standard dipping procedure; five dips/barrel, 12 dips/cistern, eight dips/washbasin, three buckets/well during monthly/ biweekly surveys | |
| Source of funding | UNDP/World Bank/WHO Special Programme for Research and Training in Tropical Diseases; National Organisation for the Control of Malaria and Other Vectorborne Diseases, Ministry of Health, Ethiopia | |
| Notes | No environmental data collected Acceptibility of fish to householders assessed by questionnaire | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Quasi-RCT: "In every other house or mosque, fish were stocked in all wells and water storage containers" |
| Site selection | Unclear risk | "A total of 54 households were selected by systematic sampling. All six mosques were also included in the study. Seven households were excluded because they had only jerrycans and buckets for water storage. They were replaced by seven other households selected by lottery system" |
| Site allocation | High risk | "In every other house or mosque, fish were stocked in all wells and water storage containers" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "During monthly or biweekly larval surveys the fish were counted and restocking was carried out as necessary to maintain the original number of fish" |
| Baseline values | Low risk | In both control and experimental groups at pre-stocking (February 1987), the proportion of sites with Anopheles larvae was 0% |
| Number of sites | Low risk | Number of sites adequate as more than 20 sites per group |
Howard 2007
| Methods | Study design: controlled interrupted time series Study location: Kisii Central District, Western Kenya Study dates: October 2003 to October 2004 Transmission intensity: endemic but highly seasonal Malaria parasite species: not specified Primary vectors: An. gambiae s. l.,An. funestus Giles Breeding sites: abandoned fishponds Baseline data: October 2003 to January 2004 | |
| Participants | Not applicable | |
| Interventions | Fish species: Oreochromis niloticus L. Indigenous fish species used: yes Fish source: local FD hatchery in Kisii town Populated sites: three abandoned fishponds, Pond A (104 m2), Pond C (128 m2), and Pond D (72 m2); 150 m distance from each other Restocked: no Co-interventions: none | |
| Outcomes | Number of immature Anopheles per pond Density of immature Anopheles per pond Method: five larval dips (2.5 L total volume) randomly from edges of each pond, at least one dip/side, five to seven days/week | |
| Source of funding | Government of Finland and BioVision | |
| Notes | Climatic data for study period obtained from Kenya Agricultural Research Institute Study started with Pond B included, but as it was destroyed during the study period, the authors were unable to collect data for it for the requisite time period | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study |
| Site selection | Low risk | "The site has three abandoned fishponds within 150 m of each other". Author communication: "We started with a Pond B but it got destroyed during the study period so we were unable to collect data for it for the requisite time" |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to treatment |
| Baseline values | Low risk | Numbers of An. gambiae s. l. and An. funestus immatures comparable in Ponds A, C, and D |
| Number of sites | High risk | Probably inadequate as < five sites per group; control = one site, experimental = two sites |
Imbahale 2011a
| Methods | Study design: controlled time series Study location: Nyalenda, Kisumu County, Kenya Study dates: February 2008 to May 2008 Transmission intensity: not stated Malaria parasite species: not specified Primary vectors: An. gambiae Giles Breeding sites: man-made habitats (ponds or water canals) Baseline data: not recorded | |
| Participants | Not applicable | |
| Interventions | Fish species: G. affinis Indigenous fish species used: no Fish source: colony at KEMRI (Kenya Medical Research Institute) established from a wild-caught population provided by Kenya Marine and Fisheries Research Institute (KEMFRI) Populated sites: man-made habitats; 30 pools (average 1 m × 1 m × 1 m deep) or water canals (15 m × 1 m × 0.3 m deep). Pond sites and water canal sites were constructed by people for the purposes of this experiment, so can be defined as "semi-field" studies Restocked: no (treatment arm: ponds fish once), fortnightly (treatment arms: pond fish only or water canal fish only) Co-interventions: Bacillus thuringiensis var. israelensis | |
| Outcomes | Density of early instars (L1 and L2) or late instars (L3 and L4) of anopheline mosquitoes Method: standard larval dipping procedure using 350 mL mosquito dipper (Bioquip, Gardena, CA, USA), maximum of 10 dips/habitat, estimated weekly | |
| Source of funding | The Dioraphte Foundation, The Netherlands | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled time series study |
| Site selection | Low risk | "Thirty man-made habitats (1 m × 1 m × 1 m) were created as mosquito larval habitats" |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen for ponds. In water canals: "Six treatments were randomly administered in canal habitats" |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to treatment |
| Baseline values | Unclear risk | Not reported |
| Number of sites | High risk | Number of sites may be inadequate: five sites per group |
Kim 2002
| Methods | Study design: controlled interrupted time series Study location: Banwol, Suwon City, Gyeonggi Province, Korea Study dates: June to October 1989 Transmission intensity: not specified Malaria parasite species: not specified Primary vectors: An. sinensis Breeding sites: rice fields Baseline data: none | |
| Participants | Not applicable | |
| Interventions | Fish species: T. m. niloticus (herbivorous) with either A. latipes or Aphyocypris chinensis Indigenous fish species used: yes, except for T. m. niloticus Fish source: A. latipes: not stated; A. chinensis: holding ponds at Ansan rice fields, 2.5 km north; T. m. niloticus: fish farm at Gwagiu, Gyeonggi Populated sites: six rice fields (three control sites, three experimental sites 500 m2, 300 m2, or 600 m2 in size) Restocked: no Co-interventions: none | |
| Outcomes | Average number and percentage of reduction An. sinensis Method: larval dips using 500 mL dipper, two to four replicates per rice field | |
| Source of funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study |
| Site selection | Unclear risk | "A confined field plot of ca. 20,000 m2 rice field located in Banwol near Suwon City, Gyeonggi Province....three of the six paddies were taken" |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen for ponds |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to treatment |
| Baseline values | Low risk | Average number of An. sinensis larvae comparable at experimental and control sites |
| Number of sites | High risk | Probably inadequate number of sites |
Kusumawathie 2008a
| Methods | Study design: controlled before-and-after study Study location: Kotmale oya, below Kotmale dam, Sri Lanka Study dates: May to August 2000 Transmission intensity: epidemic Malaria parasite species: not specified Primary vectors: An. culicifacies adanensis (national importance), An. annularis, An. subpictus, An. tessellatus (local importance) Breeding sites: pools formed in riverbed between dam and power plant Baseline data: one day before stocking | |
| Participants | Not applicable | |
| Interventions | Fish species: P. reticulata Indigenous fish species used: no Fish source: riverbed pools below the Kotmale dam and then reared in stock tanks at Regional Office Anti-Malaria Campaign, Kandy Populated sites: 60 riverbed pools, 0.25 to 1.0 m2 surface area and < 1 m depth (29 experimental, 31 control, randomly selected) Restocked: no Co-interventions: none | |
| Outcomes | Number (percentage) of pools positive for anopheline larvae Mean number of larvae per pool Mean number of larvae per 100 dips Method: larval dipping using 100 mL dipper, six dips per m2. Authors collected anopheline immatures but reported larval numbers only | |
| Source of funding | National Research Council, Sri Lanka (NRC Grant No. 99/09) | |
| Notes | Fish number monitored An. culicifacies not identified at any sites | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled before-and-after study |
| Site selection | Unclear risk | "Sixty isolated riverbed pools...were selected and labeled" |
| Site allocation | Unclear risk | "P. reticulata was stocked in 29 randomly selected pools". Method of randomization not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Visual counts of P. reticulata were made in each pool, monthly. Visual counts were possible, as the pools were small (not exceeding 1 m2 surface area), shallow (< 1 m depth) and contained clean water" |
| Baseline values | Low risk | Comparable between control and experimental sites |
| Number of sites | Low risk | Adequate numbers of sites in control (31 site) and experimental groups (29 sites) |
Kusumawathie 2008b
| Methods | Study design: controlled before-and-after study Study location: riverbeds below Laxapana, Kotmale 1, Kotmale 2, Nilambe, Rantembe and Victoria dams, Sri Lanka Study dates: September 2000 to August 2002 Transmission intensity: epidemic Malaria parasite species: not specified Primary vectors: An. culicifacies adanensis (national importance), An. annularis, An. subpictus, An. tessellatus (local importance) Breeding sites: pools formed in riverbed between dam and power plant Baseline data: September 2000 to August 2001 | |
| Participants | Not applicable | |
| Interventions | Fish species: P. reticulata Indigenous fish species used: no Fish source: not stated Populated sites: pools of six riverbeds below dams (two controls, two fish intervention) Restocked: yes, pools that had no fish were restocked at the same rate during fortnightly larval surveys Co-intervention: temephos treatment of all pools in two riverbeds | |
| Outcomes | Mean percentage of pools positive for anopheline larvae Mean number of anopheline larvae per 100 pools Mean number of anopheline larvae per 100 dips Total number of anopheline larvae Methods: larval dips, six dips per m2 surface area of water | |
| Source of funding | National Research Council of Sri Lanka (Grant No. 99/09) | |
| Notes | Cost analysis estimation and simulations performed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled before-and-after study |
| Site selection | Low risk | "Six study sites, namely Laxapana, Kotmale 1, Kotmale 2, Nilambe, Rantembe and Victoria...were selected based on the occurrence of malaria outbreaks since 1985....all the pools in the riverbeds were stocked" |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen for ponds |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to treatment. "Subsequently the pools that had no fish were restocked at the same rate" |
| Baseline values | High risk | Baseline values higher in experimental group than in control group |
| Number of sites | High risk | Probably inadequate: number of pools not specified |
Mahmoud 1985
| Methods | Study design: controlled time series Study location: Gezira irrigated area, Sudan Study dates: January to December, but the two years were not specified Transmission intensity: not specified Malaria parasite species: not specified Primary vectors: An. arabiensis Breeding sites: small temporary pools Baseline data: none | |
| Participants | Not applicable | |
| Interventions | Fish species: G. holbrooki (Note: This study refers to G. affinis holbrooki, as these fish were then considered a subspecies of G. affinis. This subspecies is now recognized as a full species) Indigenous fish species used: no Fish source: rearing ponds at Wad Medani, 20 to 25 km from trial sites Populated sites: 20 irrigation canals, 1 m in depth, 2 m in width, and 4 to 10 km in length; five control canals Restocked: yes Co-intervention: none | |
| Outcomes | Average larval density of An. arabiensis/100 dips, according to instar stage Methods: larval dipping at two sites per km in each canal, 10 dips per site | |
| Source of funding | Malaria Control Project, Ministry of Health, Sudan | |
| Notes | Flow of water from large branch canals was controlled by gates opened at certain times; this system deprived the Gambusia of free movement into the smaller canals, which usually are richer in mosquito larvae than the larger ones, where the fish had originally been stocked | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled time series study |
| Site selection | Unclear risk | "Medium size irrigation canals of about 1 m depth, 2 m width, and 4-10 km length, officially classified as minor canals, were selected as sites for the trials. Twenty such canals were seeded with Gambusia...while five others were used as control" |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen for ponds |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to treatment |
| Baseline values | Unclear risk | Not reported. Fish release in October and measurements not taken until following January |
| Number of sites | High risk | May be inadequate, as only five sites in the control group |
Menon 1978
| Methods | Study design: controlled interrupted time series study Study location: Pondicherry Town, India Study dates: January to May 1977 Transmission intensity: not specified Malaria parasite species: not specified Primary vectors: An. stephensi Breeding sites: wells, water tanks Baseline data: January 1977 | |
| Participants | Not applicable | |
| Interventions | Fish species: G. affinis or A. blockii Indigenous fish species used: G. affinis: not indigenous, A. blockii: indigenous Fish source: G. affinis: mass cultured at Vector Control Research Centre (VCRC); A. blockii: collected from ponds and stored at VCRC Populated sites: 3402 to 3438 sites stocked; 317 sites unstocked Restocked: yes; if no fish were present at sites at one, two, or three months after beginning of the trial, they were restocked with G. affinis or A. blockii Co-intervention: none | |
| Outcomes | Percentage of sites positive for anopheline larvae Methods: bucket samples taken monthly | |
| Source of funding | Not specified | |
| Notes | Number of wells where fish survived monitored Chemical analysis performed of water from wells where fish died (20) or survived (20) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study |
| Site selection | Low risk | "Every house with a well was marked in the experimental and comparison area" |
| Site allocation | Unclear risk | Unclear how treatment for each site was chosen for ponds |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Wells were marked according to whether the fish was present or absent...it was possible to visually observe movement of Gambusia on the surface" |
| Baseline values | High risk | Not comparable between control and experimental sites |
| Number of sites | Low risk | Adequate numbers of sites in control and experimental groups |
Nalim 1988
| Methods | Study design: controlled time series study Study location: Central Java Study dates: 1979 to 1984 Transmission intensity: endemic Malaria parasite species: not specified Primary vectors: not stated Breeding sites: rice fields Baseline data: not recorded | |
| Participants | Not applicable | |
| Interventions | Fish species: C. carpio and P. reticulata Indigenous fish species used: C. carpio: indigenous, P. reticulata: not indigenous Fish source: mass breeding of C. carpio in nine ponds of 6 × 4 m2 tended by fishery official in cooperation with village officials. Mass breeding of P. reticulata in two ponds of 4 × 2 m2 tended by local fishery official Populated sites: number and size of control and experimental sites was not specified. Total size of area was 24.8 hectares of wetland (rice fields), cultivated by 112 farmers Restocked: fish were restocked every new rice planting season Co-intervention: control area sprayed with fenitrothion at end of 1982 | |
| Outcomes | Average number newly emerged adult mosquitoes/m2/day collected in traps (trap area 0.25 m2) averaged per year | |
| Source of funding | TDR Grant UNDP/World Bank/WHO | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled time series study |
| Site selection | Unclear risk | Number of fields not specified. "96.4% of the total 24.8 ha were included" |
| Site allocation | Unclear risk | Numbers of control and experimental sites not specified. Size of control area not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to treatment |
| Baseline values | Unclear risk | Not reported |
| Number of sites | High risk | Probably inadequate, as number of sites not specified |
Sabatinelli 1991
| Methods | Study design: controlled interrupted time series study Study location: Grande Comore Island, Federal Islamic Republic of Comoros Study dates: November 1987 to November 1988 Transmission intensity: endemic Malaria parasite species: not specified Primary vectors: An. gambiae Breeding sites: domestic water containers Baseline data: November 1987 | |
| Participants | Not applicable | |
| Interventions | Fish species: P. reticulata Indigenous fish species used: not indigenous Fish source: imported from Mayotte Island Populated sites: domestic water containers; 20 unstocked (ablution basins) for duration of trial; 59 ablution basins and 61 tanks stocked in November 1987. Stocking of basins and tanks extended, and by April 1988, all basins and tanks were treated. Total numbers of basins and tanks stocked not specified Restocked: not clearly indicated Co-interventions: temephos (concentration: 2 cc/m3) in tanks only, last treatment March 1988 | |
| Outcomes | Percentage of containers positive for anopheline larvae Method: Surface and bottom of containers were examined for An. gambiae larvae (at least 15 cm in diameter), which were recorded monthly | |
| Source of funding | Research was undertaken with the framework of project OMS-PNUD COM/MAL/001 | |
| Notes | No environmental data collected | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study |
| Site selection | Unclear risk | Unclear how sites were selected |
| Site allocation | Unclear risk | Unclear how experimental treatment was selected. Control sites were in village of Bandamadji 3 km from experimental site |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to treatment |
| Baseline values | Low risk | Percentage of sites positive for An. gambiae larvae comparable in control and experimental groups |
| Number of sites | Low risk | Adequate numbers of sites in control and experimental groups |
Sitaraman 1976
| Methods | Study design: controlled interrupted time series study Study location: Great Hyderabad City, India Study dates: not stated Transmission intensity: endemic Malaria parasite species: not specified Primary vectors: An. stephensi Breeding sites: domestic water containers Baseline data: day 0, before release of fish | |
| Participants | Not applicable | |
| Interventions | Fish species: P. reticulata Indigenous fish species used: not indigenous Fish source: not stated Populated sites: five control and 12 experimental (50 guppies/well); four control and 10 experimental (100 guppies/well) Restocked: no Co-interventions: temephos (concentration: 2 cc/m3) | |
| Outcomes | Density of immature An. stephensi stages (larvae instars I and II, III and IV, pupae) Method: five dips per well using a 30 cm diameter net | |
| Source of funding | Not stated | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study |
| Site selection | Unclear risk | Unclear how these particular sites were selected |
| Site allocation | Unclear risk | Unclear how treatment was allocated |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to treatment |
| Baseline values | High risk | Average values not comparable between control and experimental groups |
| Number of sites | High risk | Numbers of sites may be inadequate as four control sites were used |
Yu 1989
| Methods | Study design: controlled interrupted time series study Study location: Korea Study dates: June to September 1988 Transmission intensity: not specified Malaria parasite species: not specified Primary vectors: An. sinensis Breeding sites: rice fields Baseline data: June to August 1988 | |
| Participants | Not applicable | |
| Interventions | Fish species: A. latipes andT. m. niloticus Indigenous fish species used: A. latipes: indigenous; T. m. niloticus: not indigenous Fish source: A. latipes originated from holding ponds at Ansan rice fields (2.5 km away), T. m. niloticus sourced from fishfarm in Jin-Dong of Masan City, South Kyungsang Province Populated sites: rice fields (two control sites, two experimental sites with A. latipes and T. m. niloticus, two experimental sites with A. latipes only, followed by Bacillus thuringiensis treatment after three weeks) Restocked: no Co-interventions: see above | |
| Outcomes | Density of An. sinensis larvae determined weekly Method: larval dipping performed using a 500 mL dipper, two to four replicates per rice field usually consisting of two dips pooled | |
| Source of funding | WHO Medical Research Fund of the Western Pacific Region, Manila | |
| Notes | Environmental data (temperature and rainfall) recorded | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Study design | High risk | Controlled interrupted time series study |
| Site selection | Low risk | "A confined field plot of ca 1,000 m2...the rice paddy was composed of 6 similar sized (10 × 15 × 0.3 m) plots" |
| Site allocation | Unclear risk | "2 random selection of paddies was made for each group". Method of random selection not specified |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether assessors were blinded to treatment |
| Baseline values | Low risk | Comparable between control and experimental sites |
| Number of sites | High risk | Probably inadequate number of sites |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alio 1985a | Transmission baseline data collected for less than one year pre-intervention. For larval population data, Anopheles and Culex populations not monitored separately. |
| Alio 1985b | Not a fish trial. Review article. |
| Asimeng 1993 | Not a fish trial. |
| Austen 1919 | Not a fish trial. Review article. |
| Bang 1988 | Not a fish trial. Review article. |
| Bay 1967 | Not a fish trial. Review article. |
| Bedford 1936 | Medical report, not a fish trial. |
| Beltran 1973 | Not a fish trial. Review article. |
| Bolay 1989 | No primary or secondary outcomes. |
| Borel 1926 | No primary or secondary outcomes. |
| Caillouet 2008 | Not a fish trial. |
| Carlson 2004 | Not a fish trial. |
| Carnevale 1990 | Not a fish trial. Review article. |
| Chandra 2008 | Not a fish trial. Review article. |
| Chapman 1974 | Not a fish trial. Review article. |
| Das 1991 | Anopheles and Culex populations not monitored separately. No primary outcomes. |
| De Burca 1939 | Not a fish trial. Descriptive article. |
| Dev 2008 | Not a fish trial. Descriptive article. |
| Devi 2010 | No primary or secondary outcomes. |
| Dua 1991 | Multiple interventions, cannot determine effect of fish alone. |
| Dua 1997 | Multiple interventions, cannot determine effect of fish alone. |
| Fletcher 1993 | Laboratory-based study only. |
| Gammans 1926 | Not a fish trial. |
| Ghosh 2005 | Inappropriate study design. |
| Ghosh 2007 | Not a fish trial. Review article. |
| Ghrab 1999 | Laboratory-based study only. |
| Gupta 1989 | Not a fish trial. |
| Gupta 1992 | Anopheles and Culex populations not monitored separately. No primary outcomes. |
| Haas 1984 | Not a fish trial. |
| Hackett 1938 | Not a fish trial. Review article. |
| Hadjinicolaou 1973 | Inappropriate study design. |
| Holland 1933 | No primary or secondary outcomes. |
| Homski 1994 | Laboratory-based study only. |
| Howard 1920 | Inappropriate study design. |
| Hurlbert 1972 | No primary or secondary outcomes. |
| Imbahale 2011b | Not a fish trial. Review article. |
| Inci 1992 | Inappropriate study design. |
| Jayawardana 2001 | Inappropriate study design. |
| Julvez 1987 | Inappropriate study design. |
| Kaneko 2000 | Inappropriate study design. |
| Kligler 1930 | Not a fish trial. |
| Kumar 1998 | Inappropriate study design. |
| Kusumawathie 2006 | Laboratory-based study only. |
| Lacey 1990 | Not a fish trial. Review article. |
| Legendre 1921 | Inappropriate study design. |
| Louis 1988 | Inappropriate study design. |
| Luh 1981 | Inappropriate study design. |
| Malhotra 1992 | Inappropriate study design. |
| Mandoul 1954 | Inappropriate study design. |
| Menon 1977 | Inappropriate study design. |
| Merle 1955 | Inappropriate study design. |
| Missiroli 1930 | Inappropriate study design. |
| Mohamed 2003 | Inappropriate study design. |
| Molloy 1924 | Inappropriate study design. |
| Morin 1934 | Inappropriate study design. |
| Nalim 1987 | No primary outcomes. Secondary outcomes in Nalim 1988. |
| Ossi 1984 | Inappropriate study design. |
| Panicker 1985 | Inappropriate study design. |
| Patra 2010 | Anopheles and Culex populations not monitored separately. No primary outcomes. |
| Pecori 1930 | Inappropriate study design. |
| Prasad 1993 | Inappropriate study design. Anopheles and Culex populations not monitored separately. |
| Pyke 2008 | Not a fish trial. Review article. |
| Raina 1945 | Inappropriate study design. |
| Rajnikant 1993 | Inappropriate study design. Anopheles and Culex populations not monitored separately. |
| Rao 1942 | Inappropriate study design. |
| Rimbaut 1935 | Inappropriate study design. |
| Robert 1998 | Inappropriate study design. |
| Rojas 2004 | Inappropriate study design. |
| Roule 1934 | Inappropriate study design. |
| Roy 1938 | Inappropriate study design. |
| Rupp 1996 | Inappropriate study design. |
| Russell 1942 | Inappropriate study design. |
| Sabatinelli 1988 | No primary outcomes. Secondary outcomes in Sabatinelli 1991. |
| Sella 1927 | Inappropriate study design. |
| Sella 1929 | Inappropriate study design. |
| Sergiev 1937 | Inappropriate study design. |
| Sharma 1986a | Inappropriate study design. |
| Sharma 1986b | Multiple interventions, cannot determine effect of fish alone. |
| Sharma 1989a | Inappropriate study design. |
| Sharma 1989b | Multiple interventions, cannot determine effect of fish alone. |
| Sharma 1991 | Multiple interventions, cannot determine effect of fish alone. |
| Sharma 1997 | No primary outcomes. Secondary outcome follow-up only three weeks in duration. |
| Singh 1989 | Multiple interventions, cannot determine effect of fish alone. |
| Singh 2006 | Multiple interventions, cannot determine effect of fish alone. |
| Sitaraman 1975 | Inappropriate study design. No control area. |
| Tabibzadeh 1970 | Not a fish trial. |
| Teklehaimanot 1993 | Not a fish trial. |
| Tisohlbr 1950 | Inappropriate study design. |
| Trausmiller 1932 | Inappropriate study design. |
| Ungureanu 1981 | Not a fish trial. A manual on how to evaluate fish. |
| Usenbaev 2006 | Inappropriate study design. |
| Van Dam 2007 | Inappropriate study design. Not in malaria-endemic area. |
| Velichkevich 1935 | Inappropriate study design. |
| Victor 1994 | Not a fish trial. |
| Vitlin 1987a | Inappropriate study design. |
| Vitlin 1987b | Inappropriate study design. |
| Walton 2007 | Not a fish trial. Review article. |
| Wickramasinghe 1986 | Not a fish trial. Review article. |
| Wu 1991 | Anopheles and Culex populations not monitored separately. Inappropriate study design. |
| Yadav 1992 | Inappropriate study design. Multiple interventions, cannot determine effect of fish alone. |
| Yu 1982a | Inappropriate study design. |
| Yu 1982b | Secondary outcomes in Yu 1982a. |
| Yu 1982c | Secondary outcomes in Yu 1982a. |
| Yu 1986 | Inappropriate study design. Culex monitored only. |
| Zaman 1980 | Inappropriate study design. Laboratory-based experiment only. |
Contributions of authors
TB and PG conceived the review and wrote the protocol, with input from Robert A Wirtz, Raymond Beach, Graham H Pyke, and Ahmed A Abdel-Hameed Adeel. All authors performed study screening. TB and DPW extracted all the data. DPW constructed the tables, prepared the GRADE summaries, and wrote the review. PG helped with determining study inclusion, planning how to construct the review, and summarizing the data. All authors reviewed and approved the manuscript before submission.
Declarations of interest
TB is Orchestrator of the Vector Ecology and Control Network, which receives funding to develop an analytical framework to analyse with mathematical models the effectiveness of established and novel vector control strategies on malaria transmission. TB was on the Global Fund Technical Review Panel as a non-paid adviser. PG is Director of the Evidence Building and Synthesis Research Consortium, which receives money to increase the number of evidence-informed decisions by intermediary organizations, including WHO and national decision makers, that benefit the poor in middle- and low-income countries. DPW is employed as part of this Consortium. PG is the co-ordinator of a WHO Collaborating Centre for Evidence Synthesis for Infectious and Tropical Diseases (http://apps.who.int/whocc/default.aspx; UNK234): one of the Centre's aims is to help WHO in its role as an infomediary in communicating reliable summaries of research evidence to policy makers, clinicians, teachers, and the public in developing countries. AA is a member of the WHO Expert Advisory Panel on Malaria from 2004 to date and is a member of the Technical Review Panel of the Global Fund against AIDS, TB, and Malaria from 2008 to date as a non-paid adviser.
Sources of support
Internal sources
No sources of support supplied
External sources
Department for International Development, UK.
Differences between protocol and review
DPW was added as author on the review. Robert A Wirtz and Raymond Beach stepped down as authors on the review. We added EIR as an outcome, as an effect demonstrated on this would be an extremely useful indicator of an effect on malaria transmission. We limited inclusion of studies monitoring secondary outcomes to studies with a follow-up period longer than three weeks after introduction of larvivorous fish.
Data and analyses
This review has no analyses.
References
References to studies included in this review
- Fletcher M, Teklehaimanot A, Yemane G. Control of mosquito larvae in the port city of Assab by an indigenous larvivorous fish, Aphanius dispar. Acta Tropica. 1992;52:155–66. doi: 10.1016/0001-706x(92)90032-s. [DOI] [PubMed] [Google Scholar]
- Howard AF, Zhou G, Omlin FX. Malaria mosquito control using edible fish in western Kenya: preliminary findings of a controlled study. BMC Public Health. 2007;7((199)):1–6. doi: 10.1186/1471-2458-7-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbahale SS, Mweresa CK, Takken W, Mukabana WR. Development of environmental tools for anopheline larval control. Parasites & Vectors. 2011;4:130. doi: 10.1186/1756-3305-4-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HC, Lee JH, Yang KH, Yu HS. Biological control of Anopheles sinensis with native fish predators (Aplocheilus and Aphyocypris) and herbivorous fish Tilapia in natural rice fields in Korea. Korean Journal of Entomology. 2002;32((4)):247–50. [Google Scholar]
- Kusumawathie PHD, Wickremasinghe AR, Karunaweera ND, Wijeyaratne MJS. Larvivorous potential of the guppy, Poecilia reticulata, in anopheline mosquito control in riverbed pools below the Kotmale dam, Sri Lanka. Asia-Pacific Journal of Public Health. 2008;20((1)):56–63. doi: 10.1177/1010539507308507. [DOI] [PubMed] [Google Scholar]
- Kusumawathie PHD, Wickremasinghe AR, Karunaweera ND, Wijeyaratne MJS. Costs and effectiveness of application of Poecilia reticulata (guppy) and temephos in anopheline mosquito control in river basins below the major dams of Sri Lanka. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2008;102:705–11. doi: 10.1016/j.trstmh.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Mahmoud AA. Mosquitofish Gambusia affinis holbrooki as a malaria vector control agents in Gezira irrigation canals of the Sudan. Journal of the American Mosquito Control Association. 1985;1((4)):524–6. [PubMed] [Google Scholar]
- Menon PKB, Rajagopalan PK. Control of mosquito breeding in wells by using Gambusia affinis and Aplocheilus blochii in Pondicherry town. Indian Journal of Medical Research. 1978;68:927–33. [PubMed] [Google Scholar]
- Nalim S, Boewono DT, Haliman A, Winoto E. Control demonstration of the ricefield breeding mosquito Anopheles aconitus Donitz in Central Java, using Poecilia reticulata through community participation: 3. Field trial and evaluation. Buletin Penelitian Kesehatan. 1988;16((1)):6–11. [Google Scholar]
- Sabatinelli G, Blanchy S, Majori G, Papakay M. Impact of the use of larvivorous fish Poecilia reticulata on the transmission of malaria in FIR of Comoros. Annales de Parasitologie et Humaine et Comparee. 1991;66((2)):84–8. doi: 10.1051/parasite/199166284. [Impact de l'utilisation du poisson larvivore Poecilia reticulata sur la transmission du paludisme en RFI des Comores] [DOI] [PubMed] [Google Scholar]
- Sitaraman NL, Mahadevan S, Swamidas S. Biological control of A. stephensi larvae in wells by Poecilia reticulatus in Greater Hyderabad City, India. Journal of Communicable Diseases. 1976;8((4)):315–9. [Google Scholar]
- Yu IS, Lee JH. Biological control of malaria vector (Anopheles sinensis Wied.) by combined use of larvivorous fish (Aplocheilus latipes) and herbivorous hybrid (Tilapia mossambicus niloticus) in rice paddies of Korea. Korean Journal of Applied Entomology. 1989;28((4)):229–36. [Google Scholar]
References to studies excluded from this review
- Alio AY, Isaq A, Delfini LF. Field trial on the impact of Oreochromis spilurus spilurus on malaria transmission in Northern Somalia, 1985. http://whqlibdoc.who.int/malaria/WHO_MAL_85.1017.pdf (accessed 17 June 2009). Geneva: World Health Organization.
- Alio AY, Isaq A, Delfini LF. Using fish against mosquito-borne diseases. Malaria World Heatlh Forum. 1985;6:320–1. [Google Scholar]
- Asimeng EJ, Mutinga MJ. Effect of rice husbandry on mosquito breeding at Mwea Rice Irrigation Scheme with reference to biocontrol strategies. Journal of the American Mosquito Control Association. 1993;9((1)):17–22. [PubMed] [Google Scholar]
- Austen EE. Anti-mosquito measures in Palestine during the campaigns of 1917–1918. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1919;13((4)):47–62. [Google Scholar]
- Bang YH. Vector-borne diseases associated with rice cultivation and their control in Southeast Asia. In: Bos R, Enriquez VG, Cervantes EP, Smith WH, editors. Vector-Borne Disease Control Through Rice Agrosystem Management: Proceedings of the Workshop on Research and Training Needs in the Field of Integrated Vector Borne Disease Control in Riceland Agroecosystems of Developing Countries. International Rice Research Institute, Los Baños, 9-14 March 1987. Philippines: International Rice Research Institute; 1988. pp. 93–100. [Google Scholar]
- Bay EC. Mosquito control by fish: a present-day appraisal. WHO Chronicle. 1967;21((10)):415–23. [PubMed] [Google Scholar]
- Bedford HW. Medical Entomology. 1938. pp. 77–83. Reports of the Sudan Medical Service.
- Beltran O. The Use of Larvivorous Fish in Mosquito Control. London, UK: University of London; 1973. [Google Scholar]
- Bolay FK, Trpis M, Davidson EW, Faust RM, Margalit J, Tahori AS. Control of mosquitoes with Bacillus thuringiensis var. israelensis and larvivorous fish, Tilapia nilotica, in rice fields in Liberia, West Africa. Israel Journal of Entomology. 1989;23:77–82. [Google Scholar]
- Borel M. Anopheles and malaria in the Chaudoc region (Cochin China). Results of an investigation made from 16 to 21 January 1926. Bulletin de la Societe de Pathologie Exotique et de ses Filiales. 1926;19((9)):806–11. [Anopheles et paludisme dans la region de Chaudoc (Cochinochine). Resultats d'une enquete faite du 16 au 21 janvier 1926] [Google Scholar]
- Caillouet KA, Keating J, Eisele TP. Characterization of aquatic mosquito habitat, natural enemies, and immature mosquitoes in the Artibonite Valley, Haiti. Journal of Vector Ecology. 2008;33((1)):191–7. doi: 10.3376/1081-1710(2008)33[191:coamhn]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Carlson JC, Byrd BD, Omlin FX. Field assessments in western Kenya link malaria vectors to environmentally disturbed habitats during the dry season. BMC Public Health. 2004;4:33. doi: 10.1186/1471-2458-4-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnevale P, Mouchet J. Vector control and malaria control. Medecine Tropicale. 1990;50((4)):391–8. [PubMed] [Google Scholar]
- Chandra G, Bhattacharjee I, Chatterjee SN, Ghosh A. Mosquito control by larvivorous fish. Indian Journal of Medical Research. 2008;127((1)):13–27. [PubMed] [Google Scholar]
- Chapman HC. Biological control of mosquito larvae. Annual Review of Entomology. 1974;19:33–59. doi: 10.1146/annurev.en.19.010174.000341. [DOI] [PubMed] [Google Scholar]
- Das MK, Prasad RN. Evaluation of mosquito fish Gambusia affinis in the control of mosquito breeding in rice fields. Indian Journal of Malariology. 1991;28((3)):171–7. [PubMed] [Google Scholar]
- De Burca B. Note on anti-malaria measures in Quetta Cantonment during 1938. Journal of the Malaria Institute of India. 1939;2:121–30. [Google Scholar]
- Dev V, Dash AP, Hojai D. Fishing out malaria in Assam, Northeastern India: hope or hype? Transactions of the Royal Society of Tropica Medicine and Hygiene. 2008;102((8)):839–40. doi: 10.1016/j.trstmh.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Devi NP, Jauhari RK, Hasan SF. Water quality and larvivorous activity of a killifish, Aplocheilus panchax (Ham.) against Anopheles annularis larvae in fragments of Loktak lake (Manipur) Journal of Experimental Zoology India. 2010;13((2)):509–12. [Google Scholar]
- Dua VK, Sharma SK, Sharma VP. Bioenvironmental control of malaria at the Indian Drugs and Pharmaceuticals Ltd., Rishikesh (U.P) Indian Journal of Malariology. 1991;28((4)):227–35. [PubMed] [Google Scholar]
- Dua VK, Sharma SK, Srivastava A, Sharma VP. Bioenvironmental control of industrial malaria at Bharat Heavy Electricals Ltd., Hardwar, India—results of a nine-year study (1987-95) Journal of the American Mosquito Control Association. 1997;13((3)):278–85. [PubMed] [Google Scholar]
- Fletcher M, Teklehaimanot A, Yemane G, Kassahun A, Kidane G, Beyene Y. Prospects for the use of larvivorous fish for malaria control in Ethiopia: search for indigenous species and evaluation of their feeding capacity for mosquito larvae. Journal of Tropical Medicine and Hygiene. 1993;96((1)):12–21. [PubMed] [Google Scholar]
- Gammans LD. Anti-malaria work at Port Dickson. Malayan Medical Journal. 1926;1((2)):24–8. [Google Scholar]
- Ghosh SK, Tiwari SN, Sathyanarayan TS, Sampath TR, Sharma VP, Nanda N, et al. Larvivorous fish in wells target the malaria vector sibling species of the Anopheles culicifacies complex in villages in Karnataka, India. Transaction of the Royal Society of Tropical Medicine and Hygiene. 2005;99((2)):101–5. doi: 10.1016/j.trstmh.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Ghosh SK, Dash AP. Larvivorous fish against malaria vectors: a new outlook. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2007;101((11)):1063–4. doi: 10.1016/j.trstmh.2007.07.008. [DOI] [PubMed] [Google Scholar]
- Ghrab J, Bouattour A. Experimental study of larval efficiency of Gambusia affinis holbrooki (GIRARD, 1859) (fish-Poecilidae) Archives de l'Institut Pasteur de Tunis. 1999;76((1-4)):33–8. [Etude experimental de l'efficacile larvivore de Gambusia affinis holbrooki] [PubMed] [Google Scholar]
- Gupta DK, Sharma RC, Sharma VP. Bioenvironmental control of malaria linked with edible fish production in Gujarat. Indian Journal of Malariology. 1989;26((1)):55–9. [PubMed] [Google Scholar]
- Gupta DK, Bhatt RM, Sharma RC, Gautam AS, Rajnikant Intradomestic mosquito breeding sources and their management. Indian Journal of Malariology. 1992;29((1)):41–6. [PubMed] [Google Scholar]
- Haas R, Pal R. Mosquito larvivorous fishes. Bulletin of the Entomological Society of American. 1984;30((1)):17–25. [Google Scholar]
- Hackett LW, Russell PF, Scharff JW, Senior White R. The present use of naturalistic measures in the control of malaria. Bulletin of the Health Organization of the League of Nations. 1938;7:1046–64. [Google Scholar]
- Hadjinicolaou J, Betzios B. Gambusia Fish as a Means of Biological Control of Anopheles sacharovi in Greece. Geneva: World Health Organization; 1973. pp. 1–7. [Google Scholar]
- Holland EA. An experiment in the control of malaria in New Ireland by distribution of Gambusia affinis. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1933;26((6)):529–38. [Google Scholar]
- Homski D, Goren M, Gasith A. Comparative evaluation of the larvivorous fish Gambusia affinis and Aphanius dispar as mosquito control agents. Hydrobiologia. 1994;284:137–46. [Google Scholar]
- Howard HH. Malaria control in rural communities by anti-mosquito measures. Southern Medical Journal. 1920;13((4)):260–6. [Google Scholar]
- Hurlbert SH, Zedler J, Fairbanks D. Ecosystem alternation by mosquitofish (Gambusia affinis) predation. Science. 1973;175((4022)):639–41. doi: 10.1126/science.175.4022.639. [DOI] [PubMed] [Google Scholar]
- Imbahale S. Integrated malaria vector control in different agro-ecosystems in western Kenya. Entomologische Berichten. 2011;71((4)):94–103. [Google Scholar]
- Inci R, Yildirim M, Bagei N, Inci S. Biological control of mosquito larvae by mosquitofish (Gambusia affinis) in the Batman-Siirt Area. Turkiye Parazitoloji Dergisi. 1992;16((2)):60–6. [Google Scholar]
- Jayawardana A case study on physical and biological factors in relation to mosquito emergence in abandoned gem pits in Elahera, Sri Lanka. Tropical Agricultural Research. 2001;13:401–10. [Google Scholar]
- Julvez J, Galtier J, Ali Halidi M, Henry M, Mouchet J. Epidemiology of malaria and the antimalarial campaign in Mayotte (Comoro archipelago, Indian Ocean). Development of the situation between 1976 and 1986. Outlook. Bulletin de la Societe de la Pathologie Exotique et de ses Filiales. 1987;80((3 Pt 2)):505–19. [PubMed] [Google Scholar]
- Kaneko A, Taleo G, Kalkoa M, Yamar S, Kobayakawa T, Bjorkman A. Malaria eradication on islands. Lancet. 2000;356((9241)):1560–4. doi: 10.1016/S0140-6736(00)03127-5. [DOI] [PubMed] [Google Scholar]
- Kligler IJ. The Epidemiology and Control of Malaria in Palestine. Chicago, Illinois: The University of Chicago Press; 1930. [Google Scholar]
- Kumar A, Sharma VP, Sumodan PK, Thavaselvam D. Field trials of biolarvicide Bacillus thuringiensis var. israelensis strain 164 and the larvivorous fish Aplocheilus blocki against Anopheles stephensi for malaria control in Goa, India. Journal of the American Mosquito Control Association. 1998;14((4)):457–62. [PubMed] [Google Scholar]
- Kusumawathie PH, Wickremasinghe AR, Karunaweera ND, Wijeyaratne MJ. Larvivorous potential of fish species found in river bed pools below the major dams in Sri Lanka. Journal of Medical Entomology. 2006;43((1)):79–82. doi: 10.1093/jmedent/43.1.79. [DOI] [PubMed] [Google Scholar]
- Lacey LA, Lacey CM. The medical importance of riceland mosquitoes and their control using alternatives to chemical insecticides. Journal of the American Mosquito Control Association Society. 1990;2:1–93. [PubMed] [Google Scholar]
- Legendre J. Plan of the antimalaria campaign for Madagascar. Bulletin de la Societe de Pathologie Exotique et de ses Filiales. 1921;14((2)):97–100. [Plan de campagne antipaludique pour Madagascar] [Google Scholar]
- Louis JP, Albert JP. Malaria in Djibouti. Biological control strategies, using the larvivorous indigenous fish—Aphanius dispar and bacterial toxins. Medecine Tropicale. 1988;48((2)):127–31. [PubMed] [Google Scholar]
- Luh PL. The present status of biocontrol of mosquitoes in China. In: Laird M, editor. Biocontrol of Medical and Veterinary Pests. New York: Praeger; 1981. pp. 54–77. [Google Scholar]
- Malhotra MS, Prakash A. Enhancing the efficacy of Gambusia affinis to control mosquito breeding in ponds. Indian Journal of Malariology. 1992;29((1)):65–8. [PubMed] [Google Scholar]
- Mandoul R, Rejenet J. Lessons from the sanitation of the malarial oasis of Ouargla, Algerian Sahara. Bulletin de la Societe de Pathologie Exotique et de ses Filiales. 1954;47((3)):443–52. [PubMed] [Google Scholar]
- Menon AGK. Fish and malaria control. Science and Culture. 3:110–4. 43. [Google Scholar]
- Merle F, Maillot L. Vector control campaigns against malaria in Brazzaville. Bulletin de la Societe de Pathologie Exotique et de ses Filiales. 1955;48((2)):242–69. [Campagnes de deinsectisation contre le paludisme a Brazzaville] [PubMed] [Google Scholar]
- Missiroli A. The prevention of malaria in practice. Third Report (1928-1929) Rivista di Malariologia. 1930;9((6)):667–705. [La prevenzione della malaria nel campo pratico. Terza relazione (1928-1929)] [Google Scholar]
- Mohamed AA. Study of larvivorous fish for malaria vector control in Somalia, 2002. East Mediterranean Health Journal. 2003;9((4)):618–26. [PubMed] [Google Scholar]
- Molloy DM. Some personal experiences with fish as anti-mosquito agencies in the tropics. International Health Board, Managua, Nicaragua. American Journal of Tropical Medicine. 1924;4((2)):175–94. [Google Scholar]
- Morin HGS, Martin P. General concepts on the use of fish for the fights against mosquitoes. Archives des Instituts Pasteur d'Indochine. 1936;23:443–61. [Notions generales sur l'utilisation des poissons a la lutte contre les moustiques] [Google Scholar]
- Nalim S, Tribuwono Control demonstration of the ricefield breeding mosquito Anopheles aconitus Donitz in Central Java, using Poecilia reticulata through community participation: 2. Culturing, distribution and use of fish in the field. Buletin Penelitian Kesehatan. 1987;15((4)):1–7. [Google Scholar]
- Ossi GT. Malaria in Iraq from 1980 to 1983. Bulletin of Endemic Diseases. 1984;2((4-5)):5–20. [Google Scholar]
- Panicker KN, Srinivasan R, Viswam K, Rajagopalan PK. Larvivorous potential of some cypriniformes fishes. Indian Journal of Medical Research. 1985;82:517–20. [PubMed] [Google Scholar]
- Patra AK. Laboratory trials on the feeding pattern of mosquito larvae by ornamental fishes (Mollies and Swordtails) in domestic well water. Uttar Pradesh Journal of Zoology. 2010;30((1)):47–52. [Google Scholar]
- Pecori G, Escalar G. Report on the anti-malarial campaign in 1929 in the government district of Rome. Rivista di Malariologia. 1930;9((5)):479–549. [Relazione sulla campagna antimalarica dell'anno 1929] [Google Scholar]
- Prasad H, Prasad RN, Haq S. Control of mosquito breeding through Gambusia affinis in rice fields. Indian Journal of Malariology. 1993;30((2)):57–65. [PubMed] [Google Scholar]
- Pyke GH. Plague minnow or mosquito fish? A review of the biology and impacts of introduced Gambusia species. Annual Review of Ecology, Evolution and Systematics. 2008;39:171–91. [Google Scholar]
- Raina BL. Larvicidal fish of Kangra Valley-Schizothorax Progastus. Indian Medical Gazette. 1945;80((5)):273–4. [PMC free article] [PubMed] [Google Scholar]
- Rajnikant, Bhatt RM, Gupta DK, Sharma RC, Srivastava HC, Gautam AS. Observations on mosquito breeding in wells and its control. Indian Journal of Malariology. 1993;30((4)):215–20. [PubMed] [Google Scholar]
- Rao RB, Ramoo H. Observations on the relative utility of Gambusia affinis and Panchax parvus in the control of mosquito breeding in tanks and wells. Journal of the Malaria Institute of India. 1942;4:633–4. [Google Scholar]
- Rimbaut G, Mathis M. Use of fish for biological control against Anopheles in Dakar. Bulletin de la Societe de Pathologie Exotique et de ses Filiales. 1935;28:575–81. [Utilisation des poissons millions pour la lutte biologique contre les larves d'Anopheles a Dakar] [Google Scholar]
- Robert V, Awono-Ambene HP, Thiolouse J. Ecology of larval mosquitoes, with special reference to Anopheles arabiensis (Diptera: Culicidae) in market-garden wells in urban Dakar, Senegal. Journal of Medical Entomology. 1998;35((6)):948–55. doi: 10.1093/jmedent/35.6.948. [DOI] [PubMed] [Google Scholar]
- Rojas E, Gamboa M, Villalobos S, Cruzado F. Efficacy of control of larvae of the vectors of malaria with native larvivorous fish in San Martin, Peru. Revista Peruana de Medicina Experimental y Salud Publica. 2004;21((1)):44–50. [Eficacia del control de larvas de vectores de la malaria con peces larvivoros nativos en San Martin, Peru] [Google Scholar]
- Roule S. Role des Poissons Larvivores dans la Prophylaxie du Paludisme. Paris: E. Le Francois; 1934. [Google Scholar]
- Roy DN. On the control of malaria-mosquitoes in Bengal by the use of predacious fish and on the habits of two of them. Journal of the Malaria Institute of India. 1938;1((4)):405–16. [Google Scholar]
- Rupp HR. Adverse assessments of Gambusia affinis: an alternate view for mosquito control practitioners. Journal of the American Mosquito Control Association. 1996;12((2 Pt 1)):155–9. [PubMed] [Google Scholar]
- Russell PF, Knipe FW, Rao HR. On agricultural malaria and its control with special reference to South India. Indian Medical Gazette. 1942;77:744–54. [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli G, Petarca V, Petrangeli G. Preliminary data on the An. gambiae complex in the RFI of the Comores. Parassitologia. 1988;30((Suppl)):178–9. [Donnees preliminaires sur le complexe An. gambiae dans la RFI des Comores] [Google Scholar]
- Sella M. Larvivorous fish and the experimental antimalarial campaign with Gambusia at Rovigno, Istria. Rivista di Malariologia. 1927;6((6)):881–909. [I pesci larvifagi a l'esperimento di campagna antimalarica con le Gambusie a Rovigno d'Istria] [Google Scholar]
- Sella M. Gambusia and paris green in anti-malarial work at Rovigno (report for 1928) and notes on the campaign in Istria. Rivista di Malariologia. 1929;8((4)):357–92. [Gambusia e verde di Parigi nella lotta antimalarica a Rovigno (relazione per il 1928) e cenni sulla lotta in Instria] [Google Scholar]
- Sergiev PG, Kovtun AS. Organization of the campaign against malaria in the USSR towards the twentieth anniversary of the October revolution. Meditsinskaia Parazitologiia i Parazitarnye Bolezni. 1937;6((6)):723–55. [Organisation de la lutte contre le paludisme en l'URSS vers le vingtieme anniversaire de la revolution d'octobre] [Google Scholar]
- Sharma SK, Rajagopal R. Efficacy of fish larvivore, Gambusia affinis, for the control of mosquito breeding in wells in semi-urban areas near Delhi. Journal of Communicable Diseases. 1986;18((2)):95–102. [PubMed] [Google Scholar]
- Sharma VP, Sharma RC, Gautam AS. Bio-environmental control of malaria in Nadiad, Kheda district, Gujarat. Indian Journal of Malariology. 1986;23((2)):95–117. [PubMed] [Google Scholar]
- Sharma VP, Sharma RC. Community-based integrated vector control of malaria in India. Progress in Vaccinology. 1989;2:393–9. [Google Scholar]
- Sharma VP, Sharma RC. Community based bio-environmental control of malaria in Kheda district, Gujarat. Journal of the American Mosquito Control Association. 1989;5((4)):514–21. [PubMed] [Google Scholar]
- Sharma VP, Gautam AS, Bhatt RM, Gupta DK, Sharma VP. The Kheda malaria project: the case for environmental control. Health Policy and Planning. 1991;6((3)):262–70. [Google Scholar]
- Sharma SN, Kaul SM, Lal S. Use of Gambusia affinis in different habitats as a mosquito control agent. Journal of Communicable Diseases. 1997;29((4)):371–3. [PubMed] [Google Scholar]
- Singh N, Sharma VP, Mishra AK, Singh OP. Bio-environmental control of malaria in a tribal area of Mandla District, Madhya Pradesh, India. Indian Journal of Malariology. 1989;26((2)):103–20. [PubMed] [Google Scholar]
- Singh N, Shukla MM, Mishra AK, Singh MP, Paliwal JC, Dash AP. Malaria control using indoor residual spraying and larvivorous fish: a case study in Betul, central India. Tropical Medicine and International Health. 2006;11((10)):1512–20. doi: 10.1111/j.1365-3156.2006.01703.x. [DOI] [PubMed] [Google Scholar]
- Sitaraman NL, Karim MA, Reddy GV. Observations in the use of Gambusia affinis Holbrooki to control A. stephensi in wells. Results of two years study in Greater Hyderabad City-India. Indian Journal of Medical Research. 1975;63((10)):1509–16. [PubMed] [Google Scholar]
- Tabibzadeh I, Behbehani G, Nahkai R. Use of Gambusia fish in the malaria eradication programme of Iran. Bulletin of the World Health Organization. 1970;43((4)):623–6. [PMC free article] [PubMed] [Google Scholar]
- Teklehaimanot A, Kassahun A, Fletcher M. Using fish against malaria: a local initiative. World Health Forum. 1993;14((2)):176–7. [PubMed] [Google Scholar]
- Tisohlbr W. The malaria situation and mosquito control in Montenegro. Anzeiger fur Schadlingskunde. 1950;23((5)):65–9. [Google Scholar]
- Trausmiller O. On the limits of malaria control by means of Gambusia holbrooki. Archivs fur Schiffs und Tropenhygiene. 1932;36((10)):530–9. [Uber die Grenzen der Malaria- Bekampfung mittels Gambusien] [Google Scholar]
- Ungureanu E, Pull JH, Pal R. Detailed Study Design for Field Studies Regarding the Evaluation of the Efficacy of Larvivorous Fish for the Control of Malaria [WHO/MAL/81.974] Geneva: World Health Organization; 1981. [Google Scholar]
- Usenbaev NT, Ezhov MN, Zvantsov AB, Annarbaev A, Zhoroev AA, Almerekov KSh. An outbreak of Plasmodium vivax malaria in Kyrgyzstan. Meditsinskaia Parazitologiia i Parazitarnye Bolezni. 2006;1:17–20. [PubMed] [Google Scholar]
- Van Dam AR, Walton WE. Comparison of mosquito control provided by the arroyo chub (Gila orcutti) and the mosquitofish (Gambusia affinis) Journal of the American Mosquito Control Association. 2007;23((4)):430–41. doi: 10.2987/5620.1. [DOI] [PubMed] [Google Scholar]
- Velichkevich AI. New data on the biology of Anopheles and on the epidemiology of malaria in the southern coast of the Crimea. Meditsinskaia Parazitologiia i Parazitarnye Bolezni. 1935;4((6)):481–5. [Google Scholar]
- Victor TJ, Chandrasekaran B, Reuben R. Composite fish culture for mosquito control in rice fields in southern India. Southeast Asian Journal of Tropical Medicine and Public Health. 1994;25((3)):522–7. [PubMed] [Google Scholar]
- Vitlin LM, Artem'ev MM, Bol'shakov Trials of Xenotoca eiseni (Rutter), 1896 (Cyprinodontiformes, Goodeidae) as a means of controlling mosquito larvae in the field. Meditsinskaia Parazitologiia i Parazitarnye Bolezni. 1987;3:26–9. [PubMed] [Google Scholar]
- Vitlin DM, Artem'ev MM. Trial of spawning tropical fish to control mosquito larvae in south USSR in field conditions. Meditsinskaia Parazitologiia i Parazitarnye Bolezni. 1987;4:64–8. [PubMed] [Google Scholar]
- Walton WE. Larvivorous fish including Gambusia. Journal of the American Mosquito Control Association. 2007;23(((2 Suppl))):184–220. doi: 10.2987/8756-971X(2007)23[184:LFIG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe MB, Costa HH. Mosquito control with larvivorous fish. Parasitology Today. 1986;2((8)):228–30. doi: 10.1016/0169-4758(86)90089-x. [DOI] [PubMed] [Google Scholar]
- Wu N, Liao GH, Li DF, Luo YL, Zhong GM. The advantages of mosquito biocontrol by stocking edible fish in rice paddies. Southeast Asian Journal of Tropical Medicine and Public Health. 1991;22((3)):436–42. [PubMed] [Google Scholar]
- Yadav RS, Sampath RR, Sharma VP. Pyrethroid-impregnated bednets and bioenvironmental approach for control of malaria in Orissa with special reference to community participation and intersectoral cooperation. In: Sharma VP, editor. Community Participation in Malaria Control. Malaria Research Centre, Indian Council of Medical Research; 1993. pp. 259–82. [Google Scholar]
- Yu HS. Annual progress report (1981): Biological control of mosquitoes in South Korea. pp. 1–47. Seoul, Korea: Division of Medical Entomology, National Institute of Health, Republic of Korea; 1982 13 June.
- Yu HS, Lee DK, Lee WJ. Mosquito control by the release of fish predator, Aphyocypris chinensis in natural mosquito breeding habitats of fice paddies and stream seepage in South Korea. Korean Journal of Entomology. 1982;12:61–7. [Google Scholar]
- Yu HS, Lee DK, Lee WJ. Field evaluation of fish predator Aphyocypris chinensis and Bacillus thuringiensis israelensis in mosquito breeding habitats of rice paddy, marsh and sewage effluent. Korean Journal of Entomology. 1982;12((1)):122. [Google Scholar]
- Yu HS, Ban SJ, Kim HC. Integrated control of encephalitis vector (Culex tritaeniorhynchus) by using Bacillus thuringiensis israelensis and larvivore Aphyocypris chinensis in parsley field, South Korea. Korean Journal of Entomology. 1986;16((1)):49–56. [Google Scholar]
- Zaman MS. Malaria control through fish. Pakistan Journal of Science. 1980;32((3/4)):163–8. [Google Scholar]
Additional references
- Bence JR, Murdoch WW. Prey site selection by the mosquitofish: relation to optimal diet theory. Ecology. 1986;67:324–6. [Google Scholar]
- Bond JG, Arredondo-Jimenez JI, Rodriguez MH, Quiroz-Martinez H, Williams T. Oviposition habitat selection for a predator refuge and food source in a mosquito. Ecological Entomology. 2005;30:255–63. [Google Scholar]
- Garner P, Gülmezoglu AM. Drugs for preventing malaria in pregnant women. Cochrane Database of Systematic Reviews. 2006;(4. Art. No.: CD000169) doi: 10.1002/14651858.CD000169.pub2. [DOI: 10.1002/14651858.CD000169.pub2] [DOI] [PubMed] [Google Scholar]
- Gerberich JB, Laird M. Food and Agricultural Organization of the United Nations. Vol. 75. Rome: United Nations; 1968. pp. 1–70. [Google Scholar]
- Goutam C, Anupam G, Indranil B, Ghosk SK. Use of larvivorous fish in biological and environmental control of disease vectors. In: Cameron MM, Lorenz LM, editors. Biological and Environmental Control of Disease Vectors. Wallingford, UK: CABI; 2013. [Google Scholar]
- Health and Environment Linkages Initiative. Malaria control: the power of integrated action. http://www.who.int/heli/risks/vectors/malariacontrol/en/index2.html 2005 (accessed September 2013)
- Lengeler C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database of Systematic Reviews. 2004;(2) doi: 10.1002/14651858.CD000363.pub2. [DOI: 10.1002/14651858.CD000363.pub2] [DOI] [PubMed] [Google Scholar]
- Lyimo EO, Takken W. Effects of adult body size on fecundity and the pre-gravid rate of Anopheles gambiae females in Tanzania. Medical and Veterinary Entomology. 1993;7((4)):328–32. doi: 10.1111/j.1365-2915.1993.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Meremikwu MM, Donegan S, Sinclair D, Esu E, Oringanje C. Intermittent preventive treatment for malaria in children living in areas with seasonal transmission. Cochrane Database of Systematic Reviews. 2012;(2) doi: 10.1002/14651858.CD003756.pub4. [DOI: 10.1002/14651858.CD003756.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke GH. A review of the biology of Gambusia affinis and G. holbrooki. Reviews in Fish Biology and Fisheries. 2005;15((4)):339–65. [Google Scholar]
- Raghavendra K, Sharma P, Dash AP. Biological control of mosquito populations through frogs: opportunities and constraints. Indian Journal of Medical Research. 2008;128((1)):22–5. [PubMed] [Google Scholar]
- Silver J. Mosquito Ecology—Field Sampling Methods. 3rd Edition. Dordrecht: Springer; 2008. [Google Scholar]
- Sinclair D, Zani B, Donegan S, Olliaro P, Garner P. Artemisinin-based combination therapy for treating uncomplicated malaria. Cochrane Database of Systematic Reviews. 2009;(3) doi: 10.1002/14651858.CD007483.pub2. [DOI: 10.1002/14651858.CD007483.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D, Gogtay N, Brand F, Olliaro P. Artemisinin-based combination therapy for treating uncomplicated Plasmodium vivax malaria. Cochrane Database of Systematic Reviews. 2011;(7) doi: 10.1002/14651858.CD008492.pub2. [DOI: 10.1002/14651858.CD008492.pub2] [DOI] [PubMed] [Google Scholar]
- Sinclair D, Donegan S, Isba R, Lalloo DG. Artesunate versus quinine for treating severe malaria. Cochrane Database of Systematic Reviews. 2012;(6) doi: 10.1002/14651858.CD005967.pub4. [DOI: 10.1002/14651858.CD005967.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W, Klowden MJ, Chambers GM. Effect of body size on host seeking and blood meal utilization in Anopheles gambiae sensu stricto (Diptera: Culicidae): the disadvantage of being small. Journal of Medical Entomology. 1998;35((5)):639–45. doi: 10.1093/jmedent/35.5.639. [DOI] [PubMed] [Google Scholar]
- Tanser FC, Pluess B, Lengeler C, Sharp BL. Indoor residual spraying for preventing malaria. Cochrane Database of Systematic Reviews. 2007;(3) doi: 10.1002/14651858.CD006657.pub2. [DOI: 10.1002/14651858.CD006657] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tusting LS, Thwing J, Sinclair D, Fillinger U, Gimnig J, Bonner KE, et al. Mosquito larval source management for controlling malaria. Cochrane Database of Systematic Reviews. 2013;(8) doi: 10.1002/14651858.CD008923.pub2. [DOI: 10.1002/14651858.CD008923.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Development Programme. Malaria elimination in Tajikistan for 2009-2014. http://www.undp.org/content/tajikistan/en/home/operations/projects/hiv_aids/malaria/ (accessed September 2013)
- Walker K, Lynch M. Contributions of Anopheles larval control to malaria suppression in tropical Africa: review of achievements and potential. Medical and Veterinary Entomology. 2007;21((1)):2–21. doi: 10.1111/j.1365-2915.2007.00674.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO Technical Report Series. Vol. 537. Geneva: World Health Organization; 1974. Malaria control in countries where time-limited eradication is impractical at present: report of a WHO interregional conference; p. 72. [PubMed] [Google Scholar]
- World Health Organization, Regional Office for Europe. Malaria vectors and approaches to their control in malaria affected countries of the WHO European Region: the proceedings of a regional meeting on vector biology and control, Almaty, Kazakhstan, 3-5 May 2001 [EUR/01/5027499] Copenhagen: World Health Organization, Regional Office for Europe; 2001. [Google Scholar]
- World Health Organization. Regional Office for Europe. Regional strategy: from malaria control to elimination 2006-2015 in the WHO European Region [EUR/05/5061322] Copenhagen: WHO Regional Office for Europe; 2006. [Google Scholar]
- World Health Organization. Malaria Vector Control and Personal Protection. Geneva: World Health Organization; 2006. [PubMed] [Google Scholar]
- World Health Organization. Malaria Elimination: A Field Manual for Low and Moderate Endemic Countries. Geneva: World Health Organization; 2007. [Google Scholar]
- World Health Organization. World Malaria Report 2012. Geneva: World Health Organization; 2012. [Google Scholar]
- World Health Organization Global Malaria Programme. Interim Position Statement: the role of larviciding for malaria control in sub-Saharan Africa. http://www.who.int/malaria/publications/atoz/interim_position_statement_larviciding_sub_saharan_africa.pdf (accessed April 2012)
- Wurtsbaugh WA, Cech JJ, Compton J. Effect of fish size on prey selection in Gambusia affinis. 1980;48:48–51. Proceedings and papers of the forty-eighth annual Conference of the California Mosquito and Vector Control Association, January 20-23. [Google Scholar]


